The Association Between Per- and Polyfluoroalkyl Substances Exposure and Thyroid Hormones in Men and Non-Pregnant Women: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Keywords Search
- PFAS exposure: “fluorocarbons” or “perfluorinated” or “polyfluorinated” or “polyfluoroalkyl” or “perfluoroalkyl” or “perfluorochemicals” or “PFAs” or “per-and polyfluoroalkyl substances” or “Perfluorohexane sulfonic acid” or “PFHxS” or “perfluorobutane sulfonic acid” or “Perfluorononanoic acid” or “PFNA” or “perfluorooctanoic acid” or “PFOA” or “perfluorooctane sulfonate acid” or “PFOs” or “Perfluorodecaoic acid” or “PFDA”.
- Five thyroid hormones: “thyroid hormone levels” or “T3” or “T4” or “TSH” or “thyroid dysfunction” or “hypothyroidism” or “hyperthyroidism” or “FT3” or “FT4”.
- The keywords should contain “1” and “2”.
2.2. Inclusion and Exclusion Criteria
- The study was conducted with men and non-pregnant women;
- The study design was a cohort or cross-sectional study;
- The study included an association between PFAS exposure (at least one pollutant) and thyroid health effects (at least one metric);
- The study included basic statistical data such as the estimated value beta and the 95% CI;
- English-language articles were used.
- Duplicate studies;
- Off-topic studies;
- Studies reporting only PFAS exposure or thyroid hormone levels with no association between the two;
- Literature reviews, conference reports, case reports, letters, in vivo studies, in vitro studies;
- Studies with incomplete or unaccountable data.
2.3. Data Extraction and Quality Assessment
2.4. Statistical Methods
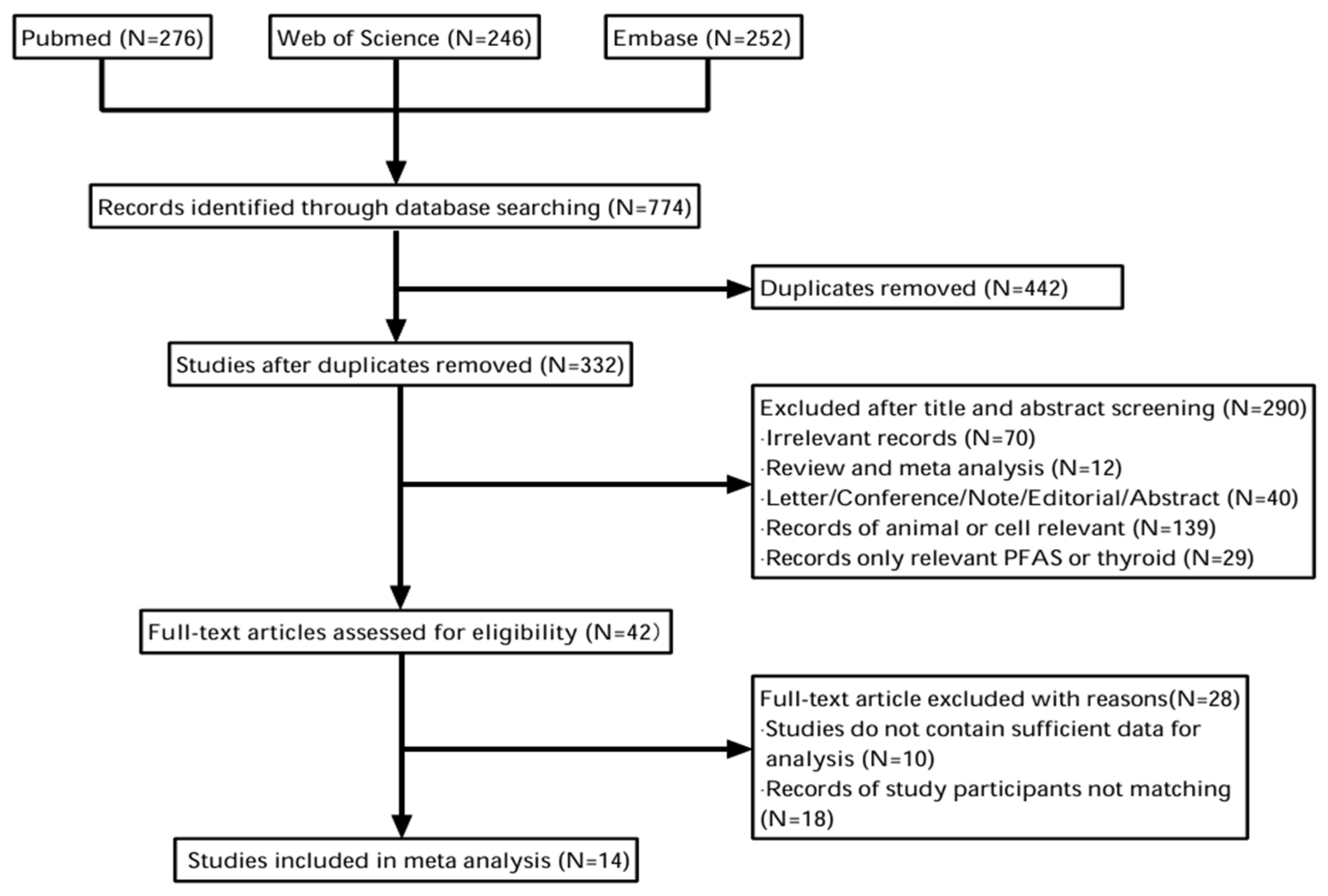
3. Results
3.1. Selected Study Results
3.2. Characteristics of the Study Included
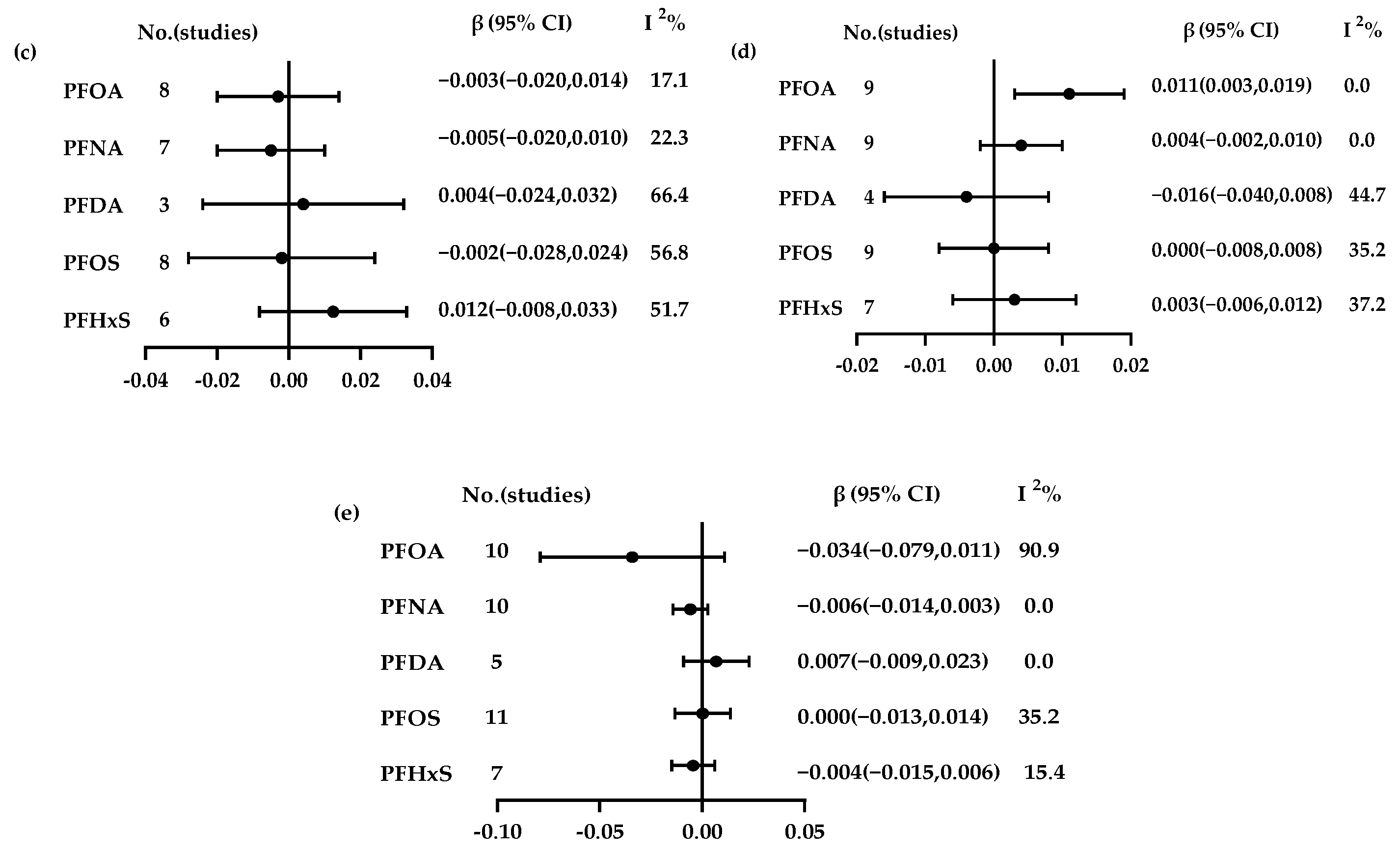
3.3. Association Between PFAs and Thyroid Hormones
3.4. Subgroup Analysis
3.4.1. PFOA
3.4.2. PFNA
3.4.3. PFDA
3.4.4. PFOS
3.4.5. PFHxS
3.5. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chaves, C.; Bruinstroop, E.; Refetoff, S.; Yen, P.; Anselmo, J. Increased Hepatic Fat Content in Patients with Resistance to Thyroid Hormone Beta. Thyroid 2021, 31, 1127–1134. [Google Scholar] [CrossRef]
- Koibuchi, N.; Jingu, H.; Iwasaki, T.; Chin, W.W. Current perspectives on the role of thyroid hormone in growth and development of cerebellum. Cerebellum 2003, 2, 279–289. [Google Scholar] [CrossRef]
- Koibuchi, N. The Role of Thyroid Hormone on Cerebellar Development. Cerebellum 2008, 7, 530–533. [Google Scholar] [CrossRef]
- Bloise, F.F.; Cordeiro, A.; Ortiga-Carvalho, T.M. Role of thyroid hormone in skeletal muscle physiology. J. Endocrinol. 2018, 236, R57–R68. [Google Scholar] [CrossRef]
- Sun, Y.; Teng, D.; Zhao, L.; Shi, X.G.; Li, Y.Z.; Shan, Z.Y.; Teng, W.P.; Thyroid Disorders, Iodine Status and Diabetes Epidemiological Survey Group (TIDE). Impaired Sensitivity to Thyroid Hormones Is Associated with Hyperuricemia, Obesity, and Cardiovascular Disease Risk in Subjects with Subclinical Hypothyroidism. Thyroid 2022, 32, 376–384. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.; Choi, S.; Lee, I.; Moon, M.K.; Choi, K.; Park, Y.J.; Cho, Y.H.; Kwon, Y.M.; Yoo, J.; et al. Association of exposure to polycyclic aromatic hydrocarbons and heavy metals with thyroid hormones in general adult population and potential mechanisms. Sci. Total Environ. 2021, 762, 9. [Google Scholar] [CrossRef]
- Liu, S.S.; Zhao, G.D.; Li, J.; Zhao, H.X.; Wang, Y.F.; Chen, J.W.; Zhao, H.D. Association of polybrominated diphenylethers (PBDEs) and hydroxylated metabolites (OH-PBDEs) serum levels with thyroid function in thyroid cancer patients. Environ. Res. 2017, 159, 1–8. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Z.C.; Yang, R.; Chen, W.N.; Zhang, J.; Li, R.X.; Lv, W.M.; Lin, B.; Luo, J.J. The interference between effects of PFAS exposure on thyroid hormone disorders and cholesterol levels: An NHANES analysis. Environ. Sci. Pollut. Res. 2023, 30, 90949–90959. [Google Scholar] [CrossRef]
- Takaguchi, K.; Nishikawa, H.; Mizukawa, H.; Tanoue, R.; Yokoyama, N.; Ichii, O.; Takiguchi, M.; Nakayama, S.M.M.; Ikenaka, Y.; Kunisue, T.; et al. Effects of PCB exposure on serum thyroid hormone levels in dogs and cats. Sci. Total Environ. 2019, 688, 1172–1183. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.E.; Ren, Q.; He, B.; Zhang, Y.; Jiang, Z.W. Fabrication of Corrosion-Resistant Superhydrophobic Coatings and Impermeable Porous Structures Using Fluorinated Microemulsions Containing Thermally Decomposable Surfactants. Coatings 2024, 14, 1176. [Google Scholar] [CrossRef]
- Galezowska, G.; Kolecka, K.; Cieszynska-Semenowicz, M.; Redko, V.; Gajewska, M. Screening of perfluoroalkyl substances and their environmental impact in sequencing batch reactors combined with nature-based solutions. Ecol. Eng. 2024, 209, 11. [Google Scholar] [CrossRef]
- Chen, D.; Hu, X.H.; Chen, C.H.; Gao, Y.M.; Zhou, Q.H.; Feng, X.; Xu, X.H.; Lin, D.H.; Xu, J. Impacts of Perfluoroalkyl Substances on Aqueous and Nonaqueous Phase Liquid Dechlorination by Sulfidized Nanoscale Zerovalent Iron. Environ. Sci. Technol. 2024, 58, 11193–11202. [Google Scholar] [CrossRef]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Djordjevic, A.B.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef]
- Sodani, K.; Ter Braak, B.; Hartvelt, S.; Boelens, M.; Jamalpoor, A.; Mukhi, S. Toxicological mode-of-action and developmental toxicity of different carbon chain length PFAS. Toxicol. Lett. 2025, 405, 59–66. [Google Scholar] [CrossRef]
- Aimuzi, R.; Luo, K.; Huang, R.; Huo, X.N.; Nian, M.; Ouyang, F.X.; Du, Y.T.; Feng, L.P.; Wang, W.Y.; Zhang, J.; et al. Perfluoroalkyl and polyfluroalkyl substances and maternal thyroid hormones in early pregnancy. Environ. Pollut. 2020, 264, 8. [Google Scholar] [CrossRef]
- Ryu, S.; Burchett, W.; Zhang, S.M.; Modaresi, S.M.S.; Areiza, J.A.; Kaye, E.; Fischer, F.C.; Slitt, A.L. Species-Specific Unbound Fraction Differences in Highly Bound PFAS: A Comparative Study across Human, Rat, and Mouse Plasma and Albumin. Toxics 2024, 12, 253. [Google Scholar] [CrossRef]
- Mussabek, D.; Ahrens, L.; Persson, K.M.; Berndtsson, R. Temporal trends and sediment-water partitioning of per- and polyfluoroalkyl substances (PFAS) in lake sediment. Chemosphere 2019, 227, 624–629. [Google Scholar] [CrossRef]
- Witt, C.C.; Gadek, C.R.; Cartron, J.L.E.; Andersen, M.J.; Campbell, M.L.; Castro-Farías, M.; Gyllenhaal, E.F.; Johnson, A.B.; Malaney, J.L.; Montoya, K.N.; et al. Extraordinary levels of per- and polyfluoroalkyl substances (PFAS) in vertebrate animals at a New Mexico desert oasis: Multiple pathways for wildlife and human exposure. Environ. Res. 2024, 249, 14. [Google Scholar] [CrossRef]
- Cioni, L.; Nikiforov, V.; Coelho, A.; Sandanger, T.M.; Herzke, D. Total oxidizable precursors assay for PFAS in human serum. Environ. Int. 2022, 170, 9. [Google Scholar] [CrossRef]
- Cappelli, F.; Bamai, Y.A.; Van Hoey, K.; Kim, D.; Covaci, A. Occurrence of short- and ultra-short chain PFAS in drinking water from Flanders (Belgium) and implications for human exposure. Environ. Res. 2024, 260, 11. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guan, R.N.; Zhu, N.L.; Hao, J.H.; Peng, H.Y.; He, A.E.; Zhao, C.Y.; Wang, Y.W.; Jiang, G.B. A critical review on the bioaccumulation, transportation, and elimination of per- and polyfluoroalkyl substances in human beings. Crit. Rev. Environ. Sci. Technol. 2024, 54, 95–116. [Google Scholar] [CrossRef]
- He, Q.R.; Yang, Q.K.; Wu, L.; He, Y.H.; Zeng, N.; Wang, Z.L. Neurotoxic effects of per- and polyfluoroalkyl substances (PFAS) mixture exposure in mice: Accumulations in brain and associated changes of behaviors, metabolome, and transcriptome. J. Hazard. Mater. 2025, 489, 12. [Google Scholar] [CrossRef]
- Ojo, A.F.; Peng, C.; Ng, J.C. Assessing the human health risks of per- and polyfluoroalkyl substances: A need for greater focus on their interactions as mixtures. J. Hazard. Mater. 2021, 407, 14. [Google Scholar] [CrossRef]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology 2022, 465, 20. [Google Scholar] [CrossRef]
- Freire, C.; Vela-Soria, F.; Castiello, F.; Salamanca-Fernández, E.; Quesada-Jiménez, R.; López-Alados, M.C.; Fernandez, M.F.; Olea, N. Exposure to perfluoroalkyl substances (PFAS) and association with thyroid hormones in adolescent males. Int. J. Hyg. Environ. Health 2023, 252, 9. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.Y.; Gao, A. Contact to perfluoroalkyl substances and thyroid health effects: A meta-analysis directing on pregnancy. Chemosphere 2023, 315, 12. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Lei, X.N.; Zhang, Y.; Shi, R.; Zhang, Q.L.; Gao, Y.; Yuan, T.; Li, J.; Tian, Y. Prenatal exposure to per- and polyfluoroalkyl substances and childhood adiposity at 7 years of age. Chemosphere 2022, 307, 8. [Google Scholar] [CrossRef]
- Matilla-Santander, N.; Valvi, D.; Lopez-Espinosa, M.J.; Manzano-Salgado, C.B.; Ballester, F.; Ibarluzea, J.; Santa-Marina, L.; Schettgen, T.; Guxens, M.; Sunyer, J.; et al. Exposure to Perfluoroalkyl Substances and Metabolic Outcomes in Pregnant Women: Evidence from the Spanish INMA Birth Cohorts. Environ. Health Perspect. 2017, 125, 11. [Google Scholar] [CrossRef]
- Chen, W.L.; Bai, F.Y.; Chang, Y.C.; Chen, P.C.; Chen, C.Y. Concentrations of perfluoroalkyl substances in foods and the dietary exposure among Taiwan general population and pregnant women. J. Food Drug Anal. 2018, 26, 994–1004. [Google Scholar] [CrossRef]
- Xing, Y.A.; Li, Z.; Wang, J.H.; Qu, Y.L.; Hu, Q.P.; Ji, S.S.; Chang, X.C.; Zhao, F.; Lv, Y.B.; Pan, Y.T.; et al. Associations between serum per- and polyfluoroalkyl substances and thyroid hormones in Chinese adults: A nationally representative cross-sectional study. Environ. Int. 2024, 184, 10. [Google Scholar] [CrossRef]
- Xie, L.N.; Wang, X.C.; Su, L.Q.; Ji, S.S.; Gu, W.; Barrett, H.; Dong, X.J.; Zhu, H.J.; Hou, S.S.; Li, Z.H.; et al. The association between per-/polyfluoroalkyl substances in serum and thyroid function parameters: A cross-sectional study on teenagers living near a Chinese fluorochemical industrial plant. Sci. Total Environ. 2024, 920, 11. [Google Scholar] [CrossRef]
- Ji, K.; Kim, S.; Kho, Y.; Paek, D.; Sakong, J.; Ha, J.; Kim, S.; Choi, K. Serum concentrations of major perfluorinated compounds among the general population in Korea: Dietary sources and potential impact on thyroid hormones. Environ. Int. 2012, 45, 78–85. [Google Scholar] [CrossRef]
- Shrestha, S.; Bloom, M.S.; Yucel, R.; Seegal, R.F.; Wu, Q.; Kannan, K.; Rej, R.; Fitzgerald, E.F. Perfluoroalkyl substances and thyroid function in older adults. Environ. Int. 2015, 75, 206–214. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.Y.; Fletcher, T.; Scott, K.; Nielsen, C.; Pineda, D.; Lindh, C.H.; Olsson, D.S.; Andersson, E.M.; Jakobsson, K. Associations between perfluoroalkyl substances and thyroid hormones after high exposure through drinking water. Environ. Res. 2021, 194, 10. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Prisma, P.G. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ-Br. Med. J. 2015, 349, 25. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Cousins, I.T.; Scheringer, M.; Hungerbuehler, K. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions. Environ. Int. 2015, 75, 172–179. [Google Scholar] [CrossRef]
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; von Holst, H.E. Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 900. [Google Scholar] [CrossRef]
- Nhu, H.D.; Tri, D.V.; Luu, T.L.; Trippel, J.; Wagner, M. Degradation of 29 per- and poly-fluoroalkyl substances (PFAS) in water using fenton-assisted electrochemical oxidation process. Sep. Purif. Technol. 2025, 362, 13. [Google Scholar] [CrossRef]
- Li, W.T.; Liu, X.Y.; Mao, H.; Wang, S.L. Concentration, distribution, and bioconcentration of short- and long-chain perfluoroalkyl substances in the water, suspended particulate matter, and surface sediment of a typical semi-enclosed bay. Sci. Total Environ. 2023, 890, 13. [Google Scholar] [CrossRef] [PubMed]
- Schneck, A. Examining publication bias-a simulation-based evaluation of statistical tests on publication bias. PeerJ 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.S.; Kannan, K.; Spliethoff, H.M.; Tao, L.; Aldous, K.M.; Vena, J.E. Exploratory assessment of perfluorinated compounds and human thyroid function. Physiol. Behav. 2010, 99, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.C.; Miller, P.; Seguinot-Medina, S.; Waghiyi, V.; Buck, C.L.; von Hippel, F.A.; Carpenter, D.O. Exposure to perfluoroalkyl substances and associations with serum thyroid hormones in a remote population of Alaska Natives. Environ. Res. 2018, 166, 537–543. [Google Scholar] [CrossRef]
- Caron-Beaudoin, É.; Ayotte, P.; Sidi, E.A.L.; McHugh, N.G.L.; Lemire, M.; Community of Lac Simon; Community of Winneway –Long Point First Nation; CSSS Tshukuminu Kanani of Nutashkuan; Community of Unamen Shipu. Exposure to perfluoroalkyl substances (PFAS) and associations with thyroid parameters in First Nation children and youth from Quebec. Environ. Int. 2019, 128, 13–23. [Google Scholar] [CrossRef]
- Gallo, E.; Amidei, C.B.; Barbieri, G.; Fabricio, A.S.C.; Gion, M.; Pitter, G.; Daprà, F.; Russo, F.; Gregori, D.; Fletcher, T.; et al. Perfluoroalkyl substances and thyroid stimulating hormone levels in a highly exposed population in the Veneto Region. Environ. Res. 2022, 203, 9. [Google Scholar] [CrossRef]
- Rodríguez-Carrillo, A.; Salamanca-Fernández, E.; den Hond, E.; Verheyen, V.J.; Fabelová, L.; Murinova, L.P.; Pedraza-Díaz, S.; Castaño, A.; García-Lario, J.; Remy, S.; et al. Association of exposure to perfluoroalkyl substances (PFAS) and phthalates with thyroid hormones in adolescents from HBM4EU aligned studies. Environ. Res. 2023, 237, 11. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, Q.Q.; Wang, Y.J.; Wang, C.F.; Hu, C.F.; Wang, L.; Liu, H.L.; Tian, Z.Q. Associations between per- and polyfluoroalkyl substances exposure and thyroid hormone levels in the elderly. Sci. Total Environ. 2024, 920, 9. [Google Scholar] [CrossRef]
- Tillaut, H.; Monfort, C.; Giton, F.; Warembourg, C.; Rouget, F.; Cordier, S.; Laine, F.; Gaudreau, E.; Garlantezec, R.; Saint-Amour, D.; et al. Persistent organic pollutant exposure and thyroid function among 12-year-old children. Neuroendocrinology 2023, 113, 1232–1247. [Google Scholar] [CrossRef]
- Webster, G.M.; Rauch, S.A.; Ste Marie, N.; Mattman, A.; Lanphear, B.P.; Venners, S.A. Cross-Sectional Associations of Serum Perfluoroalkyl Acids and Thyroid Hormones in US Adults: Variation According to TPOAb and Iodine Status (NHANES 2007-2008). Environ. Health Perspect. 2016, 124, 935–942. [Google Scholar] [CrossRef]
- Wen, L.L.; Lin, L.Y.; Su, T.C.; Chen, P.C.; Lin, C.Y. Association Between Serum Perfluorinated Chemicals and Thyroid Function in US Adults: The National Health and Nutrition Examination Survey 2007-2010. J. Clin. Endocrinol. Metab. 2013, 98, E1456–E1464. [Google Scholar] [CrossRef] [PubMed]
- Blake, B.E.; Pinney, S.M.; Hines, E.P.; Fenton, S.E.; Ferguson, K.K. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ. Pollut. 2018, 242, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Ahn, J.; Oh, H.S.; Jeon, M.J.; Kim, W.G.; Kim, W.B.; Shong, Y.K.; Kim, T.Y. Sex-Dependent Association between Weight Change and Thyroid Dysfunction: Population-Level Analysis Using the Korean National Health and Nutrition Examination Survey. Eur. Thyroid J. 2019, 8, 202–207. [Google Scholar] [CrossRef]
- Qiu, Y.K.; Liu, Q.Y.; Luo, Y.H.; Chen, J.D.; Zheng, Q.Z.; Xie, Y.P.; Cao, Y.P. Causal association between obesity and hypothyroidism: A two-sample bidirectional Mendelian randomization study. Front. Endocrinol. 2024, 14, 13. [Google Scholar] [CrossRef]
- Canova, C.; Di Nisio, A.; Barbieri, G.; Russo, F.; Fletcher, T.; Batzella, E.; Dalla Zuanna, T.; Pitter, G. PFAS Concentrations and Cardiometabolic Traits in Highly Exposed Children and Adolescents. Int. J. Environ. Res. Public Health 2021, 18, 12881. [Google Scholar] [CrossRef]
- Watkins, A.M.; Wood, C.R.; Lin, M.T.; Abbott, B.D. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol. Cell. Endocrinol. 2015, 400, 90–101. [Google Scholar] [CrossRef]
- Kinkade, C.W.; Rivera-Nunez, Z.; Thurston, S.W.; Kannan, K.; Miller, R.K.; Brunner, J.; Wong, E.Y.; Groth, S.; O’Connor, T.G.; Barrett, E.S. Per- and polyfluoroalkyl substances, gestational weight gain, postpartum weight retention and body composition in the UPSIDE cohort. Environ. Health 2023, 22, 16. [Google Scholar] [CrossRef]
- Leko, M.B.; Gunjaca, I.; Pleic, N.; Zemunik, T. Environmental Factors Affecting Thyroid-Stimulating Hormone and Thyroid Hormone Levels. Int. J. Mol. Sci. 2021, 22, 6521. [Google Scholar] [CrossRef]
- Vorst, K.L.; Saab, N.; Silva, P.; Curtzwiler, G.; Steketee, A. Risk assessment of per- and polyfluoroalkyl substances (PFAS) in food: Symposium proceedings. Trends Food Sci. Technol. 2021, 116, 1203–1211. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J. 2018, 16, 284. [Google Scholar] [CrossRef]
- Xie, Y.; Berntsen, H.F.; Zimmer, K.E.; Ropstad, E.; Verhaegen, S.; Connolly, L. Luteolin protects against adipogenic and lipogenic potency induced by human relevant mixtures of persistent organic pollutants (POPs) in the 3T3-L1 model. Food Chem. Toxicol. 2023, 173, 10. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P. The Impact of Toxins on the Developing Brain. Annu. Rev. Public Health 2015, 36, 211–230. [Google Scholar]
- Guo, P.F.; Warren, J.L.; Deziel, N.C.; Liew, Z. Exposure range matters: Considering nonlinear associations in the meta-analysis of environmental pollutant exposure using examples of per- and polyfluoroalkyl substances and birth outcomes. Am. J. Epidemiol. 2025, 9, kwae309. [Google Scholar] [CrossRef]
- Mancini, F.R.; Rajaobelina, K.; Praud, D.; Dow, C.; Antignac, J.P.; Kvaskoff, M.; Severi, G.; Bonnet, F.; Boutron-Ruault, M.C.; Fagherazzi, G. Nonlinear associations between dietary exposures to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and type 2 diabetes risk in women: Findings from the E3N cohort study. Int. J. Hyg. Environ. Health. 2018, 221, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Dunder, L.; Salihovic, S.; Elmståhl, S.; Lind, P.M.; Lind, L. Associations between per- and polyfluoroalkyl substances (PFAS) and diabetes in two population-based cohort studies from Sweden. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 748–756. [Google Scholar] [CrossRef]
- Dharpure, R.; Pramanik, S.; Pradhan, A. In silico analysis decodes transthyretin (TTR) binding and thyroid disrupting effects of per- and polyfluoroalkyl substances (PFAS). Arch. Toxicol. 2023, 97, 755–768. [Google Scholar] [CrossRef]
- Shen, Y.; Bovee, T.F.H.; Molenaar, D.; Weide, Y.; Nolles, A.; Mitrovic, C.B.; van Leeuwen, S.P.J.; Louisse, J.; Hamers, T. Optimized methods for measuring competitive binding of chemical substances to thyroid hormone distributor proteins transthyretin and thyroxine binding globulin. Arch. Toxicol. 2024, 98, 3797–3809. [Google Scholar] [CrossRef]
- Degitz, S.J.; Olker, J.H.; Denny, J.S.; Degoey, P.P.; Hartig, P.C.; Cardon, M.C.; Eytcheson, S.A.; Haselman, J.T.; Mayasich, S.A.; Hornung, M.W. In vitro screening of per- and polyfluorinated substances (PFAS) for interference with seven thyroid hormone system targets across nine assays. Toxicol. Vitro 2024, 95, 12. [Google Scholar] [CrossRef]
- Riesco-Eizaguirre, G.; Santisteban, P.; De la Vieja, A. The complex regulation of NIS expression and activity in thyroid and extrathyroidal tissues. Endocr.-Relat. Cancer 2021, 28, T141–T165. [Google Scholar] [CrossRef]
- Conti, A.; Strazzeri, C.; Rhoden, K.J. Perfluorooctane sulfonic acid, a persistent organic pollutant, inhibits iodide accumulation by thyroid follicular cells in vitro. Mol. Cell. Endocrinol. 2020, 515, 10. [Google Scholar] [CrossRef]
- Santhanam, S.D.; Ramamurthy, K.; Priya, P.S.; Sudhakaran, G.; Guru, A.; Arockiaraj, J. A combinational threat of micro- and nano-plastics (MNPs) as potential emerging vectors for per- and polyfluoroalkyl substances (PFAS) to human health. Environ. Monit. Assess. 2024, 196, 31. [Google Scholar] [CrossRef]
- Di Nisio, A.; Rocca, M.S.; De Toni, L.; Sabovic, I.; Guidolin, D.; Dall’Acqua, S.; Acquasaliente, L.; De Filippis, V.; Plebani, M.; Foresta, C. Endocrine disruption of vitamin D activity by perfluoro-octanoic acid (PFOA). Sci. Rep. 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Wielsoe, M.; Long, M.H.; Ghisari, M.; Bonefeld-Jorgensen, E.C. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2015, 129, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Region | Period | Sample Size | Age | Design |
|---|---|---|---|---|---|
| Carmen Freire (2023) | Spain | 2017–2019 | 129 | 15–17 | cross-section |
| Elisa Gallo (2022) | Veneto | 2017–2019 | 21,424 | 14–39 | cross-section |
| Yanan Xing (2024) | China | 2017–2018 | 10,853 | ≥18 | cross-section |
| Kai Tan (2024) | China | May 2023–June 2023 | 746 | >60 | cross-section |
| Andrea Rodríguez-Carrillo (2023) | Belgium, Slovakia, Spain | 2014–2020 | 733 | 12–19 | cross-section |
| Élyse Caron-Beaudoin (2019) | Quebec | May 2015–October 2015 | 186 | 3–19 | cross-section |
| Srishti Shrestha (2015) | New York | 2005 | 84 | 55–74 | cross-section |
| Samuel C. Byrne (2018) | Alaska | 2013–2014 | 85 | 18–45 | cross-section |
| Hélène Tillaut (2022) | France | 2014–2018 | 476 | 12 | cross-section |
| Michael S. Bloom (2010) | New York | 2006 | 31 | 31–45 | cross-section |
| Kyunghee Ji (2012) | Korea | July 2008–August 2008 | 633 | 12–75 | cross-section |
| Lili Wen (2013) | USA | 2007–2010 | 1181 | ≥20 | cross-section |
| Glenys M. Webster (2016) | USA | 2007–2008 | 1525 | ≥18 | cross-section |
| Linna Xie (2024) | China | 2018 | 836 | 11–15 | cross-section |
| Author (Year) | Sample Detected | Exposures | Effect Indicator | Adjustment Factors |
|---|---|---|---|---|
| Carmen Freire (2023) | serum | PFOA, PFNA, PFDA, PFOS, PFHxS | T3, FT4, TSH | Child passive smoking, alcohol intake, total fish intake, iodine intake, physician-diagnosed thyroid disease history, maternal schooling, and the use of current or recent medication |
| Elisa Gallo (2022) | serum | PFOA, PFNA, PFOS, PFHxS | TSH | BMI, time lag between the enrolment and the beginning of the study, gender, physical activity, smoking habits, food consumption, country of birth, alcohol consumption, education level, and laboratory in charge of the TSH analyses |
| Yanan Xing (2024) | serum | PFOA, PFNA, PFDA, PFOS, PFHxS | T4, T3 | Age, BMI, sex, nationality, education level, residence, marital status, annual family income, current smoking, current alcohol consumption, and multivitamin supplementation |
| Kai Tan (2024) | serum | PFOA, PFNA, PFDA, PFOS, PFHxS | TSH, T3, T4, FT3, FT4 | Sex, age, BMI, smoking, and alcohol |
| Andrea Rodríguez-Carrillo (2023) | serum | PFOA, PFNA, PFOS | TSH, FT3, FT4 | Random effect, age, sex, z-BMI, household education, and urinary creatinine concentration |
| Élyse Caron-Beaudoin (2019) | serum | PFOA, PFNA, PFOS, PFHxS | TSH, FT4 | Age, sex, studied nation, urinary iodine, urinary cotinine, parent’s education, and BMI z-score |
| Srishti Shrestha (2015) | serum | PFOA, PFOS | TSH, T3, T4, FT4 | Age, sex, years of education, and serum ∑ PCBs |
| Samuel C. Byrne (2018) | serum | PFOA, PFNA, PFOS | TSH, T3, T4 | Using total T3, fT3, total T4, fT4 or TSH as the dependent variables, adjusted for age, sex, and smoking habits |
| Hélène Tillaut (2022) | serum | PFOA, PFNA, PFDA, PFOS, PFHxS | TSH, FT3, FT4 | Namely parental history of thyroid hormonal disorders, season, and time of day for the blood draw |
| Michael S. Bloom (2010) | serum | PFOA, PFNA, PFDA, PFOS, PFHxS | TSH, FT4 | Age, gender, BMI, cigarette smoking, history of physician-diagnosed goiter or thyroid condition, race/ethnicity, the use of medication, and the consumption situation of sportfish from self-report |
| Kyunghee Ji (2012) | serum | PFOA, PFNA, PFDA, PFOS, PFHxS | TSH, T4 | Age, sex, and BMI |
| Lili Wen (2013) | serum | PFOA, PFNA, PFOS, PFHxS | TSH, T3, T4, FT3, FT4 | Age, gender, race, alcohol consumption, smoking status, and urinary iodine |
| Glenys M. Webster (2016) | serum | PFOA, PFNA, PFOS, PFHxS | TSH, T3, T4, FT3, FT4 | Rage, race, log serum cotinine, sex, parity, pregnancy, and menopause status |
| Linna Xie (2024) | serum | PFOA, PFNA, PFDA, PFOS, PFHxS | TSH, FT3, FT4 | Sex, age, BMI, and household income levels |
| Subgroup | FT3 | FT4 | |||||
|---|---|---|---|---|---|---|---|
| No | β (95% CI) | I2 | No | β (95% CI) | I2 | ||
| Sample size | <500 | 3 | −0.014 (−0.107, 0.079) | 0.0 | 4 | 0.006 (−0.028, 0.049) | 0.0 |
| >500 | 6 | 0.011 (0.001, 0.020) | 13.4 | 6 | −0.057 (−0.118, 0.005) | 94.7 | |
| Region | Asia | 2 | −0.037 (−0.124, 0.049) | 41.9 | 2 | −0.760 (−2.250, 0.730) | 98.2 |
| North America | 4 | 0.014 (0.005, 0.022) | 0.0 | 6 | −0.001 (−0.017, 0.014) | 0.0 | |
| Europe | 3 | 0.010 (−0.012, 0.032) | 0.0 | 2 | −0.045 (−0.146, 0.056) | 92.1 | |
| Adjusted for BMI | yes | 2 | −0.037 (−0.124, 0.0491) | 41.9 | 3 | −0.445 (−0.896, 0.006) | 96.4 |
| no | 7 | 0.013 (0.005, 0.021) | 0.0 | 7 | −0.017 (−0.055, 0.020) | 85.9 | |
| Sex | male | 9 | 0.001 (−0.006, 0.007) | 38.0 | 9 | −0.003 (−0.020, 0.014) | 84.3 |
| female | 9 | 0.002 (−0.003, 0.006) | 32.8 | 10 | 0.000 (−0.007, 0.007) | 64.0 | |
| Age | <19 | 5 | 0.014 (0.003, 0.026) | 0.0 | 4 | −0.152 (−0.289, −0.015) | 96.2 |
| ≥19 | 4 | −0.001 (−0.027, 0.025) | 41.5 | 7 | −0.004 (−0.017, 0.010) | 0.0 | |
| Subgroup | TSH | T3 | T4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | β (95% CI) | I2 | No | β(95% CI) | I2 | No | β (95% CI) | I2 | ||
| Age | <19 | 7 | 0.005 (−0.012, 0.021) | 0.0 | 1 | 0.020 (−0.030, 0.080) | -- | 2 | 0.278 (0.065, 0.491) | 0.0 |
| ≥19 | 5 | −0.000 (−0.061, 0.060) | 53.4 | 5 | −0.005 (−0.017, 0.008) | 0.0 | 5 | −0.005 (−0.019, 0.009) | 0.0 | |
| Subgroup | TSH | T3 | T4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | β (95% CI) | I2 | No | β(95% CI) | I2 | No | β (95% CI) | I2 | ||||
| Age | <19 | 3 | −0.018 (−0.093, 0.058) | 35.3 | 1 | 0.040 (−0.040, 0.120) | -- | 1 | −0.080 (−0.660, 0.500) | -- | ||
| ≥19 | 2 | −0.022 (−0.041, −0.003) | 0.0 | 1 | −0.001 (−0.022, 0.019) | -- | 1 | −0.007 (−0.027, 0.130) | -- | |||
| Subgroup | FT3 | FT4 | ||||||||||
| No | β(95%CI) | I2 | No | β(95%CI) | I2 | |||||||
| Sample size | <500 | 2 | −0.032 (−0.056, −0.007) | 0.0 | 3 | 0.014 (−0.012, 0.041) | 0.0 | |||||
| >500 | 2 | 0.023 (−0.068, 0.114) | 57.5 | 2 | 0.003 (−0.016, 0.023) | 0.0 | ||||||
| Region | Asia | 2 | 0.023 (−0.068, 0.114) | 57.5 | 2 | 0.003 (−0.016, 0.023) | 0.0 | |||||
| North America | 0 | -- | -- | 1 | 0.090 (−0.020, 0.210) | -- | ||||||
| Europe | 2 | −0.032 (−0.056, −0.007) | 0.0 | 2 | 0.010 (−0.017, 0.037) | 0.0 | ||||||
| Adjusted for BMI | yes | 2 | 0.023 (−0.068, 0.114) | 57.5 | 3 | 0.011 (−0.024, 0.046) | 7.6 | |||||
| no | 2 | −0.032 (−0.056, −0.007) | 0.0 | 2 | 0.010 (−0.017, 0.037) | 0.0 | ||||||
| Subgroup | FT3 | FT4 | |||||
|---|---|---|---|---|---|---|---|
| No | β (95%CI) | I2 | No | β (95%CI) | I2 | ||
| Adjusted for BMI | yes | 4 | 0.015 (−0.015, 0.044) | 67.0 | 5 | −0.001 (−0.014, 0.030) | 0.0 |
| no | 5 | 0.005 (−0.001, 0.011) | 0.0 | 3 | −0.011 (−0.021, −0.001) | 0.0 | |
| TSH | T3 | T4 | FT3 | FT4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Z-Value | p | Z-Value | p | Z-Value | p | Z-Value | p | Z-Value | p | |
| PFOA | 0.04 | 0.967 | 0.62 | 0.536 | 1.08 | 0.279 | 1.77 | 0.076 | 0.81 | 0.419 |
| PFNA | 1.22 | 0.224 | 0.24 | 0.806 | 0.52 | 0.602 | 0.00 | 1.000 | 1.62 | 0.105 |
| PFDA | 0.00 | 1.000 | 0.00 | 1.000 | 0.34 | 0.734 | 0.34 | 0.734 | 0.73 | 0.462 |
| PFOS | 0.87 | 0.386 | 0.12 | 0.902 | 0.89 | 0.371 | 0.00 | 1.000 | 0.86 | 0.390 |
| PFHxS | 0.05 | 0.956 | 0.96 | 0.339 | 0.75 | 0.454 | 0.46 | 0.649 | 2.10 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhao, M.; Cong, X.; Liu, C.; Li, C.; Qiu, Y.; Li, S.; Chen, Y.; Li, X.; Li, P. The Association Between Per- and Polyfluoroalkyl Substances Exposure and Thyroid Hormones in Men and Non-Pregnant Women: A Systematic Review and Meta-Analysis. Toxics 2025, 13, 214. https://doi.org/10.3390/toxics13030214
Zhang B, Zhao M, Cong X, Liu C, Li C, Qiu Y, Li S, Chen Y, Li X, Li P. The Association Between Per- and Polyfluoroalkyl Substances Exposure and Thyroid Hormones in Men and Non-Pregnant Women: A Systematic Review and Meta-Analysis. Toxics. 2025; 13(3):214. https://doi.org/10.3390/toxics13030214
Chicago/Turabian StyleZhang, Bin, Meizi Zhao, Xiangru Cong, Chunyu Liu, Chaofei Li, Yu Qiu, Sha Li, Yanying Chen, Xiaoxue Li, and Penghui Li. 2025. "The Association Between Per- and Polyfluoroalkyl Substances Exposure and Thyroid Hormones in Men and Non-Pregnant Women: A Systematic Review and Meta-Analysis" Toxics 13, no. 3: 214. https://doi.org/10.3390/toxics13030214
APA StyleZhang, B., Zhao, M., Cong, X., Liu, C., Li, C., Qiu, Y., Li, S., Chen, Y., Li, X., & Li, P. (2025). The Association Between Per- and Polyfluoroalkyl Substances Exposure and Thyroid Hormones in Men and Non-Pregnant Women: A Systematic Review and Meta-Analysis. Toxics, 13(3), 214. https://doi.org/10.3390/toxics13030214








