The Immunotoxicity of Chronic Exposure to High Levels of Lead: An Ex Vivo Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Sampling and Blood Analysis
2.3. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
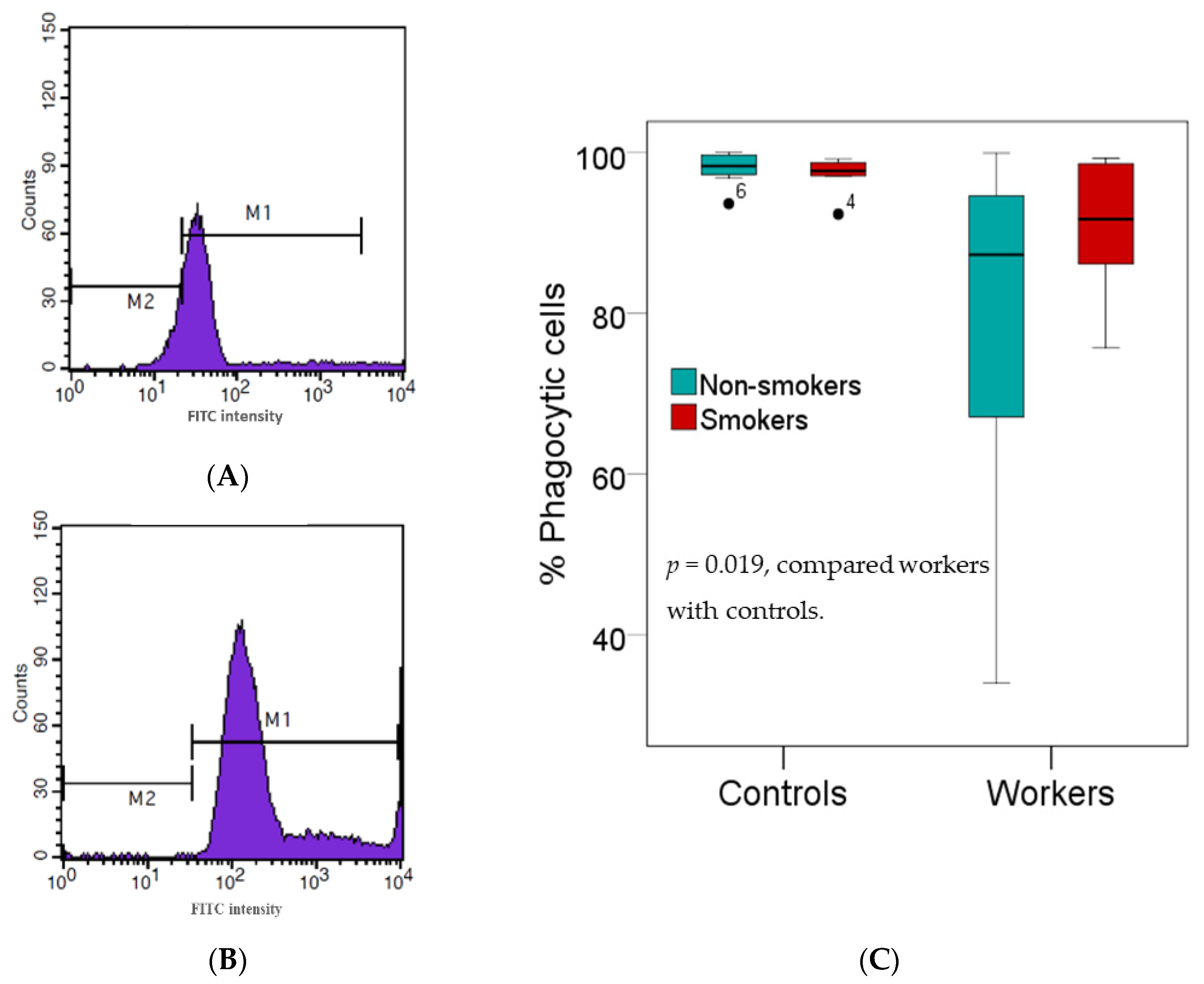
2.4. Phagocytic Activity Assay
2.5. Cytokine Assay
2.6. Proliferation Assay
2.7. Determination of T Cell Subpopulations by the Flow Cytometry
2.7.1. Helper T Lymphocytes and Cytotoxic T Lymphocytes
2.7.2. Regulatory T Lymphocytes
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Study Workers and Controls
3.2. Profiling of Cytokine Production and Other Immunologic Parameters
3.3. Effects of High Pb Exposure on Innate Immunity
3.4. Effects of High Pb Exposure on the Populations of Cytoxic and Regulatory T Lymphocytes
3.5. Blood Pb in Relation to Cytokines amd Immunologic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed] [Green Version]
- de Souza, I.D.; de Andrade, A.S.; Dalmolin, R.J.S. Lead-interacting proteins and their implication in lead poisoning. Crit. Rev. Toxicol. 2018, 48, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Caito, S.; Aschner, M. Developmental neurotoxicity of lead. Adv. Neurobiol. 2017, 18, 3–12. [Google Scholar] [PubMed]
- Li, X.; Gao, Y.; Zhang, M.; Zhang, Y.; Zhou, M.; Peng, L.; He, A.; Zhang, X.; Yan, X.; Wang, Y.; et al. In vitro lung and gastrointestinal bioaccessibility of potentially toxic metals in Pb-contaminated alkaline urban soil: The role of particle size fractions. Ecotoxicol. Environ. Saf. 2020, 190, 110151. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; McCauley, L.; Yan, C.; Shen, X.; Pinto-Martin, J.A. Low blood lead levels and hemoglobin concentrations in preschool children in China. Toxicol. Environ. Chem. 2012, 94, 423–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashem, M.A.; El-Sharkawy, N.I. The effects of low electromagnetic field and lead acetate combination on some hemato-biochemical and immunotoxicological parameters in mice. Turk. J. Hematol. 2009, 26, 181–189. [Google Scholar]
- Valcke, M.; Ouellet, N.; Dubé, M.; Laouan Sidi, E.A.; LeBlanc, A.; Normandin, L.; Balion, C.; Ayotte, P. Biomarkers of cadmium, lead and mercury exposure in relation with early biomarkers of renal dysfunction and diabetes: Results from a pilot study among aging Canadians. Toxicol. Lett. 2019, 312, 148–156. [Google Scholar] [CrossRef]
- Kim, Y.D.; Eom, S.Y.; Yim, D.H.; Kim, I.S.; Won, H.K.; Park, C.H.; Kim, G.B.; Yu, S.D.; Choi, B.S.; Park, J.D.; et al. Environmental Exposure to arsenic, lead, and cadmium in people living near Janghang copper smelter in Korea. J. Korean Med. Sci. 2016, 31, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Nanda, K.P.; Kumari, C.; Dubey, M.; Firdaus, H. Chronic lead (Pb) exposure results in diminished hemocyte count and increased susceptibility to bacterial infection in Drosophila melanogaster. Chemosphere 2019, 236, 124349. [Google Scholar] [CrossRef]
- Jorissen, A.; Plum, L.M.; Rink, L.; Haase, H. Impact of lead and mercuric ions on the interleukin-2-dependent proliferation and survival of T cells. Arch. Toxicol. 2013, 87, 249–258. [Google Scholar] [CrossRef]
- Chibowska, K.; Baranowska-Bosiacka, I.; Falkowska, A.; Gutowska, I.; Goschorska, M.; Chlubek, D. Effect of lead (Pb) on inflammatory processes in the brain. Int. J. Mol. Sci. 2016, 17, 2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baos, R.; Jovani, R.; Forero, M.G.; Tella, J.L.; Gómez, G.; Jiménez, B.; González, M.J.; Hiraldo, F. Relationships between T-cell-mediated immune response and Pb, Zn, Cu, Cd, and as concentrations in blood of nestling white storks (Ciconia Ciconia) and black kites (Milvus migrans) from Doñana (southwestern Spain) after the Aznalcóllar toxic spill. Environ. Toxicol. Chem. 2006, 25, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Ryoo, J.H.; Chang, S.J.; Kim, C.B.; Park, J.K.; Koh, S.B.; Ahn, Y.S. Blood lead levels and cause-specific mortality of inorganic lead-exposed workers in South Korea. PLoS ONE 2015, 10, e0140360. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Barry, V.; Anttila, A.; Sallmen, M.; Mueller, W.; Ritchie, P.; McElvenny, D.M.; Straif, K. Cancer incidence among workers with blood lead measurements in two countries. Occup. Environ. Med. 2019, 76, 603–610. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Li, R.; Sun, L.; Li, Z.Y.; Yang, R.L. Effect of lead exposure on the immune function of lymphocytes and erythrocytes in preschool children. J. Zhejiang Univ.-Sci. 2004, 5, 1001–1004. [Google Scholar] [CrossRef]
- Sata, F.; Araki, S.; Tanigawa, T.; Morita, Y.; Sakurai, S.; Katsuno, N. Changes in natural killer cell subpopulations in lead workers. Int. Arch. Occup. Environ. Health 1997, 69, 306–310. [Google Scholar] [CrossRef]
- Mishra, K.P.; Singh, V.K.; Rani, R.; Yadav, V.S.; Chandran, V.; Srivastava, S.P.; Seth, P.K. Effect of lead exposure on the immune response of some occupationally exposed individuals. Toxicology 2003, 188, 251–259. [Google Scholar] [CrossRef]
- Massadeh, A.M.; Al-Safi, S. Analysis of cadmium and lead: Their immunosuppressive effects and distribution in various organs of mice. Biol. Trace Elem. Res. 2005, 108, 279–285. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, F.; Shen, X.; Ouyang, W.; Liu, X.; Xu, Y.; Yu, T.; Jin, B.; Chen, J.; Luo, W. Pb exposure attenuates hypersensitivity in vivo by increasing regulatory T cells. Toxicol. Appl. Pharmacol. 2012, 265, 272–278. [Google Scholar] [CrossRef]
- McCabe, M.J., Jr.; Lawrence, D.A. Lead, a major environmental pollutant, is immunomodulatory by its differential effects on CD4+ T cells subsets. Toxicol. Appl. Pharmacol. 1991, 111, 13–23. [Google Scholar] [CrossRef]
- Yimthiang, S.; Waeyang, D.; Kuraeiad, S. Screening for elevated blood lead levels and related risk factors among Thai children residing in a fishing community. Toxics 2019, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanapop, C.; Geater, A.F.; Robson, M.G.; Phakthongsuk, P.; Viroonudomphol, D. Exposure to lead of boatyard workers in southern Thailand. J. Occup. Health Psychol. 2007, 49, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.osha.gov/OshDoc/Directive_pdf/CPL_03-00-0009.pdf (accessed on 12 August 2020).
- Trzcinka-Ochocka, M.; Brodzka, R.; Janasik, B. Useful and fast method for blood lead and cadmium determination using ICP-MS and GF-AAS; validation parameters. J. Clin. Lab. Anal. 2016, 30, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Böyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 1968, 97, 77–89. [Google Scholar]
- Shi, Z.; Zhen, S.; Orsini, N.; Zhou, Y.; Zhou, Y.; Liu, J.; Taylor, A.W. Association between dietary lead intake and 10-year mortality among Chinese adults. Environ. Sci. Pollut. Res. 2017, 24, 12273–12280. [Google Scholar] [CrossRef]
- Wang, X.; Ding, N.; Tucker, K.L.; Weisskopf, M.G.; Sparrow, D.; Hu, H.; Park, S.K. A Western diet pattern is associated with higher concentrations of blood and bone lead among middle-aged and elderly men. J. Nutr. 2017, 147, 1374–1383. [Google Scholar] [CrossRef] [Green Version]
- Koller, L.D. Effects of environmental contaminants on the immune system. Adv. Vet. Sci. Comp. Med. 1979, 23, 267–295. [Google Scholar]
- Cobbina, S.J.; Xu, H.; Zhao, T.; Mao, G.; Zhou, Z.; Wu, X.; Liu, H.; Zou, Y.; Wu, X.; Yang, L. A multivariate assessment of innate immune-related gene expressions due to exposure to low concentration individual and mixtures of four kinds of heavy metals on zebrafish (Danio rerio) embryos. Fish Shellfish Immunol. 2015, 47, 1032–1042. [Google Scholar] [CrossRef]
- Mishra, K.P. Lead exposure and its impact on immune system: A review. Toxicol. In Vitro 2009, 23, 969–972. [Google Scholar] [CrossRef]
- Lawrence, D.A. In vivo and in vitro effects of lead on humoral and cell-mediated immunity. Infect. Immun. 1981, 31, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroz, M.L.; Costa, F.F.; Bincoletto, C.; Perlingeiro, R.C.; Dantas, D.C.; Cardoso, M.P.; Almeida, M. Engulfment and killing capabilities of neutrophils and phagocytic splenic function in persons occupationally exposed to lead. Int. Immunopharmacol. 1994, 16, 239–244. [Google Scholar] [CrossRef]
- Sen, G.C. Viruses and interferons. Annu. Rev. Microbiol. 2001, 55, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Fenga, C.; Gangemi, S.; Di Salvatore, V.; Falzone, L.; Libra, M. Immunological effects of occupational exposure to lead (Review). Mol. Med. Rep. 2017, 15, 3355–3360. [Google Scholar] [CrossRef] [Green Version]
- Laschi-Loquerie, A.; Descotes, J.; Tachon, P.; Evreux, J.C. Influence of lead acetate on hypersensitivity. Experimental study. J. Immunopharmacol. 1984, 6, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, N.Y.A.; Emmrich, F.; Adham, K.; Wichmann, G.; Lehmann, I.; El-Massry, A.; Ghoneim, H.; Lehmann, J.; Sack, U. Dose-dependent modulation of the in vitro cytokine production of human immune competent cells by lead salts. Toxicol. Sci. 2005, 86, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.-C.; Hwang, Y.-S.; Chen, Y.-Y.; Liu, C.-L.; Shen, C.-N.; Hong, W.-H.; Lo, S.-M.; Shen, C.-R. Interleukin-4 supports the suppressive immune responses elicited by regulatory T Cells. Front. Immunol. 2017, 8, 1508. [Google Scholar] [CrossRef] [Green Version]
- Radbin, R.; Vahedi, F.; Chamani, J. The influence of drinking-water pollution with heavy metal on the expression of IL-4 and IFN-γ in mice by real-time polymerase chain reaction. Cytotechnology 2014, 66, 769–777. [Google Scholar] [CrossRef]
- Kasten-Jolly, J.; Lawrence, D.A. Lead modulation of macrophages causes multiorgan detrimental health effects. J. Biochem. Mol. Toxicol. 2014, 28, 355–372. [Google Scholar] [CrossRef]
- Hernández-Castro, B.; Doníz-Padilla, L.M.; Salgado-Bustamante, M.; Rocha, D.; Ortiz-Pérez, M.D.; Jiménez-Capdeville, M.E.; Portales-Pérez, D.P.; Quintanar-Stephano, A.; González-Amaro, R. Effect of arsenic on regulatory T cells. J. Clin. Immunol. 2009, 29, 461–469. [Google Scholar] [CrossRef]
- Gera, R.; Singh, V.; Mitra, S.; Sharma, A.K.; Singh, A.; Dasgupta, A.; Singh, D.; Kumar, M.; Jagdale, P.; Patnaik, S.; et al. Arsenic exposure impels CD4 commitment in thymus and suppress T cell cytokine secretion by increasing regulatory T cells. Sci. Rep. 2017, 7, 7140. [Google Scholar] [CrossRef] [PubMed]
- Burchill, M.A.; Yang, J.; Vogtenhuber, C.; Blazar, B.R.; Farrar, M.A. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 2007, 178, 280–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrew, A.S.; Jewell, D.A.; Mason, R.A.; Whitfield, M.L.; Moore, J.H.; Karagas, M.R. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environ. Health Perspect. 2008, 116, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.-J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Abu-Eid, R.; Samara, R.N.; Ozbun, L.; Abdalla, M.Y.; Berzofsky, J.A.; Friedman, K.M.; Mkrtichyan, M.; Khleif, S.N. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol. Res. 2014, 2, 1080–1089. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhengyan, Z.; Rong, L.; Hanyun, C. Decrease of CD4+T-lymphocytes in children exposed to environmental lead. Biol. Trace Elem. Res. 2005, 105, 19–25. [Google Scholar] [CrossRef]



| Descriptors | All Subjects | Controls | Workers | Odds Ratio | p-Value 1 |
|---|---|---|---|---|---|
| Number | 30 | 16 | 14 | ||
| Age, years | 53.0 ± 9.7 | 51.5 ± 8.8 | 54.0 ± 11.3 | - | 0.595 |
| Range, years | 28–69 | 36–66 | 28–69 | ||
| BMI, kg/m2 | 22.9 ± 4.1 | 23.7 ± 4.7 | 24.0 ± 4.6 | - | 0.874 |
| Range, kg/m2 | 16.9–32.5 | 19.1–32.5 | 16.9–30.3 | ||
| Hemoglobin, g/dL | 13.9 ± 1.2 | 14.1 ± 1.1 | 13.4 ± 1.2 | - | 0.550 |
| Range, g/dL | 11.7–16.4 | 12.2–16.4 | 11.7–15.6 | ||
| Hematocrit, % | 41.8 ± 3.2 | 42.4 ± 2.9 | 40.9 ± 3.0 | - | 0.698 |
| Range, % | 36.3–48 | 37.7–48 | 36.3–45.2 | ||
| Blood Pb concentration, µg/dL | 16.17 ± 18.68 | 4.28 ± 1.12 | 37.07 ± 11.09 | - | <0.001 |
| Range, µg/dL | 3.02–58.47 | 3.02–4.24 | 25.11–58.47 | ||
| Sex | - | - | |||
| Male | 25 | 16 | 9 | ||
| Female | 5 | 0 | 5 | ||
| Occupation | - | - | |||
| Caulker | 10 | 0 | 10 | ||
| Fishing net worker | 4 | 0 | 4 | ||
| Agriculturist | 16 | 16 | - | ||
| Duration of Pb exposure | - | - | |||
| 0 year | 16 | 16 | 0 | ||
| 1–5 years | 3 | 0 | 3 | ||
| 5–10 years | 4 | 0 | 4 | ||
| >10 years | 7 | 0 | 7 | ||
| Smoking history | 1.158 | 0.282 | |||
| Yes | 14 | 6 | 8 | ||
| No | 16 | 10 | 6 |
| Parameters | Group | Median | SD | Range | 95% CI | p-Value 1 |
|---|---|---|---|---|---|---|
| Th (%) | Controls | 56.3 | 10.1 | 41.3–78.4 | 51.9–68.4 | 0.395 |
| Workers | 60.7 | 9.3 | 45.8–77.2 | 56.9–69.9 | ||
| Proliferation index | Controls | 1.50 | 0.35 | 1.06–2.41 | 1.22–1.66 | 0.226 |
| Workers | 1.14 | 0.26 | 1.09–1.66 | 1.09–1.66 | ||
| IFN-γ (pg/mL) | Controls | 20.1 | 9.3 | 3.2–30.0 | 3.2–30.0 | 0.104 |
| Workers | 12.1 | 4.6 | 3.6–20.6 | 10.0–15.5 | ||
| IL-4 (pg/mL) | Controls | 155.6 | 40.0 | 103.4–252 | 112.1–163 | 0.060 |
| Workers | 175.6 | 67.2 | 99.2–334 | 114.7–215 | ||
| IL-4/IFN-γ ratio | Controls | 7.0 | 15.6 | 3.5–50.4 | 3.5–50.4 | 0.026 |
| Workers | 15.0 | 11.8 | 6.1–48.1 | 10.3–31.3 |
| Blood Pb Versus Immunologic Parameters | Phagocytic Activity | Proliferation Index | IFN-γ | IL-4 | %Th | %Tc | %Treg |
|---|---|---|---|---|---|---|---|
| Spearman’s rho | −0.209 | −0.329 | −0.319 | 0.473 | 0.358 | −0.563 | 0.843 |
| p-value (two-tailed) | 0.364 | 0.231 | 0.213 | 0.041 | 0.121 | 0.015 | <0.001 |
| Significant (α = 0.05) | No | No | No | Yes | No | Yes | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pukanha, K.; Yimthiang, S.; Kwanhian, W. The Immunotoxicity of Chronic Exposure to High Levels of Lead: An Ex Vivo Investigation. Toxics 2020, 8, 56. https://doi.org/10.3390/toxics8030056
Pukanha K, Yimthiang S, Kwanhian W. The Immunotoxicity of Chronic Exposure to High Levels of Lead: An Ex Vivo Investigation. Toxics. 2020; 8(3):56. https://doi.org/10.3390/toxics8030056
Chicago/Turabian StylePukanha, Kawinsaya, Supabhorn Yimthiang, and Wiyada Kwanhian. 2020. "The Immunotoxicity of Chronic Exposure to High Levels of Lead: An Ex Vivo Investigation" Toxics 8, no. 3: 56. https://doi.org/10.3390/toxics8030056
APA StylePukanha, K., Yimthiang, S., & Kwanhian, W. (2020). The Immunotoxicity of Chronic Exposure to High Levels of Lead: An Ex Vivo Investigation. Toxics, 8(3), 56. https://doi.org/10.3390/toxics8030056






