Investigation into the Enhancement Effects of Combined Bioremediation of Petroleum-Contaminated Soil Utilizing Immobilized Microbial Consortium and Sudan Grass
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Petroleum-Degrading Bacterial Isolation and Identification
2.3. Petroleum-Degrading Microbial Consortium and Degradation Characteristics
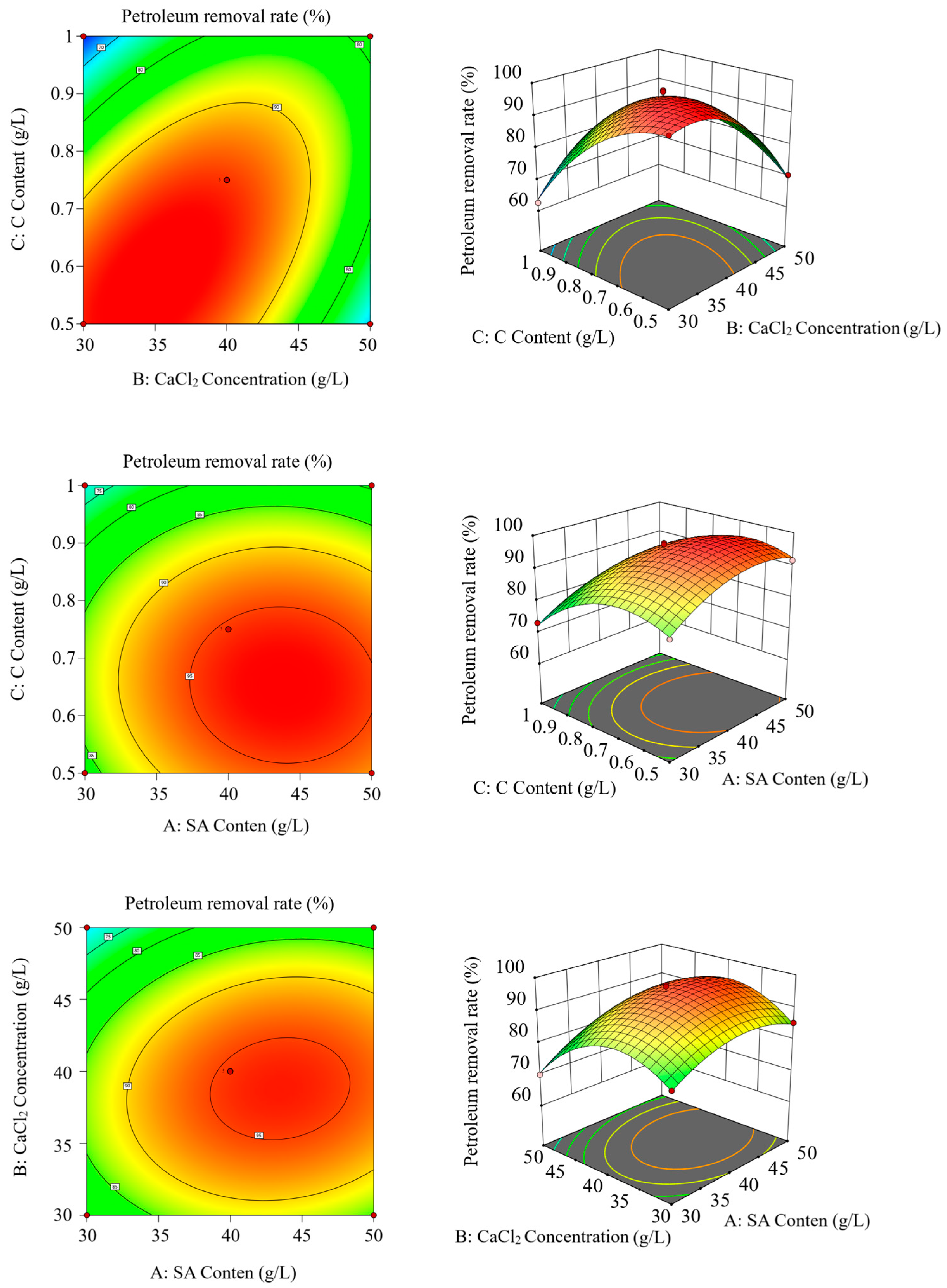
2.4. Preparation and Optimization of Immobilized Microbial Microspheres
2.5. Screening of Plants for Petroleum Remediation
2.6. Combined Immobilized Microbial Consortium and Plant Remediation of Petroleum-Contaminated Soil
2.7. Data Processing and Analysis
3. Results
3.1. Identification of Petroleum-Degrading Bacterial Strains and Phylogenetic Position
3.2. Degradation Characteristics of the Microbial Consortium
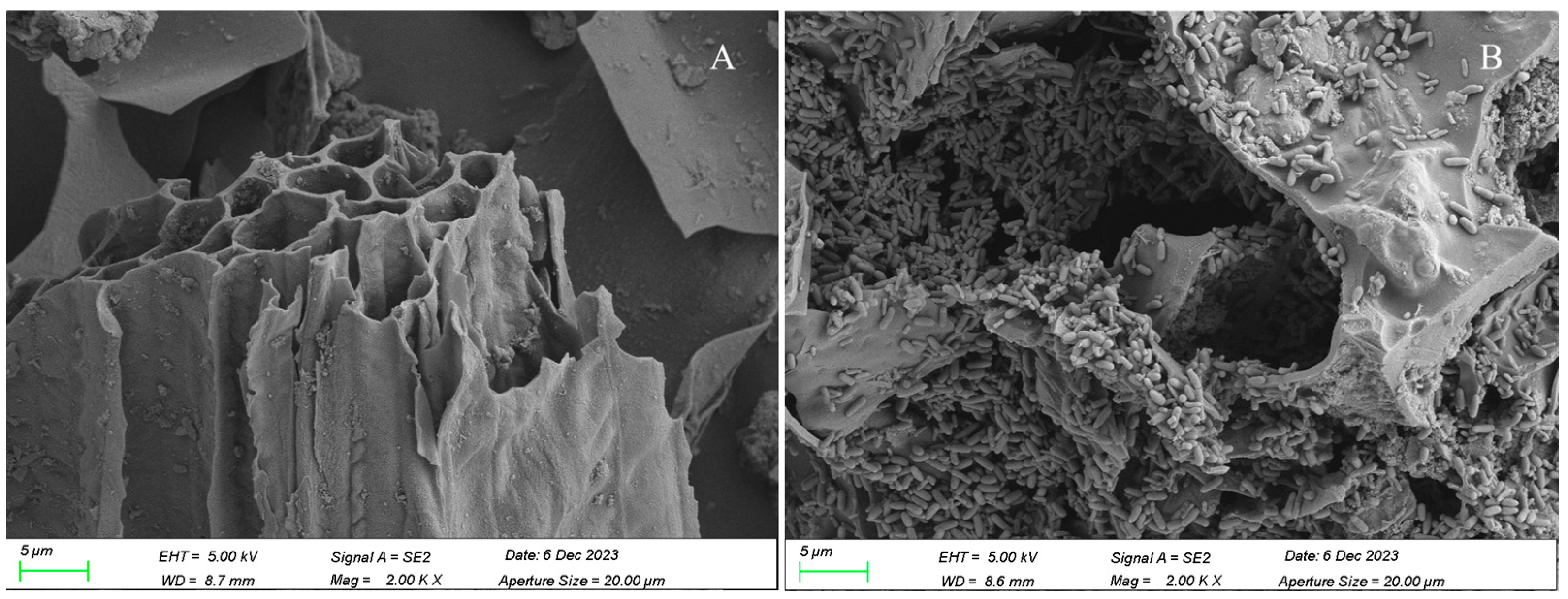
3.3. Characterization of Immobilized Microbial Microspheres
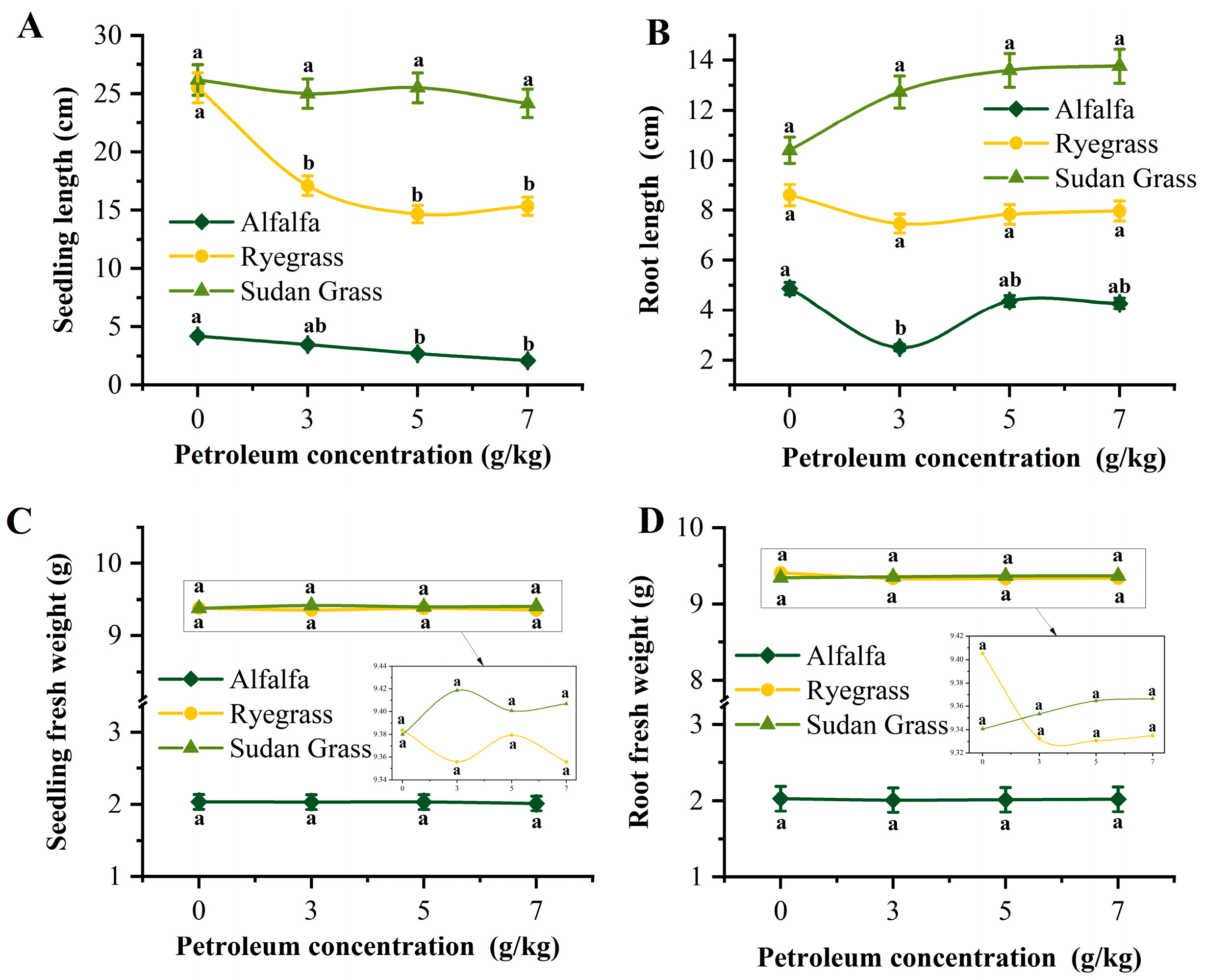
3.4. Screening of Petroleum Remediation Plants
3.5. Combined Immobilized Microbial Consortium and Sudan Grass for Enhanced Remediation of Petroleum-Contaminated Soil
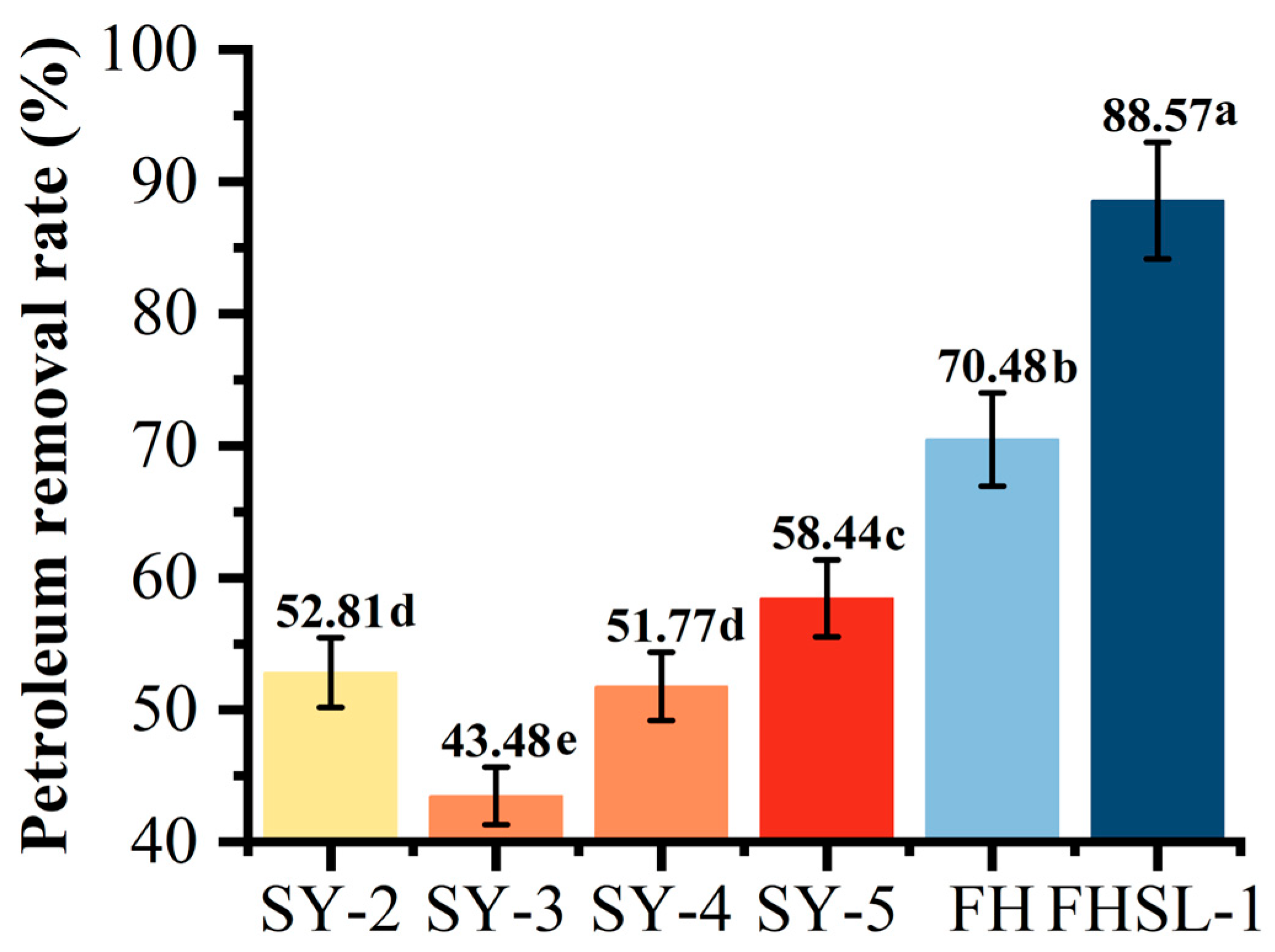
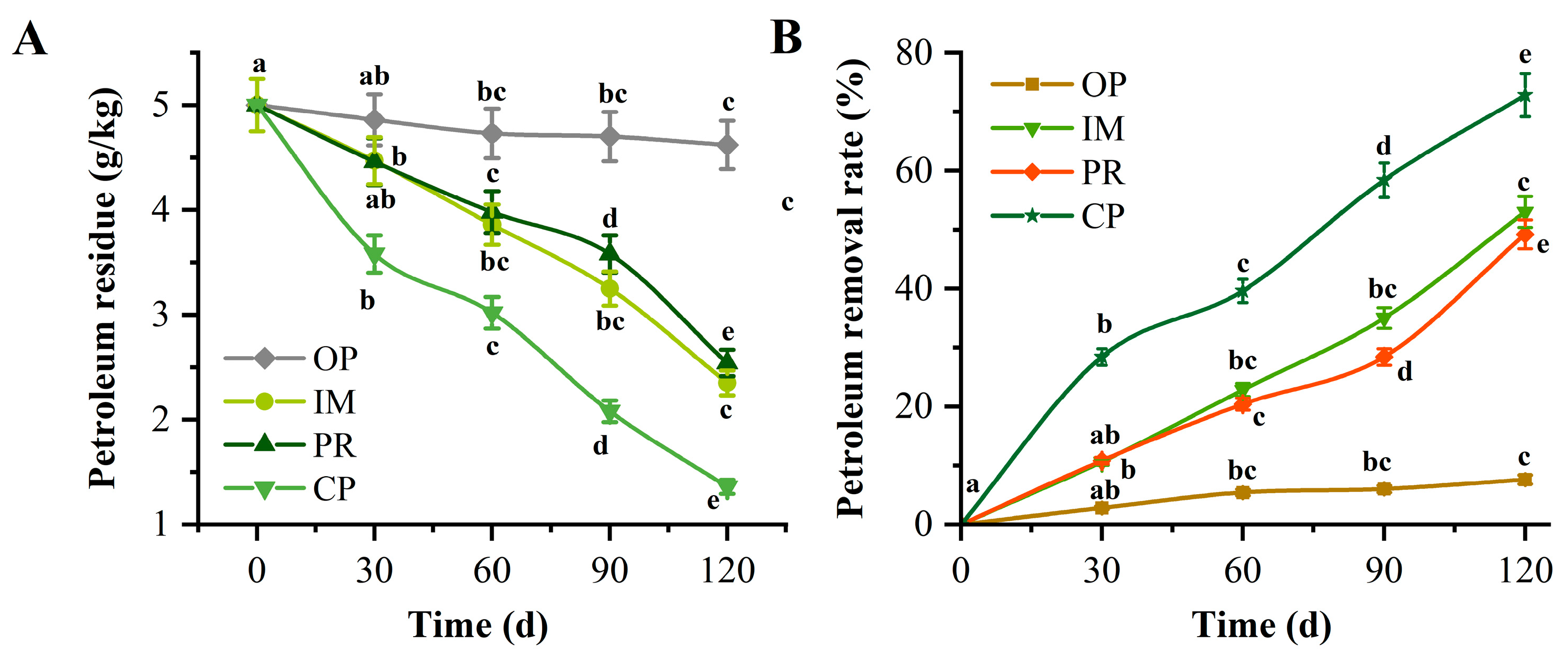
3.5.1. Petroleum Degradation Effect of Contaminated Soil
3.5.2. Soil Physicochemical Properties and Enzyme Activity
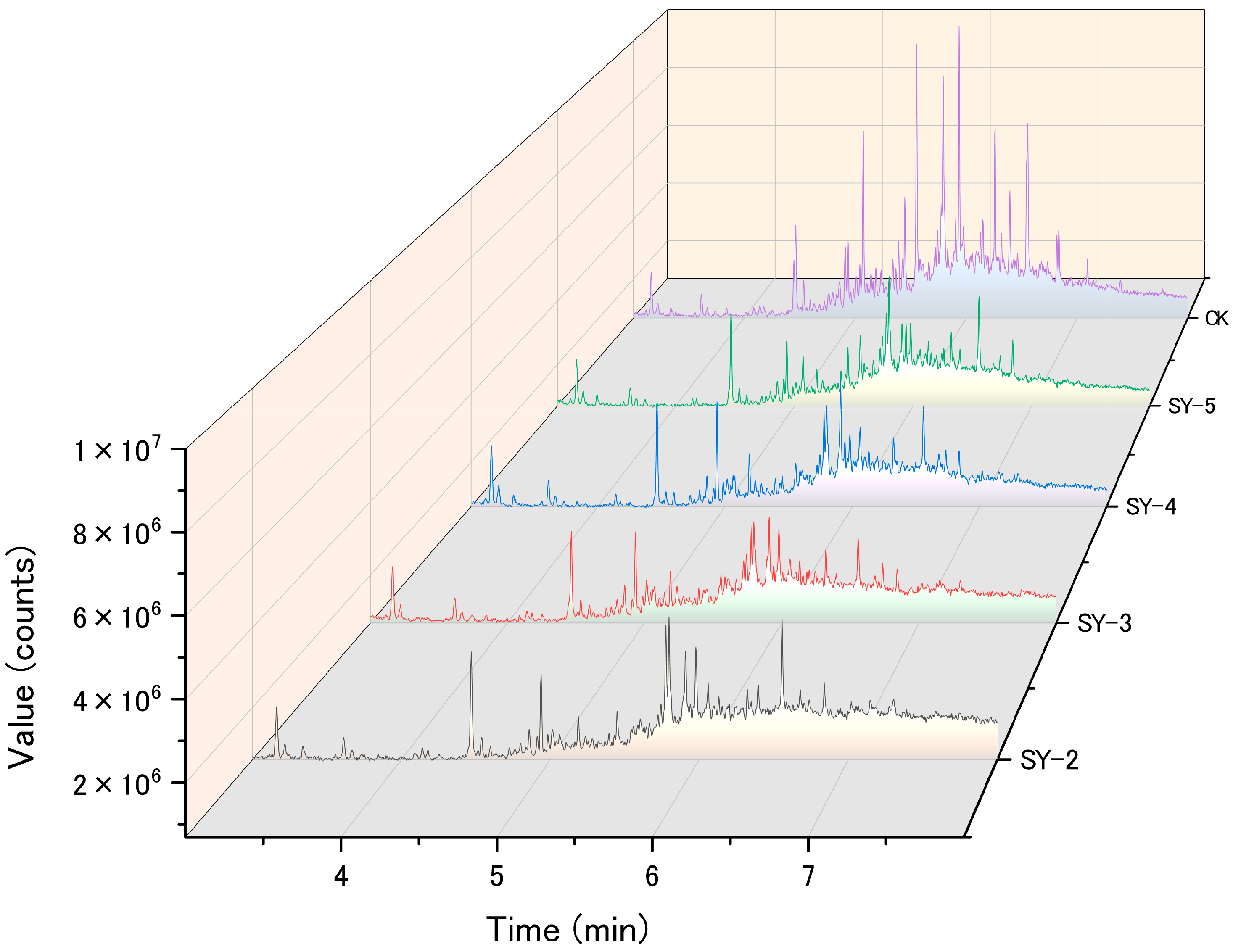
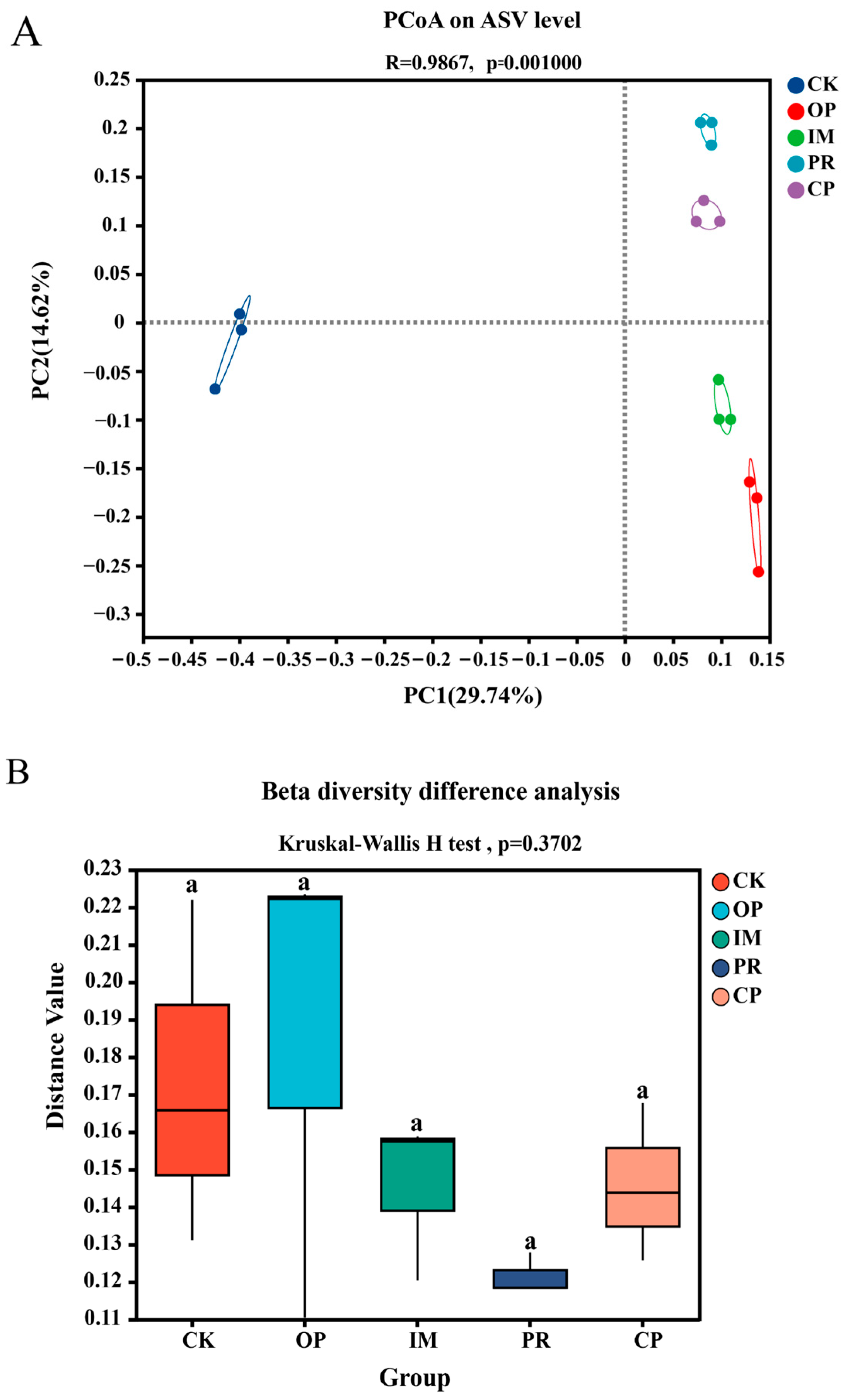
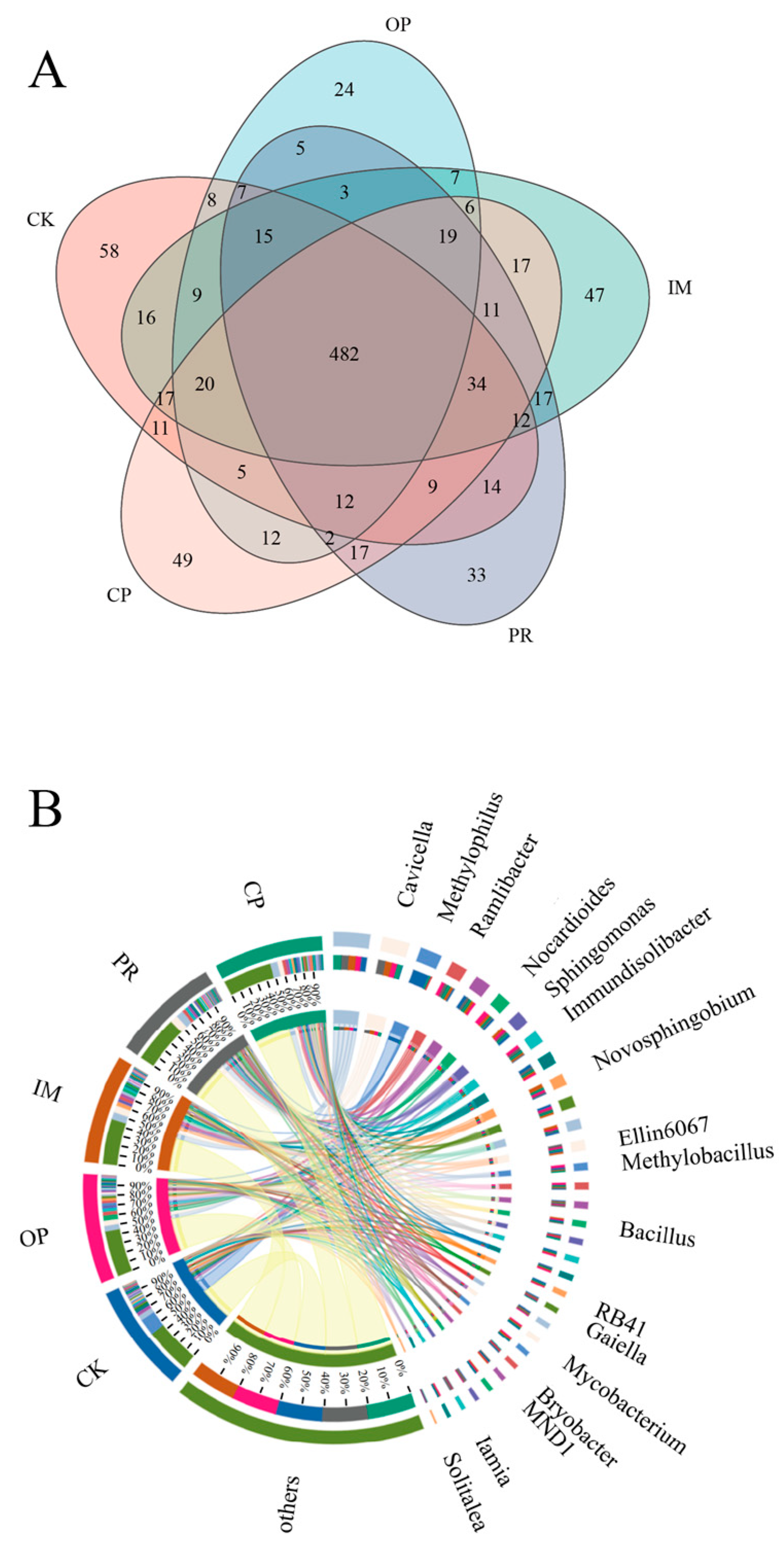
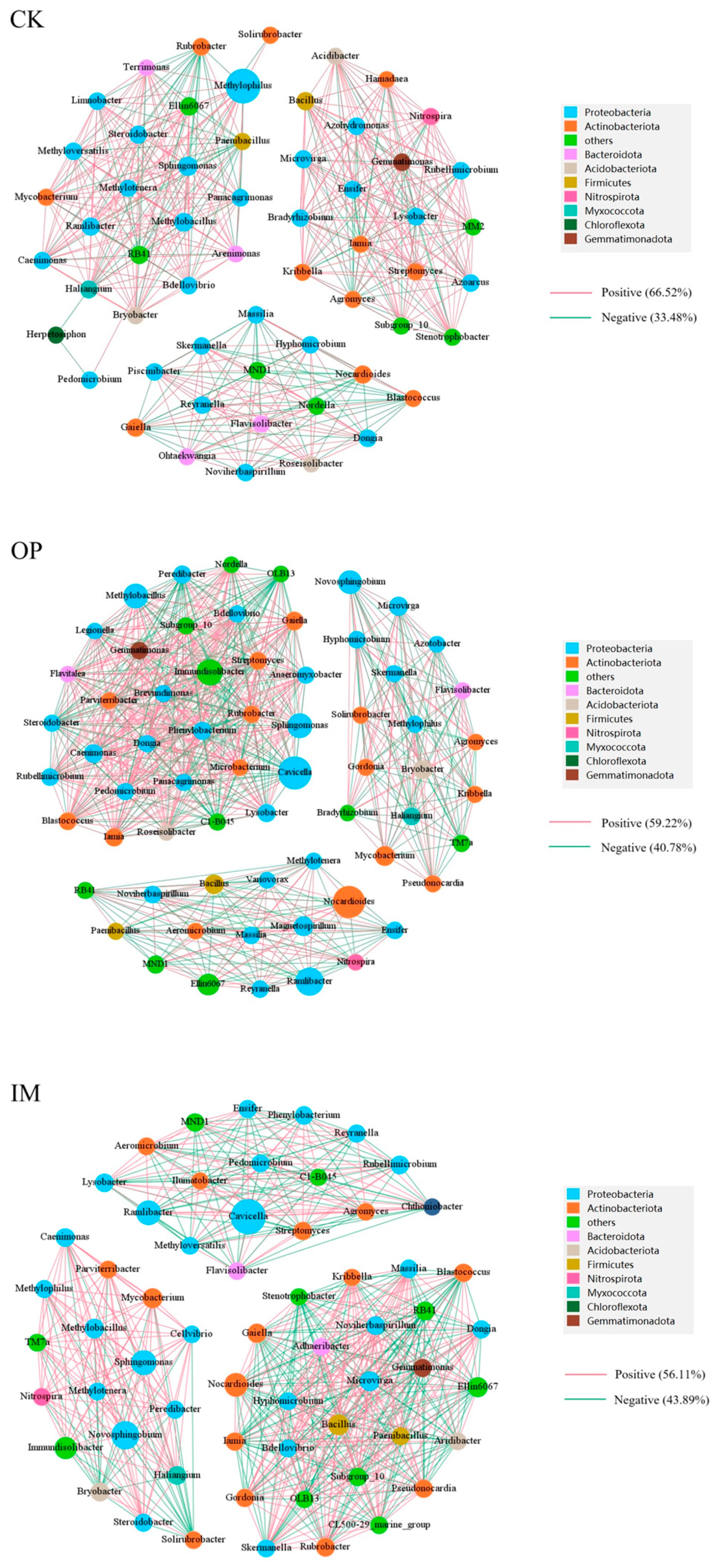
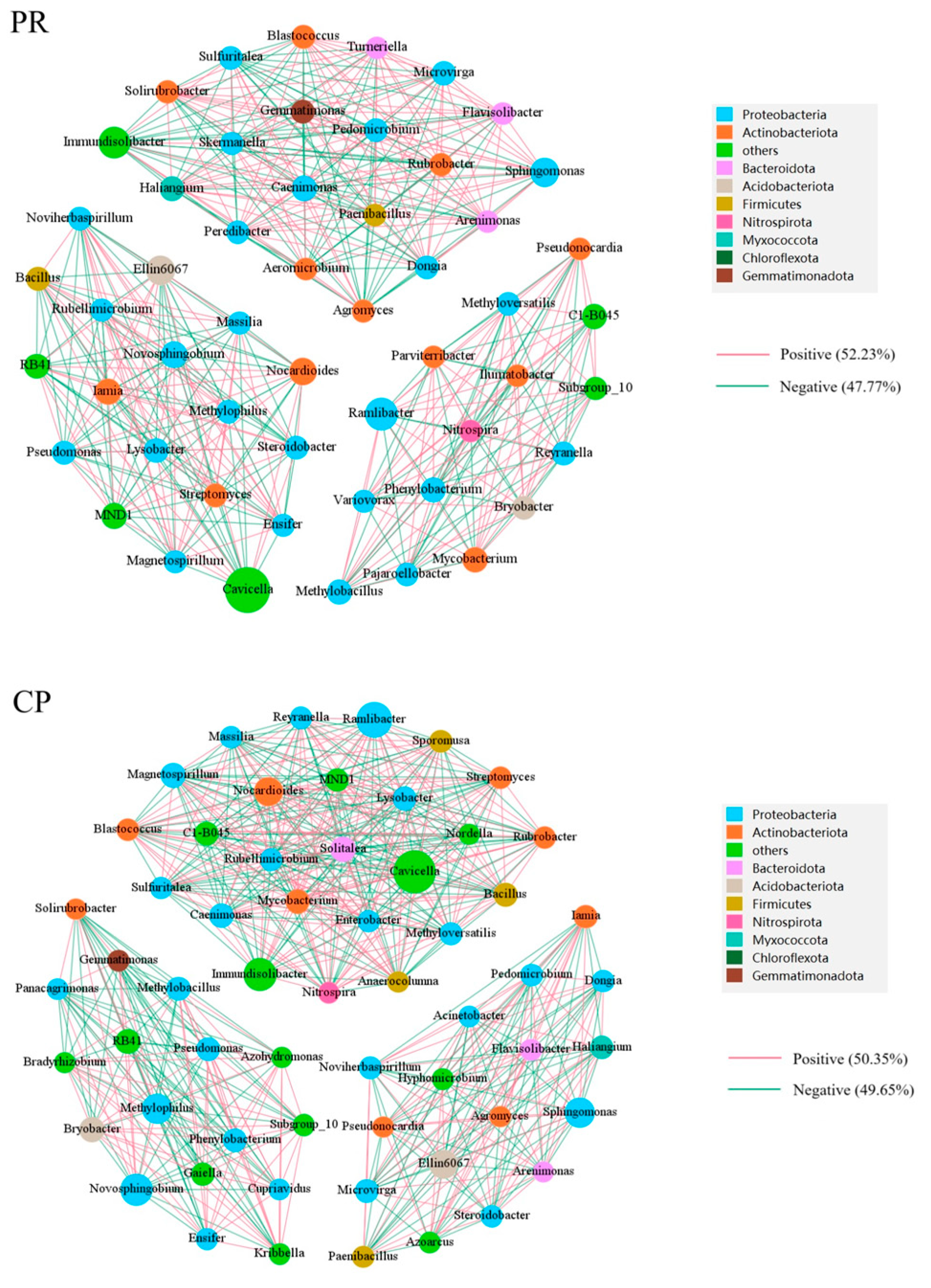
3.5.3. Bacterial Community Analysis of Petroleum-Contaminated Soil Remediation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elsayed, A.H.; Khalfaoui, R.; Naeem, S.; Gabauer, D. The impact of oil shocks on green, clean, and socially responsible markets. Energy Econ. 2024, 136, 107729. [Google Scholar] [CrossRef]
- Shahzad, A.; Zahra, A.; Li, H.Y.; Qin, M.; Wu, H.; Wen, M.Q.; Ali, M.; Iqbal, Y.; Xie, S.H.; Sattar, S.; et al. Modern perspectives of heavy metals alleviation from oil contaminated soil: A review. Ecotoxicol. Environ. Saf. 2024, 282, 116698. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Shah, G.; Singhal, K.; Soni, V. Comprehensive insights into the impact of oil pollution on the environment. Reg. Stud. Mar. Sci. 2024, 74, 103516. [Google Scholar] [CrossRef]
- Ning, Z.; Sheng, Y.; Guo, C.; Wang, S.; Yang, S.; Zhang, M. Incorporating the Soil Gas Gradient Method and Functional Genes to Assess the Natural Source Zone Depletion at a Petroleum-Hydrocarbon-Contaminated Site of a Purification Plant in Northwest China. Life 2022, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.R.; Frédéric, C.; Tamazon, C.; Ikeabiama, A.; Emmanuel, A.; Imma, B.; Ying, J. Evaluating Different Soil Amendments as Bioremediation Strategy for Wetland Soil Contaminated by Crude Oil. Sustainability 2022, 14, 16568. [Google Scholar] [CrossRef]
- Faisal, A.; Jamal, M.A.; Stephenson, L.S.; Williams, J.R. Bioremediation of petroleum wastewater and oil-polluted soils by the non-toxigenic indigenous fungi. World J. Microbiol. Biotechnol. 2024, 40, 336. [Google Scholar]
- Ambaye, T.G.; Formicola, F.; Sbaffoni, S.; Macas Lima, A.T.; Franzetti, A.; Vaccari, M. Environmental and economic performance of chemical and biological processes for treating petroleum hydrocarbon-contaminated soil: An experimental study. J. Environ. Chem. Eng. 2024, 12, 113672. [Google Scholar] [CrossRef]
- Yan, Z.F.; Huang, Q.S.; Yang, J.; Qiao, X.Y.; Xu, B.; Xia, W.; Su, L.Q. Enhanced degradation of crude oil by immobilized bacterial consortium through eliminating microbial flocculation towards crude oil. Int. Biodeterior. Biodegrad. 2025, 196, 105935. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Mohamed Hala, F.; Zheng, X.; He, J.; Lin, L.R. Rigid, eco-friendly and superhydrophobic SiO2-polyvinyl alcohol composite sponge for durable oil remediation. Chemosphere 2022, 307, 135990. [Google Scholar] [CrossRef] [PubMed]
- Vieira dos Santos, A.; Simonelli, G.; Lobato dos Santos, L.C. Review of the application of surfactants in microemulsion systems for remediation of petroleum contaminated soil and sediments. Environ. Sci. Pollut. Res. Int. 2023, 30, 32168–32183. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shi, S.; Zheng, J.; Zheng, X.; Xu, X.; Liu, K.; Wei, P.; Chen, Q.; Liu, F.; Zhao, C.; et al. An immobilized composite microbial material combined with slow release agents enhances oil-contaminated groundwater remediation. Sci. Total Environ. 2024, 919, 170762. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, J.; Fang, H. Research progress on plant-microbe combined remediation of petroleum-contaminated soil. China Resour. Compr. Util. 2024, 42, 118–120. [Google Scholar]
- Adedeji, J.A.; Tetteh, E.K.; Opoku, A.M.; AsanteSackey, D.; OforiFrimpong, S.; Armah, E.K.; Rathilal, S.; Mohammadi, A.H.; Chetty, M. Microbial bioremediation and biodegradation of petroleum products—A mini review. Appl. Sci. 2022, 12, 12212. [Google Scholar] [CrossRef]
- Njoku, K.L.; Ude, E.O.; Jegede, T.O.; Adeyanju, O.Z.; Iheme, P.O. Characterization of hydrocarbon degrading microorganisms from Glycine max and Zea mays phytoremediated crude oil contaminated soil. Environ. Anal. Health Toxicol. 2022, 37, e2022008-0. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Fu, W.; Feng, Y.; Liu, Z.; Song, D. Synergetic effects of microbial-phytoremediation reshape microbial communities and improve degradation of petroleum contaminants. J. Hazard. Mater. 2022, 429, 128396. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, T.; Deng, Z.; Wang, J.; Li, M. Neorhizobium petrolearium OS53 combined with Medicago sativa for synergistic remediation of petroleum contaminated soil. Acta Microbiol. Sin. 2024, 64, 854–868. [Google Scholar]
- Khilji, S.A.; Waseem, M.; Tariq, S.; Jabeen, S.; Jamal, A.; Alomrani, S.O.; Javed, T.; Riaz, A. Microbe-assisted phytoremediation of heavy metal contaminated soil by using Tagetes erecta L. Plant Stress 2024, 11, 100369. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Li, S.; Yang, R.; Yan, C.; Li, M.; Lu, L. Comparative effects of chemical dispersants and rhamnolipid biosurfactants on oil biodegradation and microbial community in coastal sediments. Int. Biodeterior. Biodegrad. 2025, 196, 105913. [Google Scholar] [CrossRef]
- Zhen, M.; Hang, Z.; Wu, C.; Zhao, J.; Li, Z. Performance evaluation of rhamnolipid biosurfactant produced by Pseudomonas aeruginosa and its effect on marine oil-spill remediation. Arch. Microbiol. 2024, 206, 183. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.C.; Vasconcelos, D.F.; Akram, A.H.; Zhiqiang, B. Unveiling the capacity of bioaugmentation application, in comparison with biochar and rhamnolipid for TPHs degradation in aged hydrocarbons polluted soil. Environ. Res. 2024, 252, 118880. [Google Scholar] [CrossRef] [PubMed]
- Albasri, H.M.; Almohammadi, A.A.; Alhhazmi, A.; Bukhari, D.A.; Waznah, M.S.; Mawad, A.M.M. Production and characterization of rhamnolipid biosurfactant from thermophilic Geobacillus stearothermophilus bacterium isolated from Uhud mountain. Front. Microbiol. 2024, 15, 1358175. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Khan, W.H.; Li, J.; Kubota, K.; Wang, Q.; Wang, Y. Metabolic mechanism in biosynthesis of polyhydroxyalkanoate from terephthalic acid by mixed microbial consortium. Chem. Eng. J. 2025, 515, 163695. [Google Scholar] [CrossRef]
- Guo, B.; Wei, Y.; Liu, X.; Qian, T.; Guo, J.; Yang, J.; Chen, T. Water-soluble carboxymethyl chitosan and rhamnolipids promote the remediation of Cd-contaminated soil by mediating the growth of Hylotelephium spectabile and regulating the rhizospheric ecological environment. J. Hazard. Mater. 2025, 486, 137040. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bao, H.; Pan, G.; Zhang, H.; Li, J.; Li, J.; Cai, J.; Wu, F. Combined application of rhamnolipid and agricultural wastes enhances PAHs degradation via increasing their bioavailability and changing microbial community in contaminated soil. J. Environ. Manag. 2021, 294, 112998. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, L.; Zhao, S.; Lei, Z.; Xia, M.; Zhang, C. Study of the characteristics of two immobilized microbial materials in degradation and evolution of petroleum hydrocarbon. ACS Omega 2020, 5, 19402–19408. [Google Scholar] [CrossRef] [PubMed]
- Bouabidi, Z.; El-Naas, M.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 19–31. [Google Scholar] [CrossRef]
- Du, Q.; Chen, Z.; Li, Y.; Le, L.; Li, J.; Jia, L.; Yan, X. Effects of activated carbon content on PVA-SA immobilized microspheres for treating chlorobenzene micro-polluted wastewater. J. Guangdong Univ. Ind. Technol. 2017, 34, 22–26. [Google Scholar]
- Yang, Z.; Jiang, L.; Li, X.; Ji, Q.; Wang, M.; Zhang, Y.; Cheng, Y.; Zhang, X.; Li, H.; Feng, C. Role of sludge biochar immobilized multifunctional microbiome in phytoremediation of lead-zinc composite pollution. Biochar 2025, 7, 5. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, X.; Ren, L.; Li, D.; Xiong, K.; Wang, R.; Zhang, Y.; Zuo, S. Enhanced 2,4-dichlorophenol degradation by remodeling of crude soil microbial community structure using carbon-based immobilized microorganisms. J. Environ. Chem. Eng. 2024, 12, 114339. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, Z.; Liang, W.; Niu, Q.; Wang, X. The Prevention of Bio-Organic Fertilizer Fermented from Cow Manure Compost by Bacillus sp. XG-1 on Watermelon Continuous Cropping Barrier. Int. J. Environ. Res. Public Health 2020, 17, 5714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, H.; Xiang, Y.; Wang, H.; Qian, Y.; Huang, X. Enhanced bioremediation of bensulfuron-methyl contaminated soil by Hansschlegelia zhihuaiae S113: Metabolic pathways and bacterial community structure. J. Hazard. Mater. 2024, 480, 136471. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; De, R.; Das, B. Hydrodynamic and conformational characterization of aqueous sodium alginate solutions with var ying salinity. Carbohydr. Polym. 2022, 277, 118855. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Das, B. Where do the pyrene molecules reside within the surface active ionic liquid micelles in presence of sodium alginate? Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 329, 125618. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Dong, Y.; Ji, Y.; Liu, Y.; Tian, T. Study of five petroleum degrading bacteria genes for the degradation of oil contamination. Environ. Technol. 2015, 28, 1–4. [Google Scholar]
- Olatunji, O.S.; Jimoda, L.A.; Fakinle, B.S.; Adeniran, J.A.; Sonibare, J.A. Total sulfur levels in refined petroleum products of Southwestern Nigeria using UV/VIS spectrophotometer. Pet. Sci. Technol. 2015, 33, 102–109. [Google Scholar] [CrossRef]
- Chavarín Meza, A.J.; Gómez Gil, B.; González Castillo, A. Phylogenomic analysis of the Ponticus clade: Strains isolated from the spotted rose snapper (Lutjanus guttatus). Antonie Van Leeuwenhoek 2024, 117, 59. [Google Scholar] [CrossRef] [PubMed]
- Lozoya, S.; Duarte Rico, R.; Carreón León, E.A.; López Meléndez, C.; Carreño Gallardo, C.; Aguilar Madrigal, R.M.; Monreal Romero, H.A. Evaluation of the antimicrobial effect of Ag nanoparticles on nickel–titanium archwires in the presence of Streptococcus mutans bacteria. Coatings 2024, 14, 1503. [Google Scholar] [CrossRef]
- Hong, B.; Zhu, W.; Wu, T.; Zhang, H.; Ge, H.; Hu, T.; Zhao, J.; Fang, H.; Song, C. Enhanced biodegradation of monoaromatic hydrocarbons by a novel bacterial strain Pseudomonas viridilivida FB1 and biochar. J. Clean. Prod. 2025, 498, 145194. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Ma, M.; Guo, Y.; Ma, Q.; Zhao, L. Efficient degradation of alternariol in food by a novel isolate, Bacillus pacificus ANSB901. Food Microbiol. 2025, 130, 104770. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ye, W.; Wang, G.; Xu, L.; Li, E. Isolation and identification of Sphingomonas sp. from chicken cecum and its ammonia-degrading activity. J. Biotech Res. 2021, 12, 65–73. [Google Scholar]
- Sultana, S.; Sultana, R.; Al Mansur, M.A.; Akbor, M.A.; Bhuiyan, N.A.; Ahmed, S.; Yasmin, S.; Molla Jamal, A.H.M.S.I. An industrially potent rhamnolipid-like biosurfactant produced from a novel oil-degrading bacterium, Bacillus velezensis S2. RSC Adv. 2024, 14, 24516–24533. [Google Scholar] [CrossRef] [PubMed]
- Zohaib, N.M.; Chunyan, C.; Majjid, A.Q.; Haider Syed, Z.; Khalid Hafiz, R.; Alghamdi, H.A.; Khan Iqrar, A.; Daochen, Z. Genomic and biotechnological potential of a novel oil-degrading strain Enterobacter kobei DH7 isolated from petroleum-contaminated soil. Chemosphere 2023, 340, 139815. [Google Scholar]
- Qiang, F.; Yu, Y.; Meng, Z.; Li, Z.; Si, S. Enhancing the ability of two kinds of oil-degrading bacteria to treat oily sludge by optimising the growth conditions using a response surface methodology. Water Sci. Technol. 2022, 86, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, Y.; Chen, Q.; Sun, W.; Zhu, B. Bioremediation of diesel oil polluted seawater by a hydrocarbon-degrading bacterial consortium with oleophilic nutrients. Reg. Stud. Mar. Sci. 2024, 71, 142379. [Google Scholar] [CrossRef]
- Lu, L.H.; Zue, X.; Yao, M.; Sheng, L.; Ya, C.; Yan, C.; Huai, Z.; Xiang, H.; Yu, F. Influence of rhamnolipids biosurfactant on the anodic electrochemical performance in marine sediment microbial fuel cell and the acceleration degradation of crude petroleum. J. Ocean Univ. China 2025, 24, 139–146. [Google Scholar] [CrossRef]
- Lopes, P.R.M.; Montagnolli, R.N.; Dilarri, G.; Mendes, C.R.; Cruz, J.M.; Bergamini Lopes, M.P.; Moreira, B.R.A.; Contiero, J.; Bidoia, E.D. Combination of Pseudomonas aeruginosa and rhamnolipid for bioremediation of soil contaminated with waste lubricant oil. Appl. Biochem. Microbiol. 2024, 60, 627–639. [Google Scholar] [CrossRef]
- Smita, K.; Krishna, G.; Monika, S.; Sadasivam, A.; Natesan, M. Bioremediation of polycyclic aromatic hydrocarbons in crude oil by bacterial consortium in soil amended with Eisenia fetida and rhamnolipid. Environ. Sci. Pollut. Res. Int. 2023, 30, 82517–82531. [Google Scholar]
- Sonsuphab, K.; Toomsan, W.; Soontharo, S.; Supanchaiyamat, N.; Hunt, A.J.; Ngernyen, Y.; Nasompag, S.; Kiattisaksiri, P.; Ratpukdi, T.; Siripattanakul-Ratpukdi, S. Integrated remediation and detoxification of triclocarban-contaminated water using waste-derived biochar-immobilized cells by long-term column experiments. Environ. Pollut. 2024, 357, 124456. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, D.; Wang, L.; Du, X.; Zhang, Z.; Liang, H. Bioremediation of 2,4,6-trichlorophenol by extracellular enzymes of white rot fungi immobilized with sodium alginate/hydroxyapatite/chitosan microspheres. Environ. Res. 2024, 252, 118937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, P.; Gao, J.; Li, Y.; Pu, Q.; Lyu, C.; Zhao, W. Improved microbial-plant soil bioremediation of PAHs and heavy metals through in silico methods. J. Hazard. Mater. 2024, 478, 135524. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, Z.; Wu, C.; Zhao, H.; Bao, P.; Cui, X. Water conditions and arbuscular mycorrhizal symbiosis affect the phytoremediation of petroleum-contaminated soil by Phragmites australis. Environ. Technol. Innov. 2023, 32, 103437. [Google Scholar] [CrossRef]
- Yang, C.; Li, H.; Dong, J.; Zhang, X.; Yang, Q.; Wang, Y.; Li, H.; Dang, L. Microbial agents’ effect on physicochemical indicators and bacterial community composition in chicken manure composting. J. Agric. Res. Environ. 2024, 41, 1035–1043. [Google Scholar]
- Wang, Z.; Fu, X.; Kuramae, E.E. Insight into farming native microbiome by bioinoculant in soil-plant system. Microbiol. Res. 2024, 285, 127776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, N.; Pan, Y.; Wang, H.; Guangming, Z. Immobilized microbial technology application in polycyclic aromatic hydrocarbons contaminated soil remediation. J. Environ. Eng. Technol. 2024, 14, 1617–1626. [Google Scholar]
| Indicators | SY-2 | SY-3 | SY-4 | SY-5 | Indicators | SY-2 | SY-3 | SY-4 | SY-5 |
|---|---|---|---|---|---|---|---|---|---|
| D-Ribose | + | − | − | − | D-Glucose | + | − | − | − |
| Sucrose | + | − | + | − | L-Arginine | − | + | + | + |
| Maltose | + | − | − | − | Urea | − | + | + | + |
| DL-Lactate | + | + | + | + | Tween 40 hydrolysis | + | + | + | + |
| L-Alanine | + | − | − | + | H2S generation | + | − | − | − |
| Glycogen | + | − | − | − | Tween 60 hydrolysis | + | − | − | + |
| L-Serine | + | − | − | − | Catalase | − | − | − | − |
| Mannitol | + | + | + | + | α-Glucosidase | − | − | − | − |
| Esterase (C4) | + | − | − | − | Oxidase | + | + | + | + |
| Level | Factor | ||
|---|---|---|---|
| A: SA Content (g·L−1) | B: CaCl2 Concentration (g·L−1) | C: C Content (g·L−1) | |
| −1 | 30 | 30 | 0.5 |
| 0 | 40 | 40 | 0.75 |
| 1 | 50 | 50 | 1 |
| Trial Number | Factor | Petroleum Removal Rate (%) | ||
|---|---|---|---|---|
| A: SA Content (g·L−1) | B: CaCl2 Concentration (g·L−1) | C: C Content (g·L−1) | ||
| 1 | 30 | 30 | 0.75 | 80.1 |
| 2 | 50 | 30 | 0.75 | 85.3 |
| 3 | 30 | 50 | 0.75 | 70.0 |
| 4 | 50 | 50 | 0.75 | 81.7 |
| 5 | 30 | 40 | 0.5 | 82.7 |
| 6 | 50 | 40 | 0.5 | 91.6 |
| 7 | 30 | 40 | 1 | 73.1 |
| 8 | 50 | 40 | 1 | 79.9 |
| 9 | 40 | 30 | 0.5 | 96.7 |
| 10 | 40 | 50 | 0.5 | 70.4 |
| 11 | 40 | 30 | 1 | 62.6 |
| 12 | 40 | 50 | 1 | 77.7 |
| 13 | 40 | 40 | 0.75 | 94.5 |
| 14 | 40 | 40 | 0.75 | 93.5 |
| 15 | 40 | 40 | 0.75 | 94.9 |
| 16 | 40 | 40 | 0.75 | 97.1 |
| 17 | 40 | 40 | 0.75 | 96.9 |
| Treatment | Sobs | ACE | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|
| CK | 3032 ± 437 a | 3106 ± 528 a | 3076 ± 497 a | 6.47 ± 0.28 a | 0.0309 ± 0.0140 a |
| OP | 2371 ± 154 b | 2385 ± 162 b | 2374 ± 156 b | 6.61 ± 0.06 a | 0.0060 ± 0.0016 b |
| IM | 2656 ± 112 ab | 2704 ± 129 ab | 2685 ± 128 ab | 6.73 ± 0.07 a | 0.0070 ± 0.0009 b |
| PR | 2648 ± 135 ab | 2674 ± 131 ab | 2654 ± 132 ab | 6.76 ± 0.10 a | 0.0064 ± 0.0013 b |
| CP | 2595 ± 308 ab | 2595 ± 308 ab | 2612 ± 332 ab | 6.69 ± 0.02 a | 0.0071 ± 0.0008 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-J.; Ding, Z.-Y.; Hua, Z.-W.; Yuan, Z.-W.; Niu, Q.-H.; Zhang, H. Investigation into the Enhancement Effects of Combined Bioremediation of Petroleum-Contaminated Soil Utilizing Immobilized Microbial Consortium and Sudan Grass. Toxics 2025, 13, 599. https://doi.org/10.3390/toxics13070599
Wang T-J, Ding Z-Y, Hua Z-W, Yuan Z-W, Niu Q-H, Zhang H. Investigation into the Enhancement Effects of Combined Bioremediation of Petroleum-Contaminated Soil Utilizing Immobilized Microbial Consortium and Sudan Grass. Toxics. 2025; 13(7):599. https://doi.org/10.3390/toxics13070599
Chicago/Turabian StyleWang, Tie-Jun, Zi-Yue Ding, Zi-Wei Hua, Zi-Wang Yuan, Qiu-Hong Niu, and Hao Zhang. 2025. "Investigation into the Enhancement Effects of Combined Bioremediation of Petroleum-Contaminated Soil Utilizing Immobilized Microbial Consortium and Sudan Grass" Toxics 13, no. 7: 599. https://doi.org/10.3390/toxics13070599
APA StyleWang, T.-J., Ding, Z.-Y., Hua, Z.-W., Yuan, Z.-W., Niu, Q.-H., & Zhang, H. (2025). Investigation into the Enhancement Effects of Combined Bioremediation of Petroleum-Contaminated Soil Utilizing Immobilized Microbial Consortium and Sudan Grass. Toxics, 13(7), 599. https://doi.org/10.3390/toxics13070599







