Rotenone Exposure During Development Conditions Parkinsonian Phenotype in Young Adult Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dams Breeding
2.2. Pups and Cross-Fostering
2.3. Motor Coordination Assessment
2.4. Quantification of Insoluble Forms of α-Syn (α-Synif)
2.5. Estimation of ROT Concentration in Blood Serum in Pups
2.6. Tyrosine Hydroxylase Immunocytochemistry
2.7. Quantification of Dopaminergic Neurons in the S. nigra
2.8. Global DNA Methylation (5-mC) in S. nigra
2.9. Statistical Analysis
3. Results
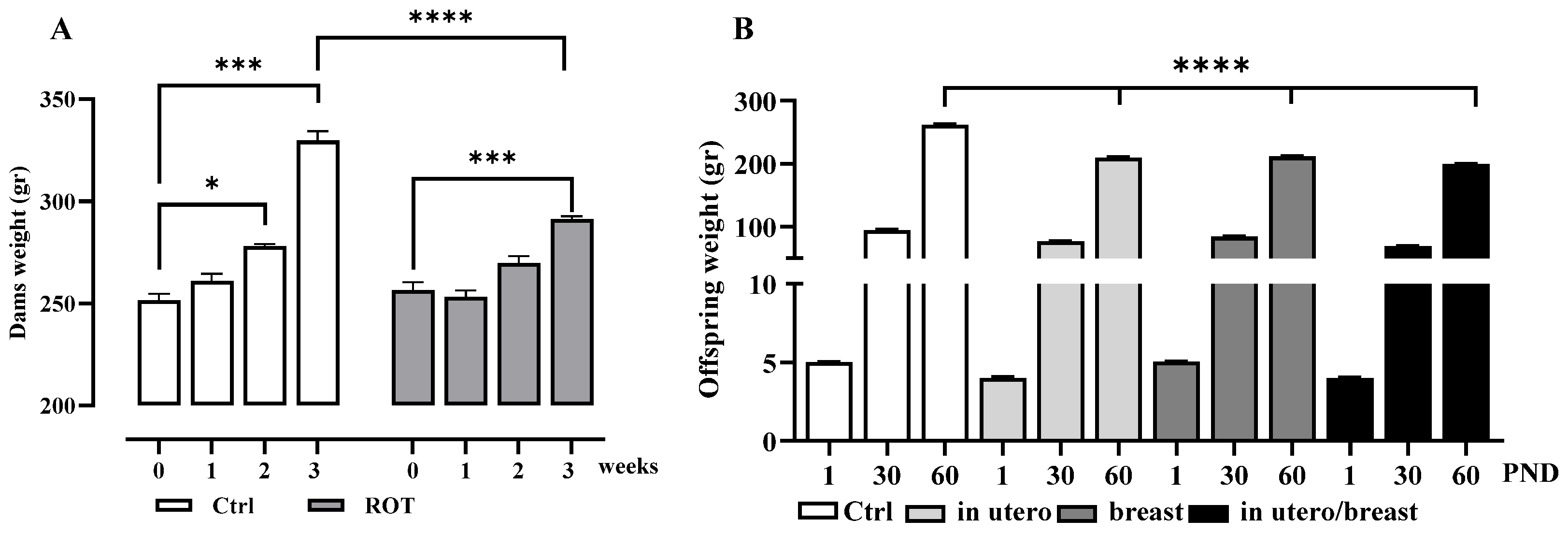
3.1. Body Weight Gain in Dams and Pups
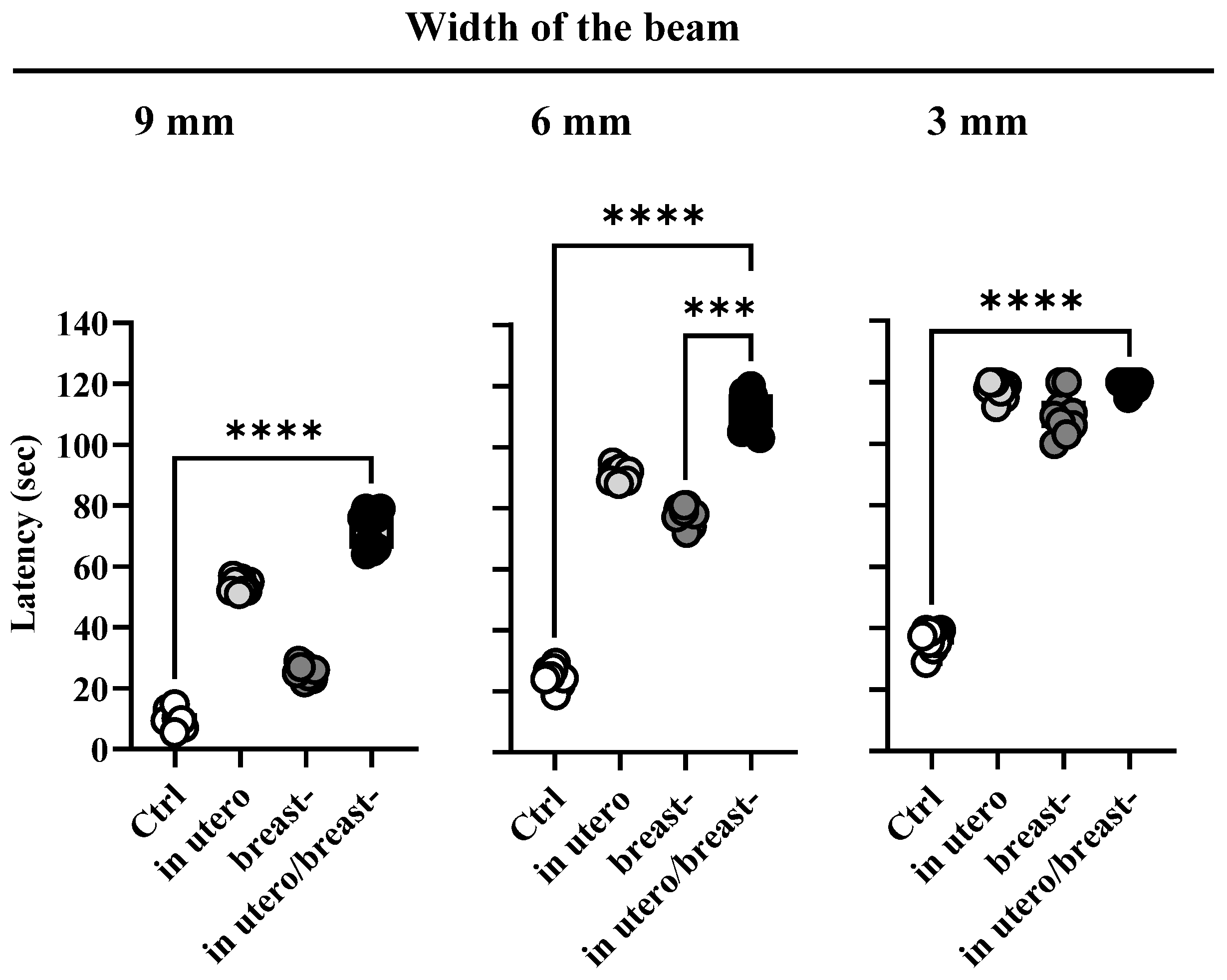
3.2. ROT Exposure In Utero and/or Breast-Impairs Motor Coordination
3.3. Exposure to Rotenone In Utero and/or Breastfeeding Decreases the Number of TH-IR Neurons in S. nigra and Increases the Concentration of α-Synif in S. nigra and C. nucleus
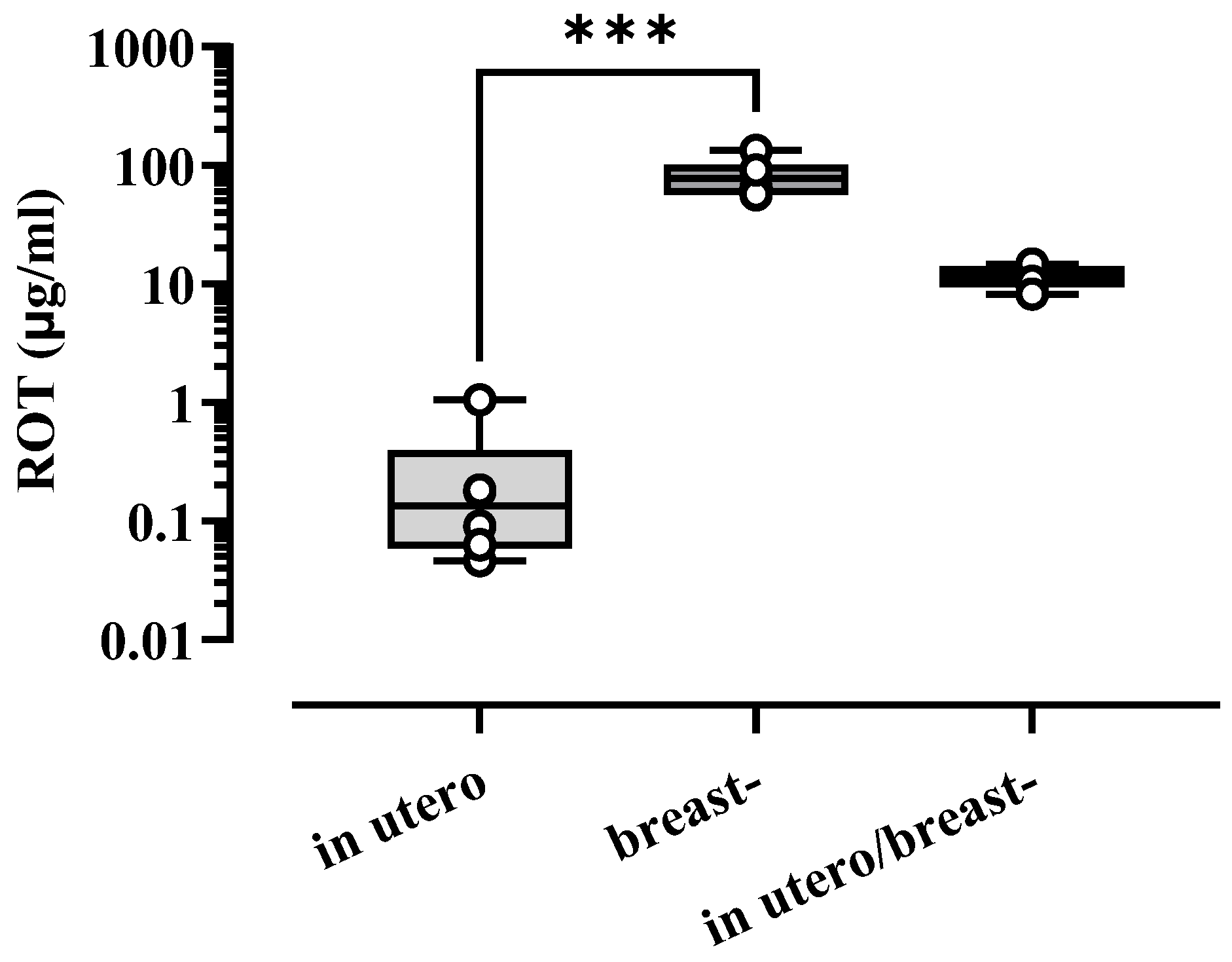
3.4. Concentration of ROT in Blood Serum in Pups
3.5. Correlation Between α-Synif Content and Dopaminergic TH-IR Neuron Number in S. nigra, andα-Synif content in C. nucleus and Motor Coordination
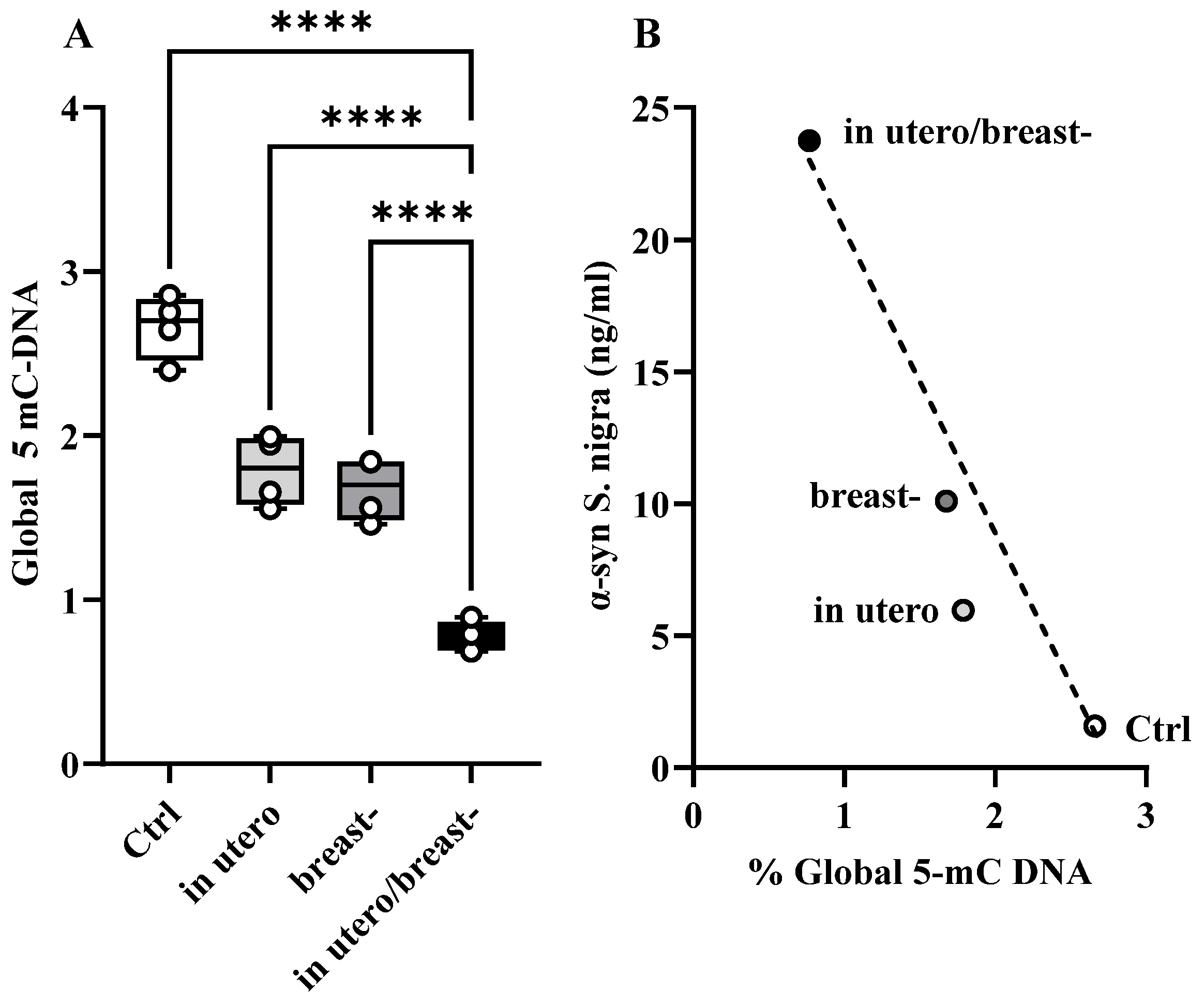
3.6. α-Synif and Global 5-mC DNA in S. nigra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114 Pt 5, 2283–2301. [Google Scholar] [CrossRef]
- McNaught, K.S.P.; Olanow, C.W.; Halliwell, B.; Isacson, O.; Jenner, P. Failure of the ubiquitin-proteasome system in Parkinson’s disease. Nat. Rev. Neurosci. 2001, 2, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liang, Z.H.; Wang, T.; Qiao, X.; Liu, H.J.; Sun, S.G. Alpha-Synuclein redistributed and aggregated in rotenone-induced Parkinson’s disease rats. Neurosci. Bull. 2006, 22, 288–293. [Google Scholar] [PubMed]
- Williams-Gray, C.H.; Foltynie, T.; Lewis, S.J.G.; A Barker, R. Cognitive deficits and psychosis in Parkinson’s disease: A review of pathophysiology and therapeutic options. CNS Drugs 2006, 20, 477–505. [Google Scholar] [CrossRef] [PubMed]
- Lonskaya, I.; Hebron, M.; Algarzae, N.; Desforges, N.; Moussa, C.H. Decreased parkin solubility is associated with impairment of autophagy in the nigrostriatum of sporadic Parkinson’s disease. Neuroscience 2013, 232, 90–105. [Google Scholar] [CrossRef]
- Hindle, J.V. Ageing, neurodegeneration and Parkinson’s disease. Age Ageing 2010, 39, 156–161. [Google Scholar] [CrossRef]
- Houlden, H.; Singleton, A.B. The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol. 2012, 124, 325–338. [Google Scholar] [CrossRef]
- Puschmann, A. Monogenic Parkinson’s disease and parkinsonism: Clinical phenotypes and frequencies of known mutations. Park. Relat. Disord. 2013, 19, 407–415. [Google Scholar] [CrossRef]
- Gorell, J.M.; Rybicki, B.A.; Johnson, C.C.; Peterson, E.L. Occupational metal exposures and the risk of Parkinson’s disease. Neuroepidemiology 1999, 18, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Moretto, A.; Colosio, C. Biochemical and toxicological evidence of neurological effects of pesticides: The example of Parkinson’s disease. Neurotoxicology 2011, 32, 383–391. [Google Scholar] [CrossRef]
- Cereda, E.; Cilia, R.; Klersy, C.; Siri, C.; Pozzi, B.; Reali, E.; Colombo, A.; Zecchinelli, A.L.; Mariani, C.B.; Tesei, S.; et al. Dementia in Parkinson’s disease: Is male gender a risk factor? Park. Relat. Disord. 2016, 26, 67–72. [Google Scholar] [CrossRef]
- Cory-Slechta, D.A.; Thiruchelvam, M.; Richfield, E.K.; Barlow, B.K.; Brooks, A.I. Developmental pesticide exposures and the Parkinson’s disease phenotype. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 136–139. [Google Scholar] [CrossRef]
- Gómez-Chavarín, M.; Diaz-Perez, R.; Morales-Espinosa, R.; Fernandez-Ruiz, J.; Roldan-Roldan, R.; Torner, C. Developmental effects of rotenone pesticide exposure on the rat nigrostriatal dopaminergic system. Salud Ment. 2013, 36, 1–7. [Google Scholar] [CrossRef]
- Perera, F.; Rauh, V.; Whyatt, R.; Tang, D.; Tsai, W.; Bernert, J.; Tu, Y.; Andrews, H.; Barr, D.; Camann, D.; et al. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology 2005, 26, 573–587. [Google Scholar] [CrossRef]
- Barlow, B.K.; Coryslechta, D.; Richfield, E.K.; Thiruchelvam, M. The gestational environment and Parkinson’s disease: Evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod. Toxicol. 2007, 23, 457–470. [Google Scholar] [CrossRef]
- Fleming, S.M. Mechanisms of Gene-Environment Interactions in Parkinson’s Disease. Curr. Environ. Health Rep. 2017, 4, 192–199. [Google Scholar] [CrossRef]
- Shan, L.; Heusinkveld, H.J.; Paul, K.C.; Hughes, S.; Darweesh, S.K.L.; Bloem, B.R.; Homberg, J.R. Towards improved screening of toxins for Parkinson’s risk. NPJ Park. Dis. 2023, 9, 169. [Google Scholar] [CrossRef]
- Luo, W.; Ruan, D.; Yan, C.; Yin, S.; Chen, J. Effects of chronic lead exposure on functions of nervous system in Chinese children and developmental rats. Neurotoxicology 2012, 33, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Stejskal, V.; Urbina, M.A.; Dadar, M.; Chirumbolo, S.; Mutter, J. Metals and Parkinson’s Disease: Mechanisms and Biochemical Processes. Curr. Med. Chem. 2018, 25, 2198–2214. [Google Scholar] [CrossRef]
- Vellingiri, B.; Suriyanarayanan, A.; Abraham, K.S.; Venkatesan, D.; Iyer, M.; Raj, N.; Gopalakrishnan, A.V. Influence of heavy metals in Parkinson’s disease: An overview. J. Neurol. 2022, 269, 5798–5811. [Google Scholar] [CrossRef]
- Mursaleen, L.R.; Stamford, J.A. Drugs of abuse and Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 209–217. [Google Scholar] [CrossRef]
- Hagberg, H.; Gressens, P.; Mallard, C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2012, 71, 444–457. [Google Scholar] [CrossRef]
- Iacono, S.; Schirò, G.; Davì, C.; Mastrilli, S.; Abbott, M.; Guajana, F.; Arnao, V.; Aridon, P.; Ragonese, P.; Gagliardo, C.; et al. COVID-19 and neurological disorders: What might connect Parkinson’s disease to SARS-CoV-2 infection. Front. Neurol. 2023, 14, 1172416. [Google Scholar] [CrossRef]
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef]
- Casper, R.C. Nutrients, neurodevelopment, and mood. Curr. Psychiatry Rep. 2004, 6, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Valleau, J.C.; Sullivan, E.L. The impact of leptin on perinatal development and psychopathology. J. Chem. Neuroanat. 2014, 61, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Betarbet, R.; Sherer, T.B.; Greenamyre, J.T. Animal models of Parkinson’s disease. Bioessays 2002, 24, 308–318. [Google Scholar] [CrossRef]
- Sherer, T.B.; Kim, J.-H.; Betarbeta, R.; Greenamyrea, J.T. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 2003, 179, 9–16. [Google Scholar] [CrossRef]
- Schmidt, W.J.; Alam, M. Controversies on new animal models of Parkinson’s disease pro and con: The rotenone model of Parkinson’s disease (PD). J. Neural Transm. Suppl. 2006, 70, 273–276. [Google Scholar] [CrossRef]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.D.; Dolatabadi, N.; Chan, S.F.; Zhang, X.; Akhtar, M.W.; Parker, J.; Soldner, F.; Sunico, C.R.; Nagar, S.; Talantova, M.; et al. Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell 2013, 155, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.D.; Davison, C.A.; Aschner, M.; Bowman, A.B. From the Cover: Manganese and Rotenone-Induced Oxidative Stress Signatures Differ in iPSC-Derived Human Dopamine Neurons. Toxicol. Sci. 2017, 159, 366–379. [Google Scholar] [CrossRef] [PubMed]
- McKnight, S.; Hack, N. Toxin-Induced Parkinsonism. Neurol. Clin. 2020, 38, 853–865. [Google Scholar] [CrossRef]
- Mexican Official Standard Norm for Animal Care. Diario Oficial, México. 1992. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 1 January 2020).
- Drucker-Colín, R.; García-Hernández, F. A new motor test sensitive to aging and dopaminergic function. J. Neurosci. Methods 1991, 39, 153–161. [Google Scholar] [CrossRef]
- Campbell, B.C.; Li, Q.X.; Culvenor, J.G.; Jäkälä, P.; Cappai, R.; Beyreuther, K.; Masters, C.L.; McLean, C.A. Accumulation of insoluble alpha-synuclein in dementia with Lewy bodies. Neurobiol. Dis. 2000, 7, 192–200. [Google Scholar] [CrossRef]
- Juraska, J.M.; Wilson, C.J.; Groves, P.M. The substantia nigra of the rat: A Golgi study. J. Comp. Neurol. 1977, 172, 585–600. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Sonawane, B.; Butler, R.N.; Trasande, L.; Callan, R.; Droller, D. Early environmental origins of neurodegenerative disease in later life. Environ. Health Perspect. 2005, 113, 1230–1233. [Google Scholar] [CrossRef]
- Wexler, E.M.; Geschwind, D.H. Out FOXing Parkinson disease: Where development meets neurodegeneration. PLoS Biol. 2007, 5, e334. [Google Scholar] [CrossRef]
- Swarnkar, S.; Goswami, P.; Kamat, P.K.; Gupta, S.; Patro, I.K.; Singh, S.; Nath, C. Rotenone-induced apoptosis and role of calcium: A study on Neuro-2a cells. Arch. Toxicol. 2012, 86, 1387–1397. [Google Scholar] [CrossRef]
- Ma, J.; Huang, C.; Ma, K.; Wu, Y.P.; Li, B.X.; Sun, Y. Effect of Wnt1 and Wnt5a on the development of dopaminergic neurons, and toxicity induced by combined exposure to paraquat and maneb during gestation and lactation. Mol. Med. Rep. 2017, 16, 9721–9728. [Google Scholar] [CrossRef] [PubMed]
- Vitalis, T.; Cases, O.; Parnavelas, J.G. Development of the dopaminergic neurons in the rodent brainstem. Exp. Neurol. 2005, (Suppl. S1), S104–S112. [Google Scholar] [CrossRef]
- Ang, S.L. Transcriptional control of midbrain dopaminergic neuron development. Development 2006, 133, 3499–3506. [Google Scholar] [CrossRef]
- Alavian, K.N.; Scholz, C.; Simon, H.H. Transcriptional regulation of mesencephalic dopaminergic neurons: The full circle of life and death. Mov. Disord. 2008, 23, 319–328. [Google Scholar] [CrossRef]
- Betarbet, R.; Canet-Aviles, R.M.; Sherer, T.B.; Mastroberardino, P.G.; McLendon, C.; Kim, J.-H.; Lund, S.; Na, H.-M.; Taylor, G.; Bence, N.F.; et al. Intersecting pathways to neurodegeneration in Parkinson’s disease: Effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol. Dis. 2006, 22, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Schieler, J.L.; Rochet, J.-C.; Regnier, F. Identification of rotenone-induced modifications in alpha-synuclein using affinity pull-down and tandem mass spectrometry. Anal. Chem. 2006, 78, 2422–2431. [Google Scholar] [CrossRef]
- Chu, Y.; Kordower, J.H. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol. Dis. 2007, 25, 134–149. [Google Scholar] [CrossRef]
- Matsumoto, L.; Takuma, H.; Tamaoka, A.; Kurisaki, H.; Date, H.; Tsuji, S.; Iwata, A. CpG demethylation enhances alpha-synuclein expression and affects the pathogenesis of Parkinson’s disease. PLoS ONE. 2010, 5, e15522. [Google Scholar] [CrossRef]
- Jowaed, A.; Schmitt, I.; Kaut, O.; Wüllner, U. Methylation regulates alpha-synuclein expression and is decreased in Parkinson’s disease patients’ brains. J. Neurosci. 2010, 30, 6355–6359. [Google Scholar] [CrossRef]
- Mpofana, T.; Daniels, W.M.U.; Mabandla, M.V. Exposure to Early Life Stress Results in Epigenetic Changes in Neurotrophic Factor Gene Expression in a Parkinsonian Rat Model. Park. Dis. 2016, 2016, 6438783. [Google Scholar] [CrossRef]
- Mattson, M.P. Methylation and acetylation in nervous system development and neurodegenerative disorders. Ageing Res. Rev. 2003, 2, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Kwok, J.B. Role of epigenetics in Alzheimer’s and Parkinson’s disease. Epigenomics 2010, 2, 671–682. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, R.; Yang, Z.; Wang, Y.; Gong, X.; Wang, X. A DNA methyltransferase inhibitor, 5-aza-2′-deoxycytidine, exacerbates neurotoxicity and upregulates Parkinson’s disease-related genes in dopaminergic neurons. CNS Neurosci. Ther. 2013, 19, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.J.; Zota, A.R.; Schwartz, J.M. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect. 2011, 119, 878–885. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Chavarín, M.; Padilla, P.; Velázquez-Paniagua, M. Rotenone Exposure During Development Conditions Parkinsonian Phenotype in Young Adult Rats. Toxics 2025, 13, 290. https://doi.org/10.3390/toxics13040290
Gómez-Chavarín M, Padilla P, Velázquez-Paniagua M. Rotenone Exposure During Development Conditions Parkinsonian Phenotype in Young Adult Rats. Toxics. 2025; 13(4):290. https://doi.org/10.3390/toxics13040290
Chicago/Turabian StyleGómez-Chavarín, Margarita, Patricia Padilla, and Mireya Velázquez-Paniagua. 2025. "Rotenone Exposure During Development Conditions Parkinsonian Phenotype in Young Adult Rats" Toxics 13, no. 4: 290. https://doi.org/10.3390/toxics13040290
APA StyleGómez-Chavarín, M., Padilla, P., & Velázquez-Paniagua, M. (2025). Rotenone Exposure During Development Conditions Parkinsonian Phenotype in Young Adult Rats. Toxics, 13(4), 290. https://doi.org/10.3390/toxics13040290





