Abstract
Industrial development has increased environmental dioxin concentrations, sparking concern about human health impacts. Examining dioxin neurotoxicity has highlighted associations with cognitive impairment and behavioral abnormality. Dioxins are ligands of the aryl hydrocarbon receptor (AHR), a ligand-activated transcription factor; it is speculated that dioxin-induced AHR activation is pivotal for toxic effects. Accurate AHR-expressing cell identification is therefore indispensable for understanding the molecular and cellular mechanisms of dioxin toxicity. Herein, current knowledge regarding AHR expression in the mammalian brain is summarized, and dioxin neurotoxicity mechanisms are discussed. Histological studies show AHR-expressing neurons in multiple brain regions, including the hippocampus and cerebral cortex. Dopaminergic and noradrenergic neurons exhibit AHR expression, suggesting possible roles in the monoaminergic system. AHR overactivation evokes dendritic arborization atrophy, whereas its deficiency increases complexity, implying that AHR-mediated signaling is crucial for neuronal growth and maturation. AHR is also involved in neurogenesis and neuronal precursor migration. Collectively, these findings support the notion that dioxin-induced AHR overactivation in individual neurons disrupts neural circuit structure, ultimately leading to impaired brain function. However, as AHR downstream signaling is intertwined with various molecules and pathways, the precise mechanisms remain unclear. Further studies on the expression, signaling, and roles of AHR are needed to clarify dioxin neurotoxicity.
1. Introduction
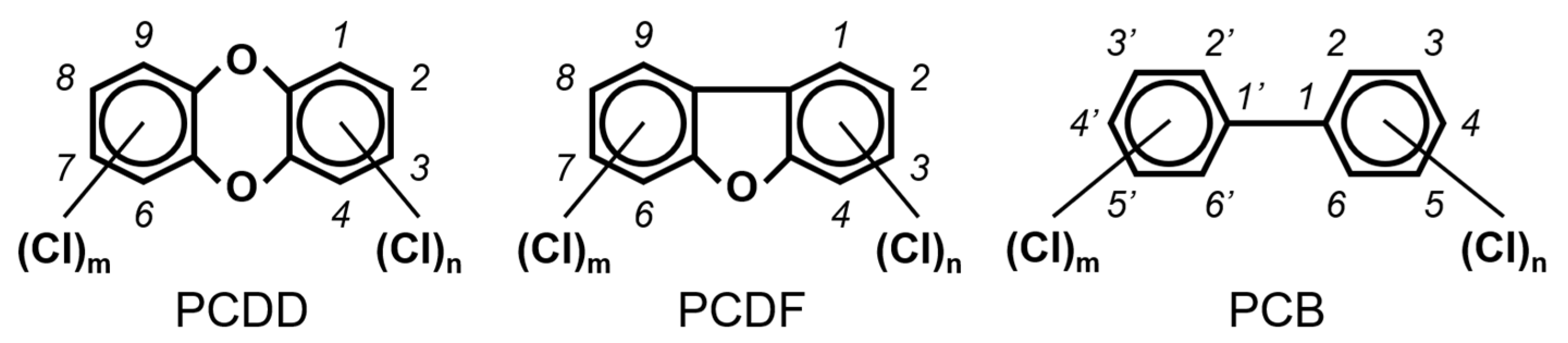
Chlorinated dioxins include two series of organohalogenated substances, polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs), which together contain 210 congeners (75 PCDDs and 135 PCDFs). Chemically, the compounds comprise two benzene rings connected by oxygen atoms, differing in the number and position of their chlorine atoms (Figure 1). Because dioxins can be formed by natural processes, such as forest fires and volcanic eruptions [1,2], they were present on Earth long before industrialization. For instance, dioxins were identified in historical soil, herbage, and sediment samples collected from England and Switzerland in the 1840s [3,4]. They were also identified in soil samples dating back to 70–40 B.C., sourced from Roman brickwork along the Lower Rhine in Germany [5]. With the development of the chlorine chemical industry in the 20th century, dioxins were produced as unwanted byproducts of industrial and thermal processes and released into the environment, substantially increasing their concentrations in our surroundings [1,3,4,6]. Polychlorinated biphenyls (PCBs), comprising 209 congeners, are also organohalogenated substances that are deeply related to dioxins (Figure 1), because some coplanar congeners have similar toxicological characteristics (i.e., dioxin-like PCBs (dl-PCBs)). In contrast to dioxins, PCBs began to be produced commercially in 1929 and had a wide range of applications, such as electronic appliances and heat transfer systems. Although their production is currently banned, they may be produced unintentionally during thermal reactions in industrial processes [2,7].
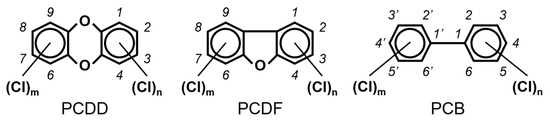
Figure 1.
The chemical structures of polychlorinated dibenzo-p-dioxin (PCDD), polychlorinated dibenzofuran (PCDF), and polychlorinated biphenyl (PCB).
Dioxins and PCBs, which are both lipophilic and hydrophobic, are chemically stable and resistant to metabolic processes, resulting in environmental persistence. Humans have a relatively high trophic level; as these compounds tend to bioaccumulate, human tissues may contain relatively high amounts of them. Therefore, humans are at risk of not only occupational and accidental exposure but also environmental (background) exposure. A plethora of epidemiological, clinical, and experimental studies has revealed a variety of toxic effects in humans and laboratory animals, including cancer, reproductive disorders, immune deficiency, endocrine disruption, and cognitive impairment [2]. Importantly, molecular studies have determined that most, if not all, of the toxic effects of exposure to dioxins and dl-PCBs are evoked through the aryl hydrocarbon receptor (AHR), a ligand-activated transcription factor. Upon binding to a ligand, AHR translocates from the cytoplasm into the nucleus, where it acts as a transcription factor, inducing target gene expression [8]. Because some dioxins and dl-PCBs function as high-affinity ligands, AHR is a pivotal molecule in their toxicity.
A meeting of the World Health Organization was held in 1997 to assess and propose toxic equivalency factor (TEF) values for a group of 29 dioxin and dl-PCB congeners based on their chemical structure, binding affinity to and reactivity with AHR, environmental persistence, and bioaccumulation in the food chain [9]. Specifically, seventeen dioxins (seven PCDDs and ten PCDFs) displaying 2,3,7,8-substituted compounds with tetra- to octa-chlorines and twelve dl-PCBs (non- and mono-ortho-substituted PCBs) were assigned TEF values indicating their toxic strength relative to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TeCDD, TEF = 1), the most toxic congener in the group. These TEF values have since been reevaluated and revised twice, in 2005 and 2022 [10,11] (Table 1), and the risk posed by dioxins is still a subject of active global debate.

Table 1.
Toxic equivalency factors (TEFs) for dioxins and dioxin-like polychlorinated biphenyls (dl-PCBs) proposed by the World Health Organization in 1998, 2005, and 2022.
Accumulating evidence suggests that a variety of chemicals, including organohalogenated substances, could potentially disrupt the development and function of the central nervous system (CNS) in children [12,13,14,15]. Because dioxin exposure reportedly evokes impaired brain function and abnormal behavior in humans and laboratory animals, dioxins are representative chemicals that contribute to the disruption of developmental processes in the CNS, such as neuronal growth and neural circuit formation. Thus, the goals of this review are to (1) outline epidemiological studies showing dioxin neurotoxicity associated with human brain function, (2) summarize experimental studies using laboratory animals that support dioxin neurotoxicity in humans, and (3) review AHR expression and signaling with an emphasis on neurons in the mammalian brain.
2. Epidemiological Studies on Dioxin Neurotoxicity
In previous decades, epidemiological studies have revealed the toxic effects of dioxins on neurodevelopment in humans. First, food poisoning caused by rice bran oil contaminated with the dioxins generated from heat-degraded PCBs was identified. Known as “oil disease,” it was reported in Yusho, Japan, in 1968 and Yu-cheng, Taiwan, in 1979. Patients suffering from oil disease exhibited various symptoms, such as hyperpigmentation and ophthalmological manifestations [16,17,18,19]. Because some neurological symptoms, including mild intellectual disability, were reported in children born to mothers consuming contaminated oil in Yusho [20], it is plausible that dioxin exposure could have disrupted the development of the CNS during the fetal and infant stages. Neurotoxic effects were also reported in the children of mothers exposed to dioxins and PCBs in Yu-cheng. Specifically, worse scores in multiple landmarks associated with mental and motor development were revealed in exposed children aged 1–82 months compared to unexposed children [21]. Later, cognitive impairment and activity disorders were found in children up to 12 years of age [22,23,24,25,26,27]. While dioxin congeners, especially 2,3,4,7,8-pentachlorodibenzofuran, are likely involved in toxic effects in patients [16,28], it is interesting to note that perinatal exposure to PCBs is relevant to cognitive and intellectual impairments in children [29,30,31]. Therefore, PCBs could partly contribute to neurotoxicity, suggesting that combined exposure is an underlying mechanism of toxicities in oil disease.
During the Vietnam War (1961–1971), the herbicide Agent Orange, which contains dioxins, was sprayed in Southern Vietnam by the United States Air Force (USAF) [32,33]. Moreover, owing to herbicide spills, dioxins have contaminated the environment (i.e., soil and sediment) surrounding former USAF bases [34]. Thus, it is reasonable to speculate that dioxins could be transferred not only to aquatic organisms, such as fish, but also to agricultural products, resulting in human exposure through the food chain. Consistent with this speculation, dioxin levels in the breast milk of mothers residing in contaminated areas around former USAF bases were higher than those of mothers in unsprayed areas [35,36], implying that their infants were perinatally exposed to dioxins. Indeed, birth cohort studies have revealed altered body growth and adverse effects on neurodevelopment, such as cognitive, language, and motor functions, in infants and children [37,38,39]. Additionally, epidemiological evidence of children exhibiting symptoms of neurodevelopmental disorders (i.e., autistic traits, learning disabilities, and hyperactivity) in contaminated areas continues to accumulate [40,41,42]. Notably, among the dioxin congeners, TeCDD is strongly associated with these symptoms. Magnetic resonance imaging scans of adult men who were estimated to have been perinatally exposed to dioxins displayed increases in gray matter volume and total brain volume, while their white matter volume was decreased [43,44,45]. Intriguingly, these studies also demonstrated relationships between brain volume and blood dioxin levels, a marker of exposure in adulthood. This suggests the possibility of dioxin exposure impacting brain structure and function even in adulthood. In line with this idea, veterans exposed to Agent Orange reportedly exhibited brain atrophy and a higher prevalence of dementia than unexposed veterans in Korea and the USA [46,47].
Epidemiological studies in European countries and the USA have revealed the effects of perinatal exposure to dioxins on neurodevelopment in children. A chemical factory explosion in Seveso, Italy, occurred in 1976, exposing residents to dioxins [48]. In the beginning, while cancer prevalence and reproductive impairment increased, no overt neurotoxicity to physical function or working memory were found [49]. Later, another study focusing on second-generation health revealed that exposure adversely affected neuropsychological functions in boys, albeit with limited evidence [50]. Notably, several studies showing dioxin neurotoxicity at a background level have uncovered that higher dioxin concentrations in mothers (i.e., in blood and breast milk) are correlated with worse scores associated with learning, attention, language skills, and motor development in the Netherlands, Denmark, Norway, Germany, and the USA [51,52,53,54,55,56,57]. Two cohort studies in Japan suggested possible effects of environmental dioxins on health conditions, including neurodevelopmental conditions [58]. Dioxin concentrations in maternal samples were inversely correlated with the mental and psychomotor development of their children at 6 months [59,60], whereas little effect was found at 18 and 42 months [61,62].
These epidemiological studies have demonstrated adverse effects of dioxins on brain development and function in humans, suggesting the CNS as a target tissue. Generally, in the case of human studies, it is extremely difficult to completely exclude interactions between other factors, such as co-exposure to multiple chemicals and the lifestyles of individuals; therefore, experimental studies using laboratory animals are helpful to confirm the toxicity of specific chemicals. In the next section, I summarize the current understanding of behavioral abnormalities in rodents exposed to dioxins to assess the neurotoxicity.
3. Experimental Animal Studies on Dioxin Neurotoxicity
In the field of neuroscience, various behavioral tests have been developed to assess multiple brain functions, such as learning and memory, emotion, and motor activity, in laboratory animals, especially in rats and mice [63,64]. Toxicological researchers actively utilized these tests to evaluate the neurotoxicity of environmental pollutants and the safety of pharmaceutical chemicals [65]. In parallel, histopathological examination focusing on neuronal morphology offers valuable insights into behavior, because neuronal growth and neural circuit structure are linked to the development and function of the CNS. For example, neural circuits in the hippocampal CA1, CA3, and dentate gyrus (DG) subregions are known to be crucial for learning and memory in both humans and rodents [66]. Moreover, aberrant neuromorphology has been found in the brains of human patients and animal models with neurodevelopmental disorders and age-related diseases [67,68,69,70]. These results indicate the deep relationship between brain functions and neural circuit structures, supporting the importance of behavioral assessment with simultaneous histopathological examination to more accurately evaluate chemical neurotoxicity.
Rodent offspring born to dams administered dioxins have been frequently used for behavioral tests, revealing the impairment of multiple brain functions later in life after in utero and lactational exposure (Table 2). Specifically, perinatal exposure to TeCDD induced learning and memory impairment in not only rodents [71,72,73,74,75,76,77,78] but also in non-human primates [79]. Furthermore, rat and mouse offspring exposed to TeCDD in utero and via lactation exhibited impairments of contextual fear memory, behavioral flexibility, operant behavior, and motor behavior [80,81,82,83,84,85], as well as hyperactivity [86], in adulthood. Atypical behaviors have been reported in not only adults but also infants. My colleagues and I revealed the suppression of ultrasonic vocalizations (USVs) emitted by the infant mice of dams treated with TeCDD [87]. Because atypical infant USV emissions have been detected in several mouse models with neurodevelopmental disorders [88,89,90], our study suggests that infant USVs are toxicologically valuable as a behavioral endpoint at an early life stage. In line with epidemiological studies, these resultant abnormalities in laboratory animals support the notion that the exposure of mothers to dioxins adversely affects the development and function of the CNS in their offspring.
Such behavioral abnormalities could potentially be caused by dioxins disrupting the neural circuit structure. Indeed, perinatal exposure to TeCDD altered the expression pattern of key genes regulating neurite elongation and synapse formation in the developing brains of mouse offspring [91,92]. Histopathological examination demonstrated the atrophy of the cerebral cortex in mice perinatally exposed to TeCDD, along with a decrease in non-GABAergic neurons [93]. Aberrant morphology was found in the hippocampal and amygdala neurons of TeCDD-exposed mouse offspring [71,94]. This is consistent with an in vitro study demonstrating TeCDD-dependent changes in neurite elongation in primary cultured cortical neurons [95]. Additionally, perinatal exposure to TeCDD inhibited neurogenesis in the cerebellar granule neurons of developing mice [96]. These findings are in accordance with the idea that in utero and lactational exposure to dioxins may disrupt brain development, including neuronal growth, in the fetal and infant stages, ultimately leading to impaired brain function later in life.
Dioxin exposure, even in adulthood, can elicit behavioral abnormalities. TeCDD exposure reduced neurogenesis in the hippocampal DG of adult mice, exhibiting the impairment of contextual fear memory [97]. This implies that the hippocampus is vulnerable to dioxins and, importantly, is consistent with the epidemiological study reporting the increased prevalence of dementia in veterans described above [47]. In another study, depression-like behavior was observed in mice exposed to TeCDD [98]. These studies suggest the potential risk of dioxins to brain function and neural circuit maintenance throughout life.
Behavioral tests using laboratory animals are valuable tools to assess brain function and evaluate dioxin neurotoxicity; however, there are large differences in behavioral styles between humans and other species. When attempting to compensate for this gap and obtain compelling evidence, a molecular biology perspective is helpful. In this context, AHR, a protein that functions as a dioxin receptor, is key to elucidating the toxic mechanisms of dioxins. Therefore, in the following section, I briefly outline the profile of AHR.

Table 2.
Behavioral abnormalities of laboratory animals exposed to 2,3,7,8-substituted dioxins.
Table 2.
Behavioral abnormalities of laboratory animals exposed to 2,3,7,8-substituted dioxins.
| Dioxins | Chemical Structure | TEFs | Behaviors (Brain Functions) | Species | References |
|---|---|---|---|---|---|
| TeCDD | 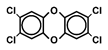 | 1 | Learning and memory | Monkey | Schantz and Bowman, 1989 [79] |
| Rat | Schantz et al., 1996 [75] | ||||
| Seo et al., 1999 [77] | |||||
| Seo et al., 2000 [76] | |||||
| Hojo et al., 2008 [73] | |||||
| Kakeyama et al., 2014 [74] | |||||
| Zhang et al., 2018 [78] | |||||
| Hattori et al., 2021 [72] | |||||
| Mouse | Gileadi et al., 2021 [71] | ||||
| Contextual fear memory | Rat | Mitsui et al., 2006 [83] | |||
| Mouse | Haijima et al., 2010 [81] | ||||
| Latchney et al., 2013 [97] | |||||
| Operant behavior | Rat | Markowski et al., 2001 [82] | |||
| Motor behavior | Rat | Nishijo et al., 2007 [85] | |||
| Socioemotional behavior | Rat | Nguyen et al., 2013 [84] | |||
| Behavioral flexibility | Mouse | Endo et al., 2012 [80] | |||
| Infant USV | Mouse | Kimura and Tohyama, 2018 [87] | |||
| Hyperactivity | Mouse | Sha et al., 2021 [86] | |||
| Depression-like behavior | Mouse | Debler et al., 2024 [98] | |||
| TeBDD | 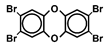 | 1 a | Learning and memory | Rat | Kakeyama et al., 2014 [74] |
| Contextual fear memory | Mouse | Haijima et al., 2010 [81] | |||
| TeBDF | 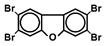 | 0.1 a | Exploratory behavior | Mouse | Kimura et al., 2020 [99] |
| Infant USV | Mouse | Kimura et al., 2020 [99] | |||
| Kimura et al., 2022 [100] | |||||
| TrBCDF | 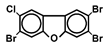 | 0.1 a | Infant USV | Mouse | Kimura et al., 2023 [101] |
a Interim values based on Van den Berg et al., 2013 [102]. TEF, toxic equivalency factor; TeBDD, 2,3,7,8-tetrabromodibenzo-p-dioxin; TeBDF, 2,3,7,8-tetrabromodibenzofuran; TeCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TrBCDF, 2,3,7-tribromo-8-chlorodibenzofuran; USV, ultrasonic vocalization.
4. Molecular Characteristics of AHR
AHR, a member of the basic helix–loop–helix (bHLH)/Per-Arnt-Sim (PAS) family, consists of multiple domains, including the PAS and transactivation domains (Figure 2A), which were cloned in humans and mice in the 1990s [103,104]. As mentioned in the introduction, AHR is localized in the cytoplasm, forming a complex with heat shock protein 90, AHR-interacting protein (also known as XAP), and p23 in the absence of its ligand [105]. Interestingly, varieties of AHR ligands have been identified not only in environmental pollutants, such as dioxins, but also in dietary and microbial metabolites [106,107]. The liganded AHR translocates to the nucleus, where it forms another complex with nuclear proteins and acts as a transcription factor, upregulating the expression of various target genes (Figure 2B). To date, several nuclear proteins have been identified as AHR-binding partners, regulating cellular activities and physiological functions through the induction of specific target genes. First, the AHR nuclear translocator (ARNT), belonging to the bHLH-PAS family, is the most well-known partner protein [108]. The AHR–ARNT heterodimer induces the expression of the cytochrome P450 (CYP) family genes, which encode enzymes that metabolize xenobiotic chemicals [109]. The heterodimer binds to xenobiotic response element (XRE) sequences in the genomic DNA, upregulating the expression of CYPs, such as CYP1A1 and 1B1, and AHR repressor (AHRR) genes. AHRR belongs also to the bHLH-PAS family and competes with AHR to bind to ARNT; thus, AHR-dependent transcriptional activity is inhibited by AHRR, likely providing negative feedback for AHR signaling pathways [110]. Second, the nuclear factor (NF)-κB subunits (i.e., RELA and RELB), which control the inflammatory response, are also known partner proteins of AHR [111,112]. Functionally, the expression of interleukin-6 (IL-6) is upregulated by the activation of both AHR and RELA [113], while the AHR–RELB complex induces the expression of IL-8 in TeCDD-exposed cells [114]. These results suggest that some inflammatory responses are intertwined with the crosstalk between AHR- and NF-κB-signaling regulators. Third, AHR binds to Krüppel-like factor 6 (KLF6) for transcriptional activity, inducing the expression of cyclin-dependent kinase inhibitor 1A (CDKN1A, also known as p21CIP1) and plasminogen activator inhibitor-1 [115,116]. CDKN1A plays an important role in cell cycle arrest via hypophosphorylation in retinoblastoma (RB) [117], suggesting the possibility that AHR is associated with cellular proliferation through preferential interactions with KLF6. In line with this idea, exposure to xenobiotic AHR ligands induces cell cycle arrest and increases the expression of CDKNs, including CDKN1A [93,118,119,120]. Furthermore, AHR forms complexes with multiple proteins, such as the RB, Maf, and estrogen receptor proteins [121,122,123], which is consistent with the fact that numerous genes are regulated in an AHR-dependent manner [124]. The reader should refer to specialized reviews for further details regarding the signaling pathways linked to AHR, ligands, and partner proteins [125,126,127,128,129,130,131].
Given these characteristics of AHR, even in situations in which AHR needs to be stably localized in the cytoplasm, dioxins may induce forced translocation into the nucleus of exposed cells, resulting in excessive transcriptional activation. The subsequent overexpression of AHR target genes could potentially perturb intracellular signaling pathways, leading to the disruption of cellular growth, tissue structure, and organ function. Specifically, TeCDD exposure evoked tissue lesions and teratogenicity, such as cleft palates and hydronephrosis, in wild type (Ahr+/+) mice but not in Ahr−/− mice [132,133], indicating the pivotal role of AHR and its downstream signaling in dioxin toxicity. While exhibiting resistance to TeCDD, albeit not to its embryonic lethality, Ahr−/− mice displayed abnormalities in developmental processes, physiological functions, aging, and lifespan [134,135,136,137]. Intriguingly, loss-of-function mutations in AHR genes are considered the genetic etiology of congenital nystagmus in both humans and mice [138,139,140], suggesting a possible role of AHR in nervous system development across species. Mice lacking AHR exhibited learning and memory impairment, neurogenesis reduction, and synaptic dysfunction, whereas no obvious gross anatomical abnormalities were reported in their brain tissues [97,141,142]. These biological and clinical studies suggest neuronal AHR expression throughout life (i.e., from the embryonic to aged stages) and highlight the importance of the spatiotemporal regulation of AHR signaling pathways in the nervous system. Therefore, the accurate identification of AHR-expressing neurons is indispensable to understanding the molecular mechanism of dioxin neurotoxicity. Next, I summarize the current insights provided by studies on AHR expression in the mammalian brain.
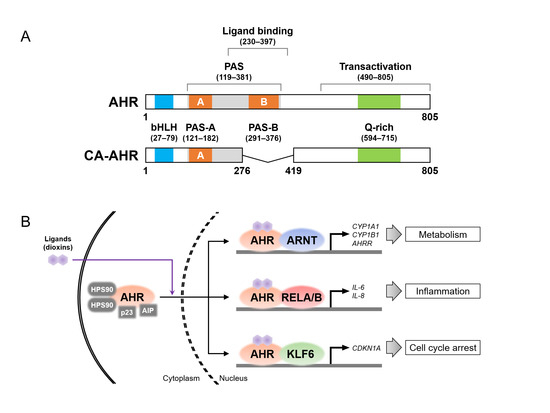
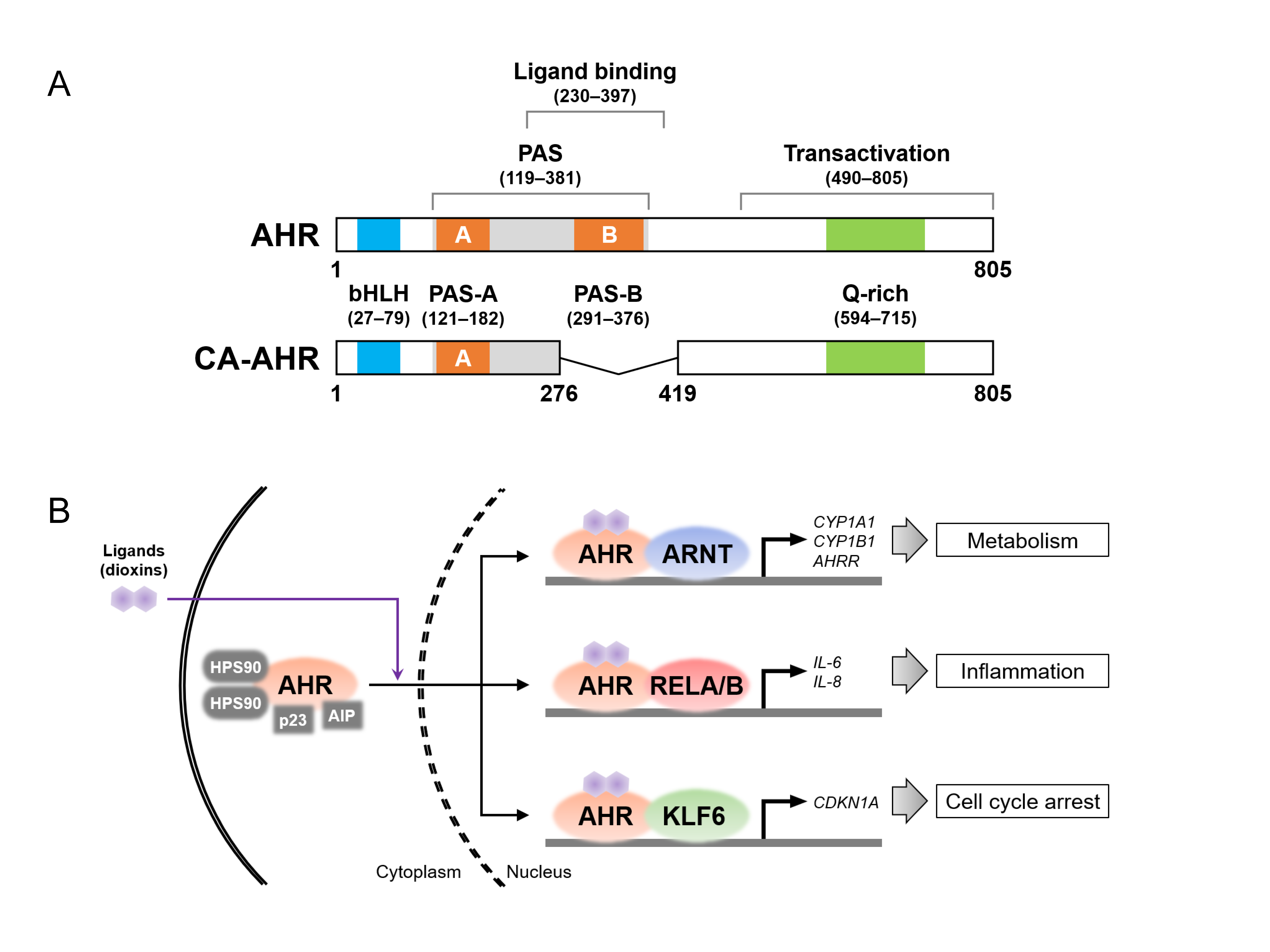
Figure 2.
Aryl hydrocarbon receptor (AHR) protein structure and its binding partners. (A) Protein structures of the full-length AHR in the C57BL/6 mouse strain (top) and constitutively active (CA) AHR (bottom). AHR consists of the functional domains, i.e., Per-Arnt-Sim (PAS, shown in gray), ligand-binding, and transactivation domains. The basic helix–loop–helix (bHLH), PAS-A and -B, and glutamine (Q)-rich motifs are shown in blue, orange, and green, respectively. The CA-AHR shows the deletion of amino acid residues 277–418, containing the ligand-binding domain and the PAS-B motif. The number of amino acid residues in each domain and motif were sourced from the references [94,125,126,143,144]. (B) Schematic image of ligand-induced nuclear translocation of AHR and AHR complexes with partner proteins (i.e., AHR nuclear translocator (ARNT), RELA/B, and Krüppel-like factor 6 (KLF6)) for the transcriptional induction of their target genes. AHRR, AHR repressor; AIP, AHR-interacting protein; CDKN1A, cyclin-dependent kinase inhibitor 1A; HSP90, heat shock protein 90.
Figure 2.
Aryl hydrocarbon receptor (AHR) protein structure and its binding partners. (A) Protein structures of the full-length AHR in the C57BL/6 mouse strain (top) and constitutively active (CA) AHR (bottom). AHR consists of the functional domains, i.e., Per-Arnt-Sim (PAS, shown in gray), ligand-binding, and transactivation domains. The basic helix–loop–helix (bHLH), PAS-A and -B, and glutamine (Q)-rich motifs are shown in blue, orange, and green, respectively. The CA-AHR shows the deletion of amino acid residues 277–418, containing the ligand-binding domain and the PAS-B motif. The number of amino acid residues in each domain and motif were sourced from the references [94,125,126,143,144]. (B) Schematic image of ligand-induced nuclear translocation of AHR and AHR complexes with partner proteins (i.e., AHR nuclear translocator (ARNT), RELA/B, and Krüppel-like factor 6 (KLF6)) for the transcriptional induction of their target genes. AHRR, AHR repressor; AIP, AHR-interacting protein; CDKN1A, cyclin-dependent kinase inhibitor 1A; HSP90, heat shock protein 90.
5. Neuronal AHR in the Mammalian Brain
AHR expression in the mammalian brain has been examined at both the transcript and protein levels. In humans, AHR transcripts have been detected in adult and fetal brains using reverse transcription PCR (RT-PCR), albeit at a lower level than that in other tissues [145]. RT-PCR also revealed Ahr transcripts in multiple brain regions in rats and mice, including the hippocampus, cerebral cortex, cerebellum, olfactory bulb, and pituitary gland [146,147]. Consistent with the transcript-level results, AHR proteins were found in these brain regions in mice by western blotting [148]. However, while these bulk approaches strongly indicate AHR expression in the brain, it is impossible to use them to identify which neurons harbor AHR in situ.
Histological experiments are useful to microscopically identify the AHR-expressing regions and neurons in a tissue sample. In situ hybridization (ISH) revealed Ahr transcript expression in various brain regions in adult rats, including the hippocampus and cerebral cortex [149], which is consistent with the RT-PCR and western blotting results [146,147,148]. More specifically, the ISH study demonstrated Ahr transcripts in the hippocampal pyramidal and granule cell layers, which make up most of the neurons in the CA1, CA2, CA3, and DG subregions [149]. These neuronal layers have more abundant Ahr transcripts than nearby areas (i.e., the stratum radiatum and oriens in the CA1, CA2 and CA3, and the hilus in the DG), suggesting that AHR is preferentially expressed in neurons. Similar to rats, mice also exhibited high Ahr transcripts in the CA1, CA3, and DG neuronal layers in both adult and developmental stages [147]. Additionally, in mice, Ahr transcripts were found in the neuroepithelium, which is the neuronal precursor of embryos [150,151]; thus, AHR may serve a function in neurogenesis. In support of this speculation, even in adults, Ahr transcripts were detected in neuronal precursors in the hippocampal DG subregion [97] and in the rostral migratory stream (RMS), where neuronal precursors migrate toward the olfactory bulb [147]. These findings suggest that AHR contributes to the growth of neuronal lineage cells from the developmental to adult stages. The detection of Ahr alongside a neuronal marker provides compelling evidence of its neuronal expression. In rats, ISH revealed Ahr transcripts in Gad1/2 transcript-positive cells (i.e., GABAergic neurons) in the preoptic area [152]; however, how AHR contributes developmentally and functionally to the GABAergic system in the mammalian brain remains unclear.
Using immunohistochemistry (IHC) to detect proteins is a more useful approach than ISH, because it allows researchers to not only identify AHR-expressing neurons but also analyze the intracellular localization of AHR at the single-neuron level. Notably, several IHC studies have shown hippocampal neurons harboring AHR across mammalian species. Consistent with ISH studies, AHR proteins were detected in the CA1, CA3, and DG neuronal layers of rodents [153,154,155], and in the CA1 neuronal layer in human brains [156]. While, in mice, AHR was found in the CA1 neuronal layer, its immunohistochemical staining concentration was dramatically attenuated by Ahr knockdown [141]. Additionally, AHR was detected in neurons expressing NeuN, a general neuronal marker, in the cerebral cortex and island of Calleja major (ICjM) in mice [148,157]. Intriguingly, in some IHC studies, AHR appears to be abundantly expressed in monoaminergic neurons. For instance, AHR was found in dopaminergic neurons expressing tyrosine hydroxylase (TH), an enzyme which is crucial for dopamine synthesis, in the ventral tegmental area and substantia nigra compacta of rodents [158,159]. In another study, AHR was evident in neurons expressing TH and dopamine-β-hydroxylase in the locus coeruleus (LC), responsible for the noradrenergic system, in Ahr+/+ mice but not in Ahr−/− mice [148]. Moreover, microscopy image analysis revealed that nuclear AHR was significantly increased in neurons of the ICjM and LC in mice orally exposed to TeCDD [148]. This provides evidence that dioxin intake activates AHR in brain neurons after intestinal absorption, blood circulation, and the crossing of the blood–brain barrier. Taken together, these histological findings indicate the existence of AHR in multiple types of brain neurons, implying its fundamental role in brain development and function in mammals.
In vitro studies using primary cultured neurons are helpful in supporting the notion of neuronal AHR expression. Immunocytochemistry (ICC) and western blotting have demonstrated AHR expression in cultured neurons from the mouse hippocampus [160,161] in accordance with histological observations in human and rodent brains. RT-PCR, western blotting, and ICC have identified Ahr transcripts and AHR proteins in cultured neurons from the cerebral cortex and cerebellum of rodents [162,163,164,165,166]. Cultured neurons from the hippocampus and cerebral cortex have been shown to exhibit the co-expression of AHR and MAP-2, a neuron-specific microtubule [161,163]. Additionally, the HT22 cell line, originating from mouse hippocampal neurons, harbors AHR [154,167]. These in vitro studies provide experimental evidence of the neuronal expression of AHR in the mammalian brain.
Table 3 summarizes AHR-expressing neurons in the mammalian brain, with AHR expression patterns organized according to neuronal subtypes, brain regions, and species. Collectively, these histological studies have revealed AHR-expressing neurons in various brain regions. This fits well with the idea that dioxins disrupt the molecular processes of cellular growth and maturation through excessive AHR activation in individual neurons, finally leading to brain functional impairment. Because neuromorphology and neural circuit structure are strongly associated with brain function as described above, I will briefly discuss the potential impacts of AHR activation on neuronal growth.

Table 3.
Aryl hydrocarbon receptor expression in brain neurons of humans and rodents.
6. Impact of Excessive AHR Activation on Neuronal Growth in the Brain
As mentioned earlier, both in vivo and in vitro studies have revealed aberrant neurite morphology in individual neurons following TeCDD exposure, as well as upregulated AHR target gene expression [71,92,94,95]. Additionally, AHR-deficient neurons have displayed altered dendritic arborization complexity [141]. In line with these findings, the TeCDD-induced nuclear translocation of AHR has been observed microscopically in brain neurons and primary cultured neurons [148,166], suggesting that AHR signaling is relevant to neuronal growth, especially neurite elongation.
Constitutively active (CA)-AHR can be used to examine the effect of AHR signaling activation on cellular growth and maturation. AHR that lacks the ligand-binding domain and the PAS-B motif functions as CA-AHR (Figure 2A), allowing the induction of transcriptional activity even in the absence of a ligand [169]. The transgenic mouse line expressing CA-AHR has been found to exhibit not only tumorigenesis and organ hypertrophy [170,171] but also the disruption of immune cells and reproductive organs [172,173], which seems at least partly similar to the toxic effects displayed by dioxin-exposed rodents [174]. In addition, in vivo electroporation is an excellent technique for transfecting plasmid vectors carrying genes encoding specific proteins, such as fluorescence proteins for cell visualization, into the neuronal lineage cells of developing mouse brains [175,176,177]. This technique allows researchers to microscopically observe neurons labeled with fluorescence proteins in brain tissues and to quantitatively analyze their morphology and location at the single-neuron level.
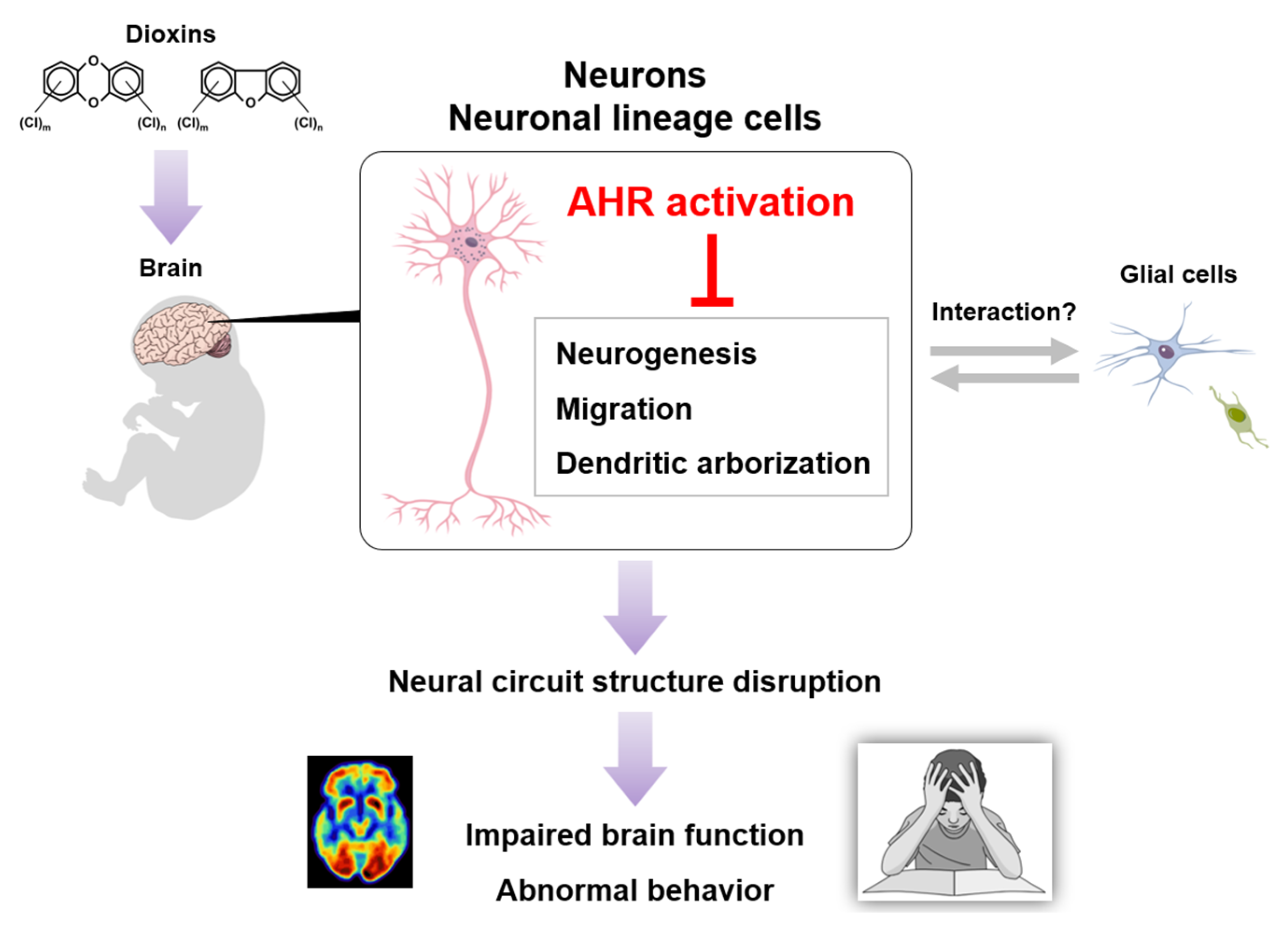
My colleagues and I utilized these two genetic tools to examine mature neurons and neuronal precursors expressing CA-AHR together with fluorescence proteins and found aberrant dendritic morphology in CA-AHR-expressing pyramidal and granule neurons in the hippocampus, cerebral cortex, and olfactory bulb of infant mice [94,178,179]. It is plausible that these dendritic abnormalities are commonly induced by excessive AHR activation across brain regions and neuronal subtypes. Intriguingly, no overt dendritic changes were observed in neurons transfected with the vector carrying the gene encoding full-length AHR (i.e., AHR overexpression); thus, excessive AHR signaling activation (i.e., its constitutively active form) is key to the observed neuromorphological abnormalities. In addition to dendritic changes, CA-AHR morphologically disrupted the initial processes of neuronal precursors in the RMS [178]. In terms of the migrating precursor phenotype, the CA-AHR-expressing neurons could not reach their destinations and finally displayed the perturbation of their distributions within several brain regions [178,179,180]. Considered together, these findings suggest a potential mechanism in which excessive AHR signaling activation, caused by dioxins, disrupts neuronal growth, such as neurite elongation and cell migration (Figure 3).
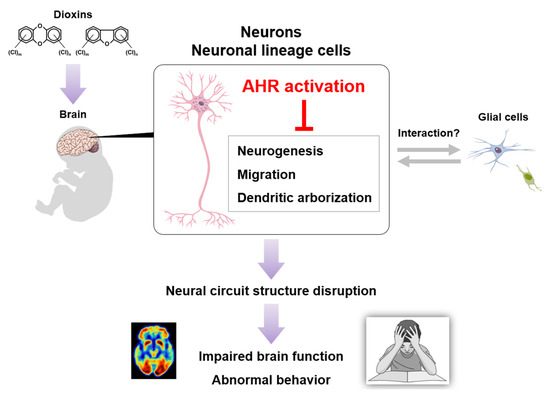
Figure 3.
A possible model of dioxin neurotoxicity. Dioxin exposure induces excessive AHR activation in brain neurons, followed by neural circuit structure disruption, finally leading to impaired brain function and abnormal behavior.
7. Glial AHR in the Mammalian Brain
Neuron–glia interactions contribute to neural circuit maintenance and activity, as well as neurological disorders [181,182,183], suggesting that glial cells could be partly involved in AHR-related neurotoxicity. Consistent with this idea, several studies have reported that AHR is detectable in glial cells, in addition to neurons, of human and rodent brains (Table 4). Initially, AHR expression was found in human glioblastoma tissues [184]; therefore, AHR is likely associated with tumor progression via the activation of its signaling in glial cells. Indeed, AHR expression was clearly detected in glioblastoma regions in a biopsy, while its expression was low in non-tumor regions [184]. Supporting this observation, correlations between AHR expression and cancer severity grades were found in human glioma and meningioma [185,186,187]. However, AHR expression in astrocytes and microglia has been demonstrated by IHC studies in human and mouse brains [156,188,189,190]. In vitro studies using primary cultured cells harvested from mice also demonstrated AHR expression in these glial cells [191,192]. Although the role of glial AHR in dioxin neurotoxicity remains poorly understood, excessive AHR signaling activation could influence neuron–glia interactions.

Table 4.
AHR expression in human and rodent glial cells.
8. Conclusions and Remarks
AHR is highly conserved, not only in mammals but also among diverse animals including avian species, fish, Drosophila, and Caenorhabditis elegans [194]. In addition to rodent models, AHR orthologs have been reported to play crucial roles in the developmental processes of the nervous system in other vertebrate and invertebrate models. For instance, zebrafish, a model which is frequently used to evaluate the neurotoxicity of chemicals [195,196], exhibited transcriptional expression of ahr2, an AHR ortholog, in the neuroepithelium of the brain and retina during development [197]. Neurite elongation and neural circuit formation were disrupted by ahr2 loss and CA-AHR2 expression, as well as dioxin exposure [198,199], which is consistent with the results of mouse studies [71,94,141,178,179]. Similarly, AHR ortholog overexpression and ablation in Drosophila and C. elegans (i.e., spineless and ahr-1, respectively) disrupted neuronal growth, including neurite elongation and the differentiation of sensory and motor neurons [200,201,202,203]. Taken together, an excess or deficiency of AHR signaling may tilt the balance of optimal neuronal growth through the dysregulation of target gene expression. Further studies on the biological roles of AHR in the nervous system across species will contribute to clarifying the molecular mechanism of AHR-dependent neurotoxicity not only in humans but also in wildlife.
In addition to chlorinated dioxins, brominated dioxins (i.e., polybrominated dibenzo-p-dioxins and dibenzofurans, PBDD/Fs) are produced as unwanted byproducts of the brominated flame retardants widely used in coatings and electrical applications [102,204]. They have been detected in indoor dust, industrial wastewater, food, and human samples [205,206,207,208,209,210,211,212]. Similar to PCDD/Fs, PBDD/Fs have been found to be highly persistent in the body [213], induce AHR transcriptional activation [99,100], and evoke teratogenicity [214] in rodents. Notably, experimental studies have revealed adverse effects of perinatal exposure to PBDD/Fs on behavior, although limited information is available (Table 2). Specifically, rodent offspring of pregnant dams exposed to brominated congeners exhibited impairments in learning and memory, contextual fear memory, exploratory behavior, and infant USV emissions [74,81,99,100,101]. In this context, toxicological studies on chlorinated and brominated dioxins will be important for establishing a more comprehensive estimation of environmental risks.
From a methodological perspective, transcriptomics at single-cell resolution is actively used in various fields, including toxicology [215]. Single-nucleus RNA sequencing (snRNA-seq) studies have revealed not only the toxic effects of dioxins but also the basal expression of AHR in the mouse liver [216,217]. Interestingly, central hepatocytes exhibited higher Ahr expression than portal hepatocytes in the lobules. This finding was in accordance with the zonal expression pattern of AHR target genes demonstrated by IHC and computational modeling [218,219]. To date, although single-cell transcriptomics for AHR expression in other organs are limited, snRNA-seq combined with spatial transcriptomics will be helpful in uncovering AHR-expressing neurons in the brain.
In conclusion, a variety of toxicological studies on AHR, demonstrating its role as a molecular intermediator between dioxins and cells, have elucidated how dioxin exposure adversely affects the functions of multiple organs and tissues, including the CNS. Accumulating evidence suggests the biological and clinical importance of AHR in regulating cellular growth and maturation, such as neurite elongation. Therefore, accurate determination of the spatio-temporal profile of AHR expression and signaling in the brain will offer valuable insights into the molecular mechanisms of dioxin neurotoxicity, advancing our understanding of the previously unreported involvement of environmental factors in brain development and function.
Funding
The author is supported in part by the Japan Society for the Promotion of Science, KAKENHI 24K15304.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
I am grateful to Chiharu Tohyama at the University of Tokyo and Muneko Nishijo at Kanazawa Medical University for their critical reading of the draft paper.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AHR | Aryl hydrocarbon receptor |
| AHRR | Aryl hydrocarbon receptor repressor |
| ARNT | Aryl hydrocarbon receptor nuclear translocator |
| bHLH | Basic helix–loop–helix |
| CA-AHR | Constitutively active aryl hydrocarbon receptor |
| CNS | Central nervous system |
| CYP | Cytochrome P450 |
| DG | Dentate gyrus |
| Dl-PCB | Dioxin-like polychlorinated biphenyl |
| GFAP | Glial fibrillary acidic protein |
| Iba1 | Ionized calcium-binding adapter molecule 1 |
| ICC | Immunocytochemistry |
| ICjM | Island of Calleja major |
| IHC | Immunohistochemistry |
| ISH | In situ hybridization |
| KLF6 | Krüppel-like factor 6 |
| LC | Locus coeruleus |
| NF-κB | Nuclear factor-κB |
| PAS | Per-Arnt-Sim |
| PBDD | Polybrominated dibenzo-p-dioxin |
| PBDF | Polybrominated dibenzofuran |
| PCB | Polychlorinated biphenyl |
| PCDD | Polychlorinated dibenzo-p-dioxin |
| PCDF | Polychlorinated dibenzofuran |
| RMS | Rostral migratory stream |
| RT-PCR | Reverse transcription PCR |
| snRNA-seq | Single-nucleus RNA sequencing |
| TeCDD | 2,3,7,8-Tetrachlorodibenzo-p-dioxin |
| TEF | Toxic equivalency factor |
| TH | Tyrosine hydroxylase |
| USAF | United States Air Force |
| USV | Ultrasonic vocalization |
| XRE | Xenobiotic response element |
References
- Fiedler, H. Sources of PCDD/PCDF and impact on the environment. Chemosphere 1996, 32, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Srogi, K. Levels and congener distributions of PCDDs, PCDFs and dioxin-like PCBs in environmental and human samples: A review. Environ. Chem. Lett. 2008, 6, 1–28. [Google Scholar] [CrossRef]
- Kjeller, L.-O.; Jones, K.C.; Johnston, A.E.; Rappe, C. Increases in the polychlorinated dibenzo-p-dioxin and -furan content of soils and vegetation since the 1840s. Environ. Sci. Technol. 1991, 25, 1619–1627. [Google Scholar] [CrossRef]
- Zennegg, M.; Kohler, M.; Hartmann, P.C.; Sturm, M.; Gujer, E.; Schmid, P.; Gerecke, A.C.; Heeb, N.V.; Kohler, H.P.; Giger, W. The historical record of PCB and PCDD/F deposition at Greifensee, a lake of the Swiss plateau, between 1848 and 1999. Chemosphere 2007, 67, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Grupe, A.; Neupert, M. Bestimmung von PCDD- und PCDF-Gehalten in Bodenproben. UWSF-Z Umweltchem Ökotox 1992, 4, 197. [Google Scholar] [CrossRef]
- Czuczwa, J.M.; McVeety, B.D.; Hites, R.A. Polychlorinated Dibenzo-p-dioxins and Dibenzofurans in Sediments from Siskiwit Lake, Isle Royale. Science 1984, 226, 568–569. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.; Kaminska, J.; Czerska, M.; Ligocka, D.; Urbaniak, M. Levels and sources of PCDDs, PCDFs and dl-PCBs in the water ecosystems of central Poland—A mini review. Int. J. Occup. Med. Environ. Health 2014, 27, 902–918. [Google Scholar] [CrossRef] [PubMed]
- Furness, S.G.; Whelan, F. The pleiotropy of dioxin toxicity--xenobiotic misappropriation of the aryl hydrocarbon receptor’s alternative physiological roles. Pharmacol. Ther. 2009, 124, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.; Birnbaum, L.; Bosveld, A.T.; Brunström, B.; Cook, P.; Feeley, M.; Giesy, J.; Hanberg, A.; Hasegawa, R.; Kennedy, S.W.; et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 1998, 106, 775–792. [Google Scholar] [CrossRef] [PubMed]
- DeVito, M.; Bokkers, B.; van Duursen, M.B.M.; van Ede, K.; Feeley, M.; Antunes Fernandes Gaspar, E.; Haws, L.; Kennedy, S.; Peterson, R.E.; Hoogenboom, R.; et al. The 2022 world health organization reevaluation of human and mammalian toxic equivalency factors for polychlorinated dioxins, dibenzofurans and biphenyls. Regul. Toxicol. Pharmacol. 2024, 146, 105525. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C.; Campbell, A. Developmental neurotoxicology. J. Neurosci. Res. 2005, 81, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet. Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Szpir, M. New thinking on neurodevelopment. Environ. Health Perspect. 2006, 114, A100–A107. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Uenotsuchi, T.; Urabe, K.; Ishikawa, T.; Kuwabara, I.; Yusho, S.G.f. Overview of Yusho. J. Dermatol. Sci. Suppl. 2005, 1, S3–S10. [Google Scholar] [CrossRef]
- Hsu, S.T.; Ma, C.I.; Hsu, S.K.; Wu, S.S.; Hsu, N.H.; Yeh, C.C.; Wu, S.B. Discovery and epidemiology of PCB poisoning in Taiwan: A four-year followup. Environ. Health Perspect. 1985, 59, 5–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuratsune, M.; Yoshimura, T.; Matsuzaka, J.; Yamaguchi, A. Epidemiologic study on Yusho, a Poisoning Caused by Ingestion of Rice Oil Contaminated with a Commercial Brand of Polychlorinated Biphenyls. Environ. Health Perspect. 1972, 1, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.W. Congenital PCB poisoning: A reevaluation. Environ. Health Perspect. 1985, 60, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Intrauterine poisoning: Clinical and epidemiological studies and significance of the problem. Bull. Inst. Constit. Med. Kumamoto Univ. 1976, 25, 38–50. [Google Scholar]
- Rogan, W.J.; Gladen, B.C.; Hung, K.L.; Koong, S.L.; Shih, L.Y.; Taylor, J.S.; Wu, Y.C.; Yang, D.; Ragan, N.B.; Hsu, C.C. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science 1988, 241, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Guo, Y.L.; Hsu, C.C.; Rogan, W.J. Cognitive development of Yu-Cheng (“oil disease”) children prenatally exposed to heat-degraded PCBs. JAMA 1992, 268, 3213–3218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Yu, M.L.; Rogan, W.J.; Gladen, B.C.; Hsu, C.C. A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. Am. J. Public Health 1994, 84, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Hsu, C.C. Effects of prenatal exposure to PCBs on the neurological function of children: A neuropsychological and neurophysiological study. Dev. Med. Child. Neurol. 1994, 36, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Lambert, G.H.; Hsu, C.C. Growth abnormalities in the population exposed in utero and early postnatally to polychlorinated biphenyls and dibenzofurans. Environ. Health Perspect. 1995, 103, 117–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, T.J.; Guo, Y.L.; Guo, N.W.; Hsu, C.C. Effect of prenatal exposure to polychlorinated biphenyls on cognitive development in children: A longitudinal study in Taiwan. Br. J. Psychiatry Suppl. 2001, 40, s49–s52. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.J.; Guo, Y.L.; Yu, M.L.; Ko, H.C.; Hsu, C.C. Cognitive development in Yucheng children. Chemosphere 1994, 29, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Lambert, G.H.; Hsu, C.C.; Hsu, M.M. Yucheng: Health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. Int. Arch. Occup. Environ. Health 2004, 77, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.L.; Jacobson, S.W. Dose-response in perinatal exposure to polychlorinated biphenyls (PCBs): The Michigan and North Carolina cohort studies. Toxicol. Ind. Health 1996, 12, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.L.; Jacobson, S.W. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N. Engl. J. Med. 1996, 335, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Patandin, S.; Lanting, C.I.; Mulder, P.G.; Boersma, E.R.; Sauer, P.J.; Weisglas-Kuperus, N. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J. Pediatr. 1999, 134, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Dai, L.C.; Papke, O.; Prange, J.; Constable, J.D.; Matsuda, M.; Thao, V.D.; Piskac, A.L. Recent dioxin contamination from Agent Orange in residents of a southern Vietnam city. J. Occup. Environ. Med. 2001, 43, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Stellman, J.M.; Stellman, S.D.; Christian, R.; Weber, T.; Tomasallo, C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature 2003, 422, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.A.; Doan, T.V.; Tarradellas, J.; de Alencastro, L.F.; Grandjean, D. Dioxin contamination in soils of Southern Vietnam. Chemosphere 2007, 67, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Vu, H.T.; Pham-The, T.; Pham, T.N.; Tran, N.N.; Nakagawa, H.; Nishijo, H. Dioxin Congener Patterns in Breast Milk Samples from Areas Sprayed with Herbicide during the Vietnam War 40 Years after the War Ended. Toxics 2022, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.T.; Nishijo, M.; Kido, T.; Nakagawa, H.; Maruzeni, S.; Naganuma, R.; Anh, N.T.; Morikawa, Y.; Luong, H.V.; Anh, T.H.; et al. Dioxin concentrations in breast milk of Vietnamese nursing mothers: A survey four decades after the herbicide spraying. Environ. Sci. Technol. 2011, 45, 6625–6632. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, G.T.; Nishijo, M.; Pham, T.N.; Ito, M.; Pham, T.T.; Tran, A.H.; Nishimaru, H.; Nishino, Y.; Nishijo, H. Adverse effects of maternal dioxin exposure on fetal brain development before birth assessed by neonatal electroencephalography (EEG) leading to poor neurodevelopment; a 2-year follow-up study. Sci. Total. Environ. 2019, 667, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Tai, P.T.; Nakagawa, H.; Maruzeni, S.; Anh, N.T.; Luong, H.V.; Anh, T.H.; Honda, R.; Morikawa, Y.; Kido, T.; et al. Impact of perinatal dioxin exposure on infant growth: A cross-sectional and longitudinal studies in dioxin-contaminated areas in Vietnam. PLoS ONE 2012, 7, e40273. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.T.; Nishijo, M.; Anh, N.T.; Maruzeni, S.; Nakagawa, H.; Van Luong, H.; Anh, T.H.; Honda, R.; Kido, T.; Nishijo, H. Dioxin exposure in breast milk and infant neurodevelopment in Vietnam. Occup. Environ. Med. 2013, 70, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Pham, T.T.; Nguyen, A.T.; Tran, N.N.; Nakagawa, H.; Hoang, L.V.; Tran, A.H.; Morikawa, Y.; Ho, M.D.; Kido, T.; et al. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin in breast milk increases autistic traits of 3-year-old children in Vietnam. Mol. Psychiatry 2014, 19, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Pham The, T.; Pham Ngoc, T.; Hoang Van, T.; Nishijo, M.; Tran Ngoc, N.; Vu Thi, H.; Hoang Van, L.; Tran Hai, A.; Nishino, Y.; Nishijo, H. Effects of perinatal dioxin exposure on learning abilities of 8-year-old children in Vietnam. Int. J. Hyg. Environ. Health 2020, 223, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Pham-The, T.; Nishijo, M.; Pham, T.N.; Vu, H.T.; Tran, N.N.; Tran, A.H.; Hoang, L.V.; Do, Q.; Nishino, Y.; Nishijo, H. Perinatal Dioxin Exposure and Attention Deficit Hyperactivity Disorder (ADHD) Symptoms in Children Living in a Dioxin Contamination Hotspot in Vietnam. Toxics 2022, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Thao, P.N.; Nishijo, M.; Tai, P.T.; Nghi, T.N.; Yokawa, T.; Hoa, V.T.; Tien, T.V.; Kien, N.X.; Anh, T.H.; Nishino, Y.; et al. Impacts of dioxin exposure on brain connectivity estimated by DTI analysis of MRI images in men residing in contaminated areas of Vietnam. Front. Neurosci. 2024, 18, 1344653. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Pham, T.N.; Nishijo, M.; Yokawa, T.; Pham The, T.; Takiguchi, T.; Nishino, Y.; Nishijo, H. Impact of dioxin exposure on brain morphometry and social anxiety in men living in the most dioxin-contaminated area in Vietnam. J. Psychiatr. Res. 2023, 166, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Pham, T.N.; Yokawa, T.; Nishijo, M.; The, T.P.; Do, Q.; Nishino, Y.; Nishijo, H. Alterations in Regional Brain Regional Volume Associated with Dioxin Exposure in Men Living in the Most Dioxin-Contaminated Area in Vietnam: Magnetic Resonance Imaging (MRI) Analysis Using Voxel-Based Morphometry (VBM). Toxics 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Kyeong, S.; Kim, D.H. Long-term effects of defoliant exposure on brain atrophy progression in humans. Neurotoxicology 2022, 92, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Yaffe, K.; Li, Y.; Byers, A.L.; Peltz, C.B.; Barnes, D.E. Agent Orange Exposure and Dementia Diagnosis in US Veterans of the Vietnam Era. JAMA Neurol. 2021, 78, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Warner, M.; Brambilla, P.; Signorini, S.; Ames, J.; Mocarelli, P. The Seveso accident: A look at 40 years of health research and beyond. Environ. Int. 2018, 121, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Ames, J.; Warner, M.; Brambilla, P.; Mocarelli, P.; Satariano, W.A.; Eskenazi, B. Neurocognitive and physical functioning in the Seveso Women’s Health Study. Environ. Res. 2018, 162, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ames, J.; Warner, M.; Siracusa, C.; Signorini, S.; Brambilla, P.; Mocarelli, P.; Eskenazi, B. Prenatal dioxin exposure and neuropsychological functioning in the Seveso Second Generation Health Study. Int. J. Hyg. Environ. Health 2019, 222, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, I.H.; Aase, H.; Biele, G.; Brantsaeter, A.L.; Haugen, M.; Kvalem, H.E.; Skogan, A.H.; Zeiner, P.; Alexander, J.; Meltzer, H.M.; et al. The influence of maternal dietary exposure to dioxins and PCBs during pregnancy on ADHD symptoms and cognitive functions in Norwegian preschool children. Environ. Int. 2016, 94, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, I.H.; Haugen, M.; Schjolberg, S.; Vejrup, K.; Knutsen, H.K.; Brantsaeter, A.L.; Meltzer, H.M.; Alexander, J.; Magnus, P.; Kvalem, H.E. Maternal dietary exposure to dioxins and polychlorinated biphenyls (PCBs) is associated with language delay in 3year old Norwegian children. Environ. Int. 2016, 91, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, T.I.; Thorsdottir, I.; Meltzer, H.M.; Strom, M.; Olsen, S.F. Dioxin-like activity in plasma among Danish pregnant women: Dietary predictors, birth weight and infant development. Environ. Res. 2009, 109, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Huisman, M.; Koopman-Esseboom, C.; Fidler, V.; Hadders-Algra, M.; van der Paauw, C.G.; Tuinstra, L.G.; Weisglas-Kuperus, N.; Sauer, P.J.; Touwen, B.C.; Boersma, E.R. Perinatal exposure to polychlorinated biphenyls and dioxins and its effect on neonatal neurological development. Early Hum. Dev. 1995, 41, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Koopman-Esseboom, C.; Weisglas-Kuperus, N.; de Ridder, M.A.; Van der Paauw, C.G.; Tuinstra, L.G.; Sauer, P.J. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants’ mental and psychomotor development. Pediatrics 1996, 97, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Jacobs, D.R.; Porta, M. Association of serum concentrations of persistent organic pollutants with the prevalence of learning disability and attention deficit disorder. J. Epidemiol. Community Health 2007, 61, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, J.; Wittsiepe, J.; Kasper-Sonnenberg, M.; Schoneck, N.; Scholmerich, A.; Wilhelm, M. The influence of low level pre- and perinatal exposure to PCDD/Fs, PCBs, and lead on attention performance and attention-related behavior among German school-aged children: Results from the Duisburg Birth Cohort Study. Int. J. Hyg. Environ. Health 2015, 218, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kishi, R.; Kobayashi, S.; Ikeno, T.; Araki, A.; Miyashita, C.; Itoh, S.; Sasaki, S.; Okada, E.; Kobayashi, S.; Kashino, I.; et al. Ten years of progress in the Hokkaido birth cohort study on environment and children’s health: Cohort profile--updated 2013. Environ. Health Prev. Med. 2013, 18, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Saijo, Y.; Kato, S.; Sasaki, S.; Uno, A.; Kanagami, N.; Hirakawa, H.; Hori, T.; Tobiishi, K.; Todaka, T.; et al. Effects of prenatal exposure to polychlorinated biphenyls and dioxins on mental and motor development in Japanese children at 6 months of age. Environ. Health Perspect. 2006, 114, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Yim, G.; Minatoya, M.; Kioumourtzoglou, M.A.; Bellavia, A.; Weisskopf, M.; Ikeda-Araki, A.; Miyashita, C.; Kishi, R. The associations of prenatal exposure to dioxins and polychlorinated biphenyls with neurodevelopment at 6 Months of age: Multi-pollutant approaches. Environ. Res. 2022, 209, 112757. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, T.; Miyashita, C.; Nakajima, S.; Kobayashi, S.; Yamazaki, K.; Saijo, Y.; Kita, T.; Sasaki, S.; Konishi, K.; Kajiwara, J.; et al. Effects of low-level prenatal exposure to dioxins on cognitive development in Japanese children at 42months. Sci. Total Environ. 2018, 618, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Saijo, Y.; Miyashita, C.; Ikeno, T.; Sasaki, S.; Kajiwara, J.; Kishi, R. Sex-specific differences in effect of prenatal exposure to dioxin-like compounds on neurodevelopment in Japanese children: Sapporo cohort study. Environ. Res. 2017, 159, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.M.; Sprengel, R.; Sanderson, D.J.; McHugh, S.B.; Rawlins, J.N.; Monyer, H.; Seeburg, P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014, 15, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.P.; Dunnett, S.B. Tests to assess motor phenotype in mice: A user’s guide. Nat. Rev. Neurosci. 2009, 10, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.C. Functional assays for neurotoxicity testing. Toxicol. Pathol. 2011, 39, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Siegelbaum, S.A. The Corticohippocampal Circuit, Synaptic Plasticity, and Memory. Cold Spring Harb. Perspect. Biol. 2015, 7, a021733. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Moser, H.W. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex 2000, 10, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.A.; Firestein, B.L. The dendritic tree and brain disorders. Mol. Cell. Neurosci. 2012, 50, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cerdeno, V. Dendrite and spine modifications in autism and related neurodevelopmental disorders in patients and animal models. Dev. Neurobiol. 2017, 77, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism spectrum disorder: Neuropathology and animal models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef] [PubMed]
- Gileadi, T.E.; Swamy, A.K.; Hore, Z.; Horswell, S.; Ellegood, J.; Mohan, C.; Mizuno, K.; Lundebye, A.K.; Giese, K.P.; Stockinger, B.; et al. Effects of Low-Dose Gestational TCDD Exposure on Behavior and on Hippocampal Neuron Morphology and Gene Expression in Mice. Environ. Health Perspect. 2021, 129, 57002. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Takeda, T.; Fujii, M.; Taura, J.; Yamada, H.; Ishii, Y. Attenuation of growth hormone production at the fetal stage is critical for dioxin-induced developmental disorder in rat offspring. Biochem. Pharmacol. 2021, 186, 114495. [Google Scholar] [CrossRef] [PubMed]
- Hojo, R.; Kakeyama, M.; Kurokawa, Y.; Aoki, Y.; Yonemoto, J.; Tohyama, C. Learning behavior in rat offspring after in utero and lactational exposure to either TCDD or PCB126. Environ. Health Prev. Med. 2008, 13, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kakeyama, M.; Endo, T.; Zhang, Y.; Miyazaki, W.; Tohyama, C. Disruption of paired-associate learning in rat offspring perinatally exposed to dioxins. Arch. Toxicol. 2014, 88, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Schantz, S.L.; Seo, B.W.; Moshtaghian, J.; Peterson, R.E.; Moore, R.W. Effects of gestational and lactational exposure to TCDD or coplanar PCBs on spatial learning. Neurotoxicol. Teratol. 1996, 18, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.W.; Powers, B.E.; Widholm, J.J.; Schantz, S.L. Radial arm maze performance in rats following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Neurotoxicol. Teratol. 2000, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.W.; Sparks, A.J.; Medora, K.; Amin, S.; Schantz, S.L. Learning and memory in rats gestationally and lactationally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Neurotoxicol. Teratol. 1999, 21, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Liu, Y.N.; Xian, P.; Ma, J.; Sun, Y.W.; Chen, J.S.; Chen, X.; Tang, N.J. Maternal exposure to TCDD during gestation advanced sensory-motor development, but induced impairments of spatial learning and memory in adult male rat offspring. Chemosphere 2018, 212, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Schantz, S.L.; Bowman, R.E. Learning in monkeys exposed perinatally to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD). Neurotoxicol. Teratol. 1989, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kakeyama, M.; Uemura, Y.; Haijima, A.; Okuno, H.; Bito, H.; Tohyama, C. Executive function deficits and social-behavioral abnormality in mice exposed to a low dose of dioxin in utero and via lactation. PLoS ONE 2012, 7, e50741. [Google Scholar] [CrossRef] [PubMed]
- Haijima, A.; Endo, T.; Zhang, Y.; Miyazaki, W.; Kakeyama, M.; Tohyama, C. In utero and lactational exposure to low doses of chlorinated and brominated dioxins induces deficits in the fear memory of male mice. Neurotoxicology 2010, 31, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Markowski, V.P.; Zareba, G.; Stern, S.; Cox, C.; Weiss, B. Altered operant responding for motor reinforcement and the determination of benchmark doses following perinatal exposure to low-level 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ. Health Perspect. 2001, 109, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Sugiyama, N.; Maeda, S.; Tohyama, C.; Arita, J. Perinatal exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin suppresses contextual fear conditioning-accompanied activation of cyclic AMP response element-binding protein in the hippocampal CA1 region of male rats. Neurosci. Lett. 2006, 398, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Nishijo, M.; Hori, E.; Nguyen, N.M.; Pham, T.T.; Fukunaga, K.; Nakagawa, H.; Tran, A.H.; Nishijo, H. Influence of Maternal Exposure to 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin on Socioemotional Behaviors in Offspring Rats. Environ. Health Insights 2013, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Kuriwaki, J.; Hori, E.; Tawara, K.; Nakagawa, H.; Nishijo, H. Effects of maternal exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin on fetal brain growth and motor and behavioral development in offspring rats. Toxicol. Lett. 2007, 173, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Chen, Y.; Wang, Y.; Luo, Y.; Liu, Y.; Ma, Y.; Li, Y.; Xu, L.; Xie, H.Q.; Zhao, B. Gestational and lactational exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in mice: Neurobehavioral effects on female offspring. Sci. Total Environ. 2021, 752, 141784. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Tohyama, C. Vocalization as a novel endpoint of atypical attachment behavior in 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-exposed infant mice. Arch. Toxicol. 2018, 92, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Chadman, K.K.; Gong, S.; Scattoni, M.L.; Boltuck, S.E.; Gandhy, S.U.; Heintz, N.; Crawley, J.N. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism. Res. 2008, 1, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, J.; Tamada, K.; Hatanaka, F.; Ise, S.; Ohta, H.; Inoue, K.; Tomonaga, S.; Watanabe, Y.; Chung, Y.J.; Banerjee, R.; et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell 2009, 137, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Winslow, J.T.; Hearn, E.F.; Ferguson, J.; Young, L.J.; Matzuk, M.M.; Insel, T.R. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm. Behav. 2000, 37, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, J.M.; Stockton, P.S.; Sieber, S.; Foley, J.; Portier, C.J. AhR-mediated gene expression in the developing mouse telencephalon. Reprod. Toxicol. 2009, 28, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Endo, T.; Yoshioka, W.; Ding, Y.; Ujita, W.; Kakeyama, M.; Tohyama, C. In utero and lactational dioxin exposure induces Sema3b and Sema3g gene expression in the developing mouse brain. Biochem. Biophys. Res. Commun. 2016, 476, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, T.; Yonemoto, J.; Sone, H.; Kosuge, Y.; Kosaki, K.; Takahashi, T. In utero exposure to dioxin causes neocortical dysgenesis through the actions of p27Kip1. Proc. Natl. Acad. Sci. USA 2010, 107, 16331–16335. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Kubo, K.I.; Matsuyoshi, C.; Benner, S.; Hosokawa, M.; Endo, T.; Ling, W.; Kohda, M.; Yokoyama, K.; Nakajima, K.; et al. Developmental origin of abnormal dendritic growth in the mouse brain induced by in utero disruption of aryl hydrocarbon receptor signaling. Neurotoxicol. Teratol. 2015, 52, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Jung, J.S.; Jin, I.; Jung, Y.W.; Ko, B.H.; Nam, K.S.; Park, I.K.; Moon, I.S. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the expression of synaptic proteins in dissociated rat cortical cells. Mol. Cells 2002, 14, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.L.; Williamson, M.A.; Thompson, B.D.; Dever, D.P.; Gasiewicz, T.A.; Opanashuk, L.A. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin exposure disrupts granule neuron precursor maturation in the developing mouse cerebellum. Toxicol. Sci. 2008, 103, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Latchney, S.E.; Hein, A.M.; O’Banion, M.K.; DiCicco-Bloom, E.; Opanashuk, L.A. Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J. Neurochem. 2013, 125, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Debler, R.A.; Gallegos, P.L.; Ojeda, A.C.; Perttula, A.M.; Lucio, A.; Chapkin, R.S.; Safe, S.; Eitan, S. TCDD (2, 3, 7, 8-tetrachlorodibenzo-p-dioxin) induces depression-like phenotype. Neurotoxicology 2024, 103, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Suzuki, G.; Uramaru, N.; Endo, T.; Maekawa, F. Behavioral impairments in infant and adult mouse offspring exposed to 2,3,7,8-tetrabromodibenzofuran in utero and via lactation. Environ. Int. 2020, 142, 105833. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Suzuki, G.; Uramaru, N.; Kakeyama, M.; Maekawa, F. Liver-specific decrease in Tff3 gene expression in infant mice perinatally exposed to 2, 3, 7, 8-tetrabromodibenzofuran or 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. J. Appl. Toxicol. 2022, 42, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Suzuki, G.; Uramaru, N.; Kakeyama, M.; Maekawa, F. 2-Chloro-3, 7, 8-tribromodibenzofuran as a new environmental pollutant inducing atypical ultrasonic vocalization in infant mice. Toxicol. Res. 2023, 12, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.; Denison, M.S.; Birnbaum, L.S.; Devito, M.J.; Fiedler, H.; Falandysz, J.; Rose, M.; Schrenk, D.; Safe, S.; Tohyama, C.; et al. Polybrominated dibenzo-p-dioxins, dibenzofurans, and biphenyls: Inclusion in the toxicity equivalency factor concept for dioxin-like compounds. Toxicol. Sci. 2013, 133, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Burbach, K.M.; Poland, A.; Bradfield, C.A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc. Natl. Acad. Sci. USA 1992, 89, 8185–8189. [Google Scholar] [CrossRef] [PubMed]
- Dolwick, K.M.; Schmidt, J.V.; Carver, L.A.; Swanson, H.I.; Bradfield, C.A. Cloning and expression of a human Ah receptor cDNA. Mol. Pharmacol. 1993, 44, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Gruszczyk, J.; Grandvuillemin, L.; Lai-Kee-Him, J.; Paloni, M.; Savva, C.G.; Germain, P.; Grimaldi, M.; Boulahtouf, A.; Kwong, H.S.; Bous, J.; et al. Cryo-EM structure of the agonist-bound Hsp90-XAP2-AHR cytosolic complex. Nat. Commun. 2022, 13, 7010. [Google Scholar] [CrossRef] [PubMed]
- Barroso, A.; Mahler, J.V.; Fonseca-Castro, P.H.; Quintana, F.J. The aryl hydrocarbon receptor and the gut-brain axis. Cell. Mol. Immunol. 2021, 18, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef] [PubMed]
- Reyes, H.; Reisz-Porszasz, S.; Hankinson, O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science 1992, 256, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Mimura, J.; Fujii-Kuriyama, Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim. Biophys. Acta 2003, 1619, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Mimura, J.; Ema, M.; Sogawa, K.; Fujii-Kuriyama, Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes. Dev. 1999, 13, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ke, S.; Denison, M.S.; Rabson, A.B.; Gallo, M.A. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J. Biol. Chem. 1999, 274, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Sciullo, E.; Li, W.; Wong, P.; Lazennec, G.; Matsumura, F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol. 2007, 21, 2941–2955. [Google Scholar] [CrossRef] [PubMed]
- Hollingshead, B.D.; Beischlag, T.V.; Dinatale, B.C.; Ramadoss, P.; Perdew, G.H. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res. 2008, 68, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Sciullo, E.; Matsumura, F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem. Biophys. Res. Commun. 2007, 363, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.P.; Li, H.; Mitchell, K.A.; Joshi, A.D.; Elferink, C.J. Ah receptor-mediated suppression of liver regeneration through NC-XRE-driven p21Cip1 expression. Mol. Pharmacol. 2014, 85, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Joshi, A.D.; Elferink, C.J. The tumor suppressor Kruppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. J. Pharmacol. Exp. Ther. 2013, 345, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.Q.; Jung, J.W.; Lee, Y.S.; Kim, J.A. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin inhibits cell proliferation through arylhydrocarbon receptor-mediated G1 arrest in SK-N-SH human neuronal cells. Neurosci. Lett. 2004, 363, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Kolluri, S.K.; Weiss, C.; Koff, A.; Gottlicher, M. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 1999, 13, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.H.; Lin, Y.H.; Lee, Y.H.; Hou, H.H.; Hsu, S.P.; Juan, S.H. Molecular mechanisms of p21 and p27 induction by 3-methylcholanthrene, an aryl-hydrocarbon receptor agonist, involved in antiproliferation of human umbilical vascular endothelial cells. J. Cell. Physiol. 2008, 215, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Quintana, F.J.; Pot, C.; Joller, N.; Xiao, S.; Kumar, D.; Burns, E.J.; Sherr, D.H.; Weiner, H.L.; Kuchroo, V.K. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010, 11, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Elferink, C.J.; Ge, N.L.; Levine, A. Maximal aryl hydrocarbon receptor activity depends on an interaction with the retinoblastoma protein. Mol. Pharmacol. 2001, 59, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Wormke, M.; Stoner, M.; Saville, B.; Walker, K.; Abdelrahim, M.; Burghardt, R.; Safe, S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol. Cell. Biol. 2003, 23, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Beischlag, T.V.; Luis Morales, J.; Hollingshead, B.D.; Perdew, G.H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 207–250. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.P.; Joshi, A.D.; Elferink, C.J. Ah Receptor Pathway Intricacies; Signaling Through Diverse Protein Partners and DNA-Motifs. Toxicol. Res. 2015, 4, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, K.; Fujii-Kuriyama, Y. Cytochrome P450 gene regulation and physiological functions mediated by the aryl hydrocarbon receptor. Arch. Biochem. Biophys. 2007, 464, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, Y.; Chen, Z.; Tan, X.; Mei, X.; Wu, Z. The role of aryl hydrocarbon receptor in vitiligo: A review. Front. Immunol. 2024, 15, 1291556. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer. 2014, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Holfelder, P.; Prentzell, M.T.; Trump, S. The complex biology of aryl hydrocarbon receptor activation in cancer and beyond. Biochem. Pharmacol. 2023, 216, 115798. [Google Scholar] [CrossRef] [PubMed]
- Sondermann, N.C.; Fassbender, S.; Hartung, F.; Hatala, A.M.; Rolfes, K.M.; Vogel, C.F.A.; Haarmann-Stemmann, T. Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway. Biochem. Pharmacol. 2023, 208, 115371. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Matsumura, F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem. Pharmacol. 2009, 77, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Salguero, P.M.; Hilbert, D.M.; Rudikoff, S.; Ward, J.M.; Gonzalez, F.J. Aryl-hydrocarbon receptor-deficient mice are resistant to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol. Appl. Pharmacol. 1996, 140, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Mimura, J.; Yamashita, K.; Nakamura, K.; Morita, M.; Takagi, T.N.; Nakao, K.; Ema, M.; Sogawa, K.; Yasuda, M.; Katsuki, M.; et al. Loss of teratogenic response to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 1997, 2, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Salguero, P.; Pineau, T.; Hilbert, D.M.; McPhail, T.; Lee, S.S.; Kimura, S.; Nebert, D.W.; Rudikoff, S.; Ward, J.M.; Gonzalez, F.J. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 1995, 268, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Salguero, P.M.; Ward, J.M.; Sundberg, J.P.; Gonzalez, F.J. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol 1997, 34, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, Y.; Inoue, T. Aryl hydrocarbon receptor biology and xenobiotic responses in hematopoietic progenitor cells. Biochem. Pharmacol. 2009, 77, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.V.; Su, G.H.; Reddy, J.K.; Simon, M.C.; Bradfield, C.A. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. USA 1996, 93, 6731–6736. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, A.; Mialot, A.; Petit, J.M.; Fernandez-Salguero, P.; Barouki, R.; Coumoul, X.; Beraneck, M. Oculomotor deficits in aryl hydrocarbon receptor null mouse. PLoS ONE 2013, 8, e53520. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.K.; Mahajnah, M.; Thomas, M.G.; Cohen, Y.; Habib, A.; Schulze, M.; Maconachie, G.D.E.; AlMoallem, B.; De Baere, E.; Lorenz, B.; et al. Homozygous stop mutation in AHR causes autosomal recessive foveal hypoplasia and infantile nystagmus. Brain 2019, 142, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, V.M.; Lasseaux, E.; Michaud, V.; Courdier, C.; Meunier, I.; Arveiler, B.; Defoort-Dhellemmes, S. Crossed VEP asymmetry in a patient with AHR-linked infantile nystagmus and foveal hypoplasia. Doc. Ophthalmol. 2024, 149, 47–52. [Google Scholar] [CrossRef] [PubMed]
- de la Parra, J.; Cuartero, M.I.; Perez-Ruiz, A.; Garcia-Culebras, A.; Martin, R.; Sanchez-Prieto, J.; Garcia-Segura, J.M.; Lizasoain, I.; Moro, M.A. AhR deletion promotes aberrant morphogenesis and synaptic activity of adult-generated granule neurons and impairs hippocampus-dependent memory. eNeuro 2018, 5, ENEURO.0370-17.2018. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, H.; Wang, X.Y.; Yu, Q.; Han, J.Y.; Huang, Y.; Zhou, W.X. Deletion of AhR attenuates fear memory leaving other types of memory intact. Behav. Brain. Res. 2023, 451, 114505. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, B.N.; Probst, M.R.; Reisz-Porszasz, S.; Hankinson, O. Identification of functional domains of the aryl hydrocarbon receptor. J. Biol. Chem. 1995, 270, 29270–29278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.B.; Ramadoss, P.; Reen, R.K.; Vanden Heuvel, J.P.; Perdew, G.H. The Q-rich subdomain of the human Ah receptor transactivation domain is required for dioxin-mediated transcriptional activity. J. Biol. Chem. 2001, 276, 42302–42310. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Ihara, K.; Nakayama, H.; Hikino, S.; Satoh, K.; Kubo, N.; Iida, T.; Fujii, Y.; Hara, T. Characteristic expression of aryl hydrocarbon receptor repressor gene in human tissues: Organ-specific distribution and variable induction patterns in mononuclear cells. Life Sci. 2004, 74, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Rannug, A.; Ahlbom, E.; Hakansson, H.; Ceccatelli, S. Effect of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin on the expression of cytochrome P450 1A1, the aryl hydrocarbon receptor, and the aryl hydrocarbon receptor nuclear translocator in rat brain and pituitary. Toxicol. Appl. Pharmacol. 2000, 169, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Tohyama, C. Embryonic and Postnatal Expression of Aryl Hydrocarbon Receptor mRNA in Mouse Brain. Front. Neuroanat. 2017, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Kohda, M.; Maekawa, F.; Fujii-Kuriyama, Y.; Tohyama, C. Neurons expressing the aryl hydrocarbon receptor in the locus coeruleus and island of Calleja major are novel targets of dioxin in the mouse brain. Histochem. Cell Biol. 2021, 156, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.L.; Curran, M.A.; Marconi, S.A.; Carpenter, C.D.; Lubbers, L.S.; McAbee, M.D. Distribution of mRNAs encoding the arylhydrocarbon receptor, arylhydrocarbon receptor nuclear translocator, and arylhydrocarbon receptor nuclear translocator-2 in the rat brain and brainstem. J. Comp. Neurol. 2000, 427, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B.D.; Birnbaum, L.S.; Perdew, G.H. Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev. Dyn. 1995, 204, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Maltepe, E.; Lu, M.M.; Simon, C.; Bradfield, C.A. Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mech. Dev. 1998, 73, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Hays, L.E.; Carpenter, C.D.; Petersen, S.L. Evidence that GABAergic neurons in the preoptic area of the rat brain are targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin during development. Environ. Health Perspect. 2002, 110, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xiao, Q.; Wen, Z.; Gong, F.; Zhan, H.; Liu, J.; Li, H.; Jiao, Y. Gut microbiome-derived indole-3-carboxaldehyde regulates stress vulnerability in chronic restraint stress by activating aryl hydrocarbon receptors. Pharmacol. Res. 2025, 213, 107654. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Hesperetin protects hippocampal neurons from the neurotoxicity of Aflatoxin B1 in mice. Ecotoxicol. Environ. Saf. 2024, 269, 115782. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yang, Z.; Shi, R.; Luo, C.; Zhang, Z. Expression of aryl hydrocarbon receptor in rat brain lesions following traumatic brain injury. Diagn. Pathol. 2016, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Garcia, N.A.; Orozco-Ibarra, M.; Estudillo, E.; Elizondo, G.; Gomez Apo, E.; Chavez Macias, L.G.; Sosa-Ortiz, A.L.; Torres-Ramos, M.A. Aryl Hydrocarbon Receptor in Post-Mortem Hippocampus and in Serum from Young, Elder, and Alzheimer’s Patients. Int. J. Mol. Sci. 2020, 21, 1983. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.I.; Ballesteros, I.; de la Parra, J.; Harkin, A.L.; Abautret-Daly, A.; Sherwin, E.; Fernandez-Salguero, P.; Corbi, A.L.; Lizasoain, I.; Moro, M.A. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation 2014, 130, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, E.; Yoshimura, S.; Uruno, S.; Ishihara-Sugano, M. Effect of dioxins on regulation of tyrosine hydroxylase gene expression by aryl hydrocarbon receptor: A neurotoxicology study. Environ. Health 2009, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Barbosa, E.; Garcia-Aguilar, R.; Vega, L.; Cabanas-Cortes, M.A.; Gonzalez, F.J.; Segovia, J.; Morales-Lazaro, S.L.; Cisneros, B.; Elizondo, G. Parkin is transcriptionally regulated by the aryl hydrocarbon receptor: Impact on alpha-synuclein protein levels. Biochem. Pharmacol. 2019, 168, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Rzemieniec, J.; Litwa, E.; Wnuk, A.; Lason, W.; Krzeptowski, W.; Kajta, M. Selective Aryl Hydrocarbon Receptor Modulator 3, 3’-Diindolylmethane Impairs AhR and ARNT Signaling and Protects Mouse Neuronal Cells Against Hypoxia. Mol. Neurobiol. 2016, 53, 5591–5606. [Google Scholar] [CrossRef] [PubMed]
- Rzemieniec, J.; Wnuk, A.; Lason, W.; Bilecki, W.; Kajta, M. The neuroprotective action of 3, 3’-diindolylmethane against ischemia involves an inhibition of apoptosis and autophagy that depends on HDAC and AhR/CYP1A1 but not ERalpha/CYP19A1 signaling. Apoptosis 2019, 24, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Dever, D.P.; Adham, Z.O.; Thompson, B.; Genestine, M.; Cherry, J.; Olschowka, J.A.; DiCicco-Bloom, E.; Opanashuk, L.A. Aryl hydrocarbon receptor deletion in cerebellar granule neuron precursors impairs neurogenesis. Dev. Neurobiol. 2016, 76, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Kajta, M.; Wnuk, A.; Rzemieniec, J.; Lason, W.; Mackowiak, M.; Chwastek, E.; Staniszewska, M.; Nehring, I.; Wojtowicz, A.K. Triclocarban Disrupts the Epigenetic Status of Neuronal Cells and Induces AHR/CAR-Mediated Apoptosis. Mol. Neurobiol. 2019, 56, 3113–3131. [Google Scholar] [CrossRef] [PubMed]
- Kajta, M.; Wojtowicz, A.K.; Mackowiak, M.; Lason, W. Aryl hydrocarbon receptor-mediated apoptosis of neuronal cells: A possible interaction with estrogen receptor signaling. Neuroscience 2009, 158, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Juan, S.H.; Wang, C.Y.; Sun, Y.Y.; Chou, C.M.; Chang, S.F.; Hu, S.Y.; Lee, W.S.; Lee, Y.H. Neuronal activity enhances aryl hydrocarbon receptor-mediated gene expression and dioxin neurotoxicity in cortical neurons. J. Neurochem. 2008, 104, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.A.; Gasiewicz, T.A.; Opanashuk, L.A. Aryl hydrocarbon receptor expression and activity in cerebellar granule neuroblasts: Implications for development and dioxin neurotoxicity. Toxicol. Sci. 2005, 83, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhang, S.; Liang, H.; Xing, Z.; Guo, L.; Shi, L.; Du, L.; Kuang, C.; Takikawa, O.; Yang, Q. Amyloid beta neurotoxicity is IDO1-Kyn-AhR dependent and blocked by IDO1 inhibitor. Signal Transduct. Target. Ther. 2020, 5, 96. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Ferrer, I.; Cuartero, M.I.; Medina, V.; Ahedo-Quero, D.; Pena-Martinez, C.; Perez-Ruiz, A.; Fernandez-Valle, M.E.; Hernandez-Sanchez, C.; Fernandez-Salguero, P.M.; Lizasoain, I.; et al. Lack of the aryl hydrocarbon receptor accelerates aging in mice. FASEB J. 2019, 33, 12644–12654. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.; Okamoto, K.; Whitelaw, M.L.; Tanaka, H.; Poellinger, L. Definition of a dioxin receptor mutant that is a constitutive activator of transcription: Delineation of overlapping repression and ligand binding functions within the PAS domain. J. Biol. Chem. 2001, 276, 41841–41849. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.; McGuire, J.; Rubio, C.; Gradin, K.; Whitelaw, M.L.; Pettersson, S.; Hanberg, A.; Poellinger, L. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 9990–9995. [Google Scholar] [CrossRef] [PubMed]
- Brunnberg, S.; Andersson, P.; Lindstam, M.; Paulson, I.; Poellinger, L.; Hanberg, A. The constitutively active Ah receptor (CA-Ahr) mouse as a potential model for dioxin exposure--effects in vital organs. Toxicology 2006, 224, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.; Ridderstad, A.; McGuire, J.; Pettersson, S.; Poellinger, L.; Hanberg, A. A constitutively active aryl hydrocarbon receptor causes loss of peritoneal B1 cells. Biochem. Biophys. Res. Commun. 2003, 302, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Brunnberg, S.; Andersson, P.; Poellinger, L.; Hanberg, A. The constitutively active Ah receptor (CA-AhR) mouse as a model for dioxin exposure—Effects in reproductive organs. Chemosphere 2011, 85, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K. Dioxin: A review of its environmental effects and its aryl hydrocarbon receptor biology. J. Comp. Physiol. B 2005, 175, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Diestel, S.; Desoeuvre, A.; Tiveron, M.C.; Cremer, H. Efficient in vivo electroporation of the postnatal rodent forebrain. PLoS ONE 2008, 3, e1883. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, A.; Kubo, K.; Hayashi, K.; Matsunaga, Y.; Ishii, K.; Nakajima, K. Hippocampal pyramidal neurons switch from a multipolar migration mode to a novel “climbing” migration mode during development. J. Neurosci. 2014, 34, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Tabata, H.; Nakajima, K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: Visualization of neuronal migration in the developing cortex. Neuroscience 2001, 103, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Ding, Y.; Tohyama, C. AhR signaling activation disrupts migration and dendritic growth of olfactory interneurons in the developing mouse. Sci. Rep. 2016, 6, 26386. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Kubo, K.I.; Endo, T.; Ling, W.; Nakajima, K.; Kakeyama, M.; Tohyama, C. Impaired dendritic growth and positioning of cortical pyramidal neurons by activation of aryl hydrocarbon receptor signaling in the developing mouse. PLoS ONE 2017, 12, e0183497. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Kubo, K.I.; Endo, T.; Nakajima, K.; Kakeyama, M.; Tohyama, C. Excessive activation of AhR signaling disrupts neuronal migration in the hippocampal CA1 region in the developing mouse. J. Toxicol. Sci. 2017, 42, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ben Haim, L.; Rowitch, D.H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 2017, 18, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, J.; Yoon, B.E. Neuron-Glia Interactions in Neurodevelopmental Disorders. Cells 2020, 9, 2176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, X.; Zhang, Y.; Zheng, X.; Cepeda, C.; Wang, Y.; Duan, S.; Tong, X. Interactions of glial cells with neuronal synapses, from astrocytes to microglia and oligodendrocyte lineage cells. Glia 2023, 71, 1383–1401. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.R.; Michelhaugh, S.K.; Klinger, N.V.; Fadel, H.A.; Kiousis, S.; Ali-Fehmi, R.; Kupsky, W.J.; Juhasz, C.; Mittal, S. Investigation of the aryl hydrocarbon receptor and the intrinsic tumoral component of the kynurenine pathway of tryptophan metabolism in primary brain tumors. J. Neurooncol. 2018, 139, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ye, L.; Zhong, C.; Li, J.; Ye, F.; Lv, L.; Yu, Y.; Jiang, S.; Zhou, P. Kynurenine produced by tryptophan 2, 3-dioxygenase metabolism promotes glioma progression through an aryl hydrocarbon receptor-dependent signaling pathway. Cell Biol. Int. 2022, 46, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.C.; Gutierrez-Vazquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Chang, L.H.; Huang, S.S.; Huang, Y.J.; Chih, C.L.; Kuo, H.C.; Lee, Y.H.; Lee, I.H. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J. Neuroinflamm. 2019, 16, 187. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Zhu, K.; Wang, D.; Zhao, X.; Zhang, H.; Wu, S.; Wang, Y.; Wang, J. Microglial aryl hydrocarbon receptor enhances phagocytic function via SYK and promotes remyelination in the cuprizone mouse model of demyelination. J. Neuroinflamm. 2023, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lin, C.H.; Hsu, P.C.; Sun, Y.Y.; Huang, Y.J.; Zhuo, J.H.; Wang, C.Y.; Gan, Y.L.; Hung, C.C.; Kuan, C.Y.; et al. Aryl hydrocarbon receptor mediates both proinflammatory and anti-inflammatory effects in lipopolysaccharide-activated microglia. Glia 2015, 63, 1138–1154. [Google Scholar] [CrossRef] [PubMed]
- Minhas, P.S.; Jones, J.R.; Latif-Hernandez, A.; Sugiura, Y.; Durairaj, A.S.; Wang, Q.; Mhatre, S.D.; Uenaka, T.; Crapser, J.; Conley, T.; et al. Restoring hippocampal glucose metabolism rescues cognition across Alzheimer’s disease pathologies. Science 2024, 385, eabm6131. [Google Scholar] [CrossRef] [PubMed]
- Filbrandt, C.R.; Wu, Z.; Zlokovic, B.; Opanashuk, L.; Gasiewicz, T.A. Presence and functional activity of the aryl hydrocarbon receptor in isolated murine cerebral vascular endothelial cells and astrocytes. Neurotoxicology 2004, 25, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.E. Aryl hydrocarbon receptors: Diversity and evolution. Chem. Biol. Interact. 2002, 141, 131–160. [Google Scholar] [CrossRef] [PubMed]
- de Esch, C.; Slieker, R.; Wolterbeek, A.; Woutersen, R.; de Groot, D. Zebrafish as potential model for developmental neurotoxicity testing: A mini review. Neurotoxicol. Teratol. 2012, 34, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Murakami, S.; Ashikawa, Y.; Sasagawa, S.; Umemoto, N.; Shimada, Y.; Tanaka, T. Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit. Anom. 2015, 55, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, E.A.; Spitsbergen, J.M.; Tanguay, R.L.; Stegeman, J.J.; Heideman, W.; Peterson, R.E. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: Effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol. Sci. 2002, 68, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.R.; Patel, R.; Kossack, M.E.; Tian, L.; Camarillo, M.A.; Cintron-Rivera, L.G.; Gawdzik, J.C.; Yue, M.S.; Nwagugo, F.O.; Elemans, L.M.H.; et al. Proper modulation of AHR signaling is necessary for establishing neural connectivity and oligodendrocyte precursor cell development in the embryonic zebrafish brain. Front. Mol. Neurosci. 2022, 15, 1032302. [Google Scholar] [CrossRef] [PubMed]
- Ton, C.; Lin, Y.; Willett, C. Zebrafish as a model for developmental neurotoxicity testing. Birth Defects Res. A Clin. Mol. Teratol. 2006, 76, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Powell-Coffman, J.A.; Jin, Y. The AHR-1 aryl hydrocarbon receptor and its co-factor the AHA-1 aryl hydrocarbon receptor nuclear translocator specify GABAergic neuron cell fate in C. elegans. Development 2004, 131, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.D.; Jan, L.Y.; Jan, Y.N. The bHLH-PAS protein Spineless is necessary for the diversification of dendrite morphology of Drosophila dendritic arborization neurons. Genes Dev. 2006, 20, 2806–2819. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Powell-Coffman, J.A. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev. Biol. 2004, 270, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; O’Brien, T.; Chatzigeorgiou, M.; Spencer, W.C.; Feingold-Link, E.; Husson, S.J.; Hori, S.; Mitani, S.; Gottschalk, A.; Schafer, W.R.; et al. Sensory neuron fates are distinguished by a transcriptional switch that regulates dendrite branch stabilization. Neuron 2013, 79, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, J. Emissions, environmental levels, sources, formation pathways, and analysis of polybrominated dibenzo-p-dioxins and dibenzofurans: A review. Environ. Sci. Pollut. Res. Int. 2018, 25, 33082–33102. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Fujimaki, T.S.; Kitamura, K.; Hashimoto, S.; Ito, H.; Suzuki, N.; Sakai, S.; Morita, M. Polybrominated dibenzo-p-dioxins, dibenzofurans, and diphenyl ethers in Japanese human adipose tissue. Environ. Sci. Technol 2003, 37, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Croes, K.; Colles, A.; Koppen, G.; De Galan, S.; Vandermarken, T.; Govarts, E.; Bruckers, L.; Nelen, V.; Schoeters, G.; Van Larebeke, N.; et al. Determination of PCDD/Fs, PBDD/Fs and dioxin-like PCBs in human milk from mothers residing in the rural areas in Flanders, using the CALUX bioassay and GC-HRMS. Talanta 2013, 113, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Haglund, P.; Malmvarn, A.; Bergek, S.; Bignert, A.; Kautsky, L.; Nakano, T.; Wiberg, K.; Asplund, L. Brominated dibenzo-p-dioxins: A new class of marine toxins? Environ. Sci. Technol. 2007, 41, 3069–3074. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Matsukami, H.; Ieda, T.; Suzuki, G. Comprehensive screening of polybromochlorodibenzo-p-dioxins, dibenzofurans as mixed halogenated compounds in wastewater samples from industrial facilities by GCxGC/ToFMS and post-data processing. Chemosphere 2021, 276, 130085. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Jiang, Q.; Yuan, W.; Hanari, N.; Okazawa, T.; Wyrzykowska, B.; So, M.K.; Lam, P.K.; Yamashita, N. Preliminary health risk assessment for polybrominated diphenyl ethers and polybrominated dibenzo-p-dioxins/furans in seafood from Guangzhou and Zhoushan, China. Mar. Pollut. Bull. 2008, 57, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Watanabe, J.; Honda, Y.; Takatsuki, H.; Aoki, I.; Futamatsu, M.; Shiozaki, K. Combustion of brominated flame retardants and behavior of its byproducts. Chemosphere 2001, 42, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Someya, M.; Takahashi, S.; Tanabe, S.; Sakai, S.; Takigami, H. Dioxin-like activity in Japanese indoor dusts evaluated by means of in vitro bioassay and instrumental analysis: Brominated dibenzofurans are an important contributor. Environ. Sci. Technol. 2010, 44, 8330–8336. [Google Scholar] [CrossRef] [PubMed]
- Zacs, D.; Rjabova, J.; Viksna, A.; Bartkevics, V. Method development for the simultaneous determination of polybrominated, polychlorinated, mixed polybrominated/chlorinated dibenzo-p-dioxins and dibenzofurans, polychlorinated biphenyls and polybrominated diphenyl ethers in fish. Chemosphere 2015, 118, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Tue, N.M.; Kimura, E.; Maekawa, F.; Goto, A.; Uramaru, N.; Kunisue, T.; Suzuki, G. Uptake, Elimination and Metabolism of Brominated Dibenzofurans in Mice. Toxics 2024, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S.; Morrissey, R.E.; Harris, M.W. Teratogenic effects of 2,3,7,8-tetrabromodibenzo-p-dioxin and three polybrominated dibenzofurans in C57BL/6N mice. Toxicol. Appl. Pharmacol. 1991, 107, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Caudle, W.M.; Pi, J.; Bhattacharya, S.; Andersen, M.E.; Kaminski, N.E.; Conolly, R.B. Embracing Systems Toxicology at Single-Cell Resolution. Curr. Opin. Toxicol. 2019, 16, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nault, R.; Fader, K.A.; Bhattacharya, S.; Zacharewski, T.R. Single-Nuclei RNA Sequencing Assessment of the Hepatic Effects of 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Nault, R.; Saha, S.; Bhattacharya, S.; Sinha, S.; Maiti, T.; Zacharewski, T. Single-cell transcriptomics shows dose-dependent disruption of hepatic zonation by TCDD in mice. Toxicol. Sci. 2023, 191, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Tritscher, A.M.; Goldstein, J.A.; Portier, C.J.; McCoy, Z.; Clark, G.C.; Lucier, G.W. Dose-response relationships for chronic exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in a rat tumor promotion model: Quantification and immunolocalization of CYP1A1 and CYP1A2 in the liver. Cancer Res. 1992, 52, 3436–3442. [Google Scholar] [PubMed]
- Yang, Y.; Filipovic, D.; Bhattacharya, S. A Negative Feedback Loop and Transcription Factor Cooperation Regulate Zonal Gene Induction by 2, 3, 7, 8-Tetrachlorodibenzo-p-Dioxin in the Mouse Liver. Hepatol. Commun. 2022, 6, 750–764. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).