The Role of Sex Steroid Hormones in the Association Between Manganese Exposure and Bone Mineral Density: National Health and Nutrition Examination Survey 2013–2018
Abstract
1. Introduction
2. Materials and Methods
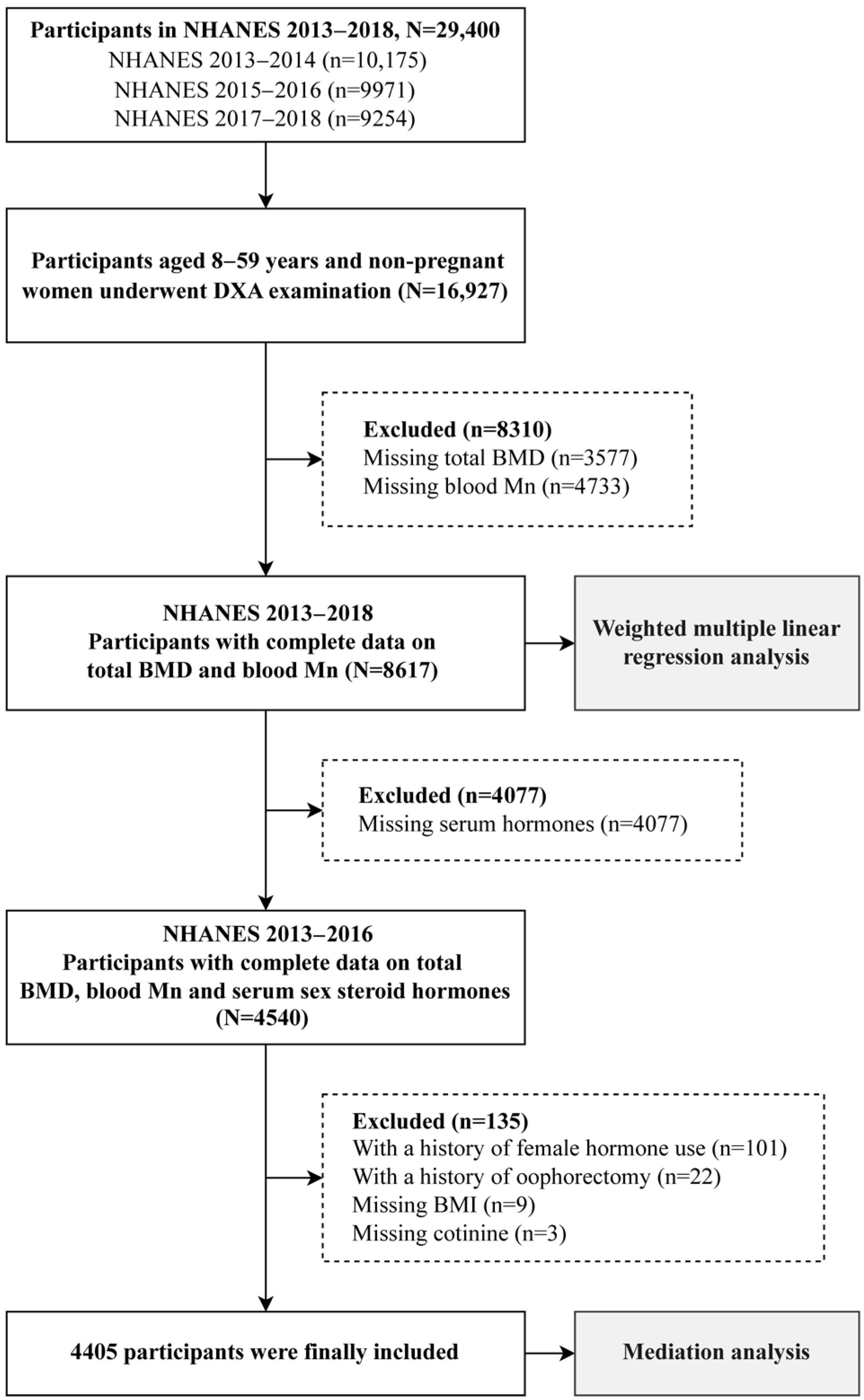
2.1. Study Population
2.2. Measurement of Variables
2.3. Menopausal Status Definitions
2.4. Covariates
2.5. Statistical Analysis
2.6. Sensitivity Analysis
3. Results
3.1. Characteristics of the Study Population
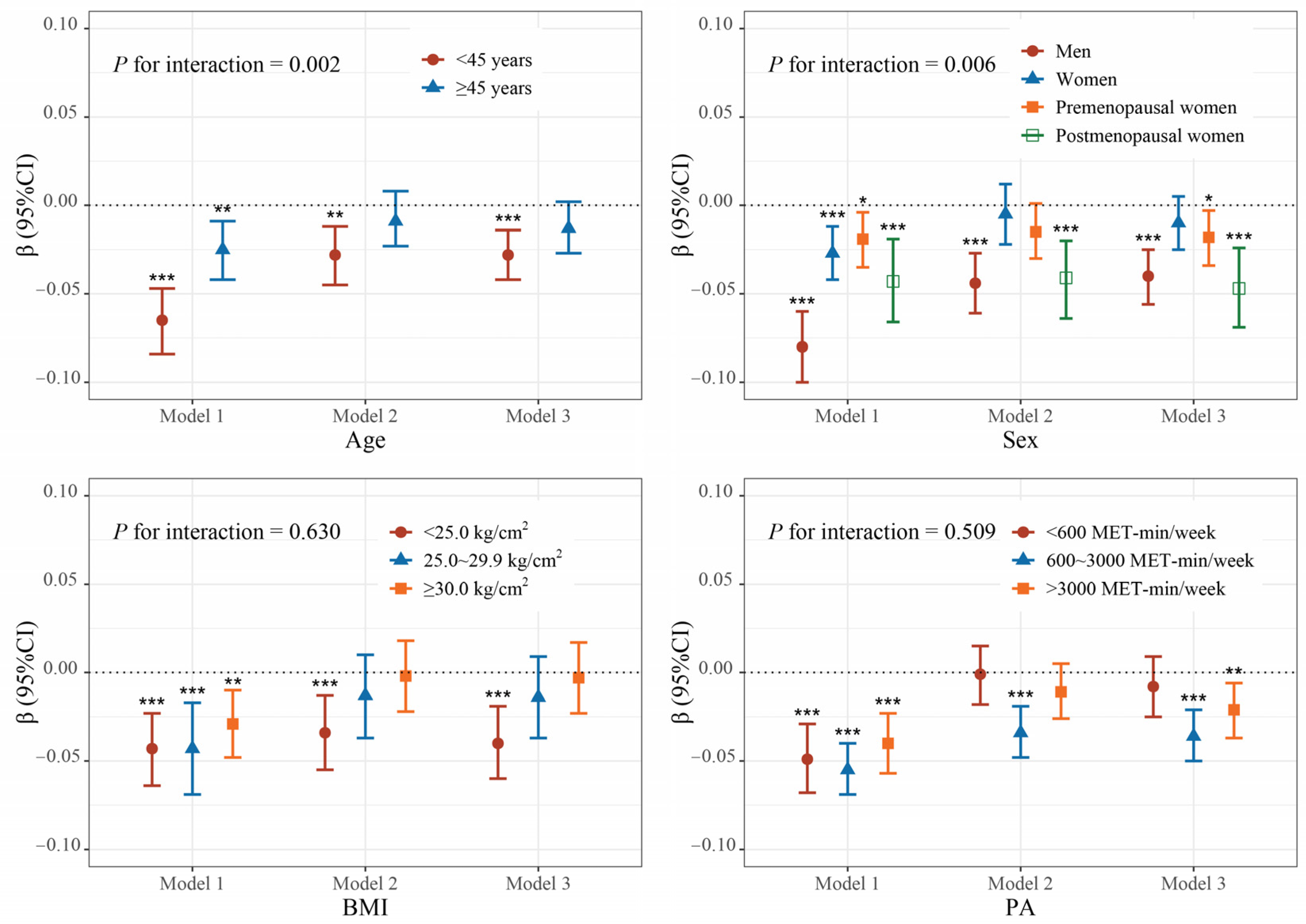
3.2. Association Between Blood Mn and Total BMD
3.3. Association Between Blood Mn and Serum Sex Steroid Hormones
3.4. Association Between Serum Sex Steroid Hormones and Total BMD
3.5. Mediation Analyses
4. Discussion
4.1. Main Study Findings
4.2. Interpretations and Comparisons with Other Studies
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Consensus, A. Consensus Development Conference: Diagnosis, Prophylaxis, and Treatment of Osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- Xiao, P.-L.; Cui, A.-Y.; Hsu, C.-J.; Peng, R.; Jiang, N.; Xu, X.-H.; Ma, Y.-G.; Liu, D.; Lu, H.-D. Global, Regional Prevalence, and Risk Factors of Osteoporosis According to the World Health Organization Diagnostic Criteria: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.E.; Shin, D.W.; Han, K.; Kim, D.; Yoon, J.W.; Lee, D.-Y. Association of Female Reproductive Factors With Incidence of Fracture Among Postmenopausal Women in Korea. JAMA Netw. Open 2021, 4, e2030405. [Google Scholar] [CrossRef]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J. Bone Min. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Morris, J.A.; Kemp, J.P.; Youlten, S.E.; Laurent, L.; Logan, J.G.; Chai, R.C.; Vulpescu, N.A.; Forgetta, V.; Kleinman, A.; Mohanty, S.T.; et al. An Atlas of Genetic Influences on Osteoporosis in Humans and Mice. Nat. Genet. 2019, 51, 258–266. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, X.; Wang, H.; Zhang, S.; Du, X.; Wei, H. Relationship of Blood Heavy Metals and Osteoporosis among the Middle-Aged and Elderly Adults: A Secondary Analysis from NHANES 2013 to 2014 and 2017 to 2018. Front. Public Health 2023, 11, 1045020. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Huang, Q.; Dai, Y.; Zhou, H.; Cui, Y.; Song, W.; Di, D.; Zhang, R.; Li, C.; Wang, Q.; et al. Manganese, Iron, Copper, and Selenium Co-Exposure and Osteoporosis Risk in Chinese Adults. J. Trace Elem. Med. Biol. 2022, 72, 126989. [Google Scholar] [CrossRef]
- Baj, J.; Flieger, W.; Barbachowska, A.; Kowalska, B.; Flieger, M.; Forma, A.; Teresiński, G.; Portincasa, P.; Buszewicz, G.; Radzikowska-Büchner, E.; et al. Consequences of Disturbing Manganese Homeostasis. Int. J. Mol. Sci. 2023, 24, 14959. [Google Scholar] [CrossRef]
- Chung, S.E.; Cheong, H.-K.; Ha, E.-H.; Kim, B.-N.; Ha, M.; Kim, Y.; Hong, Y.-C.; Park, H.; Oh, S.-Y. Maternal Blood Manganese and Early Neurodevelopment: The Mothers and Children’s Environmental Health (MOCEH) Study. Environ. Health Perspect. 2015, 123, 717–722. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, M.; Cui, J.; Du, Y.; Teng, X.; Zhang, Z. Heat Shock Proteins Took Part in Oxidative Stress-Mediated Inflammatory Injury via NF-κB Pathway in Excess Manganese-Treated Chicken Livers. Ecotoxicol. Environ. Saf. 2021, 226, 112833. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, S.; Teng, X. The Involvement of the Mitochondrial Pathway in Manganese-Induced Apoptosis of Chicken Splenic Lymphocytes. Chemosphere 2016, 153, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhu, Y.; Teng, X.; Zhang, K.; Teng, X.; Li, S. Toxicological Effect of Manganese on NF-κB/iNOS-COX-2 Signaling Pathway in Chicken Testes. Biol. Trace Elem. Res. 2015, 168, 227–234. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.L.; Hong, L.; Fu, S.; Jiang, W.; Jones, A.; Nie, L.H.; Zheng, W. Manganese Accumulation in Bone Following Chronic Exposure in Rats: Steady-State Concentration and Half-Life in Bone. Toxicol. Lett. 2014, 229, 93–100. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Chen, Y.; Zhang, X.; Xia, Y.; Geng, B. Association between Blood Manganese and Bone Mineral Density in US Adolescents. Env. Sci. Pollut. Res. 2023, 30, 29743–29754. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, Y.; Long, H.; Ou, M.; Zhao, S. Relationship between Blood Manganese and Bone Mineral Density and Bone Mineral Content in Adults: A Population-Based Cross-Sectional Study. PLoS ONE 2022, 17, e0276551. [Google Scholar] [CrossRef]
- Wei, M.-H.; Cui, Y.; Zhou, H.-L.; Song, W.-J.; Di, D.-S.; Zhang, R.-Y.; Huang, Q.; Liu, J.-A.; Wang, Q. Associations of Multiple Metals with Bone Mineral Density: A Population-Based Study in US Adults. Chemosphere 2021, 282, 131150. [Google Scholar] [CrossRef]
- Li, D.; Ge, X.; Liu, Z.; Huang, L.; Zhou, Y.; Liu, P.; Qin, L.; Lin, S.; Liu, C.; Hou, Q.; et al. Association between Long-Term Occupational Manganese Exposure and Bone Quality among Retired Workers. Environ. Sci. Pollut. Res. Int. 2020, 27, 482–489. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [PubMed]
- Zofková, I.; Nemcikova, P.; Matucha, P. Trace Elements and Bone Health. Clin. Chem. Lab. Med. (CCLM) 2013, 51, 1555–1561. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, L.; Konermann, A.; Zhou, H.; Jin, F.; Liu, W. Manganese Superoxide Dismutase Is Required to Maintain Osteoclast Differentiation and Function under Static Force. Sci. Rep. 2015, 5, 8016. [Google Scholar] [CrossRef]
- David, K.; Narinx, N.; Antonio, L.; Evenepoel, P.; Claessens, F.; Decallonne, B.; Vanderschueren, D. Bone Health in Ageing Men. Rev. Endocr. Metab. Disord. 2022, 23, 1173–1208. [Google Scholar] [CrossRef] [PubMed]
- Shigehara, K.; Izumi, K.; Kadono, Y.; Mizokami, A. Testosterone and Bone Health in Men: A Narrative Review. J. Clin. Med. 2021, 10, 530. [Google Scholar] [CrossRef]
- Spaur, M.; Nigra, A.E.; Sanchez, T.R.; Navas-Acien, A.; Lazo, M.; Wu, H.-C. Association of Blood Manganese, Selenium with Steatosis, Fibrosis in the National Health and Nutrition Examination Survey, 2017–2018. Environ. Res. 2022, 213, 113647. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Gatcombe, M.; Farris, J.; Botelho, J.C.; Caudill, S.P.; Vesper, H.W. Simultaneous Measurement of Total Estradiol and Testosterone in Human Serum by Isotope Dilution Liquid Chromatography Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2017, 409, 5943–5954. [Google Scholar] [CrossRef]
- Seccareccia, F.; Zuccaro, P.; Pacifici, R.; Meli, P.; Pannozzo, F.; Freeman, K.M.; Santaquilani, A.; Giampaoli, S. Serum Cotinine as a Marker of Environmental Tobacco Smoke Exposure in Epidemiological Studies: The Experience of the MATISS Project. Eur. J. Epidemiol. 2003, 18, 487–492. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, Y.; Ren, X.; Cai, Y.; Wang, J.; Chen, O. Association of a Body Shape Index with Femur Bone Mineral Density among Older Adults: NHANES 2007–2018. Arch. Osteoporos. 2024, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, D.; MacKinnon, D.P. RMediation: An R Package for Mediation Analysis Confidence Intervals. Behav. Res. 2011, 43, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-J.; Kim, M.-H. Manganese Supplementation Improves Mineral Density of the Spine and Femur and Serum Osteocalcin in Rats. Biol. Trace Elem. Res. 2008, 124, 28–34. [Google Scholar] [CrossRef]
- Lin, S.; Yang, F.; Ling, M.; Fan, Y. Association between Bone Trace Elements and Osteoporosis in Older Adults: A Cross-Sectional Study. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221125984. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, B.; Feng, W.; Wang, Y.-X.; Liu, A.-L.; Yue, J.; Li, Y.-F.; Lu, W.-Q. Associations of Urinary Metal Concentrations and Circulating Testosterone in Chinese Men. Reprod. Toxicol. 2013, 41, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Narinx, N.; David, K.; Walravens, J.; Vermeersch, P.; Claessens, F.; Fiers, T.; Lapauw, B.; Antonio, L.; Vanderschueren, D. Role of Sex Hormone-Binding Globulin in the Free Hormone Hypothesis and the Relevance of Free Testosterone in Androgen Physiology. Cell. Mol. Life Sci. 2022, 79, 543. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, G.; Aguirre, L.; Napoli, N.; Qualls, C.; Villareal, D.T.; Armamento-Villareal, R. Testosterone Therapy Effects on Bone Mass and Turnover in Hypogonadal Men with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, e3058–e3068. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kong, G.; Yao, X.; Zhu, Z. Association between Serum Total Testosterone Level and Bone Mineral Density in Middle-Aged Postmenopausal Women. Int. J. Endocrinol. 2022, 2022, 4228740. [Google Scholar] [CrossRef]
- Nunes, E.; Gallardo, E.; Morgado-Nunes, S.; Fonseca-Moutinho, J. Steroid Hormone Levels and Bone Mineral Density in Women over 65 Years of Age. Sci. Rep. 2023, 13, 4925. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Liu, H.; Bai, H.; Hu, K.; Zhang, R.; Liu, Q.; Fan, P. Oxidative Stress Promotes Hyperandrogenism by Reducing Sex Hormone-Binding Globulin in Polycystic Ovary Syndrome. Fertil. Steril. 2021, 116, 1641–1650. [Google Scholar] [CrossRef]
- Nkpaa, K.W.; Amadi, B.A.; Adedara, I.A.; Wegwu, M.O.; Farombi, E.O. Ethanol Exacerbates Manganese—Induced Functional Alterations along the Hypothalamic-Pituitary-Gonadal Axis of Male Rats. Neurosci. Lett. 2018, 684, 47–54. [Google Scholar] [CrossRef]
- Osmancevic, A.; Daka, B.; Michos, E.D.; Trimpou, P.; Allison, M. The Association between Inflammation, Testosterone and SHBG in Men: A Cross-Sectional Multi-Ethnic Study of Atherosclerosis. Clin. Endocrinol. 2023, 99, 190–197. [Google Scholar] [CrossRef]
- Chen, C.; Wang, N.; Nie, X.; Han, B.; Li, Q.; Chen, Y.; Zhai, H.; Zhu, C.; Chen, Y.; Xia, F.; et al. Blood Cadmium Level Associates with Lower Testosterone and Sex Hormone-Binding Globulin in Chinese Men: From SPECT-China Study, 2014. Biol. Trace Elem. Res. 2016, 171, 71–78. [Google Scholar] [CrossRef]
- Fittipaldi, S.; Bimonte, V.M.; Soricelli, A.; Aversa, A.; Lenzi, A.; Greco, E.A.; Migliaccio, S. Cadmium Exposure Alters Steroid Receptors and Proinflammatory Cytokine Levels in Endothelial Cells in Vitro: A Potential Mechanism of Endocrine Disruptor Atherogenic Effect. J. Endocrinol. Investig. 2019, 42, 727–739. [Google Scholar] [CrossRef]
- Chen, Y.; Pu, Y.; Liu, H.; Cao, A.; Du, Y.; He, S.; Ai, S.; Dang, Y. A Study on the Mediating Role of Serum Hormones in the Effects of Heavy Metals on Preeclampsia. Environ. Pollut. 2024, 360, 124721. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Xie, L.; Jing, G.; Liang, W.; He, J.; Dang, Y. The Relationship Between Heavy Metals and Missed Abortion: Using Mediation of Serum Hormones. Biol. Trace Elem. Res. 2024, 202, 3401–3412. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Luo, K.; Liu, H.; Fan, X.; Yin, X.; Zhao, Q.; Liu, X.; Yang, Y. Exposure to Metals and the Disruption of Sex Hormones in 6–19 Years Old Children: An Exploration of Mixture Effects. Ecotoxicol. Environ. Saf. 2023, 250, 114477. [Google Scholar] [CrossRef] [PubMed]
- Vasconsuelo, A.; Pronsato, L.; Ronda, A.C.; Boland, R.; Milanesi, L. Role of 17β-Estradiol and Testosterone in Apoptosis. Steroids 2011, 76, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Khosla, S.; Melton, L.J., III. Sex Steroids and the Construction and Conservation of the Adult Skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef]
- Pinsino, A.; Roccheri, M.C.; Costa, C.; Matranga, V. Manganese Interferes with Calcium, Perturbs ERK Signaling, and Produces Embryos with No Skeleton. Toxicol. Sci. 2011, 123, 217–230. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, F.; Winzenberg, T.; Jones, G. Tracking of Areal Bone Mineral Density from Age Eight to Young Adulthood and Factors Associated With Deviation From Tracking: A 17-Year Prospective Cohort Study. J. Bone Miner. Res. 2018, 33, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, C.; Cai, X.; Yang, F.; Fan, Y. The Concentrations of Bone Calcium, Phosphorus and Trace Metal Elements in Elderly Patients with Intertrochanteric Hip Fractures. Front. Endocrinol. 2022, 13, 1005637. [Google Scholar] [CrossRef]
- Ang, S.B.; Xia, J.Y.; Cheng, S.J.; Chua, M.T.; Goh, L.; Dhaliwal, S.S. A Pilot Screening Study for Low Bone Mass in Singaporean Women Using Years since Menopause and BMI. Climacteric 2022, 25, 163–169. [Google Scholar] [CrossRef]
- Qiao, D.; Li, Y.; Liu, X.; Zhang, X.; Qian, X.; Zhang, H.; Zhang, G.; Wang, C. Association of Obesity with Bone Mineral Density and Osteoporosis in Adults: A Systematic Review and Meta-Analysis. Public Health 2020, 180, 22–28. [Google Scholar] [CrossRef]
- Kennedy, M.L.; Failla, M.L.; Smith, J.C. Influence of Genetic Obesity on Tissue Concentrations of Zinc, Copper, Manganese and Iron in Mice. J. Nutr. 1986, 116, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Su, X.; Su, D.; Zeng, F.; Wang, M.H.; Huang, L.; Huang, E.; Zhu, Y.; Zhao, D.; He, D.; et al. Dietary Intake of Manganese and the Risk of the Metabolic Syndrome in a Chinese Population. Br. J. Nutr. 2016, 116, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, G.A.; Tsametis, C.P.; Goulis, D.G. Measuring Testosterone in Women and Men. Maturitas 2019, 125, 41–44. [Google Scholar] [CrossRef]
- Karyakina, N.A.; Shilnikova, N.; Farhat, N.; Ramoju, S.; Cline, B.; Momoli, F.; Mattison, D.; Jensen, N.; Terrell, R.; Krewski, D. Biomarkers for Occupational Manganese Exposure. Crit. Rev. Toxicol. 2022, 52, 636–663. [Google Scholar] [CrossRef] [PubMed]
- Battistini, B.; Greggi, C.; Visconti, V.V.; Albanese, M.; Messina, A.; De Filippis, P.; Gasperini, B.; Falvino, A.; Piscitelli, P.; Palombi, L.; et al. Metals Accumulation Affects Bone and Muscle in Osteoporotic Patients: A Pilot Study. Environ. Res. 2024, 250, 118514. [Google Scholar] [CrossRef]

| Variables | Quartile 1 (<0.931 g/cm2) | Quartile 2 (0.931–1.056 g/cm2) | Quartile 3 (1.057–0.1.144 g/cm2) | Quartile 4 (≥1.145 g/cm2) | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SE) or Percent (%) | N | Mean (SE) or Percent (%) | N | Mean (SE) or Percent (%) | N | Mean (SE) or Percent (%) | ||
| Age (years) | 2155 | 16.14 (0.77) | 2154 | 33.59 (0.50) | 2154 | 35.51 (0.38) | 2154 | 37.15 (0.30) | <0.001 |
| Sex, men | 1069 | 49.04 | 785 | 35.00 | 1013 | 46.41 | 1412 | 67.91 | <0.001 |
| Race/ethnicity | <0.001 | ||||||||
| Mexican American | 492 | 16.65 | 431 | 13.56 | 407 | 12.65 | 298 | 9.55 | |
| Other Hispanic | 247 | 8.82 | 268 | 9.72 | 227 | 7.84 | 182 | 6.56 | |
| Non-Hispanic White | 620 | 53.26 | 679 | 57.59 | 730 | 60.19 | 690 | 58.54 | |
| Non-Hispanic Black | 406 | 9.64 | 300 | 7.11 | 350 | 8.54 | 660 | 16.60 | |
| Other race | 390 | 11.62 | 476 | 12.02 | 440 | 10.78 | 324 | 8.75 | |
| BMI (kg/m2) | <0.001 | ||||||||
| <25.0 | 1770 | 79.15 | 1066 | 45.09 | 757 | 34.16 | 595 | 26.55 | |
| 25.0~29.9 | 255 | 13.78 | 565 | 27.91 | 627 | 29.49 | 653 | 33.13 | |
| ≥30.0 | 127 | 7.07 | 517 | 27.00 | 765 | 36.36 | 896 | 40.32 | |
| Cotinine level (ng/mL) | 2118 | 13.22 (2.27) | 2126 | 46.09 (3.49) | 2128 | 53.58 (4.82) | 2135 | 64.66 (5.93) | <0.001 |
| PA (MET-min/week) | <0.001 | ||||||||
| <600 | 1901 | 82.29 | 987 | 40.55 | 744 | 30.23 | 597 | 24.59 | |
| 600~3000 | 154 | 10.98 | 598 | 29.93 | 635 | 32.64 | 600 | 28.66 | |
| >3000 | 100 | 6.73 | 569 | 29.51 | 775 | 37.13 | 957 | 46.75 | |
| Mn (ng/mL) | 2155 | 10.99 (0.14) | 2154 | 10.71 (0.12) | 2154 | 10.14 (0.11) | 2154 | 9.60 (0.10) | <0.001 |
| TT (ng/dl) | 1285 | 57.53 (6.93) | 1103 | 175.33 (8.1) | 1089 | 220.23 (9.67) | 1063 | 301.13 (7.88) | <0.001 |
| E2 (pg/mL) | 1285 | 17.15 (1.71) | 1103 | 54.35 (2.77) | 1089 | 52.32 (2.92) | 1063 | 48.54 (2.71) | <0.001 |
| SHBG (nmol/L) | 1285 | 81.71 (3.16) | 1103 | 60.23 (1.43) | 1089 | 55.04 (1.90) | 1063 | 48.16 (1.46) | <0.001 |
| Model a | n | β (95% CI) b | % Change (95% CI) c | Mn, IQR (ng/mL) | |
|---|---|---|---|---|---|
| Model 1 | Total | 8617 | −0.065 (−0.077, −0.052) * | −24.9% (−28.9%, −20.7%) * | 4.43 |
| Men | 4279 | −0.080 (−0.100, −0.060) * | −26.8% (−32.3%, −20.8%) * | 3.91 | |
| Women | 4338 | −0.024 (−0.0.37, −0.011) * | −11.1% (−16.6%, −5.2%) * | 4.88 | |
| Premenopausal | 2445 | −0.019 (−0.035, −0.004) * | −9.5% (−16.6%, −1.9%) * | 5.22 | |
| Postmenopausal | 641 | −0.043 (−0.066, −0.019) * | −17.0% (−25.0%, −8.1%) * | 4.35 | |
| Model 2 | Total | 8617 | −0.021 (−0.034, −0.009) * | −9.0% (−13.9%, −3.9%) * | 4.43 |
| Men | 4279 | −0.044 (−0.061, −0.027) * | −15.8% (−21.3%, −9.9%) * | 3.91 | |
| Women | 4338 | −0.021 (−0.035, −0.008) * | −9.8% (−15.5%, −3.7%) * | 4.88 | |
| Premenopausal | 2445 | −0.015 (−0.030, 0.001) | −7.5% (−14.6%, 0.3%) | 5.22 | |
| Postmenopausal | 641 | −0.041 (−0.064, −0.020) * | −16.5% (−24.3%, −7.8%) * | 4.35 | |
| Model 3 | Total | 7441 | −0.022 (−0.033, −0.011) * | −9.3% (−13.5%, −4.9%) * | 4.46 |
| Men | 3675 | −0.037 (−0.053, −0.021) * | −13.4% (−18.6%, −7.8%) * | 3.88 | |
| Women | 3766 | −0.027 (−0.040, −0.014) * | −12.4% (−17.7%, −6.7%) * | 4.86 | |
| Premenopausal | 2168 | −0.019 (−0.034, −0.004) * | −9.5% (−16.2%, −2.2%) * | 5.23 | |
| Postmenopausal | 631 | −0.045 (−0.068, −0.023) * | −17.9% (−25.6%, −9.6%) * | 4.36 |
| Mn, IQR (ng/mL) | Mediator | Direct Effect | Indirect Effect | Proportion Mediated % (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) a | % Change (95% CI) b | p Value | β (95% CI) | % Change (95% CI) | p Value | ||||
| Total | 4.47 | TT | −0.026 (−0.036, −0.016) | −10.9% (−14.8%, −6.3%) | <0.001 | 0.001 (−0.002, 0.006) | 0.7% (−1.4%, 2.6%) | 0.492 | −5.99 (−31.66, 9.23) |
| E2 | −0.029 (−0.039, −0.020) | −12.1% (−15.8%, −8.4%) | <0.001 | 0.005 (0.000, 0.009) | 2.1% (−0.1%, 4.2%) | 0.056 | −18.91 (−54.92, −0.08) | ||
| SHBG | −0.028 (−0.039, −0.017) | −11.8% (−16.2%, −7.6%) | <0.001 | 0.004 (0.002, 0.006) | 1.7% (1.0%, 2.6%) | <0.001 | −15.70 (−33.05, −8.03) | ||
| Men | 3.87 | TT | −0.043 (−0.056, −0.029) | −15.2% (−19.4%, −10.6%) | <0.001 | 0.004 (−0.004, 0.001) | 1.4% (−1.4%, 4.0%) | 0.298 | −9.01 (−39.93, 7.56) |
| E2 | −0.043 (−0.057, −0.029) | −15.3% (−19.5%, −10.8%) | <0.001 | 0.004 (−0.003, 0.011) | 1.4% (−1.2%, 4.3%) | 0.306 | −9.37 (−38.00, 6.98) | ||
| SHBG | −0.054 (−0.069, −0.039) | −18.7% (−23.2%, −14.2%) | <0.001 | 0.015 (0.010, 0.019) | 5.8% (4.2%, 7.6%) | <0.001 | −37.16 (−66.07, −21.66) | ||
| Women | 5.20 | TT | −0.029 (−0.043, −0.014) | −14.1% (−20.7%, −7.2%) | <0.001 | 0.000 (−0.002, 0.001) | −0.2% (−0.8%, 0.3%) | 0.452 | 1.07 (−1.75, 5.94) |
| E2 | −0.029 (−0.043, −0.013) | −13.8% (−20.7%, −6.9%) | <0.001 | −0.001 (−0.003, 0.001) | −0.5% (−1.3%, 0.3%) | 0.246 | 2.96 (−1.99, 10.14) | ||
| SHBG | −0.029 (−0.045, −0.013) | −14.1% (−20.4%, −7.3%) | <0.001 | 0.000 (−0.001, 0.001) | −0.1% (−0.5%, 0.3%) | 0.704 | 0.35 (−2.01, 3.86) | ||
| Premenopausal | 5.37 | TT | −0.025 (−0.042, −0.010) | −12.5% (−19.7%, −5.2%) | <0.001 | −0.001 (−0.002, 0.000) | −0.4% (−1.3%, 0.1%) | 0.158 | 3.27 (−1.09, 12.55) |
| E2 | −0.026 (−0.041, −0.009) | −12.8% (−20.0%, −5.3%) | 0.002 | 0.000 (−0.001, 0.001) | −0.1% (−0.6%, 0.4%) | 0.778 | 0.49 (−4.09, 5.39) | ||
| SHBG | −0.026 (−0.042, −0.010) | −12.8% (−19.9%, −4.8%) | 0.002 | 0.000 (−0.001, 0.000) | −0.1% (−0.5%, 0.3%) | 0.750 | 0.44 (−2.12, 4.44) | ||
| Postmenopausal | 4.16 | TT | −0.034 (−0.076, 0.004) | −13.2% (−27.9%, 2.2%) | 0.084 | 0.001 (−0.004, 0.006) | 0.3% (−1.5%, 2.8%) | 0.764 | −2.01 (−45.69, 38.25) |
| E2 | −0.026 (−0.068, 0.016) | −10.3% (−24.6%, 6.7%) | 0.222 | −0.007 (−0.021, −0.000) | −3.0% (−8.0%, −0.2%) | 0.026 | 21.90 (−71.78, 208.69) | ||
| SHBG | −0.034 (−0.074, 0.007) | −13.1% (−25.8%, 2.6%) | 0.102 | 0.000 (−0.003, 0.005) | 0.2% (−1.3%, 1.8%) | 0.082 | −1.30 (−24.29, 19.85) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Li, J.; Yu, J.; Shi, Y.; Tang, M. The Role of Sex Steroid Hormones in the Association Between Manganese Exposure and Bone Mineral Density: National Health and Nutrition Examination Survey 2013–2018. Toxics 2025, 13, 296. https://doi.org/10.3390/toxics13040296
Zhao X, Li J, Yu J, Shi Y, Tang M. The Role of Sex Steroid Hormones in the Association Between Manganese Exposure and Bone Mineral Density: National Health and Nutrition Examination Survey 2013–2018. Toxics. 2025; 13(4):296. https://doi.org/10.3390/toxics13040296
Chicago/Turabian StyleZhao, Xiang, Jiayi Li, Jincong Yu, Yinhui Shi, and Mengling Tang. 2025. "The Role of Sex Steroid Hormones in the Association Between Manganese Exposure and Bone Mineral Density: National Health and Nutrition Examination Survey 2013–2018" Toxics 13, no. 4: 296. https://doi.org/10.3390/toxics13040296
APA StyleZhao, X., Li, J., Yu, J., Shi, Y., & Tang, M. (2025). The Role of Sex Steroid Hormones in the Association Between Manganese Exposure and Bone Mineral Density: National Health and Nutrition Examination Survey 2013–2018. Toxics, 13(4), 296. https://doi.org/10.3390/toxics13040296






