1. Introduction
Despite the significant decline in youth e-cigarette usage since the Federal Drug Enforcement Agency’s (FDA) flavored e-cigarette enforcement policy which was enacted in February 2020, youth e-cigarette use within the United States remains significantly high [
1]. Moreover, according to a cross-sectional study conducted by the Centers for Disease Control and Prevention (CDC), in 2020, 4.7% of middle school students (550,000) and 19.6% of high school students (3.02 million) reported current e-cigarette use [
1]. The prevalence of e-cigarette usage in the United States, especially amongst its youth, is partly due to the switch many cartridge-based e-cigarette users made to using disposable e-cigarettes; the FDA’s 2020 e-cigarette flavoring enforcement policy prompted this action [
1]. Further, the FDA’s flavoring enforcement policy only applies to flavoring for cartridge-based Electronic Nicotine Delivery System (ENDS) products; these products include cartridge-based e-cigarettes and pre-filled pod devices [
1]. More specifically, the FDA’s February 6th, 2020 e-cigarette enforcement policy for cartridge-based ENDS products applies to all flavors with nicotine, excluding menthol and tobacco [
1]. Moreover, the FDA’s enforcement policy involves requiring all manufacturers and retailers in the United States to remove all flavored cartridge-based ENDS products with nicotine from the market except tobacco-flavored and menthol-flavored cartridge-based ENDS products [
1]. All flavored products without nicotine (zero nicotine) are still available in the market. Furthermore, products exempt from the previously mentioned enforcement policy include disposable e-cigarettes with or without nicotine in certain states within the United States. A disposable e-cigarette is a type of ENDS product which can be discarded or thrown away once it runs out of e-liquid or charge. According to the 2020 National Youth Tobacco Survey (NYTS) conducted by the CDC, the use of disposable e-cigarettes (e-cigs) by high-school students who were already e-cig users had increased significantly from 2.4% in 2019 to 26.5% in 2020. Additionally, according to the 2020 NYTS, the number of middle-school e-cig users who specifically used disposable e-cigs increased from 3.3% in 2019 to 15.2% in 2020 [
1]. One aspect of disposable e-cigs which is attractive to youth e-cigarette users is the convenience at which they can be used; they do not require recharging or refilling with e-liquids like cartridge-based products. Additionally, disposable devices are much cheaper and practical to use than their refillable counterparts.
With the substantial rise in the availability of different e-liquid flavors in recent years, investigating the role that e-liquid flavoring chemicals have in inducing pulmonary pathophysiological effects has become more complicated [
2]. Further, the long-term effects of e-cigarette vapor exposure on human health require further investigation. However, studies so far have shown that e-cigarette aerosol production involves generating reactive oxygen species (ROS) [
3]. ROS can be generated either intracellularly (via mitochondrial oxidative phosphorylation) or may arise from exogenous sources (cigarette smoke, e-cigarette aerosols, and environmental pollution,) [
4]. Specific ROS include hydrogen peroxide (H
2O
2), hydroxyl radical (
•OH), and superoxide radical (O
2•−) [
5]. ROS plays a crucial role in modulating the immune-inflammatory system and activating different signal transduction pathways and cell signaling processes for inflammatory responses [
6].
The normal physiological balance between ROS and antioxidants can be disturbed through the inhalation of exogenous sources of ROS, thus leading to the damage of cellular structures. Further, an excess in intracellular ROS levels causes oxidative damage to the cellular membrane, intracellular lipids, intracellular enzymes, and intracellular DNA (iDNA). Moreover, excess ROS can also induce a vicious cycle of chronic inflammation in the lungs due to excessive ROS leading to the activation of specific immune cells, polymorphonuclear neutrophils (PMNs); activated PMNs can, in turn, generate more ROS in pulmonary cells [
7]. This subsequent chronic inflammation leads to airways becoming more thickened and prone to mucus secretion, also known as airway modeling, this later resulting in lung dysfunction [
8]. Regarding exogenous ROS sources, studies in the past have shown that tobacco smoke-generated ROS can induce DNA damage within lung epithelial cells and premature pulmonary cell death, leading to the development of lung cancer and COPD/emphysema, respectively [
9]. Additionally, one study had shown that through activating the heating element of an e-cigarette and then aerosolizing its e-liquid component, ROS is produced; which can be drawn from the device into the lungs, directly causing inflammatory response [
10].
Despite the well-known adverse health effects of conventional cigarette smoking, one of the main factors driving both youth and adult appeal for e-cigarettes is the availability of many different flavors. These flavors add to the allure many have for e-cigarettes by creating sensory perceptions of palatable tastes, which conceal the bitter taste of nicotine [
11]. Further, one survey found that the availability of fruit and candy e-liquid flavors significantly contributes to the prevalence of youth e-cigarette usage in the United States; adults seem to prefer more traditional flavors, such as tobacco [
11]. Likewise, according to a Morbidity and Mortality Weekly Report by the CDC conducted in September 2020, among current users of flavored disposable e-cigarettes, the most commonly used flavor type was those under the fruit classification (82.7%; 650,000 [
1]). Additionally, according to the same Morbidity and Mortality Weekly Report by the CDC, the following three most widely used vape bar flavors were those falling under the mint classification (51.9%; 410,000), those falling under the sweet categorizations (candy, desserts, etc.) (41.7%; 330,000), and those falling under the menthol (cool/iced) classification (23.3%; 180,000), respectively [
1]. Accordingly, with the recent surge in flavored disposable e-cig use during this past year, more research should be conducted which investigates how ROS content within aerosols generated from disposable e-cigarettes are modulated by flavoring chemicals.
In addition to flavor, another factor contributing to the prevalence of disposable e-cigarette usage in this country is the range of nicotine concentrations which are available for these devices. Nicotine is a highly addictive alkaloid present within the aerosol generated by e-cigarettes as well as within the smoke generated from conventional cigarettes [
12]. For disposable e-cigarettes sold within the United States, nicotine content ranges from 0 mg/mL (0%, nicotine-free option) to 68 mg/mL (6.8%). Furthermore, nicotine is extremely addictive and can harm the neural development of those under the age of 25, which is most troubling given the prevalence of e-cigarette use among adolescents in this country [
13]. Exposure to nicotine through inhaling e-cigarette generated aerosols has contributed to prolonging e-cigarette usage amongst a significant portion of the country, especially those under the age of 25 [
14]. Despite youth e-cigarette usage continuing to be a rising health concern in the U.S, studies investigating how exogenous ROS generation varies as a function of nicotine concentration in ENDS products are lacking. Additionally, with the recent surge in flavored disposable e-cig use and the wide range of nicotine content available for these products, research should be conducted to determine how ROS or free radical generation among disposable e-cigarettes varies as a function of nicotine concentration. Consequently, in our study, we hypothesize that ROS levels within the aerosols generated from disposable e-cigarettes will vary with different flavors as well with different nicotine concentrations. Furthermore, disposable e-cigarettes with a wide range of salt nicotine concentrations (0–6.8%) and within six main flavor categories (Tobacco, Minty Fruit, Fruity, Minty/Cool (Iced), Desserts, and Drinks/Beverages) from different vendors were analyzed. Additionally, we analyzed vape bars of identical flavors manufactured from the same company, but with varying concentrations of nicotine. The company (vendor) that produced these bars that we subsequently analyzed were Bolt, Flair Plus, and SMOQ. Bolt and Flair Plus disposable bars, which contain a solution comprising Propylene Glycol (PG) and Vegetable Glycerin (VG) mixed in a 1:1 ratio; likewise, these bars use a 1.6 and 1.8 Ohm coil, respectively, to aerosolize their component e-liquid. Accordingly, our subsequent comparative acellular ROS analyses included semi-quantified ROS content within aerosols produced from our PG:VG controls heated using 1.6 and 1.8 Ohm coils; the controls were made using a 1:1 (i.e., 50:50 ratio) ratio of PG and VG in this pilot/preliminary screening study.
2. Materials and Methods
2.1. Vape Bar Procurement
Vape bars were purchased from various locations and manufacturers locally within Rochester, NY and from various online websites/vendors. The disposable e-cigarettes used in this experiment contained a wide range of salt nicotine concentrations (0–6.8%) and were categorized into six main flavor categories (Tobacco, Minty Fruit, Fruity, Minty/Cool (Iced), Desserts, and Drinks/Beverages). The commercial manufacturers of the disposable vape bars used were Blu, Bolt, Cyclone, Eonsmoke, Flair Plus, Fling, Fliq, FreshBar, Hyde, Hyppe Bar, Jolly, Lit, NJOY, Phantom, Puff Bar, SMOQ, SOL, Tsunami Twin, Vice, Zaero, and Zero Disposable.
2.2. Generation of Vape Bar Aerosols
A fluorogenic dye was made using 0.01N NaOH, 2′7′ dichlorofluorescein diacetate (H2DCF-DA) (EMD Biosciences, San Diego, CA, USA) (Cat # 287810), phosphate (PO4) buffer, and horseradish peroxidase (Thermo Fisher Scientific, Waltham, MA, USA (Cat# 31491). The PO4 buffer was made using dibasic sodium phosphate (Sigma-Aldrich, St. Louis, MO, USA) (Cat# S0876) and sodium phosphate monobasic (JT Baker, Phillipsburg, NJ, USA) (Cat # 02-004-215). Afterward, i.e., upon bubbling, the resulting fluorogenic dye was analyzed via fluorescence spectroscopy with a maximum excitation and emission spectra of 475 and 535 nm, respectively. The standards used in this experiment ranged from 0 to 50 μM, each made from 1.25 mM H2O2 solution, which was prepared from 30% H2O2 (H2O2) (Thermo Fischer Scientific, Waltham, MA, USA) (Cat# H323-500) and double-distilled water (ddH2O). To enumerate, 1.25 mM H2O2 was diluted to 0.90 mM H2O2 using ddH2O, and that resulting 0.90 mM hydrogen peroxide solution was used in preparing the previously mentioned standards. Further, to ensure the desired concentration of H2O2 had been prepared using the 30% H2O2 solution (1.25 mM), Ultraviolet/Visible (UV/Vis) spectroscopy was used. To further explain, after adding 113 μL of 30% H2O2 to 999.887 mL of ddH2O, 1 mL of the resulting solution was added to a quartz cuvette (Sigma-Aldrich, St. Louis, MO, USA) (Cat # C-9542). The quartz cuvette, which has a 10 mm light path, was then inserted into a UV/Vis Spectrophotometer (Beckman Colter, Brea, CA, USA) (Cat# DU 250) and exposed to 240 nm light. Afterward, the absorbance was read and divided by 0.0436 (the extinction coefficient); the extinction coefficient was determined through previous H2O2 standardization tests and calibration curves generated. The resulting calculation should produce 1.25 mM; this signifying the correct concentration of H2O2 was produced using double distilled water and 30% H2O2. Further, before adding 1 mL of the resulting H2O2 solution into a quartz cuvette to then be inserted into the UV/Vis Spectrophotometer, ddH2O was pipetted into the same quartz cuvette and used as a blank.
Regarding the puff generation protocol itself, using a standard lab vacuum and a Buxco Individual Cigarette Puff Generator (Data Sciences International (DSI), St. Paul, MN, USA) (Cat#601-2055-001), the aerosol generated from each vape bar was individually bubbled through 10 mL of H
2DCF-DA solution within a 50 mL conical tube, at 1.5 L/min (
Figure 1). Moreover, two lime glass Pasteur pipettes (VWR, Radnor, PA, USA) (Cat # 14672-380) were inserted into the fluorogenic dye within a 50 mL conical tube via a two-hole stopper. Regarding the two Pasteur pipettes inserted into the respective 50 mL conical tube, the fine tip of one of the pipettes was manually broken (or shortened) before being inserted into the two-hole stopper; the fine tip of this pipette did not touch the fluorogenic dye. Next, the end of the same Pasteur pipette, the end usually attached to a rubber bulb, was connected to a vacuum using rubber tubing. Regarding the second Pasteur pipette inserted into the two-hole stopper on the 50 mL conical tube containing the dye, its fine tip was also shortened (via manual breaking), but not as much as the previously mentioned pipette (“shorter” Pasteur pipette). Moreover, the “longer” Pasteur pipette had its fine tip immersed within the fluorogenic dye inside the conical tube. Subsequently, the “shorter” Pasteur pipette was connected to a Fume Hood vacuum, and the “longer” Pasteur pipette was connected to the Puff Generator machine; specifically, rubber tubing was used for connecting the pipettes to the vacuum and Puff Generator. To be more specific, the ends of each pipette (the ends of lime glass Pasteur pipettes which are usually connected to a rubber bulb) were connected to the rubber tubing. Furthermore, the entirety of the puffing protocol for each vape bar and control was conducted in a fume hood; additionally, surrounding lights were turned off to reduce exposure of the fluorogenic dye to light. Furthermore, each 50 mL conical tube containing 10 mL of fluorogenic dye was wrapped with aluminum foil to minimize the dye’s exposure to light. A red light was used to see whether the vape bar generated aerosols were indeed being bubbled through the fluorogenic dye; this is due to H
2DCF-DA not absorbing red light.
In conjunction with the aforementioned puff generation set-up, once a vape bar was inserted into the Buxco Puff Generator, aerosol was generated and bubbled into the fluorogenic dye under a specific puff profile regiment. Under the particular puff regiment used in the study, a total of 20 puffs was generated through the Puff Generator apparatus; the puffing frequency was two puffs/min, and each puff had a volume of 55 mL and lasted 3.0 s. Different components making up the interior of the Puff Generator (the artificial lung, inhalation actuator, and exhalation actuator) worked together simultaneously to smoke the vape bar to the puff regiment inputted by the user. Further, the Puff Generator smoked each vape bar for ten minutes; the resulting aerosols then traveled from the tubing attached to the Puff Generator to the Pasteur pipette inserted into the 50 mL conical tube. Moreover, once ten minutes of a specific puff regiment had passed for one particular vape bar, the 50 mL conical tube containing the dye which had just been bubbled through with the aerosol of that specific vape bar was inverted several times and then put in ice. Additionally, tubing which connected the Puff Generator to the 10 mL fluorogenic dye within a respective 50 mL conical tube was rinsed with 70% Ethanol and then sterile ddH2O in between replicates for a bar of a specific flavor, vendor, and nicotine concentration and in between puffing regiments for different vape bars. After bubbling all vape bars in duplicates, each resulting fluorogenic dye sample was given 15 min to react within a 37 °C degree water bath (VWR 1228 Digital Water Bath); the resulting solution was then immediately analyzed via fluorescence spectroscopy.
2.3. Generation of Aerosols from Propylene Glycol: Vegetable Glycerin (PG:VG) Solutions, Negative Controls, and Positive Controls
The same puff generator device and puffing regiment used for bubbling the aerosols generated from the vape bars analyzed were used when bubbling solutions consisting of Propylene Glycol (PG) (Sigma-Aldrich, St. Louis, MO, USA) (Cat # P4347) and Vegetable Glycerin (VG) (Sigma-Aldrich, St. Louis, MO, USA) (Cat # G5516). In other words, a PG:VG control (humectant control) was used in conjunction with our vape bar analyses. To further explain, a PG:VG solution was prepared in a 15 mL conical tube; PG and VG were added together in a 1:1 ratio. Subsequently, the prepared PG:VG solution was vortexed for one minute, inverted several times, and then left on a laboratory shaker (Labnet, Edison, NJ, USA) (Mo: Gyrotwister GX-1000) at ten revolutions per minute (10 rpm) overnight before being used in an acellular ROS assay the following day. On the day of the acellular ROS analysis, 700μL of the PG:VG solution was pipetted into a new empty refillable JUUL pod with a 1.8 Ohm cotton wick atomizer (OVNStech, Shenzen, GD, China) (Mo: WO1 JUUL Pods). Subsequently, the PG:VG solution was allowed to sit in the pod for three to five minutes before being inserted into a rechargeable e-cigarette device (JUUL Labs Inc., Washington, DC, USA) (Mo: Rechargeable JUUL Device w/USB charger). Next, the JUUL device was inserted into the Puff Generator and was smoked under the same puff regiment as the disposable vape bars which were analyzed. Similar to the 1.8 Ohm coil PG:VG control described, the same process was used with a refillable cartridge using a 1.6 Ohm coil; in this case, Eleaf Elven pod cartridges (Eleaf Elven, Shenzen, GD, China) (Mo: Eleaf Elven Pod Cartridge) were used and inserted into a different rechargeable e-cigarette device (Eleaf Elven, Shenzen, GD, China) (Mo: Eleaf Elven Pod System).
For our negative control, air was bubbled through the fluorogenic dye; this was achieved by using the Puff Generator under the same puffing regiment as before but without inserting a disposable vape bar into the machine. For our positive control, cigarette smoke generated through burning conventional research cigarettes (Kentucky Tobacco Research & Development Center in the University of Kentucky, Lexington, KY, USA) (Mo: 3R4F) was bubbled through the fluorogenic dye. Also, the fluorogenic dye through which the 3R4F research cigarette smoke was bubbled through was diluted four-fold with freshly made dye. Each control (PG:VG heated with a 1.6 Ohm coil, PG:VG heated with a 1.8 Ohm coil, air, and the 3R4F cigarette) was run in duplicates.
2.4. Fluorescence Spectroscopy and ROS Quantification
After bubbling aerosols from every vape bar during a specific day in which an acellular ROS assay was conducted, 100 μL of each prepared standard and each bubbled dye solution was added to 3.0 mL of fluorogenic dye.Further, 3.0 mL of dye was first added to a 16 × 100 mm Durex Borosilicate Glass culture tube (VWR) (Cat #: 47729-576), and then 100 μL of the bubbled dye solution and each standard was individually added to these culture tubes. Next, each culture tube was vortexed gently. Subsequently, each culture tube was placed within a 37 °C water bath for 15 min. Further, during the 15-min incubation period, surrounding lights were turned off, and only red lights were used. Afterward, standards were measured on a spectrofluorometer (Thermo Fisher Scientific, Waltham, MA, USA) (Mo. FM109535) in fluorescence intensity units (FIU); the same was carried out with the fluorogenic dye samples through which vape bar aerosols were bubbled; all of which was performed using the previously mentioned culture tubes. Additionally, readings displayed on the fluorometer (concentration in μM) were based on the generated hydrogen peroxide standard curve and measured as hydrogen peroxide, H2O2 equivalents.
2.5. Statistical Analysis
Statistical analyses of significance were calculated using one-way ANOVA as well as Tukey’s post-hoc test for multiple pair-wise comparisons by GraphPad Prism Software version 8.1.1. Samples were run in duplicates and experiments were repeated until consistent data were obtained. The results are shown as mean ± SEM with duplicates analyses. Data were considered to be statistically significant for p values < 0.05.
4. Discussion
When analyzing the ROS content emitted by vape bars within each flavor category (Tobacco, Fruity, Minty Fruit, Minty/Cool (Iced), Drinks/Beverages, and Desserts), we observed differential ROS production among the different flavored bars. In addition, within each of the six flavor categories analyzed, different flavored disposable vape bars with the same nicotine content produced variable levels of ROS relative to the respective air control. The Tobacco, Fruity, Minty Fruit, Minty/Cool (Iced), Drinks/Beverages, and Dessert flavor categories with and without nicotine were selected for our analyses due to the popularity of these flavor categories among e-cigarette users, especially among e-cigarette users in middle and high school, after the FDA’s 2020 e-cigarette flavoring enforcement policy [
1]. Furthermore, the FDA’s 2020 flavoring enforcement policy prohibits companies from selling cartridge-based e-cigarettes with dessert, candy, fruit, mint flavors with nicotine, as well as any flavor excluding tobacco or menthol [
1]. Any flavors without nicotine (zero nicotine) are still sold in the United States without any regulations, which are used in the present study.
Vape bars under the Minty Fruit flavor category were analyzed due to their recent rise in popularity among youth e-cigarette users. Further, around the same time that disposable e-cigarette sales surged following the FDA’s flavored e-cigarette enforcement policy on nicotine in 2020, a significantly high number of minty fruit e-cigarette flavors had entered marketplaces [
15]. Further, the increased usage of minty (cool/iced) fruit flavors among e-cigarette users in the country necessitated us to analyze these flavors because of their potential to make further regulatory action more complicated due to Iced Fruit flavors not fitting into existing flavor categorizations [
15]. Research investigating how flavoring chemicals affect ROS generation in e-cigarette generated aerosols has been explored minimally; however, a few recent studies have delved into the dependence that ROS generation from e-cigarettes may have on flavoring chemicals. Our study found that ROS levels generated from cigar/cigarillo smoke varied among different flavors [
16]. Regarding studies conducted with e-cigarettes, another study found that ROS generation within the aerosols generated from cartridge-based e-cigarette devices was highly dependent on the vendor, puffing pattern, voltage, and the flavor of the cartridge-based e-cigarette device used [
3]. Moreover, our lab’s previous study found that the flavorings used in e-liquids can induce an inflammatory response in monocytes; the study further found that this response is mediated through ROS production [
17].
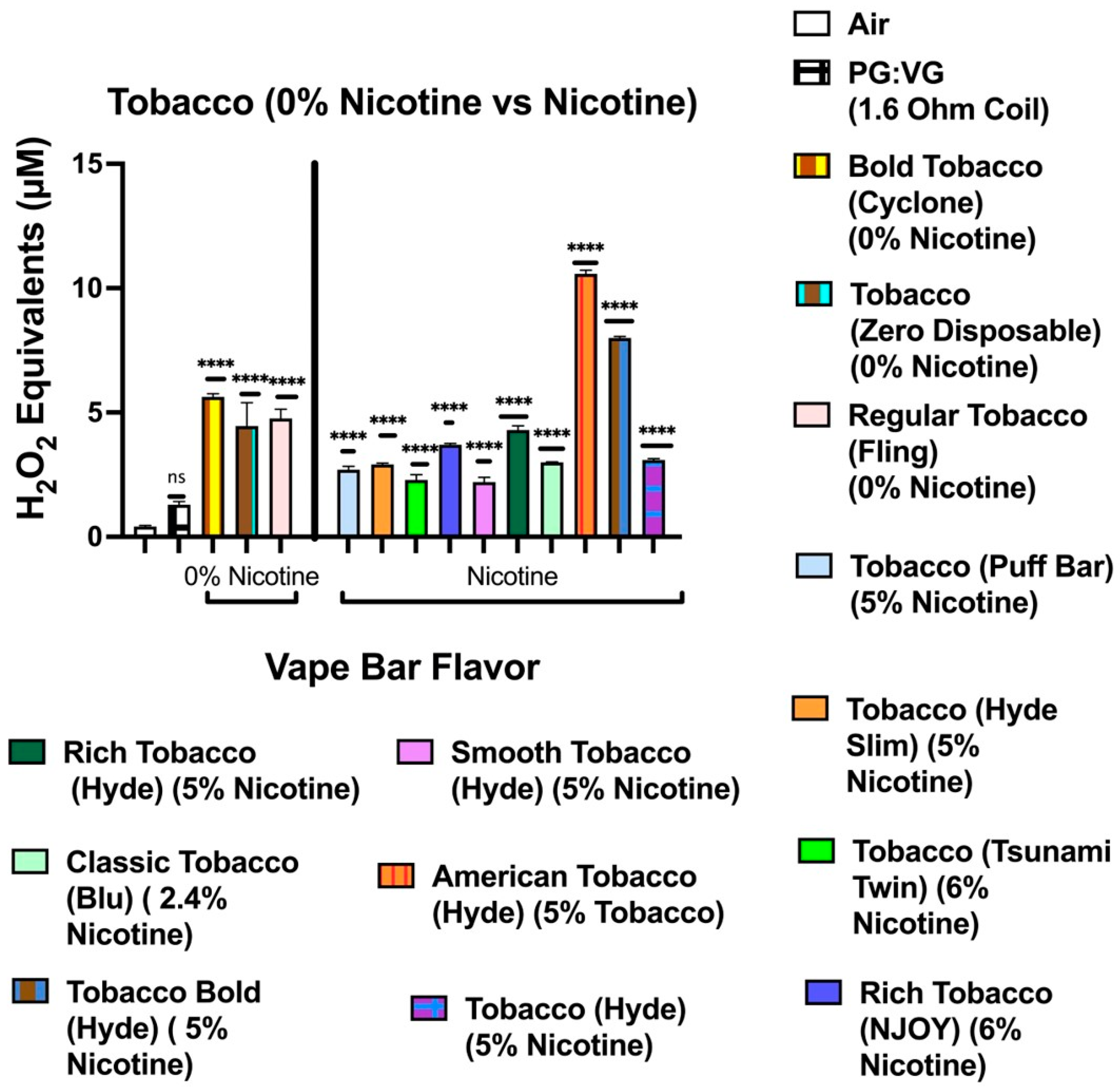
Additionally, we show that ROS content in aerosols generated in vape bars of identical flavors (Blue Razz, Peach Ice, Lychee Ice, Mango Ice, Orange Pop, Melon Ice Cream, Cool Mint, and O.M.G) varied among identically flavored bars of different nicotine content. However, only five out of eight flavors mentioned showed the corresponding nicotine-containing bar generating significantly higher ROS levels than its respective 0% nicotine-containing bar. Here, our data showed that the nicotine-containing Mango Ice and Lychee Ice bars contained significantly lower ROS levels than their corresponding 0% nicotine-containing bar. These results observed among the Mango Ice and Lychee Ice bars of differing nicotine content may have occurred because the pairwise comparisons between these identically flavored bars did not control for the vendor. In the pairwise comparisons between the 0% nicotine-containing Mango Ice bar and the 5% nicotine Mango Ice bar, each bar was made by a different manufacturer. Additionally, because each Mango Ice bar was manufactured by a different vendor, the PG:VG content within each bar may have also been different between the identically flavored bars. Previous studies have shown that the ratio of PG and VG used within an e-liquid led to significant alterations in ROS levels within generated aerosols. Similarly, when analyzing and comparing ROS content among the aerosols generated from the different Lychee Ice bars (0% nicotine and 5% nicotine-containing bars), each bar was made by a different vendor. Further, this means that among the Lychee Ice, Peach Ice, Mango Ice, and Blue Razz bars analyzed, pairwise comparisons between the 0% nicotine-containing bar and the 5% nicotine-containing bar did not control for the vendor. Similarly, this may explain the reason for a consistent relationship between increasing nicotine content and ROS generation is not seen among the Lychee Ice, Mango Ice, Peach Ice, and Blue Razz flavored bars. The ROS generated from the nicotine-containing Mango Ice and Lychee Ice bars was significantly lower than that within the aerosols generated from the corresponding 0% nicotine bar. In contrast, our data analyzing the Blue Razz and Peach Ice bars showed a direct relationship between increasing nicotine content and ROS production.
Consequently, to control for the vendor in determining how nicotine affects ROS generation, one could analyze the ROS content within aerosols generated by vape bars of the same flavor and vendor but of differing nicotine content. Correspondingly, we did this by determining the ROS concentration within aerosols generated from the Flair Plus: Cool Mint, Bolt: Orange Pop, Bolt: Melon Ice Cream, and SMOQ: O.M.G bars. Further, among three of the four vendor-specific flavors, we found that the aerosol from the respective nicotine-containing bar contained a significantly higher level of ROS than the corresponding 0% nicotine-containing bar. For instance, the 5% nicotine Flair Plus: Cool Mint bar generated an aerosol that contained a significantly higher level of ROS than that within its respective 0% nicotine bar; this suggests that nicotine contributes to this significant difference in ROS levels. Similar results were also observed when comparing the 0 and 5% nicotine-containing Orange Pop bars manufactured by Bolt and the O.M.G flavored bars (0 and 6% nicotine) manufactured by SMOQ. Further, we realized that to better elucidate the role nicotine in affecting ROS generated from flavored vape bars, more comparative acellular ROS analyses are needed between bars of the same specific flavor and vendor, but different nicotine concentrations. Further work is required to analyze more vape bars with differing nicotine concentrations made from the same vendor and of the same specific flavor.
Previous studies have shown that nicotine and the other constituents of e-liquids (flavoring agents, propylene glycol (PG), and vegetable glycerin (VG)) contribute to ROS production [
5,
18]. Similarly, Haddad et al. have shown that the ROS emission from aerosolized e-liquids was significantly affected by the PG:VG ratio of the e-liquid [
19]. Propylene Glycol (PG) and Vegetable Glycerol (VG) are humectants, substances used to maintain moisture. Furthermore, PG and VG’s ability to attract and retain moisture allows e-cigarette users to feel what is known as a “throat hit”. A “Throat hit” refer to the sensation one who uses ENDS products feels in their throat caused by nicotine inhalation. Regarding one of the specific findings from Haddad Et al., the study found that increasing the percentage of VG within the base PG:VG liquid component of an e-liquid used within a rechargeable e-cigarette significantly increased ROS flux [
19]. Similarly, another study by Bitzer et al. found increases in the PG content of an e-liquid used in rechargeable e-cigarettes led to heavy increases in free-radical production within the resulting aerosolized e-liquid [
18].
These previous studies compelled us to determine the ROS concentration with the PG:VG base solution used within the vape bars analyzed in our study. We reasoned that by semi-quantifying the ROS content within the aerosols produced from the PG:VG component of vape bars we analyzed, the role flavoring chemicals and nicotine have in contributing to ROS production during e-liquid heating and aerosolization can be further elucidated. However, out of every vape bar analyzed in our study, the only two companies which provided the PG:VG content online were Bolt and Flair Plus; for both companies, the e-liquid component of the vape-bar contained a 1:1 ratio PG:VG solution. Accordingly, we prepared a 1:1 ratio PG:VG solution to be smoked and aerosolized using the Puff Generator in tandem with the other analyzed vape bars. Additionally, when looking into other specifications of the Flair Plus and Bolt bars analyzed, we saw that each vendor used a coil of a different resistance: 1.6 and 1.8 Ohms, respectively. Accordingly, we analyzed the ROS content within a 1:1 ratio PG:VG solution aerosolized using a 1.6 Ohm coil (via Eleaf Elven cartridges) and a 1.8 Ohm coil (via OVN: W01 JUUL cartridges).
Regarding how flavoring chemicals used in vape bars contribute to ROS emissions from vape bar-generated aerosols, research delving into how the interactions between different components of e-liquids contribute to ROS generation is lacking. However, a study by Son, Yeongkwon et al. found that the flavoring chemicals within flavoring agents (those including maltol, benzyl acetate, and anethole, etc.) may undergo redox cycling with transition metal ions found with e-liquids and produce
•OH [
5]. Additionally, a previous study from our lab (Lerner et. al.) found that the oxidative nature of non-vaporized e-liquids is dependent on the flavoring additives used in an e-liquid [
10]. For example, e-liquids containing fruity or sweet flavors were stronger oxidizing agents than corresponding tobacco flavored e-liquids [
10]. Together, Lerner et al. findings and the present study suggest that flavoring chemicals themselves influence ROS production during e-liquid aerosolization. Our results comparing the ROS content within aerosols generated from different bars within each of the six major flavor categories (Tobacco, Minty Fruit, Minty/Cool (Iced), Fruity, Drinks/Beverages, and Desserts) suggest that ROS generation varies among different flavored bars. However, comparative acellular ROS analyses between a 0% nicotine-containing flavored vape bar and the PG:VG solution making up that same vape bar are needed to further investigate the role of flavoring agents in ROS production within vape bars. To further explain, a PG:VG solution heated and aerosolized using a coil of the same resistance as the flavored vape bars of interest is needed. By comparing the ROS generated between a 0% nicotine-containing flavored vape bar and an accurate PG:VG control, one can see whether the flavoring agents themselves play a role in changing the ROS levels generated upon a vape bar aerosolization.
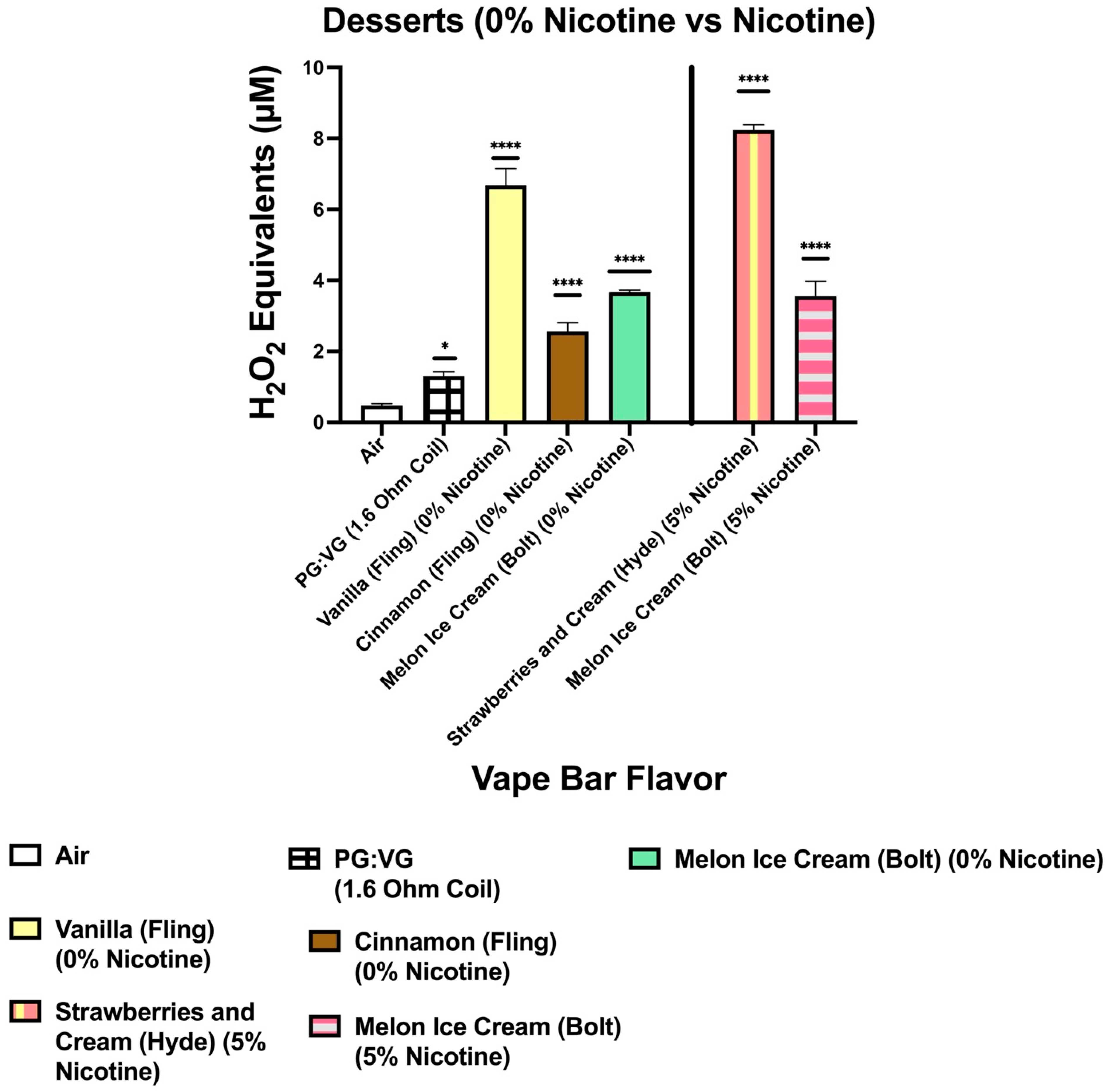
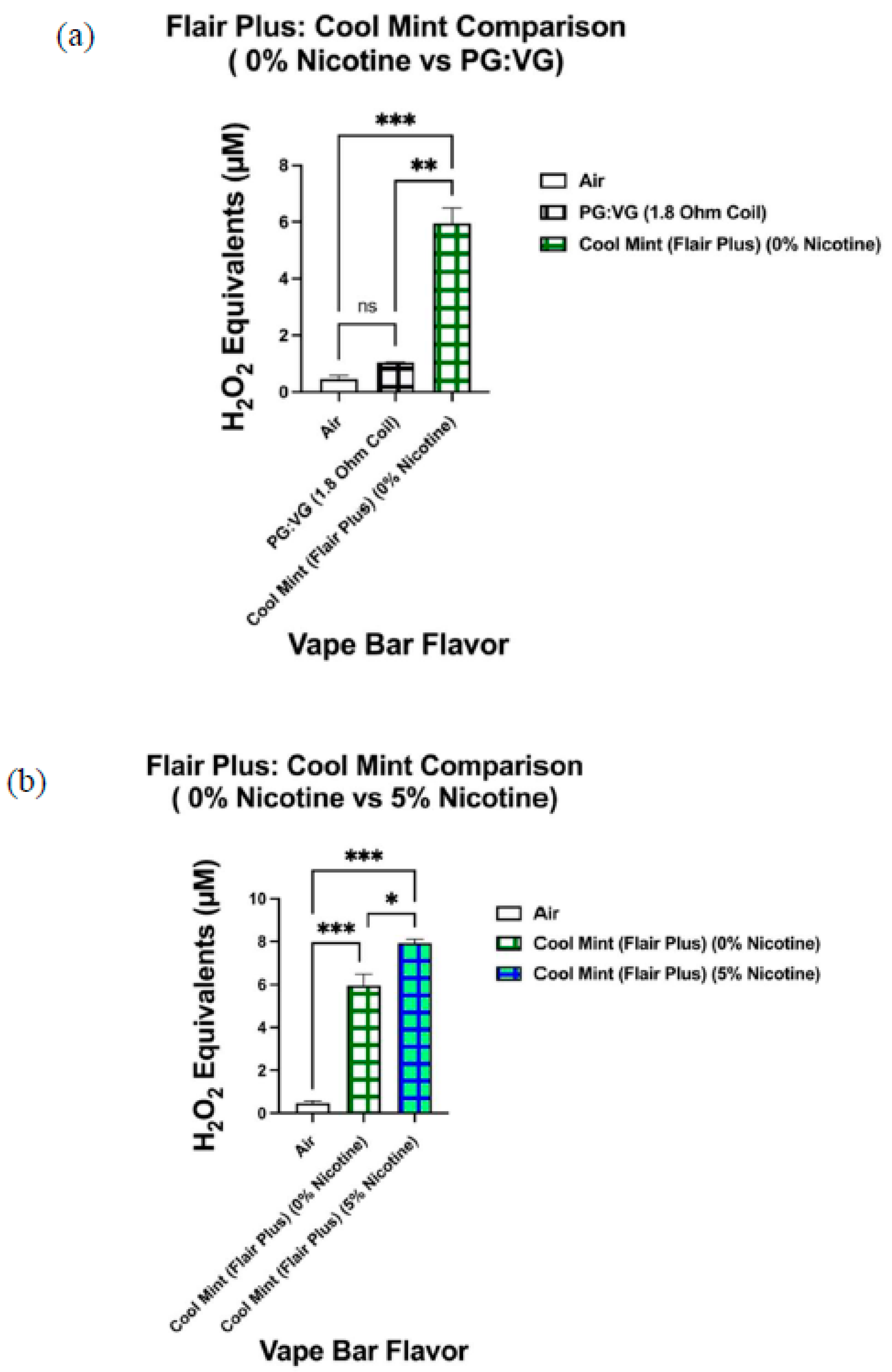
Consequently, we conducted pairwise comparisons between ROS levels produced from three 0% nicotine-containing bars and their respective PG:VG controls. These three aforementioned 0% nicotine bars were manufactured by Flair Plus and Bolt; two of which were manufactured by Bolt and one of which was manufactured by Flair Plus. Next, when comparing the ROS content within the aerosol generated from the 0% nicotine Flair Plus: Cool Mint bar with that within the aerosol produced from its respective PG:VG control, the ROS content generated from the 0% nicotine bar was significantly higher than that within aerosolized PG:VG control. Moreover, the PG:VG ratio and the coil’s resistance used in the PG:VG control were the same as that used in the 0% nicotine-containing Flair Plus: Cool Mint bar; this specific pairwise comparison minimized PG:VG content and coil resistance as potential confounding influences. Accordingly, the previously mentioned results suggest that flavoring chemicals themselves (in particular the ones used to make the Cool Mint flavor) significantly contribute to ROS generation upon e-liquid heating and subsequent aerosolization.
Similarly, pairwise comparisons between the 0% nicotine Bolt: Orange Pop bar and its respective PG:VG control and between the 0% nicotine Bolt: Melon Ice Cream bar and its PG:VG control also suggest the same conclusion we reached upon our analysis of the 0% nicotine-containing Cool Mint bar from Flair Plus and its PG:VG control. To clarify Bolt disposable bars have a PG:VG ratio of 1:1 and use a 1.6 Ohm coil to heat their e-liquid component. Consequently, the PG:VG control used in the pairwise comparisons with the aforementioned Bolt 0% nicotine bars contained a PG:VG ratio of 1:1 and was aerosolized using a 1.6 Ohm coil. Subsequently, our data showed that both 0% Bolt bars (Orange Pop and Melon Ice Cream) contain a significantly higher ROS content than their corresponding PG:VG controls. These results further suggest that flavoring agents (in this case, the ones used to make Orange Pop and Melon Ice Cream flavors) significantly contribute to ROS generation by flavored vape bars.
Regarding a limitation of our study, the only PG:VG controls we used were those with a 1:1 ratio composition of both PG and VG. These were heated using 1.6- and 1.8-Ohm coils. PG:VG controls utilizing this specific ratio of PG and VG (1:1) and which were heated using 1.6- and 1.8-Ohm coils. This was used because the PG:VG ratio and the resistance of the coils used in the Flair Plus and Bolt bars we had analyzed. Flair Plus and Bolt were the only two commercial manufacturers of the disposable vape bars used in this study that provided information on their PG: VG content, coil resistance, and that manufactured both non-nicotine-containing and nicotine-containing bars. Further, we could not find the resistance of the coils used in many of the other vape bars we analyzed, nor could we find the PG:VG ratio used within those bars. Consequently, our PG:VG controls were modeled after the specifications of the Flair Plus and Bolt bars analyzed. Additionally, in our data comparing the ROS generated from every single vape bar within each of the six major flavor categories analyzed, we only included the PG:VG control heated using a 1.6 Ohm coil. We did this because information on the resistance of the coils used in many of the other vape bars included in this study was not provided by the respective vendors of those bars. Secondly, we realized that out of all the vape bars we analyzed whose vendors provided information on their coil resistance, the highest number of bars used a 1.6 Ohm coil. Consequently, to maintain consistency among the first six graphs provided in the paper, we only included the 1.6 Ohm PG:VG control within each of those six graphs. However, acellular ROS assays and comparative analyses between different flavored vape bars in future studies should only be conducted once the resistance of coils used in the vape bars one plans to analyze is known. This is because coil resistance is a key part of the heating and aerosolization process within ENDS [
20], and possibly in vape bars. Accordingly, future related studies must include PG:VG controls that are aerosolized using coils of the same resistance of each vape bars analyzed in the respective study.
Similarly, regarding another limitation of this study, the only two commercial manufacturers of the vape bars we analyzed in our study that provided information on PG:VG ratios used in component e-liquids were Flair Plus and Bolt. Consequently, the PG:VG controls we used consisted only of a 1:1 ratio of PG:VG as these were the PG and VG ratios used in bars from Bolt and Flair Plus. The other commercial manufacturers of the vape bars we analyzed in our study did not provide information on the ratio of PG and VG contained in their vape bars. Consequently, we could not semi-quantify the ROS within aerosols produced from solutions of the same PG:VG ratio as those used in many of the vape bars we analyzed in this study. For these reasons, when producing graphs and including pairwise comparisons between the Blue Razz, Peach Ice, Mango Ice, and Lychee Ice bars of differing nicotine content, we did not include the ROS generated from our PG:VG controls. Our reasoning for this was because we did not know the ratio of the PG:VG used within the Blue Razz, Peach Ice, Mango Ice, and Lychee Ice bars; consequently, conducting pairwise comparisons between the PG:VG controls we used and each of the Blue Razz, Peach Ice, Mango Ice, and Lychee Ice bars of varying nicotine content would not have been scientifically sound. Furthermore, NMR spectroscopy using the e-liquids isolated from all the vape-bar we analyzed will determine each bar’s specific PG:VG ratio. In the future, when conducting acellular ROS analyses of flavored vape bars, we will use NMR spectroscopy to determine each bar’s PG:VG ratio to make an accurate PG:VG control for subsequent acellular ROS assays (both for bars whose manufactures provide information of PG:VG ratios and those which do not).
Moreover, assessing the ROS generation due to ‘cooling agents’ in ENDS is vital in determining the toxicity of vape bars with dual and multi flavors. Studies have found variations in the levels of synthetic cooling agents, such as WS-3 and WS-23, in cool (iced) flavors among e-cigarettes manufactured by various companies [
21,
22]. These cooling agents induce cytotoxicity in BEAS-2B lung epithelial cells, suggesting their adverse toxic effects upon inhalation [
21]. Furthermore, future studies assessing the acellular ROS generation by cooling agents should consider the confounding factors, such as flavor category and nicotine concentration, as these constituents form secondary reactive species upon heating. Further, these future acellular ROS analyses must include a fruity-flavored vape bar (e.g., apple), its respective cool (iced) flavor (e.g., apple ice), an appropriate PG:VG control, and an appropriate salt nicotine control (using a PG:VG solvent) [
23]. Additionally, acellular ROS assays conducted to investigate the effects cooling agents have in ROS generation from vape bars must include fruity flavored and respective cool (iced) flavored vape bars manufactured by various vendors. This may include flavored bars with or without nicotine (tobacco and mint/menthol flavors) [
24].
Overall, our results suggest that different flavoring chemicals used in vape bars contribute to variations in the breakdown of the chemical bonds holding together the components of the e-liquid within a vape bar during thermal degradation, leading to differential ROS levels in generated aerosols. Additionally, our pairwise comparisons made between vape bars with different nicotine concentrations but the same specific flavor and vendor suggest nicotine itself has a role in influencing ROS generation within aerosolizing vape bars. In general, cool (iced) flavors generated differential ROS than their counterpart non-cool (iced) flavors. However, further assays are needed to elucidate how both the flavor of a vape bar and its corresponding nicotine concentration affect ROS generation within vape bars, and immune-inflammatory responses in mouse model as seen previously [
25]. Future studies can use Gas Chromatography–Mass Spectrometry (GC–MS) to analyze the compounds within flavoring agents within flavored vape bars. For example, using GC-MS to analyze the e-liquids extracted from minty and cool (iced) vape bars can provide more insight on the cooling agents used within these specific flavored vape bars. In addition, Electron Paramagnetic Resonance (EPR) Spectroscopy can analyze the relative proportions of specific ROS (H
2O
2, O
2•−, and
•OH) and free radicals within the aerosol generated from vape bars.
Future studies involving acellular ROS analyses using different flavored vape bars should also include a PG:VG control which includes nicotine (either free-base or nicotine benzoate). Further, when analyzing the ROS generated from vape bars of the same flavor and vendor but different nicotine concentrations, in addition to making a PG:VG control made up of the same ratio of PG and VG and heated using a coil of the same resistance as that used in the vape bars of interest, one can also make another control consisting of PG:VG and nicotine. Further, one can make a PG:VG control which includes the same percentage of nicotine salt used in the e-liquid component of their bars of interest. Subsequently, acellular ROS analyses among bars of the same flavor and vendor but different nicotine concentrations, a PG:VG control and PG:VG control with nicotine may show whether or not ROS generated from vape bars varies as a function of nicotine content. However, due limitations in our inventory, we could not produce a PG:VG w/nicotine control and aerosolize it to semi-quantify its ROS content.