Evaluation of Existing Models to Estimate Sorption Coefficients for Ionisable Pharmaceuticals in Soils and Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluated Models
Generation of Chemical Descriptors for Inclusion in the Models
2.2. Statistical Analysis
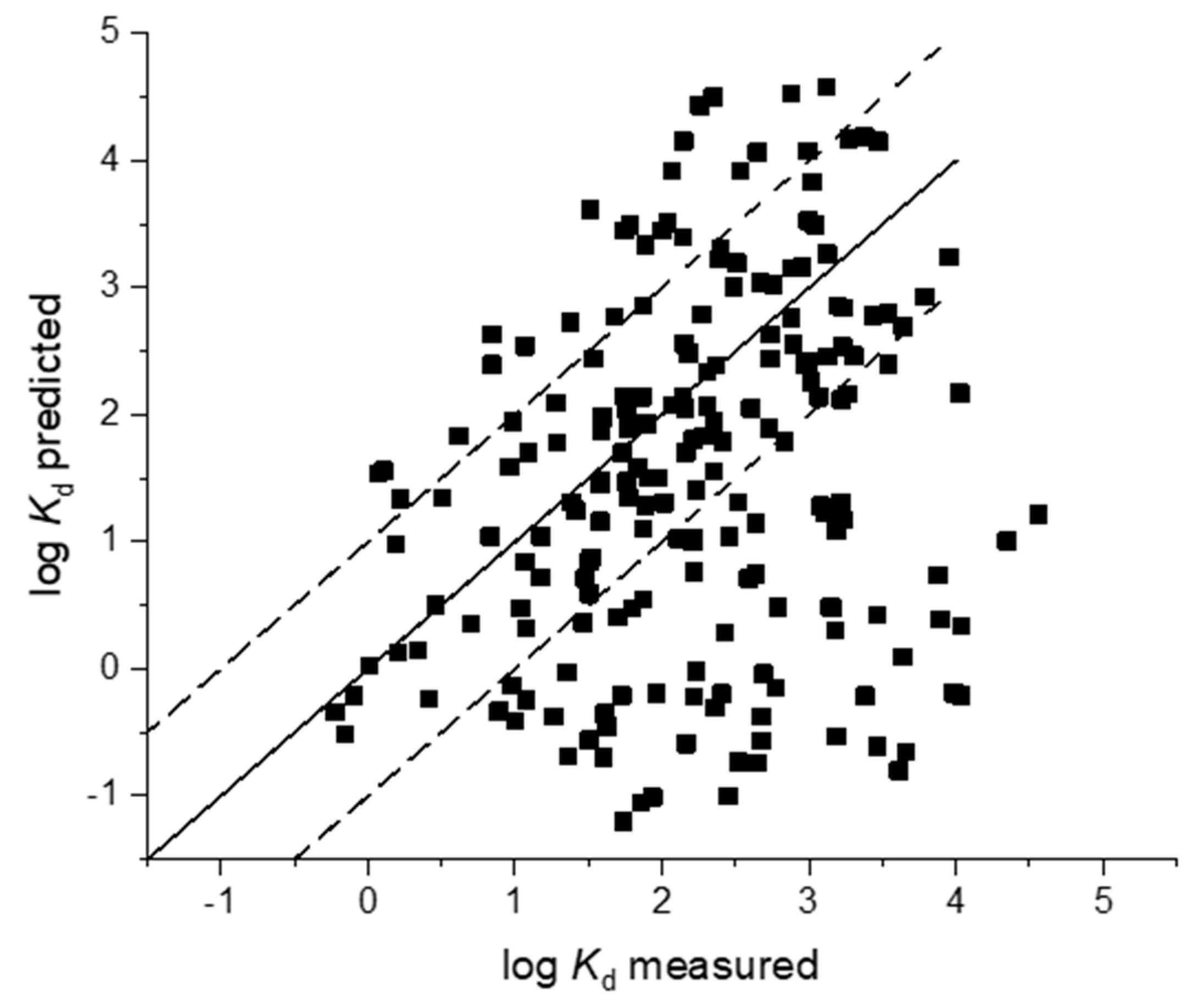
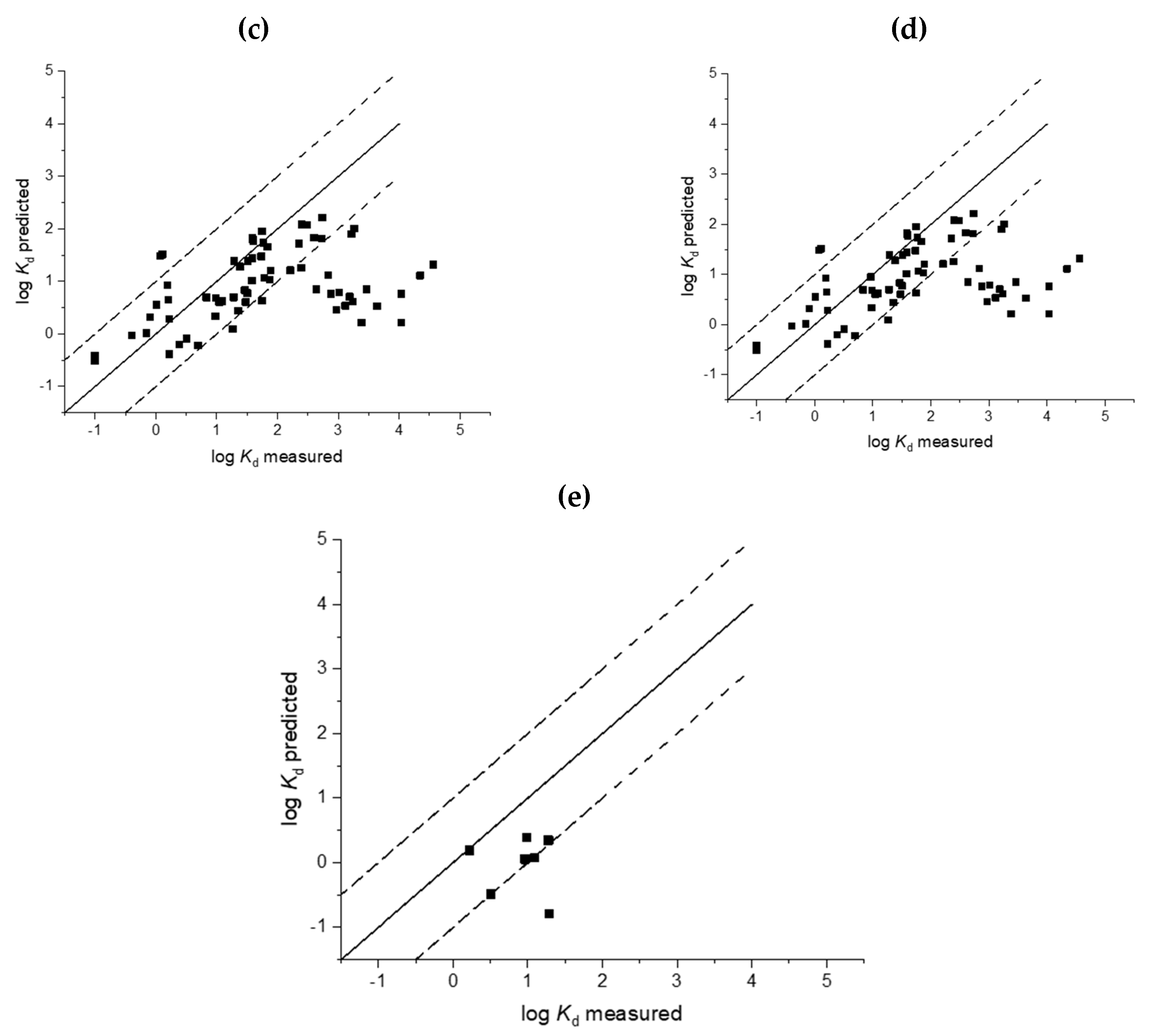
3. Results
3.1. Soil Sorption
3.2. Sludge Sorption
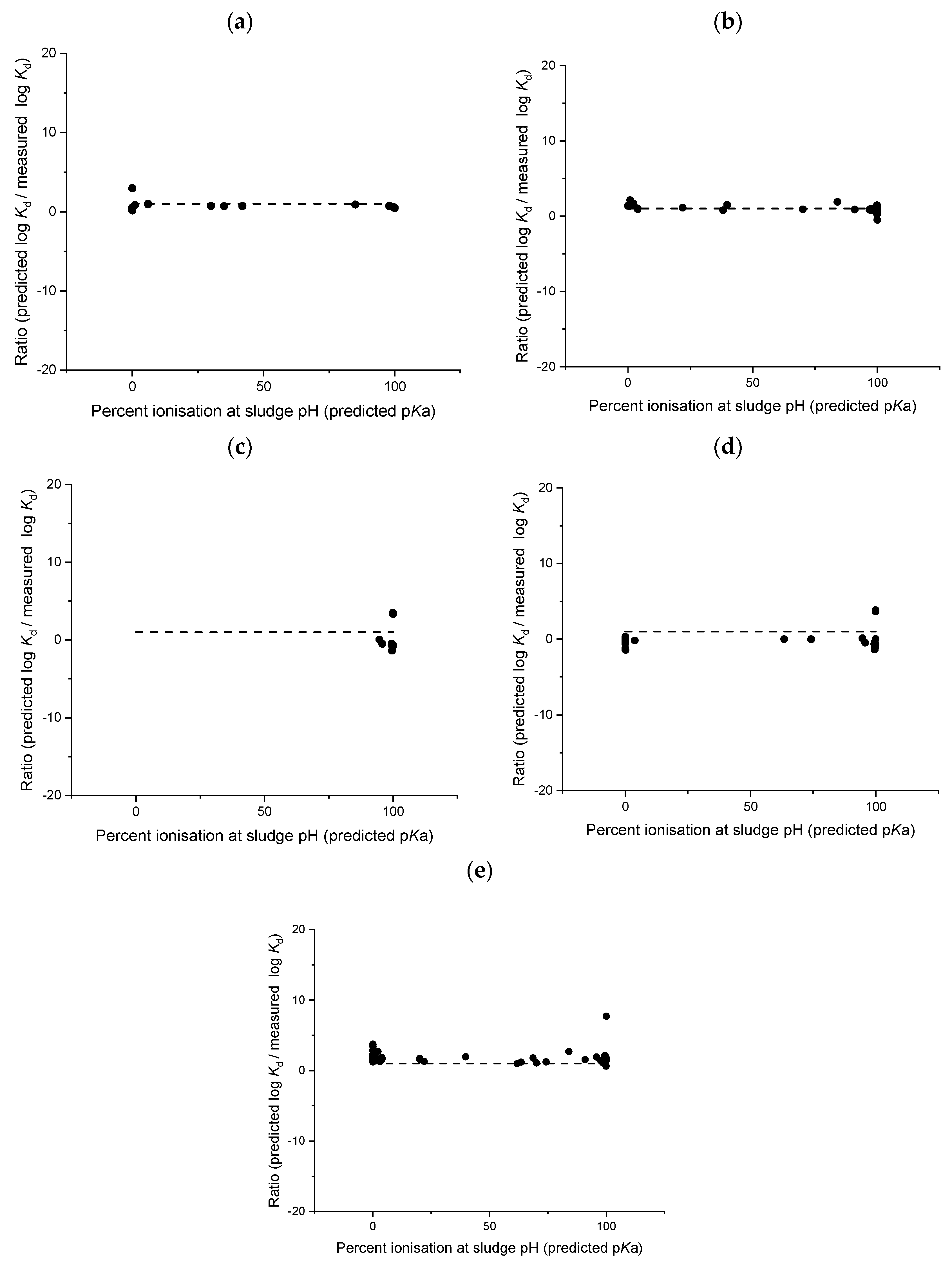
4. Discussion
4.1. Deviation from Neutrality
4.2. Regulatory Implications
4.3. Future Model Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chefetz, B.; Mualem, T.; Ben-Ari, J. Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere 2008, 73, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Hörsing, M.; Ledin, A.; Grabic, R.; Fick, J.; Tysklind, M.; la Cour Jansen, J.; Andersen, H.R. Determination of sorption of seventy-five pharmaceuticals in sewage sludge. Water Res. 2011, 45, 4470–4482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Khazrajy, O.S.A.; Boxall, A.B.A. Impacts of compound properties and sediment characteristics on the sorption behaviour of pharmaceuticals in aquatic systems. J. Hazard. Mater. 2016, 317, 198–209. [Google Scholar] [CrossRef] [Green Version]
- EMEA. Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use; CPMP/SWP/4; European Medicines Agency: London, UK, 2006. [Google Scholar]
- Kim, Y.; Lim, S.; Han, M.; Cho, J. Sorption characteristics of oxytetracycline, amoxicillin, and sulfathiazole in two different soil types. Geoderma 2012, 185, 97–101. [Google Scholar] [CrossRef]
- Kodešová, R.; Grabic, R.; Kočárek, M.; Klement, A.; Golovko, O.; Fér, M.; Nikodem, A.; Jakšík, O. Pharmaceuticals’ sorptions relative to properties of thirteen different soils. Sci. Total Environ. 2015, 511, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Ong, P.L.; Williams, D.B.; Kookana, R.S. Estimating the sorption of pharmaceuticals based on their pharmacological distribution. Environ. Toxicol. Chem. 2009, 28, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Droge, S.T.J.; Goss, K. Sorption of Organic Cations to Phyllosilicate Clay Minerals: CEC- Normalization, Salt Dependency, and the Role of Electrostatic and Hydrophobic Effects. Environ. Sci. Technol. 2013, 47, 14224–14232. [Google Scholar] [CrossRef]
- Droge, S.T.J.; Goss, K.U. Ion-exchange affinity of organic cations to natural organic matter: Influence of amine type and nonionic interactions at two different pHs. Environ. Sci. Technol. 2013, 47, 798–806. [Google Scholar] [CrossRef]
- Wegst-Uhrich, S.R.; Navarro, D.A.; Zimmerman, L.; Aga, D.S. Assessing antibiotic sorption in soil: A literature review and new case studies on sulfonamides and macrolides. Chem. Cent. J. 2014, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Tolls, J. Sorption of Veterinary Pharmaceuticals in Soils: A Review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef]
- Manallack, D.T. The acid-base profile of a contemporary set of drugs: Implications for drug discovery. Sar Qsar Environ. Res. 2009, 20, 611–655. [Google Scholar] [CrossRef]
- Rybacka, A.; Andersson, P.L. Considering ionic state in modeling sorption of pharmaceuticals to sewage sludge. Chemosphere 2016, 165, 284–293. [Google Scholar] [CrossRef]
- Bostrom, M.L.; Berglund, O. Influence of pH-dependent aquatic toxicity of ionizable pharmaceuticals on risk assessments over environmental pH ranges. Water Res. 2015, 72, 154–161. [Google Scholar] [CrossRef]
- Neuwoehner, J.; Escher, B.I. The pH-dependent toxicity of basic pharmaceuticals in the green algae Scenedesmus vacuolatus can be explained with a toxicokinetic ion-trapping model. Aquat. Toxicol. 2011, 101, 266–275. [Google Scholar] [CrossRef]
- Franco, A.; Fu, W.; Trapp, S. Influence of soil pH on the sorption of ionizable chemicals: Modeling advances. Environ. Toxicol. Chem. 2009, 28, 458–464. [Google Scholar] [CrossRef]
- Karlsson, M.V.; Carter, L.J.; Agatz, A.; Boxall, A.B.A. Novel Approach for Characterizing pH-Dependent Uptake of Ionizable Chemicals in Aquatic Organisms. Environ. Sci. Technol. 2017, 51, 6965–6971. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.; Ning, P.; Xing, B. Part V—sorption of pharmaceuticals and personal care products. Environ. Sci. Pollut. Res. 2009, 16, 106–116. [Google Scholar] [CrossRef]
- Semple, K.T.; Morriss, A.W.J.; Paton, G.I. Bioavailability of hydrophobic organic contaminants in soils: Fundamental concepts and techniques for analysis. Eur. J. Soil Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Tülp, H.C.; Fenner, K.; Schwarzenbach, R.P.; Goss, K.U. pH-dependent sorption of acidic organic chemicals to soil organic matter. Environ. Sci. Technol. 2009, 43, 9189–9195. [Google Scholar] [CrossRef]
- Figueroa, R.A.; Leonard, A.; Mackay, A.A. Modeling Tetracycline Antibiotic Sorption to Clays. Environ. Sci. Technol. 2004, 38, 476–483. [Google Scholar] [CrossRef]
- Figueroa, R.A.; MacKay, A.A. Sorption of Oxytetracycline to Iron Oxides and Iron Oxide-Rich Soils. Environ. Sci. Technol. 2005, 39, 6664–6671. [Google Scholar] [CrossRef]
- Vasudevan, D.; Bruland, G.L.; Torrance, B.S.; Upchurch, V.G.; MacKay, A.A. pH-dependent ciprofloxacin sorption to soils: Interaction mechanisms and soil factors influencing sorption. Geoderma 2009, 151, 68–76. [Google Scholar] [CrossRef]
- Kah, M.; Brown, C.D. Prediction of the Adsorption of Ionizable Pesticides in Soils. J. Agric. Food Chem. 2007, 55, 2312–2322. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Environ. Sci. Pollut. Res. 2009, 16, 106–116.
- Guo, J.H.; Sinclair, C.J.; Selby, K.; Boxall, A.B.A. Toxicological and ecotoxicological risk-based prioritization of pharmaceuticals in the natural environment. Environ. Toxicol. Chem. 2016, 35, 1550–1559. [Google Scholar] [CrossRef] [Green Version]
- Nolte, T.M.; Ragas, A.M. A review of quantitative structure-property relationships for the fate of ionizable organic chemicals in water matrices and identification of knowledge gaps. Env. Sci. Process Impacts 2017, 19, 221–246. [Google Scholar] [CrossRef]
- Bintein, S.; Devillers, J. Qsar for organic-chemical sorption in soils and sediments. Chemosphere 1994, 28, 1171–1188. [Google Scholar] [CrossRef]
- Ter Laak, T.L.; Gebbink, W.A.; Tolls, J. Estimation of soil sorption coefficients of veterinary pharmaceuticals from soil properties. Environ. Toxicol. Chem. 2006, 25, 933–941. [Google Scholar] [CrossRef]
- Droge, S.T.J.; Goss, K.-U. Development and Evaluation of a New Sorption Model for Organic Cations in Soil: Contributions from Organic Matter and Clay Minerals. Environ. Sci. Technol. 2013, 47, 14233–14241. [Google Scholar] [CrossRef]
- Franco, A.; Trapp, S. Estimation of the soil–water partition coefficient normalized to organic carbon for ionizable organic chemicals. Environ. Toxicol. Chem. 2008, 27, 1995–2004. [Google Scholar] [CrossRef]
- Sathyamoorthy, S.; Ramsburg, C.A. Assessment of quantitative structural property relationships for prediction of pharmaceutical sorption during biological wastewater treatment. Chemosphere 2013, 92, 639–646. [Google Scholar] [CrossRef]
- Franco, A.; Struijs, J.; Gouin, T.; Price, O.R. Evolution of the sewage treatment plant model SimpleTreat: Applicability domain and data requirements. Integr. Environ. Assess. Manag. 2013, 9, 560–568. [Google Scholar] [CrossRef]
- Berthod, L.; Whitley, D.C.; Roberts, G.; Sharpe, A.; Greenwood, R.; Mills, G.A. Quantitative structure-property relationships for predicting sorption of pharmaceuticals to sewage sludge during waste water treatment processes. Sci. Total Environ. 2017, 579, 1512–1520. [Google Scholar] [CrossRef] [Green Version]
- Barron, L.; Havel, J.; Purcell, M.; Szpak, M.; Kelleher, B.; Paull, B. Predicting sorption of pharmaceuticals and personal care products onto soil and digested sludge using artificial neural networks. Analyst 2009, 134, 663–670. [Google Scholar] [CrossRef]
- Jolin, W.C.; Goyetche, R.; Carter, K.; Medina, J.; Vasudevan, D.; Mackay, A.A. Predicting Organic Cation Sorption Coefficients: Accounting for Competition from Sorbed Inorganic Cations Using a Simple Probe Molecule. Environ. Sci. Technol. 2017, 51, 6193–6201. [Google Scholar] [CrossRef]
- Sabljić, A.; Güsten, H.; Verhaar, H.; Hermens, J. QSAR modelling of soil sorption. Improvements and systematics of log KOCvs. log KOWcorrelations. Chemosphere 1995, 31, 4489–4514. [Google Scholar] [CrossRef]
- European Commission. Technical Guidance Document on Risk Assessment Part II. Environ. Risk Assess; EUR 20418; Institute for Health and Consumer Protection—European Chemicals Bureau: Luxembourg, 2003. [Google Scholar]
- MacKay, A.A.; Vasudevan, D. Polyfunctional Ionogenic Compound Sorption: Challenges and New Approaches To Advance Predictive Models. Environ. Sci. Technol. 2012, 46, 9209–9223. [Google Scholar] [CrossRef]
- Celle-Jeanton, H.; Schemberg, D.; Mohammed, N.; Huneau, F.; Bertrand, G.; Lavastre, V.; Le Coustumer, P. Evaluation of pharmaceuticals in surface water: Reliability of PECs compared to MECs. Environ. Int. 2014, 73, 10–21. [Google Scholar] [CrossRef]
- Struijs, J. SimpleTreat 4.0, a Model to Predict Fate and Emission of Chemicals in Wastewater Treatment Plants. Background Report Describing the Equations; RIVM (National Institute for Public Health and the Environment): Bilthoven, The Netherlands, 2014. [Google Scholar]
- Delle Site, A. Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants. A review. J. Phys. Chem. Ref. Data 2001, 30, 187–439. [Google Scholar] [CrossRef] [Green Version]
- Aristilde, L.; Marichal, C.; Miehe-Brendle, J.; Lanson, B.; Charlet, L. Interactions of Oxytetracycline with a Smectite Clay: A Spectroscopic Study with Molecular Simulations. Environ. Sci. Technol. 2010, 44, 7839–7845. [Google Scholar] [CrossRef]
- Aristilde, L.; Sposito, G. Binding of ciprofloxacin by humic substances: A molecular dynamics study. Environ. Toxicol. Chem. 2010, 29, 90–98. [Google Scholar] [CrossRef]
- Carrasquillo, A.J.; Bruland, G.L.; Mackay, A.A.; Vasudevan, D. Sorption of Ciprofloxacin and Oxytetracycline Zwitterions to Soils and Soil Minerals: Influence of Compound Structure. Environ. Sci. Technol. 2008, 42, 7634–7642. [Google Scholar] [CrossRef]
- Weber, J.B.; Wilkerson, G.G.; Reinhardt, C.F. Calculating pesticide sorption coefficients (Kd) using selected soil properties. Chemosphere 2004, 55, 157–166. [Google Scholar] [CrossRef]






| Reference | Model | Specified Chemical Range of Applicability | Model Training Set |
|---|---|---|---|
| Bintein and Devillers [28] a | Log Kd = 0.93 log Kow + 1.09 log ƒoc + 0.32 CFa − 0.55 CFb’ + 0.25 Where: CFa = log (1(/1 + 10pH−pKa)) CFb’ = log (1(/1 + 10pKa−(pH−2))) | 3.07 ≤ pKa ≤ 8.85 b 0.12 ≤ log Kow ≤ 6.42 b | Organic chemicals (not including pharmaceuticals) (n = 229, r2 = 0.96) |
| Sabljic et al. [37] (TGD) | Log KOC = 0.10 + (0.81) log Kow | 1 ≤ log Kow ≤ 7.5 | Hydrophobic chemicals (n = 81, r2 = 0.94) |
| Sabljic et al. [37] (TGD) | Log KOC = 0.32 + (0.60) log Kow | 1 ≤ log Kow ≤ 7.5 | Organic acids (n = 23, r2 = 0.87) |
| Kah and Brown [24] | Log Kd = 0.13 Log D + 1.02 Log OC − 1.51 | 1.97 ≤ pKa ≤ 4.94 b 1.2 ≤ log Kow ≤ 4.3 b | Ionisable pesticides (n = 90, r2 = 0.39) |
| Franco and Trapp [31] | Log KOC = log (ƒneutral 100.54·logPn+1.11 + ƒion 100.11·logPn+1.54) | 0 < pKa < 12 −2.18 < log Pn < 8.50 | Organic acids (including 5 basic pharmaceuticals) (n = 62, r2 = 0.54) |
| Franco and Trapp [31] | Log KOC = log (ƒneutral 100.37·logPn+1.70 + ƒion 10pKa0.65·ƒ0.14) | 2 < pKa < 12 −1.66 < log Pn < 7.03 | Organic bases (including 5 basic pharmaceuticals) (n = 43, r2 = 0.76) |
| Franco et al. [16] | KOC = (100.54·logPn+1.11)/(1 + 10(pHsoil−0.6−pKa)) + (100.11·logPn+1.54)/(1 + 10(pKa-pHsoil+0.6) | Monovalent acids pKa < 12 −2.18 < log Pn < 8.50 | Organic acids (r2 = 0.70) |
| Droge and Goss [30] | Kd = KCEC,CLAY CECCLAY + fOC ·DOC,IE = KCEC,CLAY · (CECSOIL − 3.4 fOC) + fOC ·DOC,IE Where: Log KCEC, CLAYS = 1.22 (±0.15) Vx − 0.22 (±0.05) NAi + 2.09 (±0.05) | Strong bases (monovalent) | Organic cations (including pharmaceuticals) c |
| Reference | Model | Specified Range of Chemical Applicability | Model Training Set |
|---|---|---|---|
| Franco et al. [33] | KOC = ƒn 100.54·logKown + 1.11 + ƒion100.11·logKown + 1.54 | Monovalent acids pKa < 10 | |
| Franco et al. [33] | KOC base = 100.31·log D + 2.78 | Monovalent bases pKa > 4 | |
| Sathyamoorthy and Ramsburg [32] | Log Kd = [5.88 ± 1.69] + [(0.37 ± 0.05)log D] + [(0.30 ± 0.05)nHBA] + [(–3.56 ± 0.78)log MV] | a | Negatively charged pharmaceuticals (n = 44, r2 = 0.60) |
| Sathyamoorthy and Ramsburg [32] | Log Kd = (4.54 ± 1.36) + [(0.39 ± 0.04)log D] + [(0.32 ± 0.04) nHBA] + [(–2.41 ± 0.59)log MV] + [(−0.86 ± 0.25)log TPSA | a | Negatively charged and uncharged pharmaceuticals (n = 109, r2 = 0.64) |
| Berthod et al. [34] | Artificial Neural Network (ANN) | –4.55 ≤ log Kow ≤ 7.05 b | Ionisable pharmaceuticals |
| Reference | Charge Group Relevant to Model | Model Performance | Number of Data within Model Applicability Domain | ||||
|---|---|---|---|---|---|---|---|
| r2 | NSE | RMSE | RMSE/MAE | % within a Factor of 10 | |||
| Bintein and Devillers [28] | Acids | 0.005 | −4.43 | 0.39 | 0.18 | 26 | 38 a |
| Bintein and Devillers [28] | Bases | 0.08 | −9.65 | 0.32 | 0.12 | 24 | 85 a |
| Sabljic et al. [37] (TGD) b | Hydrophobic chemicals | 0.07 | −1.54 | 0.62 | 0.51 | 55 | 194 c |
| Sabljic et al. [37] (TGD) b | Acids | 0.04 | −1.25 | 0.62 | 0.50 | 48 | 77 |
| Kah and Brown [24] | Acids | 0.003 | −7.71 | 0.91 | 0.97 | 71 | 7 a |
| Franco and Trapp [31] b | Acids | 0.17 | −0.26 | 0.70 | 0.67 | 68 | 68 |
| Franco and Trapp [31] b | Bases | 0.07 | −0.31 | 0.65 | 0.58 | 55 | 114 |
| Franco and Trapp [16] b | Acids | 0.17 | −0.26 | 0.70 | 0.67 | 68 | 68 |
| Droge and Goss [30] | Bases | 0.29 | 0.18 | 0.79 | 0.93 | 71 | 66 |
| Reference | Charge Group Relevant to Model | Model Performance | Number of Data within Model Applicability Domain | ||||
|---|---|---|---|---|---|---|---|
| r2 | NSE | RMSE | RMSE/MAE | % within a Factor of 10 | |||
| Franco et al. [33] a | Acids | 0.07 | 0.99 | 0.89 | 0.94 | 60 | 15 b |
| Franco et al. [33] a | Bases | 0.04 | −0.07 | 0.76 | 0.90 | 67 | 28 |
| Sathyamoorthy and Ramsburg [32] | Acids (with a negative charge at sludge pH) | 0.08 | −12.68 | 0.31 | 0.10 | 11 | 10 |
| Sathyamoorthy and Ramsburg [32] | Acids | 0.04 | −9.28 | 0.32 | 0.11 | 4 | 23 |
| Berthod et al. [34] | All | 0.21 | −2.38 | 0.54 | 0.32 | 21 | 66 c |
| Berthod et al. [34] | Acids | 0.28 | −4.42 | 0.56 | 0.34 | 19 | 21 |
| Berthod et al. [34] | Bases | 0.21 | −1.76 | 0.52 | 0.30 | 19 | 32 |
| Berthod et al. [34] | Multiple ionisable groups | 0.01 | −17.50 | 0.52 | 0.33 | 30 | 13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, L.J.; Wilkinson, J.L.; Boxall, A.B.A. Evaluation of Existing Models to Estimate Sorption Coefficients for Ionisable Pharmaceuticals in Soils and Sludge. Toxics 2020, 8, 13. https://doi.org/10.3390/toxics8010013
Carter LJ, Wilkinson JL, Boxall ABA. Evaluation of Existing Models to Estimate Sorption Coefficients for Ionisable Pharmaceuticals in Soils and Sludge. Toxics. 2020; 8(1):13. https://doi.org/10.3390/toxics8010013
Chicago/Turabian StyleCarter, Laura J., John L. Wilkinson, and Alistair B. A. Boxall. 2020. "Evaluation of Existing Models to Estimate Sorption Coefficients for Ionisable Pharmaceuticals in Soils and Sludge" Toxics 8, no. 1: 13. https://doi.org/10.3390/toxics8010013
APA StyleCarter, L. J., Wilkinson, J. L., & Boxall, A. B. A. (2020). Evaluation of Existing Models to Estimate Sorption Coefficients for Ionisable Pharmaceuticals in Soils and Sludge. Toxics, 8(1), 13. https://doi.org/10.3390/toxics8010013






