Fish Reproduction Is Disrupted upon Lifelong Exposure to Environmental PAHs Fractions Revealing Different Modes of Action
Abstract
:1. Introduction
2. Material and Methods
2.1. PAH-Contaminated Diet Preparation
2.2. Reproduction Monitoring
2.3. Histological Analysis
2.4. qPCR Analysis
2.5. Statistical Analysis
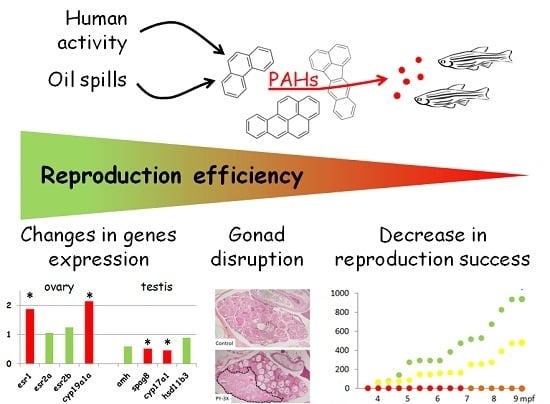
3. Results
3.1. Reproduction Monitoring
3.2. Histological Analysis
3.3. Genes Expression Analysis
4. Discussion
4.1. Reproductive Disruptions
4.2. Underlying Mechanisms
4.3. Environmental Relevance
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rochette, S.; Rivot, E.; Morin, J.; Mackinson, S.; Riou, P.; Le Pape, O. Effect of nursery habitat degradation on flatfish population: Application to Solea solea in the eastern channel (western europe). J. Sea Res. 2010, 64, 34–44. [Google Scholar] [CrossRef]
- Latimer, J.S.; Zheng, J. The sources, transport, and fate of PAHs in the marine environment. In PAHs: An Ecotoxicological Perspective; John Wiley & Sons, Ltd.: Chichester, UK, 2003; pp. 7–33. [Google Scholar]
- Shen, H.; Huang, Y.; Wang, R.; Zhu, D.; Li, W.; Shen, G.; Wang, B.; Zhang, Y.; Chen, Y.; Lu, Y.; et al. Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future predictions. Environ. Sci. Technol. 2013, 47, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
- Hylland, K. Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. J. Toxicol. Environ. Health Part A 2006, 69, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Baumard, P.; Budzinski, H.; Garrigues, P.; Sorbe, J.C.; Burgeot, T.; Bellocq, J. Concentrations of PAHs (polycyclic aromatic hydrocarbons) in various marine organisms in relation to those in sediments and to trophic level. Mar. Pollut. Bull. 1998, 36, 951–960. [Google Scholar] [CrossRef]
- Cachot, J.; Geffard, O.; Augagneur, S.; Lacroix, S.; Le Menach, K.; Peluhet, L.; Couteau, J.; Denier, X.; Devier, M.H.; Pottier, D.; et al. Evidence of genotoxicity related to high PAH content of sediments in the upper part of the seine estuary (Normandy, France). Aquat. Toxicol. 2006, 79, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Benlahcen, K.T.; Chaoui, A.; Budzinski, H.; Bellocq, J.; Garrigues, P. Distribution and sources of polycyclic aromatic hydrocarbons in some mediterranean coastal sediments. Mar. Pollut. Bull. 1997, 34, 298–305. [Google Scholar] [CrossRef]
- Johnson, L.L.; Ylitalo, G.M.; Arkoosh, M.R.; Kagley, A.N.; Stafford, C.; Bolton, J.L.; Buzitis, J.; Anulacion, B.F.; Collier, T.K. Contaminant exposure in outmigrant juvenile salmon from pacific northwest estuaries of the united states. Environ. Monit. Assess. 2007, 124, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, U.; Casillas, E.; Arkoosh, M.R.; Hom, T.; Misitano, D.; Brown, D.W.; Chan, S.-L.; Collier, T.K.; McCain, B.B.; Stein, J.E. Contaminant Exposure and Associated Biological Effects in Juvenile Chinook Salmon (Oncorhynchus tshawytscha) from Urban and Nonurban Estuaries of Puget Sound; NOAA Fisheries: Seattle, WA, USA, 1993; p. 112. [Google Scholar]
- Yanagida, G.K.; Anulacion, B.F.; Bolton, J.L.; Boyd, D.; Lomax, D.P.; Paul Olson, O.; Sol, S.Y.; Willis, M.; Ylitalo, G.M.; Johnson, L.L. Polycyclic aromatic hydrocarbons and risk to threatened and endangered chinook salmon in the lower columbia river estuary. Arch. Environ. Contam. Toxicol. 2012, 62, 282–295. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Gao, J.M.; Huang, C.J.; Li, C.Q. Zebrafish models for assessing developmental and reproductive toxicity. Neurotoxicol. Teratol. 2014, 42, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.E.; Kullman, S.W.; Hardman, R.C.; Volz, D.C.; Chen, P.-J.; Carney, M.; Bencic, D.C. Resolving mechanisms of toxicity while pursuing ecotoxicological relevance? Mar. Pollut. Bull. 2005, 51, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Leon-Olea, M.; Martyniuk, C.J.; Orlando, E.F.; Ottinger, M.A.; Rosenfeld, C.S.; Wolstenholme, J.T.; Trudeau, V.L. Current concepts in neuroendocrine disruption. Gen. Comp. Endocrinol. 2014, 203, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Kloas, W.; Urbatzka, R.; Opitz, R.; Wurtz, S.; Behrends, T.; Hermelink, B.; Hofmann, F.; Jagnytsch, O.; Kroupova, H.; Lorenz, C.; et al. Endocrine disruption in aquatic vertebrates. Ann. N. Y. Acad. Sci. 2009, 1163, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Seruto, C.; Sapozhnikova, Y.; Schlenk, D. Evaluation of the relationships between biochemical endpoints of PAH exposure and physiological endpoints of reproduction in male california halibut (Paralichthys californicus) exposed to sediments from a natural oil seep. Mar. Environ. Res. 2005, 60, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Horng, C.Y.; Lin, H.C.; Lee, W. A reproductive toxicology study of phenanthrene in medaka (Oryzias latipes). Arch. Environ. Contam. Toxicol. 2010, 58, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Tollefsen, K.E.; Sundt, R.C.; Beyer, J.; Meier, S.; Hylland, K. Endocrine modulation in atlantic cod (Gadus morhua L.) exposed to alkylphenols, polyaromatic hydrocarbons, produced water, and dispersed oil. J. Toxicol. Environ. Health A 2011, 74, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Tilghman Hall, A.; Oris, J.T. Anthracene reduces reproductive potential is maternally transferred during long-term exposure in fathead minnows. Aquat. Toxicol. 1991, 19, 249–264. [Google Scholar] [CrossRef]
- Bugel, S.M.; White, L.A.; Cooper, K.R. Impaired reproductive health of killifish (Fundulus heteroclitus) inhabiting Newwark bay, NJ, a chronically contaminated estuary. Aquat. Toxicol. 2010, 96, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Casillas, E.; Misitano, D.; Johnson, L.L.; Rhodes, L.D.; Collier, T.K.; Stein, J.E.; McCain, B.B.; Varanasi, U. Inducibility of spawning and reproductive success of female english sole (Praphrys vetulus) from urban and nonurban areas of Puget Sound, Washington. Mar. Environ. Res. 1991, 31, 99–122. [Google Scholar] [CrossRef]
- Johnson, L.L.; Stein, J.E.; Collier, T.K.; Casillas, E.; Varanasi, U. Indicators of reproductive development in prespawning female winter flounder (Pleuronectes americanus) from urban and non-urban estuaries in the northeast United States. Sci. Total Environ. 1994, 141, 241–260. [Google Scholar] [CrossRef]
- Kiceniuk, J.W.; Khan, R.A. Effect of petroleum hydrocarbons on atlantic cod, Gadus morhua, following chronic exposure. Can. J. Zool. 1987, 65, 490–494. [Google Scholar] [CrossRef]
- Ridgway, L.L.; Chapleau, F.; Comba, M.E.; Backus, S.M. Population characteristics and contaminant burdens of the white sucker (Catostomus commersoni) from the St. Lawrence river near Cornwall, Ontario and Massena, New York. J. Great Lakes Res. 1999, 25, 567–582. [Google Scholar] [CrossRef]
- Tetreault, G.R.; McMaster, M.E.; Dixon, D.G.; Parrott, J.L. Using reproductive endpoints in small forage fish species to evaluate the effects of Athabasca oil sands activities. Environ. Toxicol. Chem. 2003, 22, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.R.; Reis-Henriques, M.A.; Coimbra, J. Plasma steroid levels in female flounder (Platichthys flesus) after chronic dietary exposure to single polycyclic aromatic hydrocarbons. Mar. Environ. Res. 2000, 49, 453–467. [Google Scholar] [CrossRef]
- Arukwe, A.; Nordtug, T.; Kortner, T.M.; Mortensen, A.S.; Brakstad, O.G. Modulation of steroidogenesis and xenobiotic biotransformation responses in zebrafish (Danio rerio) exposed to water-soluble fraction of crude oil. Environ. Res. 2008, 107, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Evanson, M.; Van Der Kraak, G.J. Stimulatory effects of selected PAHs on testosterone production in goldfish and rainbow trout and possible mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 249–258. [Google Scholar] [CrossRef]
- Sol, S.Y.; Johnson, L.L.; Horness, B.H.; Collier, T.K. Relationship between oil exposure and reproductive parameters in fish collected following the Exxon Valdez oil spill. Mar. Pollut. Bull. 2000, 40, 1139–1147. [Google Scholar] [CrossRef]
- Truscott, B.; Walsh, J.M.; Burton, M.P.; Payne, J.F.; Idler, D.R. Effect of acute exposure to crude petroleum on some reproductive hormones in salmon and flounder. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1983, 75, 121–130. [Google Scholar] [CrossRef]
- Larcher, T.; Perrichon, P.; Vignet, C.; Ledevin, M.; Le Menach, K.; Lyphout, L.; Landi, L.; Clerandeau, C.; Lebihanic, F.; Menard, D.; et al. Chronic dietary exposure of zebrafish to PAH mixtures results in carcinogenic but not genotoxic effects. Environ. Sci. Pollut. Res. Int. 2014, 21, 13833–13849. [Google Scholar] [CrossRef] [PubMed]
- Vignet, C.; Le Menach, K.; Lyphout, L.; Guionnet, T.; Frere, L.; Leguay, D.; Budzinski, H.; Cousin, X.; Begout, M.L. Chronic dietary exposure to pyrolytic and petrogenic mixtures of PAHs causes physiological disruption in zebrafish—Part II: Behavior. Environ. Sci. Pollut. Res. Int. 2014, 21, 13818–13832. [Google Scholar] [CrossRef] [PubMed]
- Vignet, C.; Le Menach, K.; Mazurais, D.; Lucas, J.; Perrichon, P.; Le Bihanic, F.; Devier, M.H.; Lyphout, L.; Frere, L.; Begout, M.L.; et al. Chronic dietary exposure to pyrolytic and petrogenic mixtures of PAHs causes physiological disruption in zebrafish—Part I: Survival and growth. Environ. Sci. Pollut. Res. Int. 2014, 21, 13804–13817. [Google Scholar] [CrossRef] [PubMed]
- Daouk, T.; Larcher, T.; Roupsard, F.; Lyphout, L.; Rigaud, C.; Ledevin, M.; Loizeau, V.; Cousin, X. Long-term food-exposure of zebrafish to PCB mixtures mimicking some environmental situations induces ovary pathology and impairs reproduction ability. Aquat. Toxicol. 2011, 105, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Spitsbergen, J.M.; Tsai, H.W.; Reddy, A.; Miller, T.; Arbogast, D.; Hendricks, J.D.; Bailey, G.S. Neoplasia in zebrafish (Danio rerio) treated with 7,12-dimethylbenz[a]anthracene by two exposure routes at different developmental stages. Toxicol. Pathol. 2000, 28, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Koç, N.D.; Aytekin, Y.; Yüce, R. Ovary maturatıon stages and histological investigation of ovary of the zebrafish (Danio rerio). Braz. Arch. Biol. Technol. 2008, 51, 513–522. [Google Scholar]
- Gerlach, G.; Lysiak, N. Kin recognition and inbreeding avoidance in zebrafish, Danio rerio, is based on phenotype matching. Anim. Behav. 2006, 71, 1371–1377. [Google Scholar] [CrossRef]
- Siegfried, K.R.; Nusslein-Volhard, C. Germ line control of female sex determination in zebrafish. Dev. Biol. 2008, 324, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Brown-Peterson, N.J.; Manning, C.S.; Brouwer, M.; Griffitt, R.J. Effects of pyrene exposure on sheepshead minnow (Cyprinodon variegatus) reproduction. J. Toxicol. Environ. Health Part A 2013, 76, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zuo, Z.; Chen, M.; Chen, Y.; Wang, C. Reproductive and transgenerational toxicities of phenanthrene on female marine medaka (Oryzias melastigma). Aquat. Toxicol. 2015, 162, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Pollino, C.A.; Georgiades, E.; Holdway, D.A. Physiological changes in reproductively active rainbowfish (Melanotaenia fluviatilis) following exposure to naphthalene. Ecotoxicol. Environ. Saf. 2009, 72, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.L.; Oris, J.T. Altered gene expression: A mechanism for reproductive toxicity in zebrafish exposed to benzo[a]pyrene. Aquat. Toxicol. 2006, 78, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Booc, F.; Thornton, C.; Lister, A.; Maclatchy, D.; Willett, K.L. Benzo[a]pyrene effects on reproductive endpoints in Fundulus heteroclitus. Toxicol. Sci. 2014, 140, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sundt, R.C.; Bjorkblom, C. Effects of produced water on reproductive parameters in prespawning atlantic cod (Gadus morhua). J. Toxicol. Environ. Health A 2011, 74, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zuo, Z.; Luo, H.; Chen, M.; Zhong, Y.; Chen, Y.; Wang, C. Chronic exposure to phenanthrene influences the spermatogenesis of male Sebastiscus marmoratus: U-shaped effects and the reason for them. Environ. Sci. Technol. 2011, 45, 10212–10218. [Google Scholar] [CrossRef] [PubMed]
- Holth, T.F.; Nourizadeh-Lillabadi, R.; Blaesbjerg, M.; Grung, M.; Holbech, H.; Petersen, G.I.; Alestrom, P.; Hylland, K. Differential gene expression and biomarkers in zebrafish (Danio rerio) following exposure to produced water components. Aquat. Toxicol. 2008, 90, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Sommers, F.C.; Ylitalo, G.M.; Sloan, C.A. Altered growth and related physiological responses in juvenile chinook salmon (Oncorhynchus tshawytscha) from dietary exposure to polycyclic aromatic hydrocarbons (PAHs). Can. J. Fish. Aquat. Sci. 2006, 63, 2364–2376. [Google Scholar] [CrossRef]
- Rice, C.A.; Myers, M.S.; Willis, M.L.; French, B.L.; Casillas, E. From sediment bioassay to fish biomarker—Connecting the dots using simple trophic relationships. Mar. Environ. Res. 2000, 50, 527–533. [Google Scholar] [CrossRef]
- Kennedy, C.J.; Smyth, K.R. Disruption of the rainbow trout reproductive endocrine axis by the polycyclic aromatic hydrocarbon benzo[a]pyrene. Gen. Comp. Endocrinol. 2015, 219, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Rodas-Ortiz, J.P.; Ceja-Moreno, V.; Chan-Cocom, M.E.; Gold-Bouchot, G. Vitellogenin induction and increased plasma 17beta-estradiol concentrations in male nile tilapia, Oreochromis niloticus, exposed to organochlorine pollutants and polycyclic aromatics hydrocarbons. Bull. Environ. Contam. Toxicol. 2008, 81, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Tintos, A.; Gesto, M.; Miguez, J.M.; Soengas, J.L. Beta-naphthoflavone and benzo(a)pyrene treatment affect liver intermediary metabolism and plasma cortisol levels in rainbow trout oncorhynchus mykiss. Ecotoxicol. Environ. Saf. 2008, 69, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Menuet, A.; Le Page, Y.; Torres, O.; Kern, L.; Kah, O.; Pakdel, F. Analysis of the estrogen regulation of the zebrafish estrogen receptor (ER) reveals distinct effects of eralpha, erbeta1 and erbeta2. J. Mol. Endocrinol. 2004, 32, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Ariazi, E.; Kim, K.; Khan, S.; Barhoumi, R.; Burghardt, R.; Liu, S.; Hill, D.; Finnell, R.; Wlodarczyk, B.; et al. 3-methylcholanthrene and other aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha. Cancer Res. 2006, 66, 2459–2467. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Abdelrahim, M.; Khan, S.; Ariazi, E.; Jordan, V.C.; Safe, S. Aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha in mcf-7 breast cancer cells. Biol. Chem. 2006, 387, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Hinfray, N.; Nobrega, R.H.; Caulier, M.; Baudiffier, D.; Maillot-Marechal, E.; Chadili, E.; Palluel, O.; Porcher, J.M.; Schulz, R.; Brion, F. Cyp17a1 and cyp19a1 in the zebrafish testis are differentially affected by oestradiol. J. Endocrinol. 2013, 216, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, Y.; Miao, S.; Zhang, C.; Zong, S.; Koide, S.S.; Wang, L. Sperm associated antigen 8 (SPAG8), a novel regulator of activator of crem in testis during spermatogenesis. FEBS Lett. 2010, 584, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reyero, N.; Villeneuve, D.L.; Kroll, K.J.; Liu, L.; Orlando, E.F.; Watanabe, K.H.; Sepulveda, M.S.; Ankley, G.T.; Denslow, N.D. Expression signatures for a model androgen and antiandrogen in the fathead minnow (Pimephales promelas) ovary. Environ. Sci. Technol. 2009, 43, 2614–2619. [Google Scholar] [CrossRef] [PubMed]
- Van den Belt, K.; Wester, P.W.; van der Ven, L.T.; Verheyen, R.; Witters, H. Effects of ethynylestradiol on the reproductive physiology in zebrafish (Danio rerio): Time dependency and reversibility. Environ. Toxicol. Chem. 2002, 21, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.P.; Hill, R.L., Jr.; Janz, D.M. Developmental estrogenic exposure in zebrafish (Danio rerio): II. Histological evaluation of gametogenesis and organ toxicity. Aquat. Toxicol. 2003, 63, 431–446. [Google Scholar] [CrossRef]
- Forsgren, K.L.; Young, G. Stage-specific effects of androgens and estradiol-17beta on the development of late primary and early secondary ovarian follicles of coho salmon (Oncorhynchus kisutch) in vitro. Biol. Reprod. 2012, 87, 64. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, T.; Perez, G.I.; Jurisicova, A.; Pru, J.K.; Schlezinger, J.J.; Ryu, H.Y.; Laine, J.; Sakai, T.; Korsmeyer, S.J.; Casper, R.F.; et al. Aromatic hydrocarbon receptor-driven bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat. Genet. 2001, 28, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Mattison, D.R. Morphology of oocyte and follicle destruction by polycyclic aromatic hydrocarbons in mice. Toxicol. Appl. Pharmacol. 1980, 53, 249–259. [Google Scholar] [CrossRef]
- Johnson, L.L.; Arkoosh, M.R.; Bravo, C.F.; Collier, T.K.; Krahn, M.M.; Meador, J.P.; Myers, M.S.; Reichert, W.L.; Stein, J.E. The effects of polycyclic aromatic hydrocarbons in fish from Puget Sound, Washington. In The Toxicology of Fishes; CRC Press: Boca Raton, FL, USA, 2008; pp. 877–923. [Google Scholar]
- Peterson, C.H.; Rice, S.D.; Short, J.W.; Esler, D.; Bodkin, J.L.; Ballachey, B.E.; Irons, D.B. Long-term ecosystem response to the Exxon Valdez oil spill. Science 2003, 302, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Collier, T.K.; Anulacion, B.F.; Arkoosh, M.R.; Dietrich, J.P.; Incardona, J.P.; Johnson, L.L.; Ylitalo, G.M.; Myers, M.S. Effects on fish of polycyclic aromatic hydrocarbons (PAHs) and naphthenic acid exposures. In Organic Chemical Toxicology of Fishes; Tierney, K.B., Ed.; Elsevier: London, UK, 2014; Volume 33, pp. 195–255. [Google Scholar]
- Cailleaud, K.; Forget-Leray, J.; Souissi, S.; Hilde, D.; LeMenach, K.; Budzinski, H. Seasonal variations of hydrophobic organic contaminant concentrations in the water-column of the seine estuary and their transfer to a planktonic species Eurytemora affinis (Calanoida, Copepoda). Part 1: PCBs and PAHs. Chemosphere 2007, 70, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, J.; Gallois, J.; Pelhuet, L.; Devier, M.H.; Budzinski, H.; Pottier, D.; André, V.; Cachot, J. DNA adduct measurements in zebra mussels, Dreissena polymorpha, Pallas: Potential use for genotoxicant biomonitoring of fresh water ecosystems. Aquat. Toxicol. 2006, 79, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Jeanneret, H.; Chantereau, S.; Belliaeff, B.; Ratiskol, G.; Allenou, J.-P.; Piclet, G. Suivi sanitaire et environnemental des conséquences de la marée noire de l’Erika. In Proceedigns of the Colloque SAFERSEAS: Les Leçons Techniques de l’Erika et des Autres Accidents, Brest, France, 11–16 March 2002.
- Payne, J.R.; Driskell, W.B.; Short, J.W.; Larsen, M.L. Long term monitoring for oil in the Exxon Valdez spill region. Mar. Pollut. Bull. 2008, 56, 2067–2081. [Google Scholar] [CrossRef] [PubMed]
- Sol, S.; Johnson, L.; Boyd, D.; Olson, O.; Lomax, D.; Collier, T. Relationships between anthropogenic chemical contaminant exposure and associated changes in reproductive parameters in male english sole (Parophrys vetulus) collected from Hylebos waterway, Puget Sound, Washington. Arch. Environ. Contam. Toxicol. 2008, 55, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.L.; Landahl, J.T.; Kubin, L.A.; Horness, B.H.; Myers, M.S.; Collier, T.K.; Stein, J.E. Proceedings of the third international symposium on flatfish ecology, part II: Assessing the effects of anthropogenic stressors on puget sound flatfish populations. J. Sea Res. 1998, 39, 125–137. [Google Scholar] [CrossRef]
- Heintz, R.A. Chronic exposure to polynuclear aromatic hydrocarbons in natal habitats leads to decreased equilibrium size, growth, and stability of pink salmon populations. Integr. Environ. Assess. Manag. 2007, 3, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Vignet, C.; Joassard, L.; Lyphout, L.; Guionnet, T.; Goubeau, M.; Le Menach, K.; Brion, F.; Kah, O.; Chung, B.C.; Budzinski, H.; et al. Exposures of zebrafish through diet to three environmentally relevant mixtures of PAHs produce behavioral disruptions in unexposed F1 and F2 descendant. Environ. Sci. Pollut. Res. Int. 2015, 22, 16371–16383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
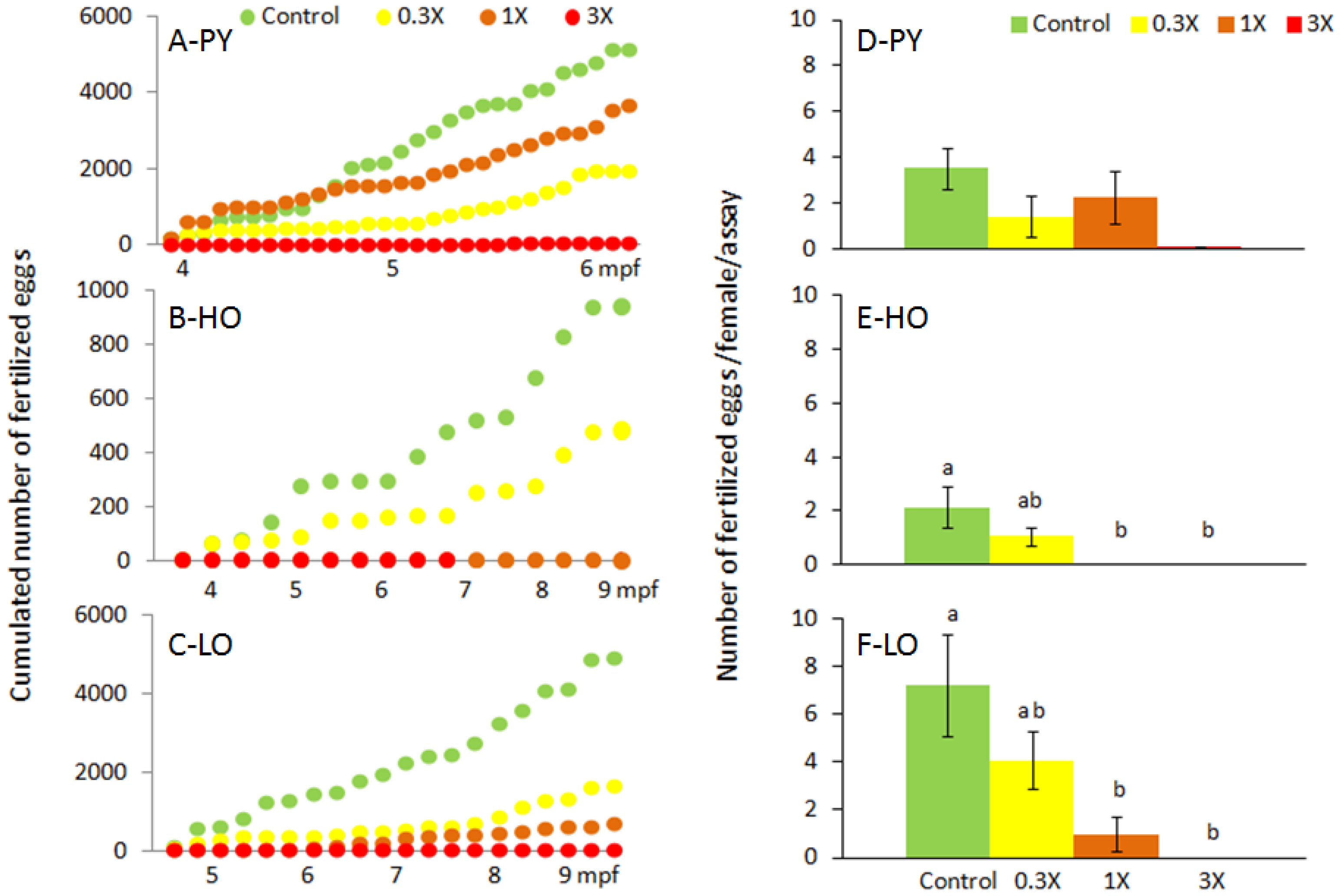
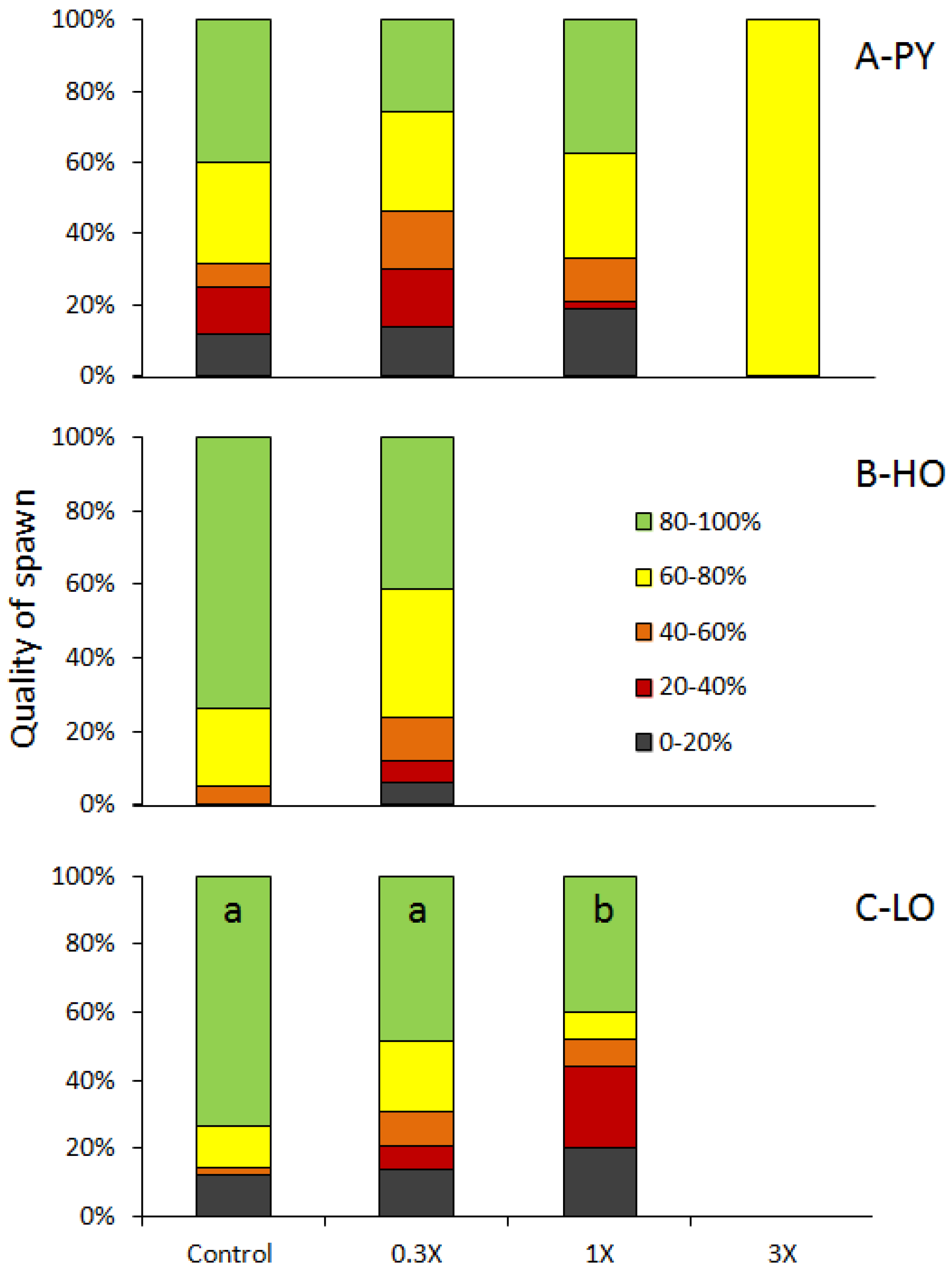
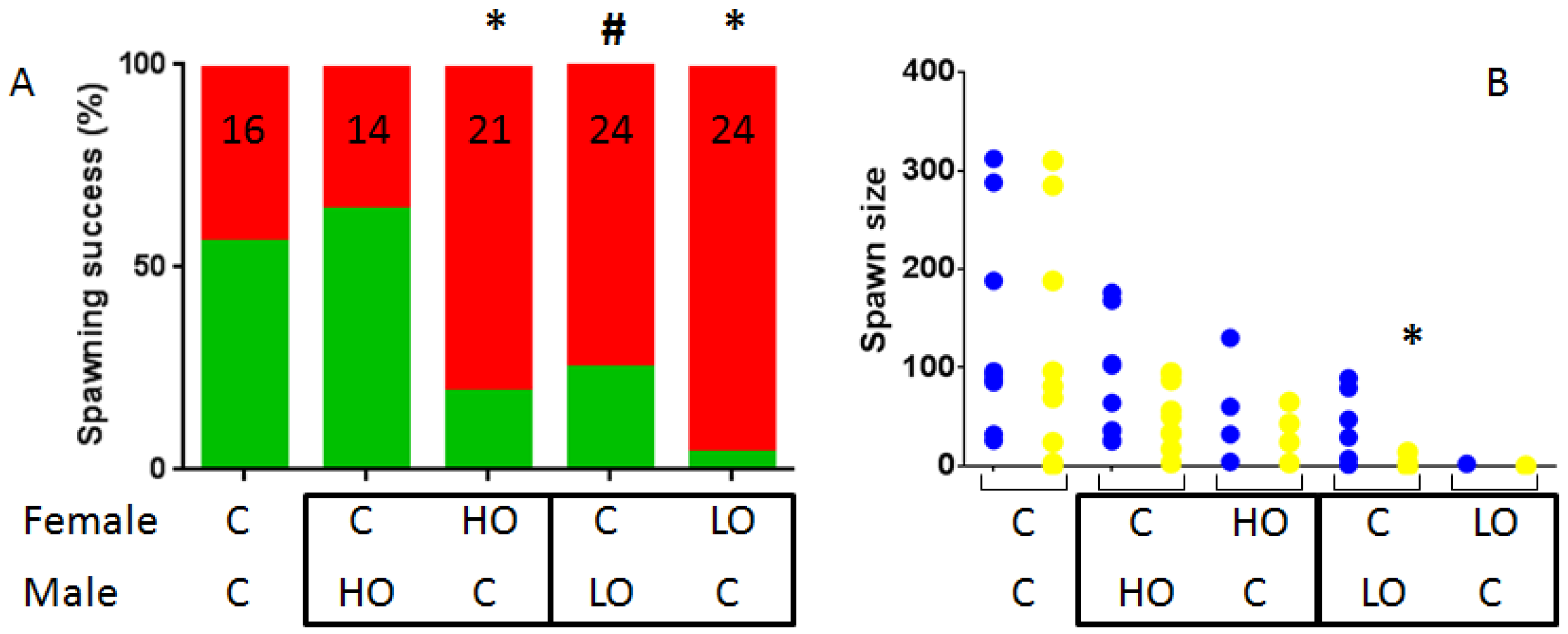
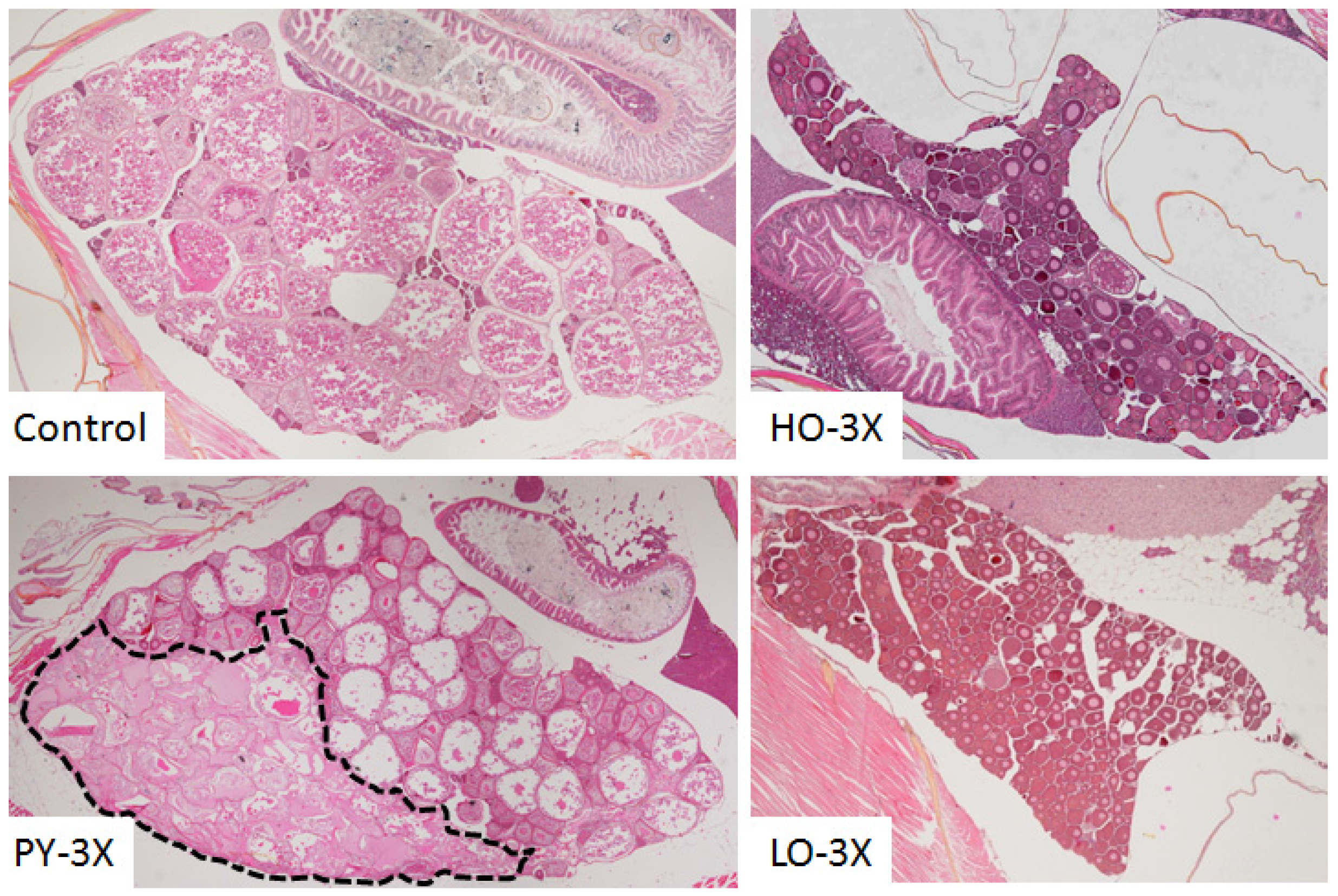
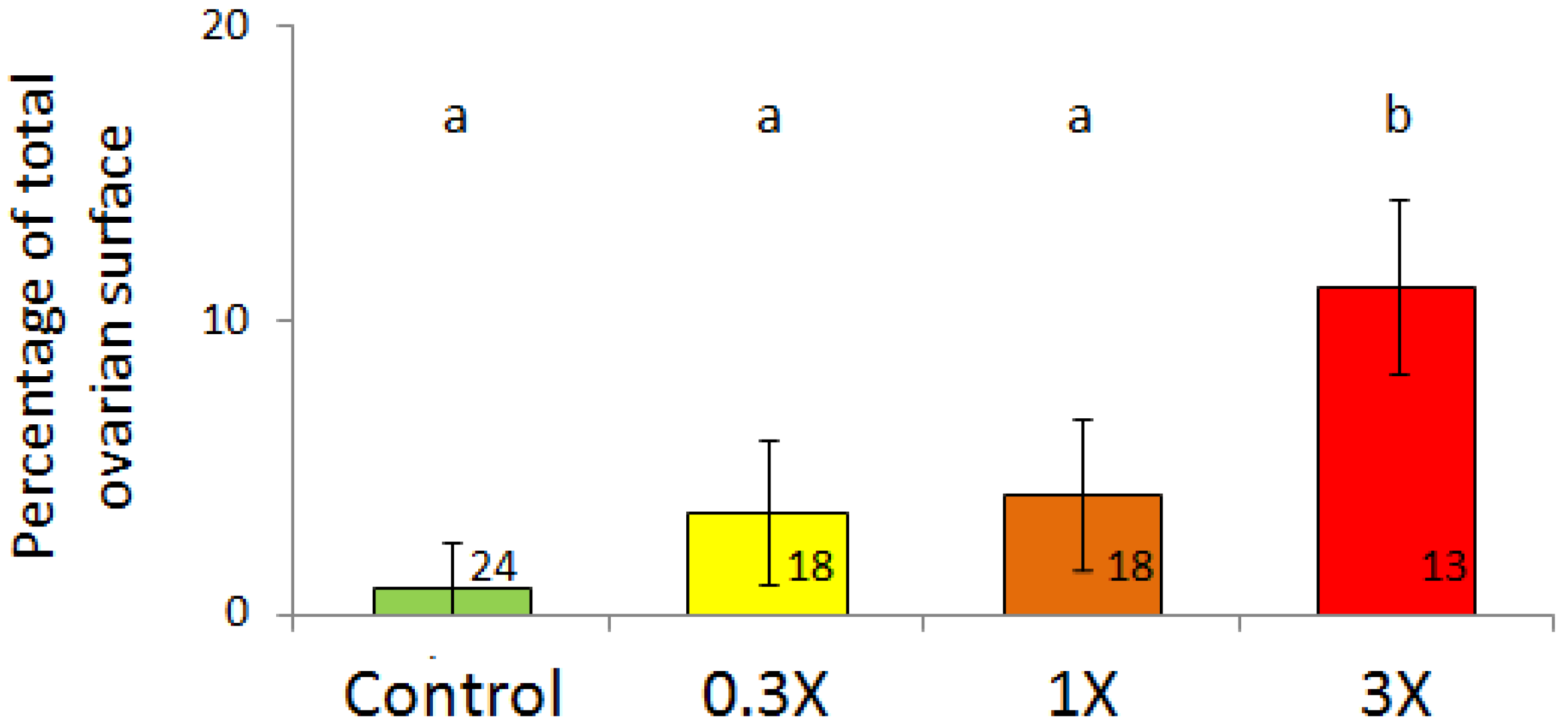
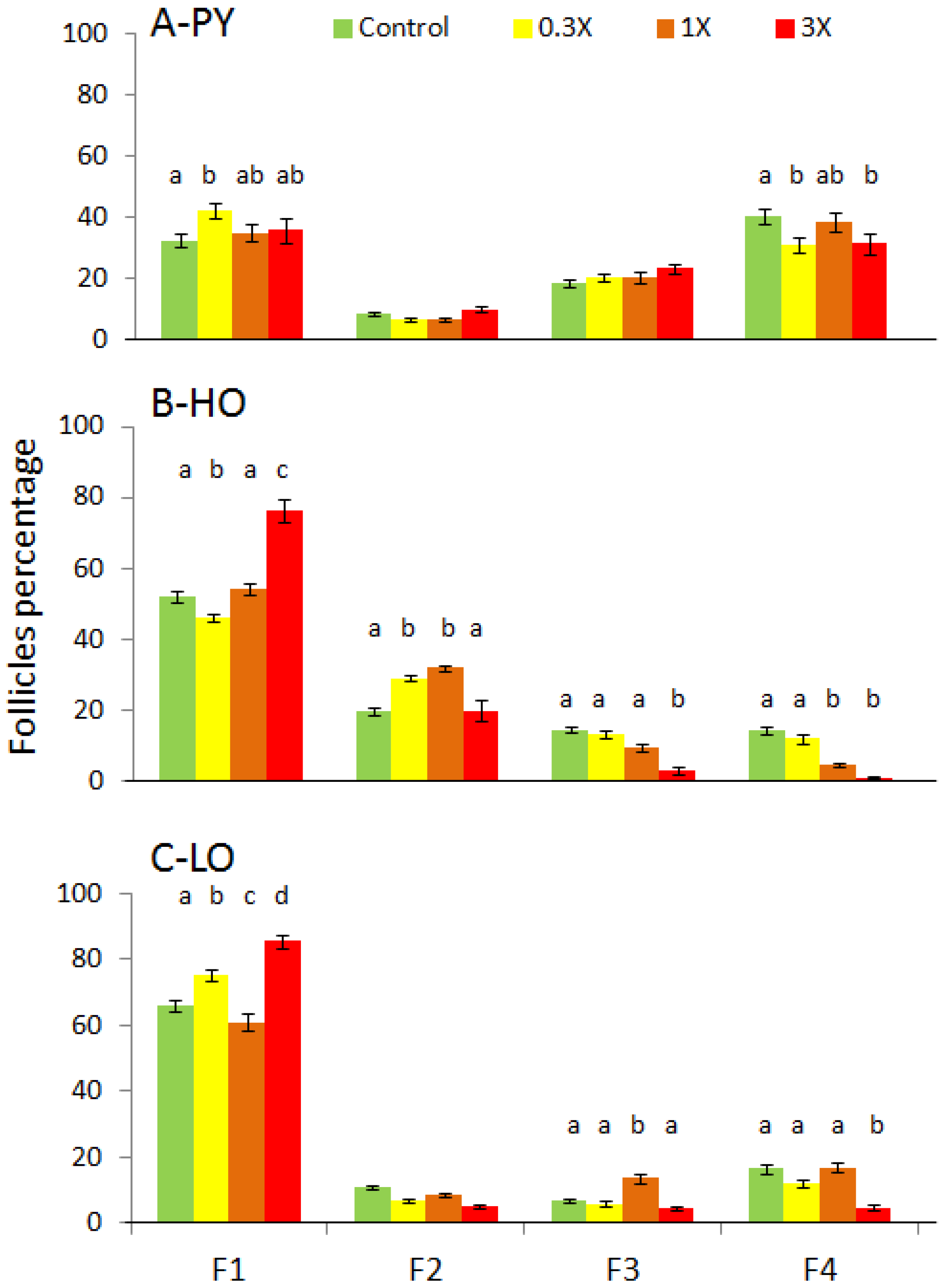

| Figures | PY 1 | HO 2 | LO 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.3X | 1X | 3X | Control | 0.3X | 1X | 3X | Control | 0.3X | 1X | 3X | |
| Males | 19 | 31 | 36 | 43 | 46 | 39 | 47 | ND | 31 | 55 | 25 | 27 |
| Females | 63 | 39 | 44 | 38 | 25 | 30 | 24 | ND | 37 | 29 | 37 | 25 |
| Assays | 87 | 87 | 87 | 87 | 37 | 37 | 37 | 19 | 53 | 53 | 53 | 53 |
| Spawns | 60 | 43 | 35 | 1 | 19 | 17 | 0 | 0 | 41 | 29 | 25 | 0 |
| Eggs (total) | 6880 | 2921 | 4836 | 48 | 1065 | 582 | 0 | 0 | 6254 | 2498 | 1307 | 0 |
| Eggs (fert.) | 5144 | 1927 | 3673 | 37 | 476 | 582 | 0 | 0 | 4894 | 1656 | 670 | 0 |
| Fert. rate (%) | 74.8 | 66.0 | 76.0 | 77.1 | 88.1 | 81.8 | - | - | 78.3 | 66.3 | 51.3 | - |
| PY | 0.3X | 1X | 3X | |||
| Fold Change | 95% CI | Fold Change | 95% CI | Fold Change | 95% CI | |
| esr1 | 0.866 | 0.467–1.523 | 1.871 | 0.738–5.330 | 1.52 | 0.471–6.460 |
| esr2a | 1.225 | 0.329–3.330 | 1.047 | 0.254–3.257 | 0.798 | 0.214–2.638 |
| esr2b | 0.906 | 0.329–2.009 | 1.24 | 0.531–2.990 | 1.028 | 0.269–4.046 |
| cyp19a1a | 1.265 | 0.373–5.167 | 2.142 | 0.632–7.352 | 1.094 | 0.287–4.970 |
| HO | 0.3X | 1X | 3X | |||
| Fold Change | 95% CI | Fold Change | 95% CI | Fold Change | 95% CI | |
| esr1 | 3.133 | 1.517–9.697 | 1.257 | 0.488–4.096 | Not done | - |
| esr2a | 5.029 | 3.323–9.452 | 3.043 | 1.182–5.624 | Not done | |
| esr2b | Not done | Not done | Not done | |||
| cyp19a1a | 1.666 | 0.573–3.221 | 1.265 | 0.686–3.414 | Not done | |
| LO | 0.3X | 1X | 3X | |||
| Fold Change | 95% CI | Fold Change | 95% CI | Fold Change | 95% CI | |
| esr1 | 0.89 | 0.339–2.869 | 0.599 | 0.182–2.501 | 0.642 | 0.220–2.272 |
| esr2a | 0.847 | 0.120–8.124 | 0.951 | 0.354–4.418 | 1.26 | 0.378–8.276 |
| esr2b | 0.747 | 0.315–2.530 | 0.455 | 0.125–1.870 | 0.486 | 0.185–1.661 |
| cyp19a1a | 1.013 | 0.041–5.064 | 0.483 | 0.054–3.590 | 0.336 | 0.061–1.206 |
| PY | 0.3X | 1X | 3X | |||
| Fold Change | 95% CI | Fold Change | 95% CI | Fold Change | 95% CI | |
| amh | 0.777 | 0.484–1.189 | 0.594 | 0.439–0.803 | 1.422 | 0.729–3.459 |
| spag8 | 0.449 | 0.280–0.776 | 0.509 | 0.382–0.677 | 0.751 | 0.483–1.109 |
| cyp17a1 | 0.528 | 0.207–1.623 | 0.447 | 0.329–0.609 | 0.948 | 0.385–1.948 |
| hsd11b3 | 0.632 | 0.337–1.241 | 0.885 | 0.552–1.437 | 0.763 | 0.472–1.334 |
| HO | 0.3X | 1X | 3X | |||
| Fold Change | 95% CI | Fold Change | 95% CI | Fold Change | - | |
| amh | 0.885 | 0.614–1.534 | 0.739 | 0.191–1.600 | Not done | - |
| spag8 | 1.496 | 0.764–2.857 | 1.381 | 0.600–3.074 | Not done | |
| cyp17a1 | 0.23 | 0.091–0.708 | 0.354 | 0.208–0.750 | Not done | |
| hsd11b3 | 0.917 | 0.578–1.644 | 0.93 | 0.603–1.611 | Not done | |
| LO | 0.3X | 1X | 3X | |||
| Fold Change | 95% CI | Fold Change | 95% CI | Fold Change | 95% CI | |
| amh | 2.109 | 1.031–3.978 | 1.723 | 1.369–2.061 | 1.864 | 1.173–3.549 |
| spag8 | 1.127 | 0.800–1.653 | 1.078 | 0.995–1.178 | 0.667 | 0.311–1.074 |
| cyp17a1 | 0.977 | 0.470–2.123 | 1.39 | 1.008–1.971 | 1.104 | 0.574–2.174 |
| hsd11b3 | 1.362 | 0.700–2.230 | 1.331 | 0.943–1.995 | 0.918 | 0.541–1.530 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignet, C.; Larcher, T.; Davail, B.; Joassard, L.; Le Menach, K.; Guionnet, T.; Lyphout, L.; Ledevin, M.; Goubeau, M.; Budzinski, H.; et al. Fish Reproduction Is Disrupted upon Lifelong Exposure to Environmental PAHs Fractions Revealing Different Modes of Action. Toxics 2016, 4, 26. https://doi.org/10.3390/toxics4040026
Vignet C, Larcher T, Davail B, Joassard L, Le Menach K, Guionnet T, Lyphout L, Ledevin M, Goubeau M, Budzinski H, et al. Fish Reproduction Is Disrupted upon Lifelong Exposure to Environmental PAHs Fractions Revealing Different Modes of Action. Toxics. 2016; 4(4):26. https://doi.org/10.3390/toxics4040026
Chicago/Turabian StyleVignet, Caroline, Thibaut Larcher, Blandine Davail, Lucette Joassard, Karyn Le Menach, Tiphaine Guionnet, Laura Lyphout, Mireille Ledevin, Manon Goubeau, Hélène Budzinski, and et al. 2016. "Fish Reproduction Is Disrupted upon Lifelong Exposure to Environmental PAHs Fractions Revealing Different Modes of Action" Toxics 4, no. 4: 26. https://doi.org/10.3390/toxics4040026