Evaluation of the Skin Sensitization Potential of Carbon Nanotubes Using Alternative In Vitro and In Vivo Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carbon Nanotubes
2.2. Preparation of CNT Suspensions
2.3. Cell Culture
2.4. CNT Treatments and KeratinoSens™ Assay Methods
2.5. Animals
2.6. CNTs Treatments and LLNA: BrdU-FCM Assay Methods
3. Results
3.1. Physicochemical Characteristic of CNTs
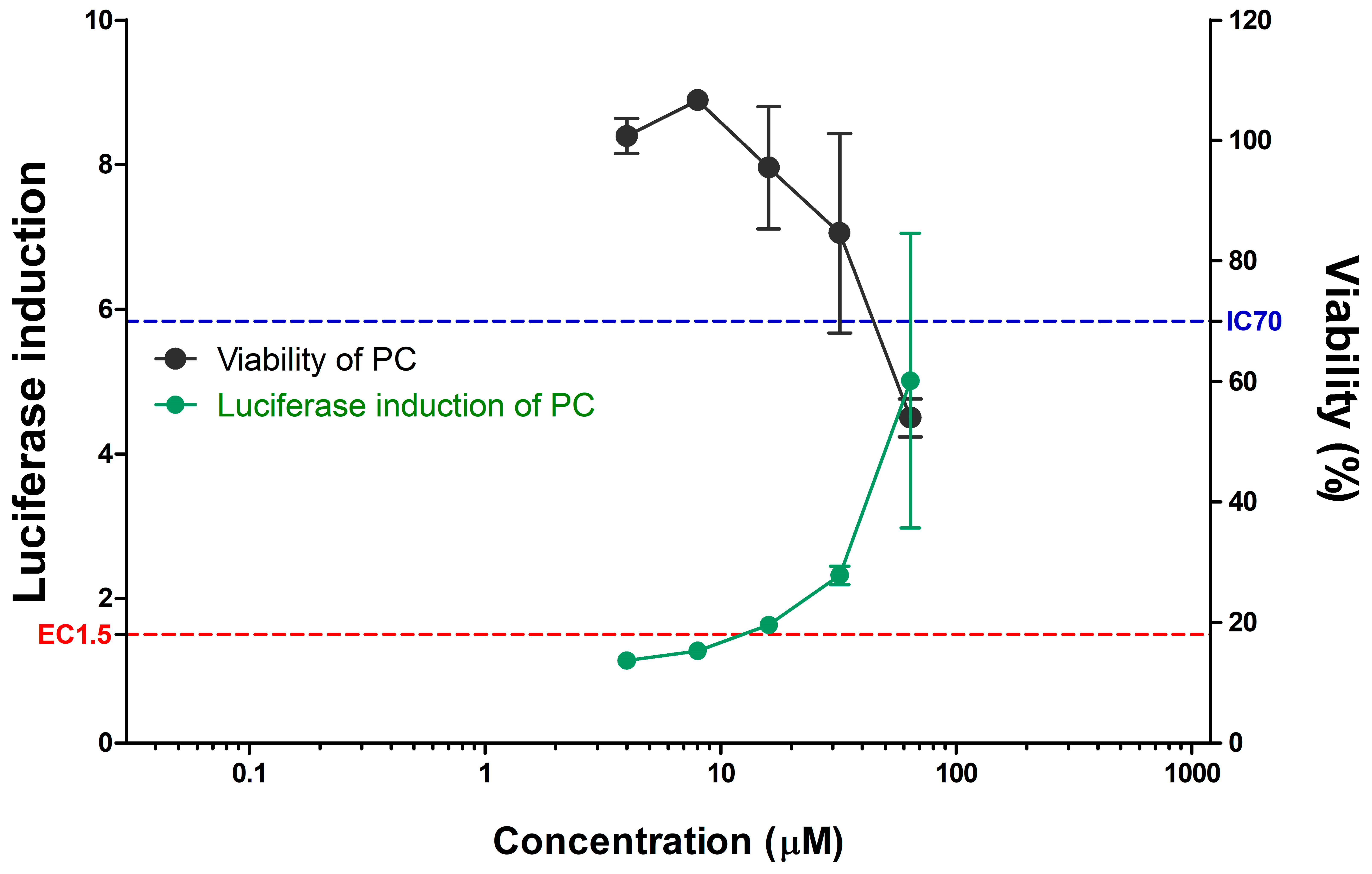
3.2. Evaluation of CNTs in the KeratinoSens™ Assay
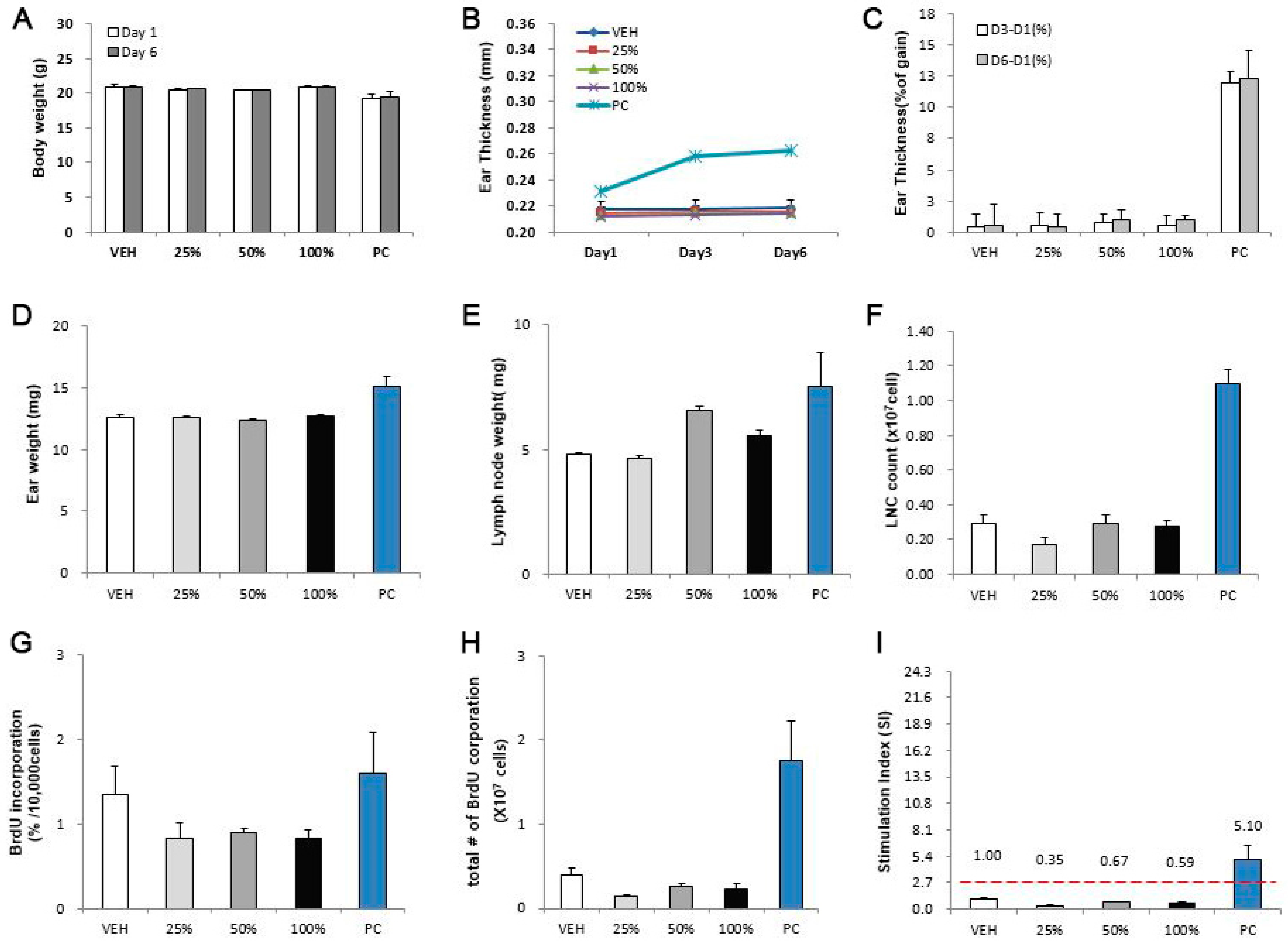
3.3. Evaluation of CNTs in the LLNA: BrdU-FCM Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AOP | Adverse outcome pathway |
| CNT | Carbon nanotube |
| DMF | N,N-Dimethylformamide |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPRA | Direct peptide reactivity assay |
| FBS | Fetal bovine serum |
| FCM | Flow cytometry |
| IACUC | Institutional Animal Care and Use Committee |
| LLNA | Local lymph node assay |
| LNC | Lymph node cells |
| MFDS | Ministry of Food and Drug Safety |
| MWCNTs | Multi-walled carbon nanotubes |
| SI | Stimulation index |
| OECD | Organization for Economic Cooperation and Development |
| SWCNTs | Single-wall carbon nanotubes |
| TEM | Transmission electron microscopy |
References
- Bandaru, P.R. Electrical Properties and Applications of Carbon Nanotube Structures. J. Nanosci. Nanotechnol. 2007, 7, 1239–1267. [Google Scholar] [CrossRef]
- Kostarelos, K.; Bianco, A.; Prato, M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat. Nanotechnol. 2009, 4, 627–633. [Google Scholar] [CrossRef] [PubMed]
- ISO (International Organization for Standardization). Nanotechnologies-Terminology and Definitions for Nanoobjects-Nanoparticle, Nanofiber and Nanoplate; ISO: Geneva, Switzerland, 2008; ISO/TS 27687. [Google Scholar]
- Oberdörster, G.; Maynard, A.D.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.D.; Carter, J.M.; Karn, B.; Kreyling, W.; Lai, D.Y.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Schinwald, A.; Murphy, F.; Cho, W.-S.; Duffin, R.; Tran, L.; Poland, C.A. The Biologically Effective Dose in Inhalation Nanotoxicology. Acc. Chem. Res. 2013, 46, 723–732. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Park, M.V.D.Z.; Gosens, I.; De Jong, W.H.; Cassee, F.R. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol. 2014, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, W.-S.; Duffin, R.; Thielbeer, F.; Bradley, M.; Megson, I.L.; MacNee, W.; Poland, C.A.; Tran, C.L.; Donaldson, K. Zeta Potential and Solubility to Toxic Ions as Mechanisms of Lung Inflammation Caused by Metal/Metal Oxide Nanoparticles. Toxicol. Sci. 2012, 126, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.-S.; Kang, B.-C.; Lee, J.K.; Jeong, J.; Che, J.-H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.; Kim, S.-H.; Lee, S.; Lee, D.-K.; Han, Y.; Jeon, S.; Cho, W.-S. Differential Contribution of Constituent Metal Ions to the Cytotoxic Effects of Fast-Dissolving Metal-Oxide Nanoparticles. Front. Pharmacol. 2018, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filon, F.L.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef]
- Journeay, W.S.; Goldman, R. Occupational handling of nickel nanoparticles: A case report. Am. J. Ind. Med. 2014, 57, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Yapar, E.A.; İnal, Ö.; Yapar, E.A.; Yapar, Ö.İ.E.A.; Inal, Ö. Nanomaterials and cosmetics. İstanbul Üniversitesi Eczacılık Fakültesi Derg. 2012, 42, 43–70. [Google Scholar]
- Rusche, B. The 3Rs and animal welfare-conflict or the way forward? Altex 2003, 20, 63–76. [Google Scholar] [PubMed]
- Kaluzhny, Y.; Kandárová, H.; Hayden, P.; Kubilus, J.; D’Argembeau-Thornton, L.; Klausner, M. Development of the EpiOcular™ Eye Irritation Test for Hazard Identification and Labelling of Eye Irritating Chemicals in Response to the Requirements of the EU Cosmetics Directive and REACH Legislation. Altern. Lab. Anim. 2011, 39, 339–364. [Google Scholar] [CrossRef] [PubMed]
- OECD. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins, OECD Series on Testing and Assessment, No. 168; OECD Publishing: Paris, France, 2014. [Google Scholar] [CrossRef] [Green Version]
- OECD. Test No. 442C: In Chemico Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins. Available online: https://www.oecd-ilibrary.org/environment/test-no-442c-in-chemico-skin-sensitisation_9789264229709-en (accessed on 26 June 2020).
- OECD. Test No. 442D: In Vitro Skin Sensitisation: Are-Nrf2 Luciferase Test Method, OECD Guidelines for the Testing of Chemicals. Available online: https://www.oecd-ilibrary.org/environment/test-no-442d-in-vitro-skin-sensitisation_9789264229822-en (accessed on 25 June 2018).
- OECD. Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation. Available online: https://www.oecd-ilibrary.org/environment/test-no-442e-in-vitro-skin-sensitisation_9789264264359-en (accessed on 25 June 2018).
- OECD. Test No. 442B: Skin Sensitization: Local Lymph Node Assay: BrdU-Elisa or -Fcm, Oecd Guidelines for the Testing of Chemicals. Available online: https://www.oecd-ilibrary.org/environment/test-no-442b-skin-sensitization_9789264090996-en (accessed on 25 June 2018).
- Park, Y.-H.; Jeong, S.H.; Yi, S.M.; Choi, B.H.; Kim, Y.-R.; Kim, I.-K.; Kim, M.-K.; Son, S.W. Analysis for the potential of polystyrene and TiO2 nanoparticles to induce skin irritation, phototoxicity, and sensitization. Toxicol. Vitr. 2011, 25, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kuroda, E.; Hirai, T.; Tsutsumi, Y.; Ishii, K.J. Allergic Responses Induced by the Immunomodulatory Effects of Nanomaterials upon Skin Exposure. Front. Immunol. 2017, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Lee, D.H.; Lee, J.H.; Yang, J.Y.; Seok, J.H.; Jung, K.; Lee, J.K. Evaluation of the skin sensitization potential of metal oxide nanoparticles using the ARE-Nrf2 Luciferase KeratinoSens™ assay. Toxicol. Res. 2020, unpublished. [Google Scholar]
- Lee, D.-K.; Jeon, S.; Han, Y.; Kim, S.-H.; Lee, S.; Yu, I.J.; Song, K.S.; Kang, A.; Yun, W.S.; Kang, S.-M.; et al. Threshold Rigidity Values for the Asbestos-like Pathogenicity of High-Aspect-Ratio Carbon Nanotubes in a Mouse Pleural Inflammation Model. ACS Nano 2018, 12, 10867–10879. [Google Scholar] [CrossRef]
- Jung, K.-M.; Bae, I.; Kim, B.-H.; Kim, W.-K.; Chung, J.-H.; Park, Y.-H.; Lim, K.-M. Comparison of flow cytometry and immunohistochemistry in non-radioisotopic murine lymph node assay using bromodeoxyuridine. Toxicol. Lett. 2010, 192, 229–237. [Google Scholar] [CrossRef]
- Ahn, I.; Kim, T.-S.; Jung, E.-S.; Yi, J.-S.; Jang, W.-H.; Jung, K.-M.; Park, M.; Jung, M.-S.; Jeon, E.-Y.; Yeo, K.-U.; et al. Performance standard-based validation study for local lymph node assay: 5-bromo-2-deoxyuridine-flow cytometry method. Regul. Toxicol. Pharmacol. 2016, 80, 183–194. [Google Scholar] [CrossRef]
- Parra, J.; Abad-Somovilla, A.; Mercader, J.V.; Taton, T.A.; Abad-Fuentes, A. Carbon nanotube-protein carriers enhance size-dependent self-adjuvant antibody response to haptens. J. Control. Release 2013, 170, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Natsch, A.; Ryan, C.A.; Foertsch, L.; Emter, R.; Jaworska, J.; Gerberick, F.; Kern, P. A dataset on 145 chemicals tested in alternative assays for skin sensitization undergoing prevalidation. J. Appl. Toxicol. 2013, 33, 1337–1352. [Google Scholar] [CrossRef]
- EURL-ECVAM. Recommendation on the KeratinoSens™ Assay for Skin Sensitisation Testing. 2014. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/eurl-ecvam-recommendation-keratinosenstm-assay-skin-sensitisation-testing (accessed on 22 October 2020).
- Andres, E.; Sá-Rocha, V.M.; Barrichello, C.; Haupt, T.; Ellis, G.; Natsch, A. The sensitivity of the KeratinoSens™ assay to evaluate plant extracts: A pilot study. Toxicol. Vitro 2013, 27, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Settivari, R.S.; Gehen, S.C.; Amado, R.A.; Visconti, N.R.; Boverhof, D.R.; Carney, E.W. Application of the KeratinoSens™ assay for assessing the skin sensitization potential of agrochemical active ingredients and formulations. Regul. Toxicol. Pharmacol. 2015, 72, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A.G.; Reichel, C.A.; Coester, C.; Tuomi, T.; Rehberg, M.; Krombach, F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 2008, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, W.-S.; Thielbeer, F.; Duffin, R.; Johansson, E.M.V.; Megson, I.L.; MacNee, W.; Bradley, M.; Donaldson, K. Surface functionalization affects the zeta potential, coronal stability and membranolytic activity of polymeric nanoparticles. Nanotoxicology 2013, 8, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hwang, S.-H.; Jeong, J.; Han, Y.; Kim, S.-H.; Lee, D.-K.; Lee, H.-S.; Chung, S.-T.; Jeong, J.; Roh, C.; et al. Nickel oxide nanoparticles can recruit eosinophils in the lungs of rats by the direct release of intracellular eotaxin. Part. Fibre Toxicol. 2016, 13, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ema, M.; Matsuda, A.; Kobayashi, N.; Naya, M.; Nakanishi, J. Evaluation of dermal and eye irritation and skin sensitization due to carbon nanotubes. Regul. Toxicol. Pharmacol. 2011, 61, 276–281. [Google Scholar] [CrossRef]
- OECD. Single Walled Carbon Nanotubes (SWCNTs): Summary of the Dossier, Series on the Safety of Manufactured Nanomaterials No. 70. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)22&doclanguage=en (accessed on 7 July 2016).
- OECD. Multi Walled Carbon Nanotubes (MWCNT): Summary of the Dossier, Series on the Safety of Manufactured Nanomaterials No. 68. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)20&doclanguage=en (accessed on 30 May 2016).
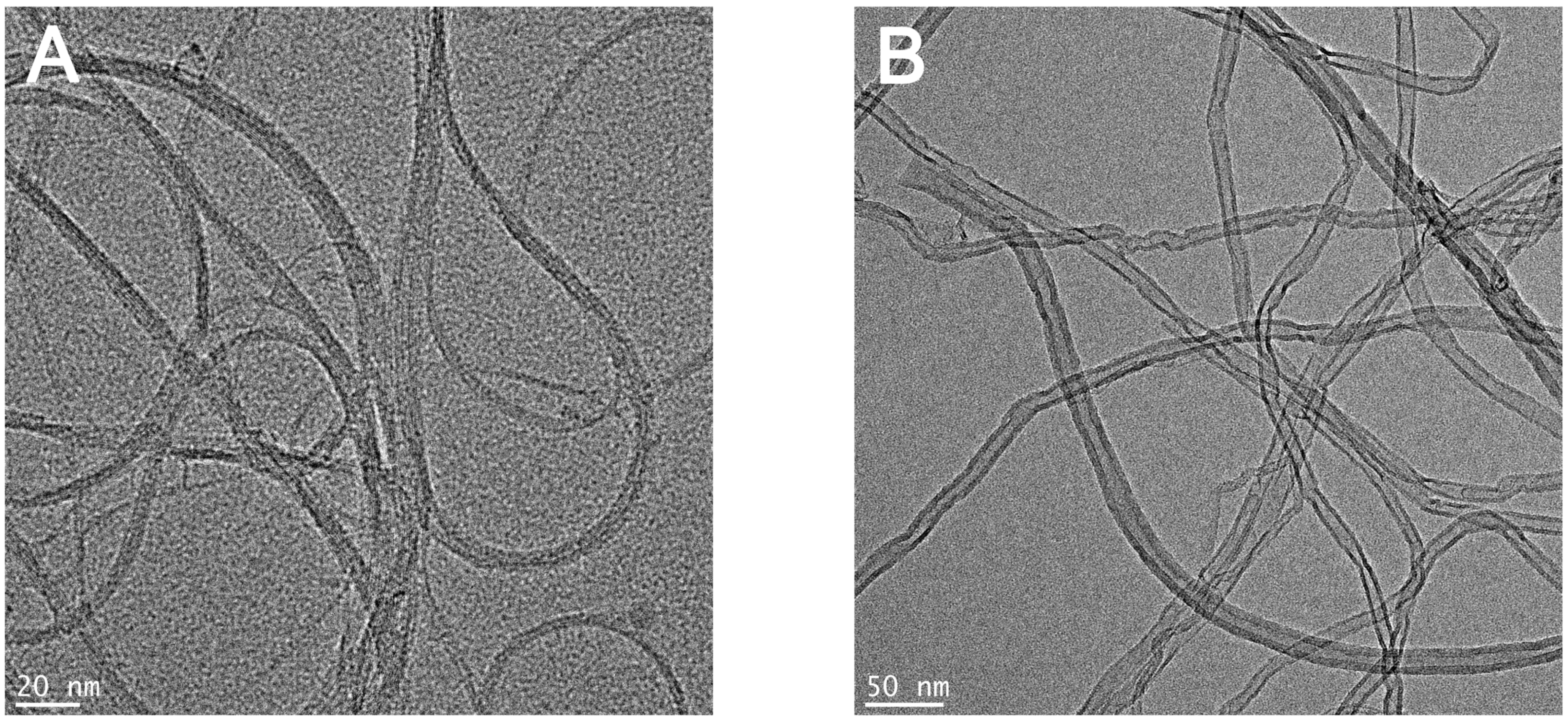



| Characteristic | KeratinoSens™ | LLNA: BrdU-FCM | ||
|---|---|---|---|---|
| SWCNT | MWCNT | SWCNT | MWCNT | |
| Average diameter (nm) | 5.97 ± 1.48 | 12.30 ± 2.18 | 5.97 ± 1.48 | 12.30 ± 2.18 |
| Average length (µm) | 1 | 10 | 1 | 10 |
| Surface area (m2/g) | ≥700 | 216 | ≥700 | 216 |
| Zeta potential (mV) | ||||
| in DW | −27.40 ± 1.59 | −34.99 ± 0.80 | −27.40 ± 1.59 | −33.99 ± 0.80 |
| in working solution * | −29.23 ± 1.79 | −26.99 ± 3.07 | −18.80 ± 0.93 | −38.38 ± 1.41 |
| CNT purity (%) | ≥77 | 99 | ≥77 | 99 |
| Carbon purity (%) | ≥90 | ≥98 | ≥90 | ≥98 |
| Endotoxin (EU/mL) | <0.1 | |||
| Nanomaterials | CAS RN | Physical Form | KeratinoSens™ Assay Results | ||||
|---|---|---|---|---|---|---|---|
| Imax | EC1.5 (µg/mL) | Cell Viability (%) a | IC50 (µg/mL) | Classification | |||
| SWCNT | 308068-56-6 | Solid | 1.07 | >1000 | >70 | 185.90 | Negative |
| MWCNT | 308068-56-6 | Solid | 1.39 | >1000 | >70 | 234.98 | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Lee, D.H.; Lee, J.H.; Yang, J.-Y.; Shin, H.-S.; Lee, J.; Jung, K.; Jeong, J.; Oh, J.-H.; Lee, J.K. Evaluation of the Skin Sensitization Potential of Carbon Nanotubes Using Alternative In Vitro and In Vivo Assays. Toxics 2020, 8, 122. https://doi.org/10.3390/toxics8040122
Kim S-H, Lee DH, Lee JH, Yang J-Y, Shin H-S, Lee J, Jung K, Jeong J, Oh J-H, Lee JK. Evaluation of the Skin Sensitization Potential of Carbon Nanotubes Using Alternative In Vitro and In Vivo Assays. Toxics. 2020; 8(4):122. https://doi.org/10.3390/toxics8040122
Chicago/Turabian StyleKim, Sung-Hyun, Dong Han Lee, Jin Hee Lee, Jun-Young Yang, Hyo-Sook Shin, JeongPyo Lee, Kikyung Jung, Jayoung Jeong, Jae-Ho Oh, and Jong Kwon Lee. 2020. "Evaluation of the Skin Sensitization Potential of Carbon Nanotubes Using Alternative In Vitro and In Vivo Assays" Toxics 8, no. 4: 122. https://doi.org/10.3390/toxics8040122
APA StyleKim, S.-H., Lee, D. H., Lee, J. H., Yang, J.-Y., Shin, H.-S., Lee, J., Jung, K., Jeong, J., Oh, J.-H., & Lee, J. K. (2020). Evaluation of the Skin Sensitization Potential of Carbon Nanotubes Using Alternative In Vitro and In Vivo Assays. Toxics, 8(4), 122. https://doi.org/10.3390/toxics8040122





