Solutions to the Dilemma of Antibiotics Use in Livestock and Poultry Farming: Regulation Policy and Alternatives
Abstract
1. Introduction
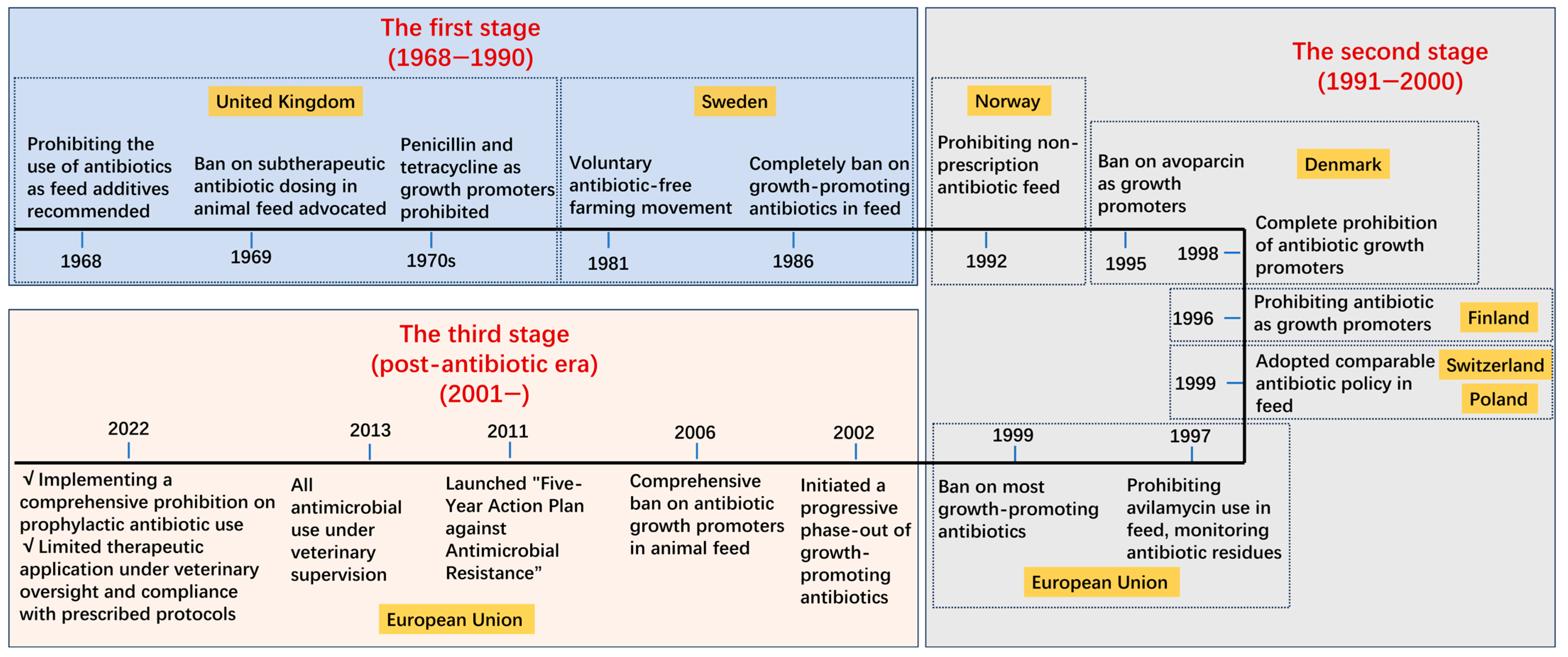
2. Regulation Policy from Different Countries and Organizations
2.1. Regulation Policy from the European Union and Its Member States
2.2. Regulation Policy from the United States
2.3. Regulation Policy from China
2.4. Other Countries and Organizations
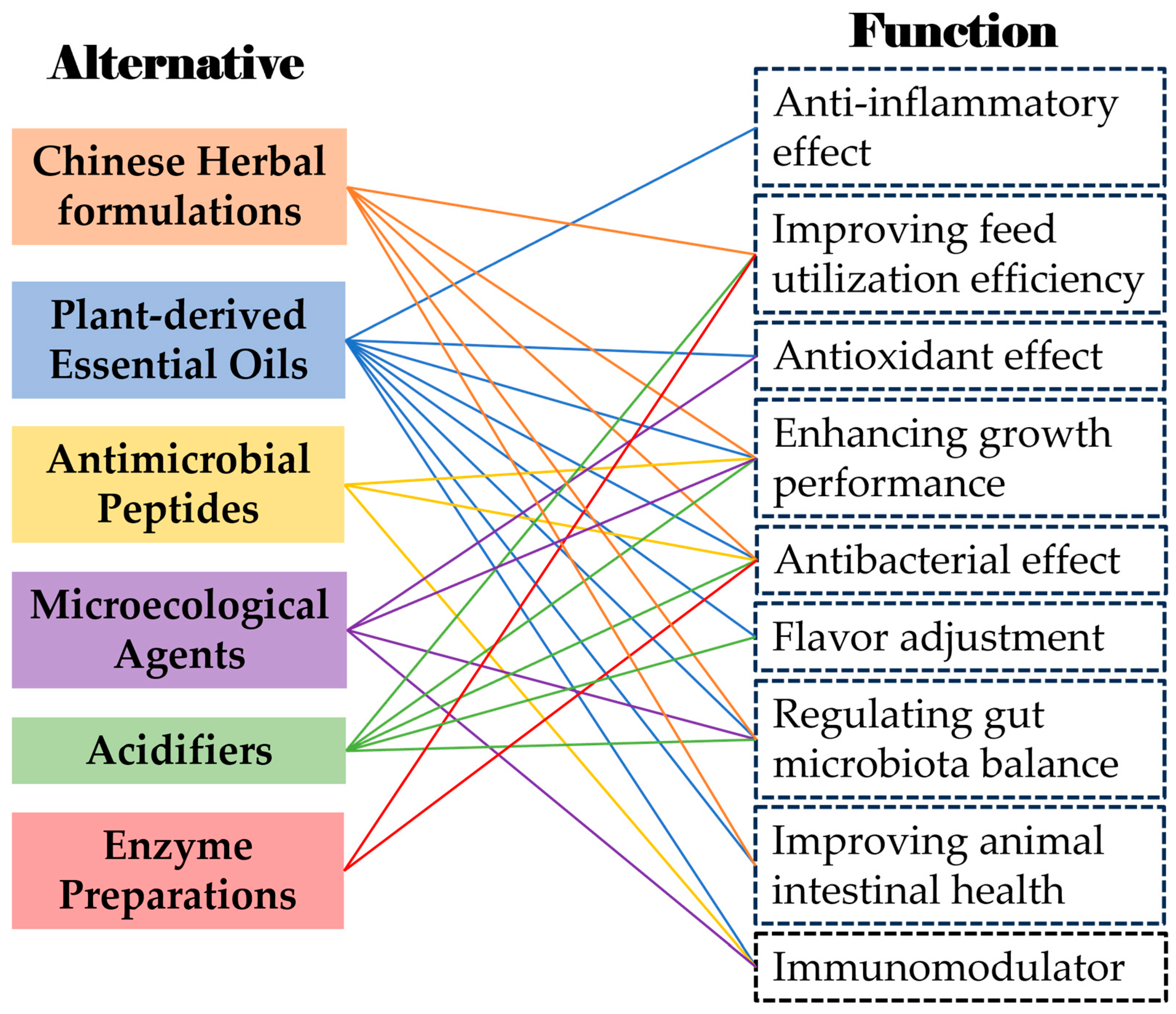
3. Antibiotic Alternatives
3.1. Chinese Herbal Formulations
3.2. Plant-Derived Essential Oils
3.3. Antimicrobial Peptides
3.4. Microecological Agents
3.5. Acidifiers
3.6. Enzyme Preparations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002, 34 (Suppl. S3), S93–S106. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.M.; Levy, S.B. The impact of antibiotic use on resistance development and persistence. Drug Resist. Update 2000, 3, 303–311. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Barnes, E.M. The effect of antibiotic supplements on the faecal streptococci (Lancefield group D) of poultry. Br. Vet. J. 1958, 114, 333–344. [Google Scholar] [CrossRef]
- Elliott, S.D.; Barnes, E.M. Changes in serological type and antibiotic resistance of Lancefield group D streptococci in chickens receiving dietary chlortetracycline. Microbiology 1959, 20, 426–433. [Google Scholar] [CrossRef]
- Swann, M.M. Report of the Joint Committee on the Use of Antibiotics in Animal Husbandry and Veterinary Medicine; HM Stationery Office: London, UK, 1969. [Google Scholar]
- Piva, A.; Bach, K.E.; Lindberg, J.E. Gut Environment of Pigs; Nottingham University Press: Nottingham, UK, 2001; pp. 219–230. [Google Scholar]
- Roe, M.T.; Pillai, S.D. Monitoring and identifying antibiotic resistance mechanisms in bacteria. Poultry Sci. 2003, 82, 622–626. [Google Scholar] [CrossRef]
- He, Y.; Zheng, Y.Y.; Liu, C.; Zhang, H.C.; Shen, J. Citric acid cross-linked β-cyclodextrins: A review of preparation and environmental/biomedical application. Carbohydr. Polym. 2024, 323, 121438. [Google Scholar] [CrossRef]
- Ronquillo, M.G.; Hernandez, J.C.A. Antibiotic and synthetic growth promoters in animal diets: Review of impact and analytical methods. Food Control 2017, 72, 255–267. [Google Scholar] [CrossRef]
- Brown, K.; Uwiera, R.R.E.; Kalmokoff, M.L.; Brooks, S.P.J.; Inglis, G.D. Antimicrobial growth promoter use in livestock: A requirement to understand their modes of action to develop effective alternatives. Int. J. Antimicrob. Agents 2017, 49, 12–24. [Google Scholar] [CrossRef]
- Selvarajan, R.; Obize, C.; Abia, A.L.K.; Sibanda, T.; Long, H.J. Evolution and emergence of antibiotic resistance in given ecosystems: Possible strategies for addressing the challenge of antibiotic resistance. Antibiotics 2022, 12, 28. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, L.Y.; Sun, Y.F.; Xiong, Y.Y.; Li, R.C.; Sui, M.X.; Gao, Z.Z.; Wang, W.; Sun, H.; Dai, J.K. Antifungal activity and multi-target mechanism of action of methylaervine on Candida albicans. Molecules 2024, 29, 4303. [Google Scholar] [CrossRef]
- Teillant, A.; Brower, C.H.; Laxminarayan, R. Economics of antibiotic growth promoters in livestock. Annu. Rev. Resour. Econ. 2015, 7, 349–374. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 16 December 2024).
- O′Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://apo.org.au/node/63983 (accessed on 6 January 2025).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. No Time to Wait: Securing the Future from Drug-Resistant Infections; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Cao, Z.; Zhou, T.T.; Ma, X.R.; Shen, Y.L.; Deng, Q.B.; Zhang, W.; Zhao, Y.F. Hydrogen production from urea sewage on NiFe-based porous electrocatalysts. ACS Sustain. Chem. Eng. 2020, 8, 11007–11015. [Google Scholar] [CrossRef]
- Shao, C.X.; Li, W.Z.; Tan, P.; Shan, A.S.; Dou, X.J.; Ma, D.Y.; Liu, C.Y. Symmetrical modification of minimized dermaseptins to extend the spectrum of antimicrobials with endotoxin neutralization potency. Int. J. Mol. Sci. 2019, 20, 1417. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.P.; Huang, Y.X.; Liu, L. Structural, spectroscopic and computational studies of a new hydrogen-bonded organic framework containing carbazole moiety. J. Mol. Struct. 2024, 1309, 138214. [Google Scholar] [CrossRef]
- Waluszewski, A.; Cinti, A.; Perna, A. Antibiotics in pig meat production: Restrictions as the odd case and overuse as normality? Experiences from Sweden and Italy. Hum. Soc. Sci. Commun. 2021, 8, 172. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Qin, Z.H.; Zhao, T.Q.; Li, Y.Y.; Hou, Z.S.; Hu, H.; Su, X.F.; Gao, Y.N. Crosslinked biodegradable hybrid hydrogels based on poly (ethylene glycol) and gelatin for drug controlled release. Molecules 2024, 29, 4952. [Google Scholar] [CrossRef]
- Liu, D.W.; Li, L.Y.; Shan, L.L.; Zhang, Q.; Yu, H.R. Dietary iron affects lipid deposition, nutritional element, and muscle quality in coho salmon (Oncorhynchus kisutch). Food Chem. X 2022, 15, 100405. [Google Scholar] [CrossRef]
- Krömker, V.; Leimbach, S. Mastitis treatment: Reduction in antibiotic usage in dairy cows. Reprod. Domest. Anim. 2017, 52, 21–29. [Google Scholar] [CrossRef]
- Aarestrup, F.M. Occurrence of glycopeptide resistance among Enterococcus faecium isolates from conventional and ecological poultry farms. Microb. Drug Resist. 1995, 1, 255–257. [Google Scholar] [CrossRef]
- Sun, Z.W.; Liu, Y.H.; Srinivasakannan, C. Green preparation and environmental application of porous carbon microspheres. ChemistrySelect 2020, 5, 9308–9312. [Google Scholar] [CrossRef]
- Kahn, L.H. One Health and the Politics of Antimicrobial Resistance; Johns Hopkins University Press: Baltimore, MD, USA, 2016. [Google Scholar]
- Hayes, D.J.; Jensen, H.H.; Backstrom, L.; Fabiosa, J. Economic impact of a ban on the use of over the counter antibiotics in US swine rations. Int. Food Agribus. Man. Rev. 2001, 4, 81–97. [Google Scholar]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef]
- Sneeringer, S.; Bowman, M.; Clancy, M. The U. S. and EU Animal Pharmaceutical Industries in the Age of Antibiotic Resistance; ERR-264; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2019. [Google Scholar]
- Grave, K.; Greko, C.; Kvaale, M.K.; Torren-Edo, J.; Mackay, D.; Muller, A.; Moulin, G. Sales of veterinary antibacterial agents in nine European countries during 2005-09: Trends and patterns. J. Antimicrob. Chemother. 2012, 67, 3001–3008. [Google Scholar] [CrossRef]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2022. Available online: https://op.europa.eu/en/publication-detail/-/publication/59eda847-b429-11ee-b164-01aa75ed71a1/language-en (accessed on 10 December 2024).
- Nunan, C. Ending Routine farm Antibiotic Use in Europe. 2022. Available online: https://epha.org/wp-content/uploads/2022/02/report-ending-routine-farm-antibiotic-use-in-europe-final-2022.pdf (accessed on 23 December 2024).
- Jensen, H.H.; Hayes, D.J. Impact of Denmark’s ban on antimicrobials for growth promotion. Curr. Opin. Microbiol. 2014, 19, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Belay, D.G.; Jensen, J.D. Quantitative input restriction and farmers’ economic performance: Evidence from Denmark’s yellow card initiative on antibiotics. J. Agric. Econ. 2022, 73, 155–171. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Jensen, V.F.; Emborg, H.-D.; Jacobsen, E.; Wegener, H.C. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. Am. J. Vet. Res. 2010, 71, 726–733. [Google Scholar] [CrossRef]
- Wierup, M. The Swedish experience of the 1986 year ban of antimicrobial growth promoters, with special reference to animal health, disease prevention, productivity, and usage of antimicrobials. Microb. Drug Resist. 2001, 7, 183–190. [Google Scholar] [CrossRef]
- Schuff, S. Congress takes new look at livestock antibiotics. Feedstuffs 2008, 80, 13. [Google Scholar]
- Steede, G.M.; Meyers, C.T.; Li, N.T.; Irlbeck, E.T.; Gearhart, S.T. A Content Analysis of Antibiotic Use in Livestock in National U. S. Newspapers; New Prairie Press: Manhattan, KS, USA, 2019; Volume 103, p. 16. [Google Scholar]
- Johnson, R. Potential Trade Implications of Restrictions on Antimicrobial Use in Animal Production; Congressional Research Service Reports; UNT Digital Library: Washington, DC, USA, 2011. [Google Scholar]
- United States Food and Drug Administration. Guidance for Industry #213: New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with Gfi #209; United States Food and Drug Administration: Rockville, MD, USA, 2013. [Google Scholar]
- Bowman, M.; Marshall, K.K.; Kuchler, F.; Lynch, L. Raised without antibiotics: Lessons from voluntary labeling of antibiotic use practices in the broiler industry. Am. J. Agric. Econ. 2016, 98, 622–642. [Google Scholar] [CrossRef]
- Zhang, L.J.; Du, F.; Wang, Y.; Li, Y.D.; Yang, C.; Li, S.S.; Huang, X.D.; Wang, C.Y. Oil fingerprint identification technology using a simplified set of biomarkers selected based on principal component difference. Can. J. Chem. Eng. 2022, 100, 23–34. [Google Scholar] [CrossRef]
- Jiao, C.P.; Pang, J.X.; Shen, L.; Lu, W.J.; Zhang, P.P.; Liu, Y.Y.; Li, J.; Jia, X.H.; Wang, Y.F. A weak acid and weak base type fluorescent probe for sensing pH: Mechanism and application in living cells. RSC Adv. 2019, 9, 20982–20988. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. Guidance for Industry #263: Recommendations for Sponsors of Medically Important Antimicrobial Drugs Approved for Use in Animals to Voluntarily Bring under Veterinary Oversight All Products That Continue to Be Available Over-The-Counter; United States Food and Drug Administration: Rockville, MD, USA, 2021. [Google Scholar]
- Sneeringer, S.; Macdonald, J.M.; Key, N.; Mcbride, W.; Mathews, K. Economics of Antibiotic Use in U.S. Livestock Production; United States Department of Agriculture: Washington, DC, USA, 2017. [Google Scholar]
- Sneeringer, S.; Short, G.; Maclachlan, M.; Bowman, M. Impacts on livestock producers and veterinarians of FDA policies on use of medically important antibiotics in food animal production. Appl. Econ. Perspect. Pol. 2020, 42, 674–694. [Google Scholar] [CrossRef]
- Sterk, R.U.S. production of red meat and poultry again record High. Food Business News 2019, 15, 128. [Google Scholar]
- United States Food and Drug Administration. From an Idea to the Marketplace: The Journey of an Animal Drug through the Approval Process; United States Food and Drug Administration: Rockville, MD, USA, 2014. [Google Scholar]
- Thomas, N.; Lo, C.Y. The macrosecuritization of antimicrobial resistance in China. J. Glob. Secur. Stud. 2020, 5, 361–378. [Google Scholar] [CrossRef]
- Announcement No. 307 of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Veterinary Drugs Other than Anticoccidial and Chinese Medicines Permitted by the Ministry for Use in Commercial Feed Shall Not Be Added; Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2020.
- The Use of Veterinary Antimicrobials in China Continues to Decline; Ministry of Agriculture and Rural Affairs of the People′s Republic of China: Beijing, China, 2022.
- Lees, P.; Pelligand, L.; Giraud, E.; Toutain, P.-L. A history of antimicrobial drugs in animals: Evolution and revolution. J. Vet. Pharmacol. Therap. 2021, 44, 137–171. [Google Scholar] [CrossRef]
- Flynn, D. South Korea Bans Antibiotics in Animal Feed; Food Safety News: Seattle, WA, USA, 2011. [Google Scholar]
- Cheng, A.C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of fluoroquinolone resistance through successful regulation, Australia. Emerg. Infect. Dis. 2012, 18, 1453–1460. [Google Scholar] [CrossRef]
- Gerbin, C.S. Enhancing US-Japan cooperation to combat antimicrobial resistance. Biosec. Bioterror Biodef. Strat. Pract. Sci. 2014, 12, 337–345. [Google Scholar] [CrossRef]
- Rahman, M.; Fliss, I.; Biron, E. Insights in the development and uses of alternatives to antibiotic growth promoters in poultry and swine production. Antibiotics 2022, 11, 766. [Google Scholar] [CrossRef]
- Biswas, S.; Ahn, J.M.; Kim, I.H. Assessing the potential of phytogenic feed additives: A comprehensive review on their effectiveness as a potent dietary enhancement for nonruminant in swine and poultry. J. Anim. Physiol. Anim. Nutr. 2024, 108, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, J.; Yao, Z.; Li, M. A review on the alternatives to antibiotics and the treatment of antibiotic pollution: Current development and future prospects. Sci. Total Environ. 2024, 926, 171757. [Google Scholar] [CrossRef]
- Tabashsum, Z.; Scriba, A.; Biswas, D. Alternative approaches to therapeutics and subtherapeutics for sustainable poultry production. Poultry Sci. 2023, 102, 102750. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Li, W.; Qin, H.; Yun, J.; Sun, X. The use of Chinese skullcap (Scutellaria baicalensis) and its extracts for sustainable animal production. Animals 2021, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Krauze, M.; Cendrowska-Pinkosz, M.; Matuseviĉius, P.; Stępniowska, A.; Jurczak, P.; Katarzyna, O. The effect of administration of a phytobiotic containing cinnamon oil and citric acid on the metabolism, immunity, and growth performance of broiler chickens. Animals 2021, 11, 399. [Google Scholar] [CrossRef]
- Thuekeaw, S.; Angkanaporn, K.; Nuengjamnong, C. Microencapsulated basil oil (Ocimum basilicum Linn.) enhances growth performance, intestinal morphology, and antioxidant capacity of broiler chickens in the tropics. Anim. Biosci. 2022, 35, 752–762. [Google Scholar] [CrossRef]
- Li, H.; Qiang, J.; Song, C.; Xu, P. Transcriptome profiling reveal Acanthopanax senticosus improves growth performance, immunity and antioxidant capacity by regulating lipid metabolism in GIFT (Oreochromis niloticus). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100784. [Google Scholar] [CrossRef]
- Chen, G.; Li, Z.; Liu, S.; Tang, T.; Chen, Q.H.; Yan, Z.M.; Peng, J.; Yang, Z.K.; Zhang, G.F.; Liu, Y.T.; et al. Fermented Chinese herbal medicine promoted growth performance, intestinal health, and regulated bacterial microbiota of weaned piglets. Anim. 2023, 13, 476. [Google Scholar] [CrossRef]
- Guo, F.C.; Williams, B.A.; Kwakkel, R.P.; Li, H.S.; Li, X.P.; Luo, J.Y.; Li, W.K.; Verstegen, M.W.A. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poultry Sci. 2004, 83, 175–182. [Google Scholar] [CrossRef]
- Hao, Y.; Huo, J.; Wang, T.; Sun, G.; Wang, W. Chemical profiling of Coptis rootlet and screening of its bioactive compounds in inhibiting Staphylococcus aureus by UPLC-Q-TOF/MS. J. Pharm. Biomed. Anal. 2020, 180, 113089. [Google Scholar] [CrossRef]
- Yang, J.L.; Ha, T.K.Q.; Dhodary, B.; Pyo, E.; Oh, W.K. Oleanane triterpenes from the flowers of Camellia japonica inhibit porcine epidemic diarrhea virus (PEDV) replication. J. Med. Chem. 2015, 58, 1268–1280. [Google Scholar] [CrossRef]
- Naz, G.; Sarwar, A.; Mahmood, S.; Sultana, S.; Sarwar, F.; Rasheed, M.; Ali, H.; Hameed, A.; Zahra, H. Plant essential oils as an alternative antimicrobial therapy. In Complementary and Alternative Medicine: Essential Oils; Zafar, M.A., Abbas, R.Z., Imran, M., Tahir, S., Qamar, W., Eds.; Unique Scientific Publishers: Faisalabad, Pakistan, 2024; pp. 114–122. [Google Scholar]
- Jadid, N.; Widodo, A.F.; Ermavitalini, D.; Sa’adah, N.N.; Gunawan, S.; Nisa, C. The medicinal umbelliferae plant fennel (Foeniculum vulgare Mill.): Cultivation, traditional uses, phytopharmacological properties, and application in animal husbandry. Arabian J. Chem. 2023, 16, 104541. [Google Scholar] [CrossRef]
- Lahucky, R.; Nuernberg, K.; Kovac, L.; Bucko, O.; Nuernberg, G. Assessment of the antioxidant potential of selected plant extracts—In vitro and in vivo experiments on pork. Meat Sci. 2010, 85, 779–784. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, P.F.; Ma, X.K.; Shang, Q.H.; Long, S.F.; Wu, Y.; Wang, W.; Piao, X.S. Forsythia suspensa extract protects broilers against breast muscle oxidative injury induced by corticosterone mimicked pre-slaughter acute stress. Poultry Sci. 2018, 97, 2095–2105. [Google Scholar] [CrossRef]
- Mohyuddin, S.G.; Qamar, A.; Hu, C.Y.; Li, Y.; Chen, S.W.; Wen, J.Y.; Bao, M.L.; Ju, X.H. Terpinen4-ol inhibits heat stress induced inflammation in colonic tissue by activating Occludin, Claudin-2 and TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2021, 99, 107727. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; Omari, N.E.; Salehi, B.; Goloshvili, T.; Silva, N.C.C.; Bouyahya, A.; Vitalini, S.; et al. Cinnamomum species: Bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Front. Pharmacol. 2021, 12, 600139. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ma, Y. The application and prospects of antimicrobial peptides in antiviral therapy. Amino Acids 2024, 56, 68. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Y.; Yuan, S.; Mao, M. A comprehensive review of antimicrobial peptides: Mechanisms, classifications and designations. Theor. Nat. Sci. 2024, 46, 131–141. [Google Scholar] [CrossRef]
- Kumari, A.; Mathur, G.; Sharma, G. Antimicrobial peptides in Tuberculosis: Insights into the immunomodulatory mechanisms. Chem. Biol. Lett. 2025, 12, 1253. [Google Scholar] [CrossRef]
- Kraaij, M.D.; van Dijk, A.; Haagsman, H.P. CATH-2 and LL-37 increase mannose receptor expression, antigen presentation and the endocytic capacity of chicken mononuclear phagocytes. Mol. Immunol. 2017, 90, 118–125. [Google Scholar] [CrossRef]
- Nazeer, N.; Uribe-Diaz, S.; Rodriguez-Lecompte, J.C.; Ahmed, M. Antimicrobial peptides as an alternative to relieve antimicrobial growth promoters in poultry. Br. Poultry Sci. 2021, 62, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bai, Y.; Xia, X.; Zhang, M.; Wu, X.; Wu, Y.; Bai, Y.; Liu, S.; Zhang, G.; Hu, J.; et al. Effects of the antimicrobial peptide mastoparan X on the performance, permeability and microbiota populations of broiler chickens. Animals 2022, 12, 3462. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ma, X.; Jiang, X. Effects of immobilized antimicrobial peptides on growth performance, serum biochemical index, inflammatory factors, intestinal morphology, and microbial community in weaning pigs. Front. Immunol. 2022, 13, 872990. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial mechanisms and linical application prospects of antimicrobial peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Shrivastava, S.; Gogoi, P.; Saxena, S.; Srivastava, S.; Singh, R.J.; Godara, B.; Kumar, N.; Gaur, G.K. Wasp venom peptide (polybia mp-1) shows antimicrobial activity against multi drug resistant bacteria isolated from mastitic cow milk. Int. J. Pept. Res. Ther. 2022, 28, 44. [Google Scholar] [CrossRef]
- Bennett, S.; Ben Said, L.; Lacasse, P.; Malouin, F.; Fliss, I. Susceptibility to nisin, bactofencin, pediocin and reuterin of multidrug resistant Staphylococcus aureus, Streptococcus dysgalactiae and Streptococcus uberis causing bovine mastitis. Antibiotics 2021, 10, 1418. [Google Scholar] [CrossRef]
- Fu, J.; Wang, T.; Xiao, X.; Cheng, Y.; Wang, F.; Jin, M.; Wang, Y.; Zong, X. Clostridium Butyricum ZJU-F1 benefits the intestinal barrier function and immune response associated with its modulation of gut microbiota in weaned piglets. Cells 2021, 10, 527. [Google Scholar] [CrossRef]
- Biswas, S.; Kim, M.H.; Baek, D.H.; Kim, I.H. Probiotic mixture (Bacillus subtilis and Bacillus licheniformis) apotential in-feed additive to improve broiler production efficiency, nutrient digestibility, caecal microflora, meat quality and to diminish hazardous odour emission. J. Anim. Physiol. Anim. Nutr. 2023, 107, 1065–1072. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Y.; Kang, X.; Liu, P.; Wang, G. Research progress on the association between mastitis and gastrointestinal microbes in dairy cows and the effect of probiotics. Microb. Pathog. 2022, 173, 105809. [Google Scholar] [CrossRef]
- Urakawa, M.; Zhuang, T.; Sato, H.; Takanashi, S.; Yoshimura, K.; Endo, Y.; Katsura, T.; Umino, T.; Tanaka, K.; Watanabe, H. Prevention of mastitis in multiparous dairy cows with a previous history of mastitis by oral feeding with probiotic Bacillus subtilis. Anim. Sci. J. 2022, 93, e13764. [Google Scholar] [CrossRef]
- Suda, Y.; Kagawa, K.; Fukuyama, K.; Elean, M.; Zhou, B.; Tomokiyo, M.; Islam, M.A.; Rajoka, M.S.R.; Kober, A.K.M.H.; Shimazu, T.; et al. Soymilk-fermented with Lactobacillus delbrueckii subsp. Delbrueckii TUA4408Limproves immune-health in pigs. Benef. Microbes 2022, 13, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, Y.H.; Yang, G.; Duan, J.; Yang, L.Y.; Chen, L.X.; Hou, S.L.; Huang, X.G. Oral administration of Lactobacillus delbrueckii enhances intestinal immunity through inducing dendritic cell activation in suckling piglets. Food Funct. 2022, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Wang, Y.; Lin, X.; Gou, Z.; Fan, Q.; Ye, J.; Jiang, S. Potential effects of acidifier and amylase as substitutes for antibiotic on the growth performance, nutrient digestion and gut microbiota in yellow-feathered broilers. Animals 2020, 10, 1858. [Google Scholar] [CrossRef]
- Chen, Y.M.; Limaye, A.; Chang, H.W.; Liu, J.R. Screening of lactic acid bacterial strains with antiviral activity against porcine epidemic diarrhea. Probiotics Antimicrob. Proteins 2022, 14, 546–559. [Google Scholar] [CrossRef]
- Jones, H.; Gilson, D.; Gosling, R.J.; Oastler, C.; Davies, R.H.; Smith, R.P. The effectiveness of short-duration in-feed organic acid use in finisher pigs for Salmonella control at slaughter. Prev. Vet. Med. 2022, 209, 105772. [Google Scholar] [CrossRef] [PubMed]
- Hervo, F.; Létourneau-Montminy, M.P.; Même, N.; Méda, B.; Duclos, M.J.; Narcy, A. Effect of phytase and limestone particle size on mineral digestibility, performance, eggshell quality, and bone mineralization in laying hens. Poultry Sci. 2023, 102, 102613. [Google Scholar] [CrossRef]
- Cao, S.C.; Gong, J.; Wang, J.; Yan, H.L.; Zhang, H.F.; Liu, J.B. The impact of the interaction between dietary total phosphorus level and efficacy of phytase on the performance of growing-finishing pigs. Anim. Feed Sci. Technol. 2023, 298, 115605. [Google Scholar] [CrossRef]
- Bugoni, M.; Takiya, C.S.; Grigoletto, N.T.S.; Vittorazzi Júnior, P.C.; Nunes, A.T.; Chesini, R.G.; da Silva, G.G.; Durman, T.; Pettigrew, J.E.; Rennó, F.P. Feeding amylolytic and proteolytic exogenous enzymes: Effects on nutrient digestibility, ruminal fermentation, and performance in dairy cows. J. Dairy Sci. 2023, 106, 3192–3202. [Google Scholar] [CrossRef]
- Dang, D.X.; Chung, Y.H.; Kim, I.H. E. coli-expressed human lysozyme supplementation improves growth performance, apparent nutrient digestibility, and fecal microbiota in weaning pigs. Livest. Sci. 2022, 263, 105004. [Google Scholar] [CrossRef]
- Singh, A.K.; Mandal, R.K.; Bedford, M.R.; Jha, R. Xylanase improves growth performance, enhances cecal short-chain fatty acids production, and increases the relative abundance of fiber fermenting cecal microbiota in broilers. Anim. Feed Sci. Technol. 2021, 277, 114956. [Google Scholar] [CrossRef]
| Veterinary Drug Formulations | Notice Number | Description of Functions |
|---|---|---|
| Ligustrum lucidum extract powder | No. 187 in 2019 | Enhancing immunity and promoting growth; to promote the growth of chicken. |
| Macleaya cordata powder | No. 246 in 2019 | Antibacterial, anti-inflammatory, appetizing, and growth promoting; to promote the growth of pigs, chickens, meat ducks, freshwater fish, shrimp, crabs, and turtles. |
| Scutellaria baicalensis extract powder | No. 246 in 2019 | Anti-inflammatory, antibacterial, and growth promoting; to promote the growth of broiler chickens and weaned piglets. |
| Callicarpa nudiflora powder | No. 327 in 2020 | Anti-inflammatory, antibacterial, hemostatic, and growth promoting; to promote the growth of pigs. |
| Leonurus japonicus extract powder | No. 408 in 2021 | Promoting blood circulation and removing blood stasis; decreased egg production rate in the late stage of laying hens. |
| Qiweng Huangbo Powder | No. 520 in 2022 | Anti-inflammatory, anti-diarrheal, and growth promoting; to prevent piglet diarrhea and improve growth performance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Li, Y.; Chen, C.; Wang, N.; Yang, F. Solutions to the Dilemma of Antibiotics Use in Livestock and Poultry Farming: Regulation Policy and Alternatives. Toxics 2025, 13, 348. https://doi.org/10.3390/toxics13050348
Zheng S, Li Y, Chen C, Wang N, Yang F. Solutions to the Dilemma of Antibiotics Use in Livestock and Poultry Farming: Regulation Policy and Alternatives. Toxics. 2025; 13(5):348. https://doi.org/10.3390/toxics13050348
Chicago/Turabian StyleZheng, Shimei, Yongchao Li, Cuihong Chen, Naiyu Wang, and Fengxia Yang. 2025. "Solutions to the Dilemma of Antibiotics Use in Livestock and Poultry Farming: Regulation Policy and Alternatives" Toxics 13, no. 5: 348. https://doi.org/10.3390/toxics13050348
APA StyleZheng, S., Li, Y., Chen, C., Wang, N., & Yang, F. (2025). Solutions to the Dilemma of Antibiotics Use in Livestock and Poultry Farming: Regulation Policy and Alternatives. Toxics, 13(5), 348. https://doi.org/10.3390/toxics13050348







