Mercury Chloride Impacts on the Development of Erythrocytes and Megakaryocytes in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Hg Treatment
2.2. Routine Blood Test (RBT)
2.3. Hg Measurement
2.4. Antibodies (Ab) and Flow Cytometry
2.5. RBC Clearance Assay
2.6. CFU Assay
2.7. Hematoxylin-Eosin (H&E) Staining
2.8. EMPs Intervention and Differentiation Assay In Vitro
2.9. Real-Time Quantitative Polymerase Chain Reaction (q-PCR)
2.10. Confocol Imaging
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Statistics
3. Results
3.1. Hg Concentration in the Blood and BM
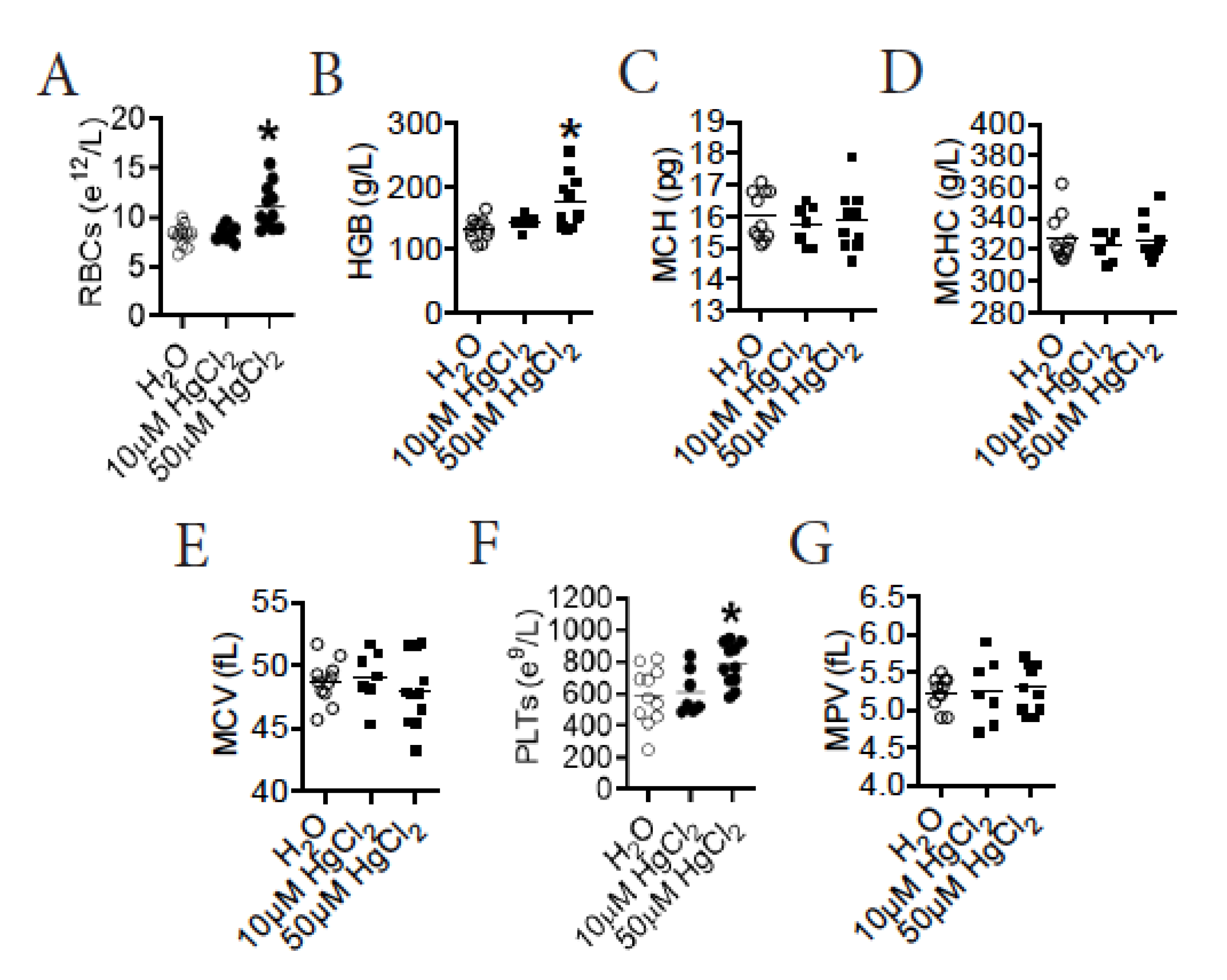
3.2. HgCl2 Increases the Number of Mature RBCs and Platelets in the Blood of B10.S Mice
3.3. HgCl2 Does Not Impact the Clearance of Mature RBCs in B10.S Mice
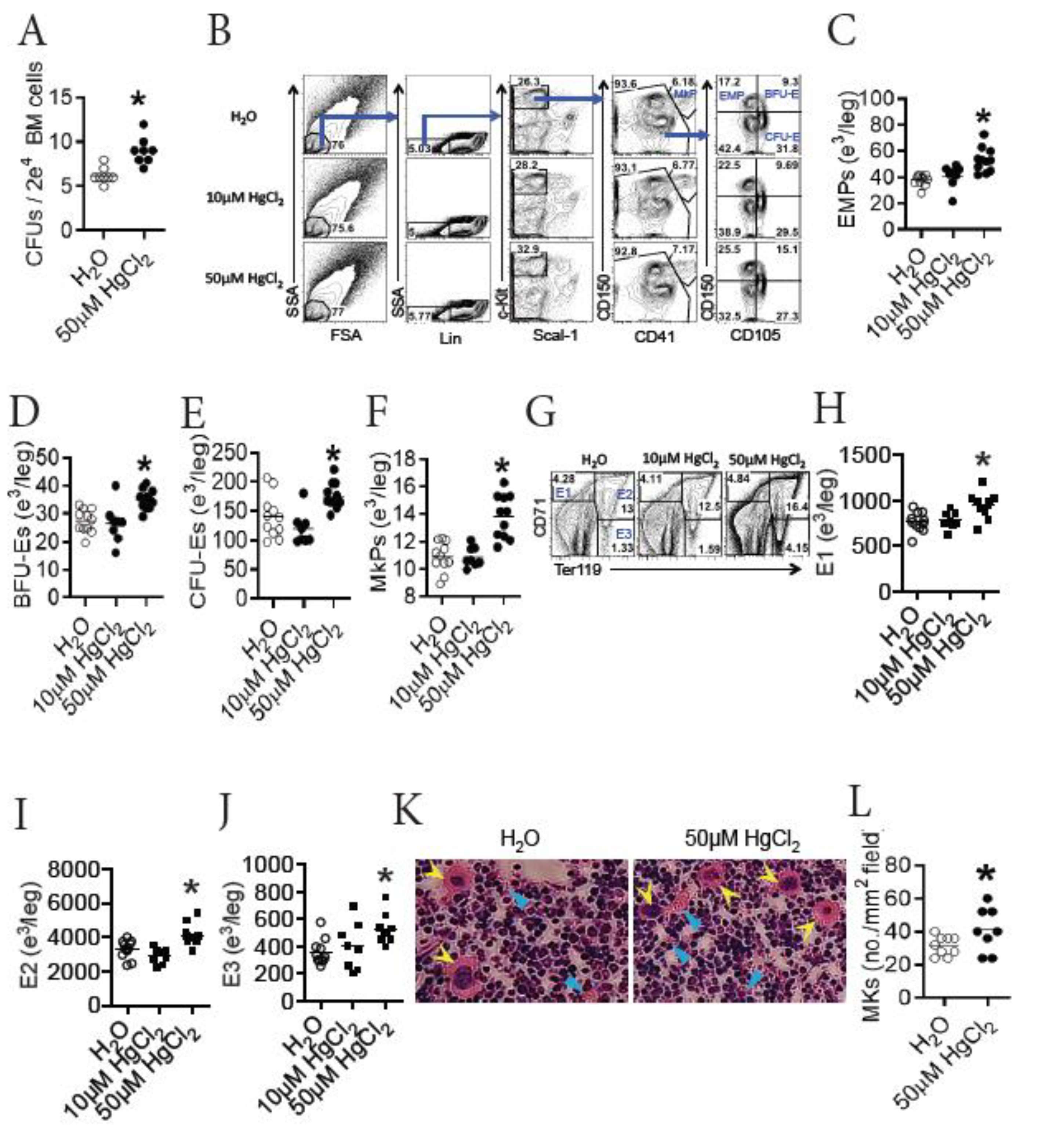
3.4. HgCl2 Increases the Development of Erythrocytes and Megakaryocytes/Platelets in the BM of B10.S Mice
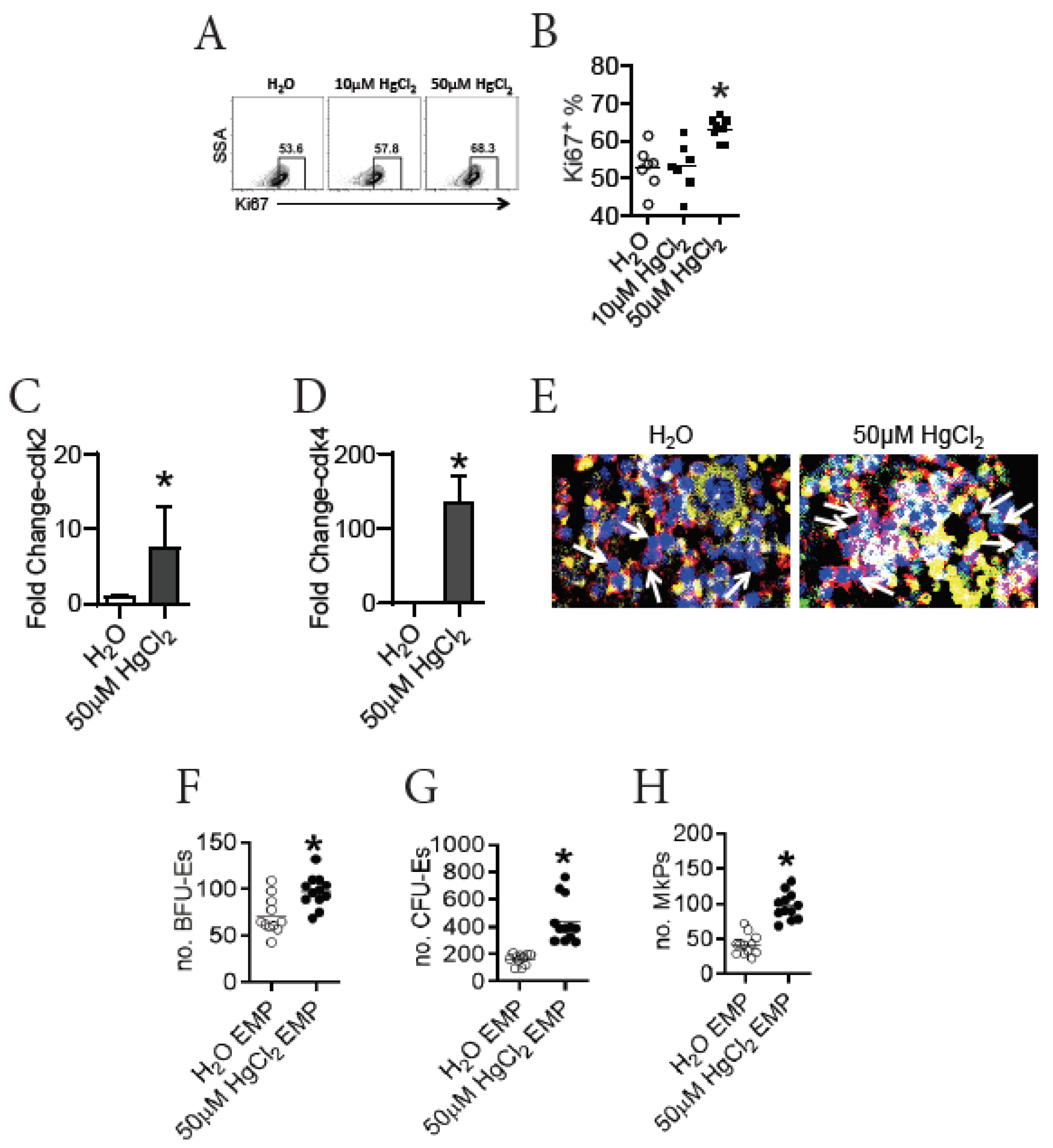
3.5. HgCl2 Drives the Proliferation and Differentiation of EMPs in the BM of B10.S Mice
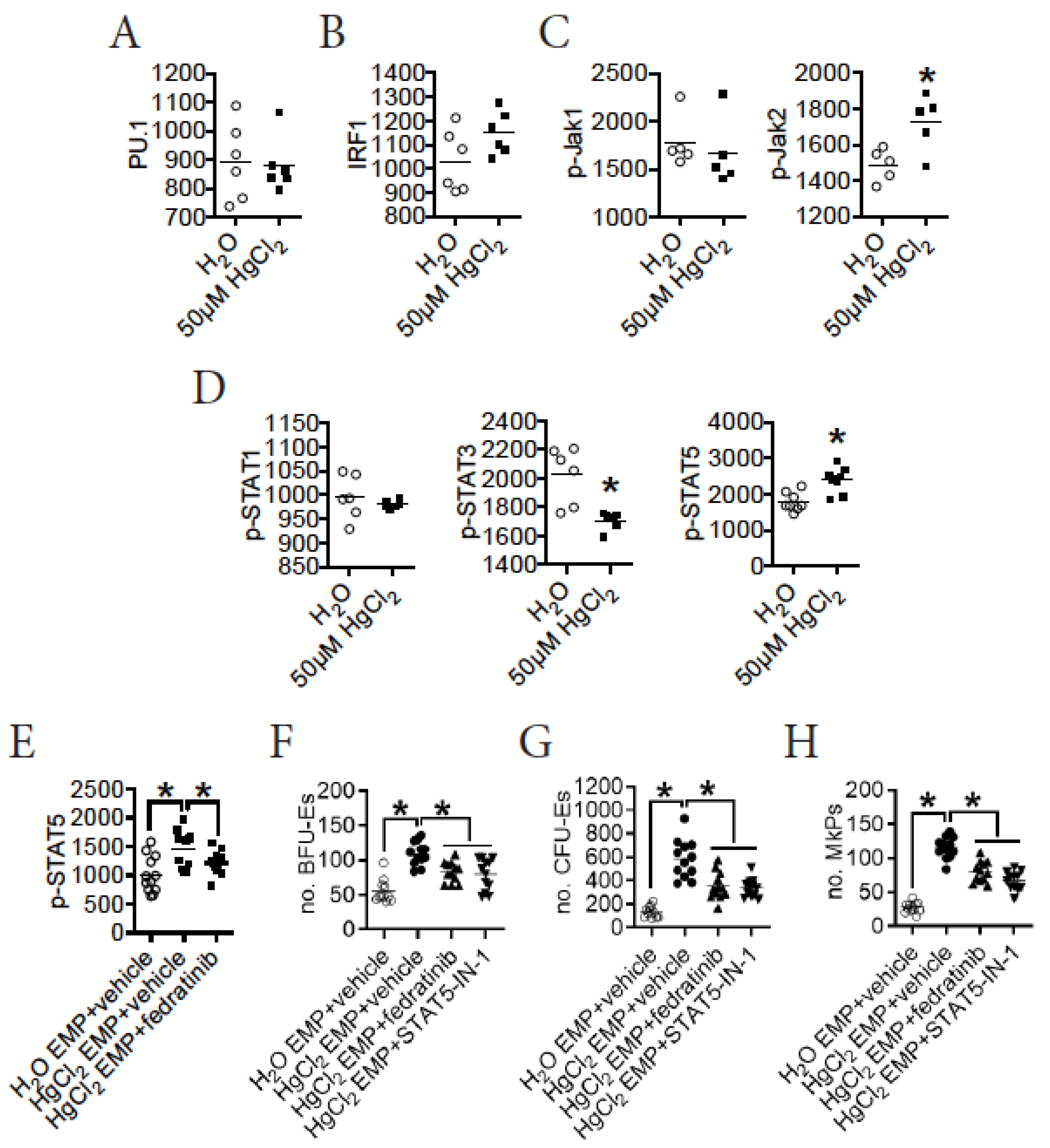
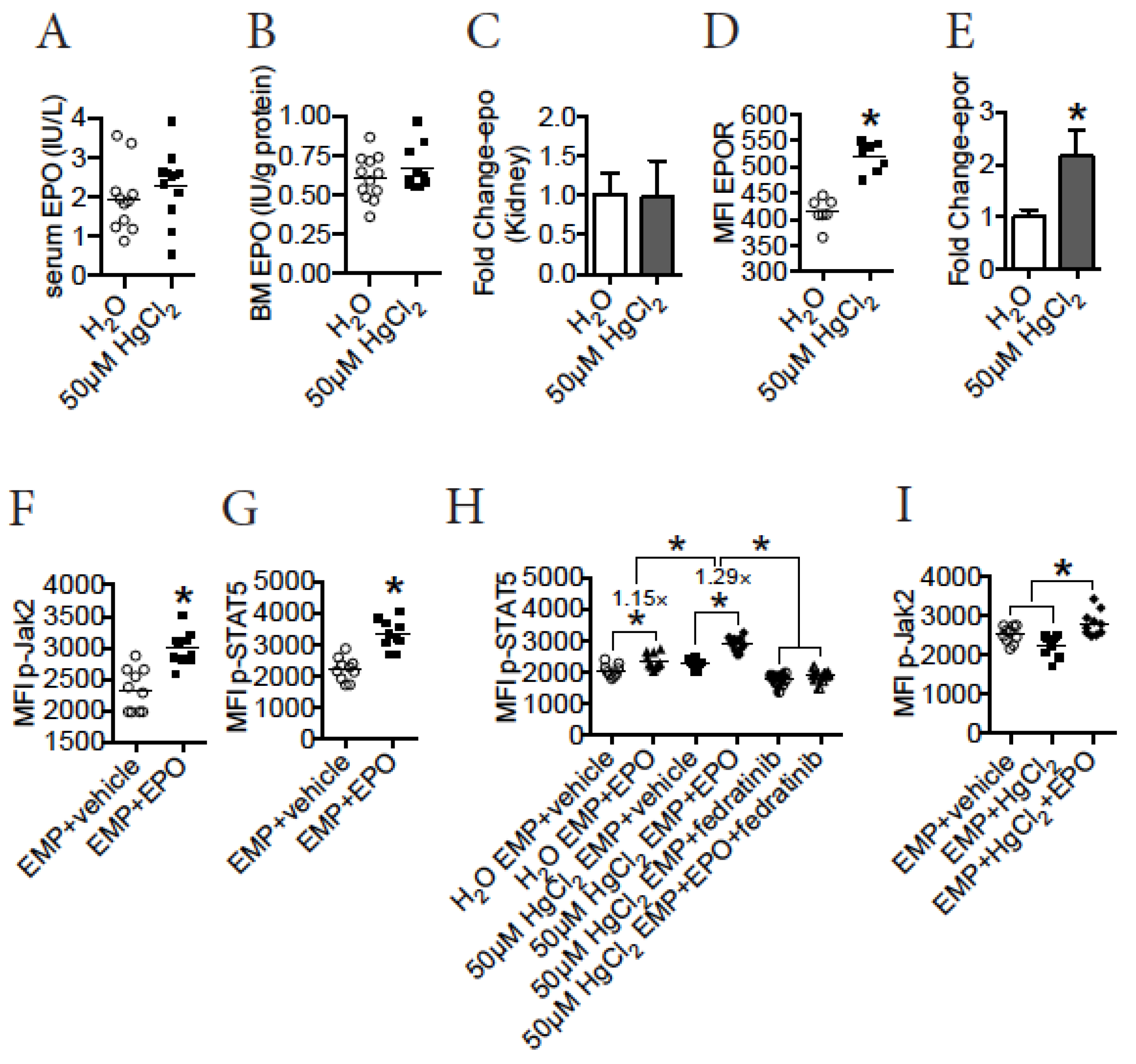
3.6. HgCl2 Activates the Jak2/STAT5 Signaling Pathway to Promote EMP Differentiation in the BM of B10.S Mice
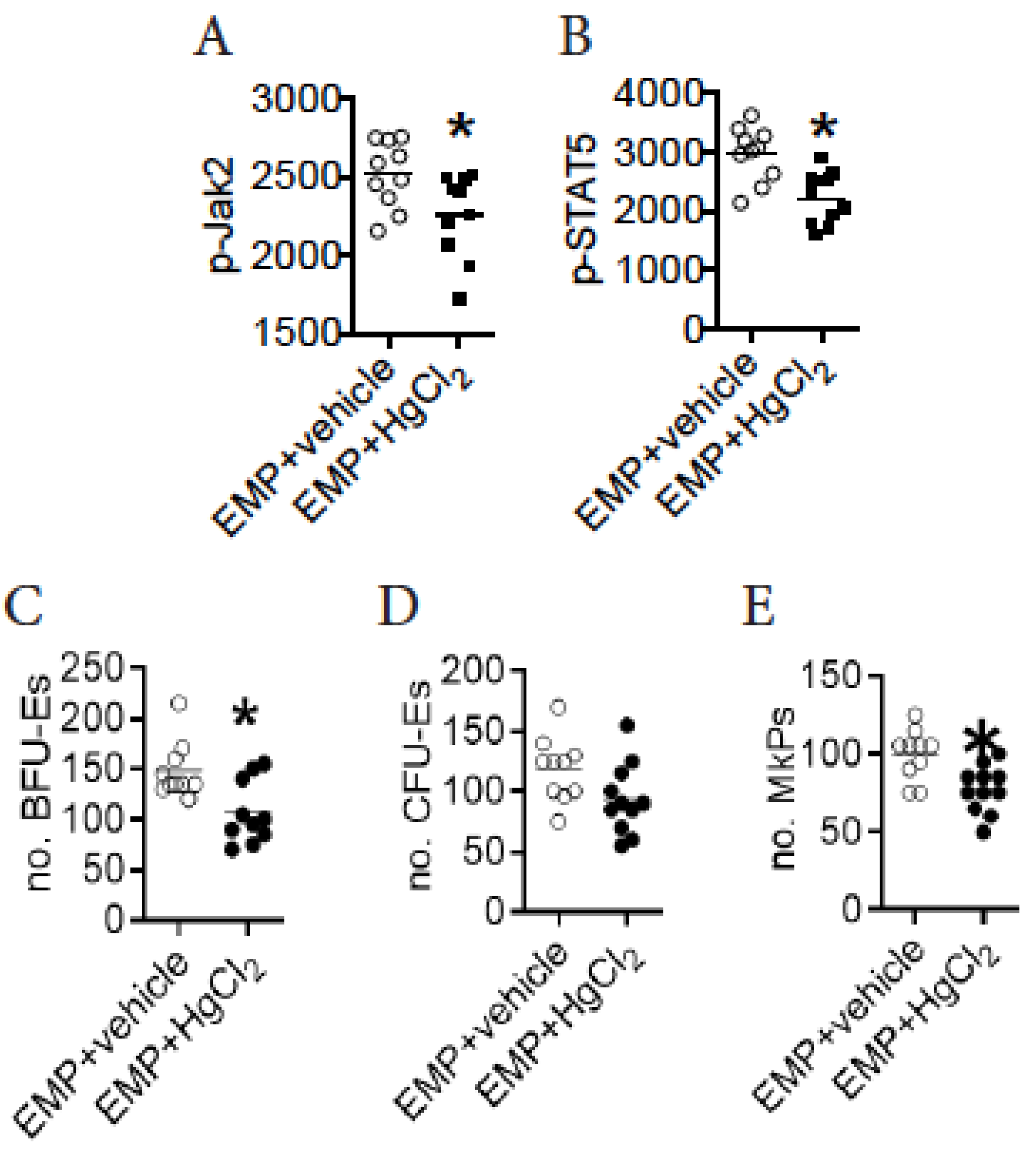
3.7. A Direct Action of HgCl2 on EMPs Suppresses Their Differentiation in the BM of B10.S Mice
3.8. HgCl2 Does Not Impact EPO Production, but Increases the Expression of EPOR to Enhance the Jak2/STAT5 Signaling Pathway in EMPs in the BM of B10.S Mice
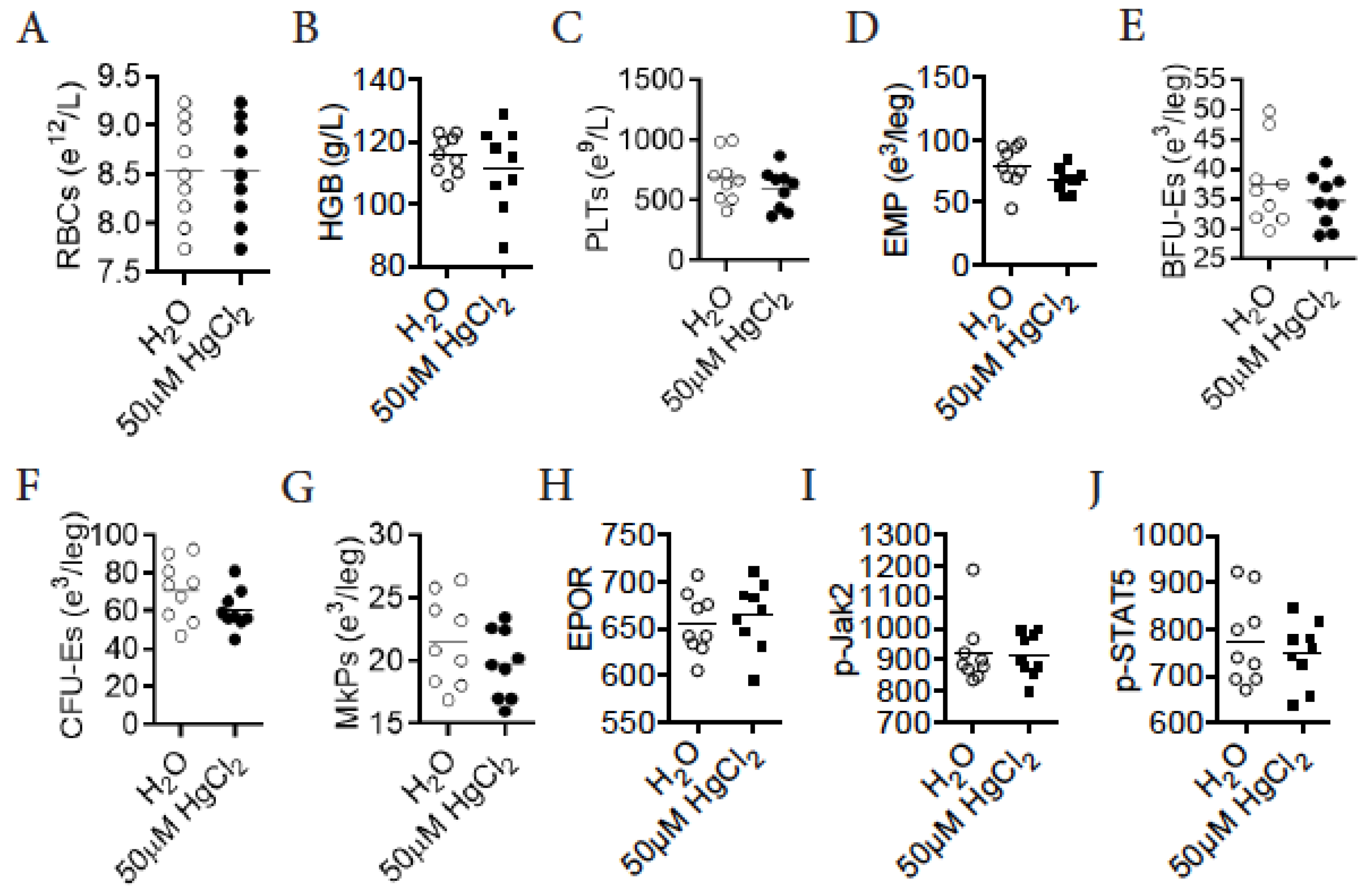
3.9. HgCl2 Does Not Impact Erythro-Megakaryopoiesis in DBA/2 Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef]
- Park, J.D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Dadar, M.; Mutter, J.; Aaseth, J. The toxicology of mercury: Current research and emerging trends. Environ. Res. 2017, 159, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Mahmood, R. Mercury chloride toxicity in human erythrocytes: Enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environ. Sci. Pollut. Res. Int. 2019, 26, 5645–5657. [Google Scholar] [CrossRef]
- Temel, Y.; Taysi, M.S. The Effect of Mercury Chloride and Boric Acid on Rat Erythrocyte Enzymes. Biol. Trace Elem. Res. 2019, 191, 177–182. [Google Scholar] [CrossRef]
- Boujbiha, M.A.; Ben Salah, G.; Ben Feleh, A.; Saoudi, M.; Kamoun, H.; Bousslema, A.; Ommezzine, A.; Said, K.; Fakhfakh, F.; El Feki, A. Hematotoxicity and genotoxicity of mercuric chloride following subchronic exposure through drinking water in male rats. Biol. Trace Elem. Res. 2012, 148, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Purton, L.E.; Scadden, D.T. The Hematopoietic Stem Cell Niche; StemBook: Cambridge, MA, USA, 2008. [Google Scholar]
- Wang, L.D.; Wagers, A.J. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.M.; Ravid, K. Matrix Mechanosensation in the Erythroid and Megakaryocytic Lineages. Cells 2020, 9, 894. [Google Scholar] [CrossRef]
- Deleschaux, C.; Moras, M.; Lefevre, S.D.; Ostuni, M.A. An Overview of Different Strategies to Recreate the Physiological Environment in Experimental Erythropoiesis. Int. J. Mol. Sci. 2020, 21, 5263. [Google Scholar] [CrossRef]
- Raghuwanshi, S.; Dahariya, S.; Musvi, S.S.; Gutti, U.; Kandi, R.; Undi, R.B.; Sahu, I.; Gautam, D.; Paddibhatla, I.; Gutti, R.K. MicroRNA function in megakaryocytes. Platelets 2019, 30, 809–816. [Google Scholar] [CrossRef]
- Kuhrt, D.; Wojchowski, D.M. Emerging EPO and EPO receptor regulators and signal transducers. Blood 2015, 125, 3536–3541. [Google Scholar] [CrossRef]
- Lai, X.; Nikolov, S.; Wolkenhauer, O.; Vera, J. A multi-level model accounting for the effects of JAK2-STAT5 signal modulation in erythropoiesis. Comput. Biol. Chem. 2009, 33, 312–324. [Google Scholar] [CrossRef]
- Chang, H.C.; Huang, D.Y.; Wu, M.S.; Chu, C.L.; Tzeng, S.J.; Lin, W.W. Spleen tyrosine kinase mediates the actions of EPO and GM-CSF and coordinates with TGF-beta in erythropoiesis. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 687–696. [Google Scholar] [CrossRef]
- Lappin, T.R.; Lee, F.S. Update on mutations in the HIF: EPO pathway and their role in erythrocytosis. Blood Rev. 2019, 37, 100590. [Google Scholar] [CrossRef]
- Libregts, S.F.; Gutiérrez, L.; de Bruin, A.M.; Wensveen, F.M.; Papadopoulos, P.; van Ijcken, W.; Özgür, Z.; Philipsen, S.; Nolte, M.A. Chronic IFN-gamma production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood 2011, 118, 2578–2588. [Google Scholar] [CrossRef]
- Worley, J.R.; Parker, G.C. Effects of environmental stressors on stem cells. World J. Stem Cells 2019, 11, 565–577. [Google Scholar] [CrossRef]
- Scharf, P.; Broering, M.F.; Oliveira da Rocha, G.H.; Farsky, S.H.P. Cellular and Molecular Mechanisms of Environmental Pollutants on Hematopoiesis. Int. J. Mol. Sci. 2020, 21, 6996. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, Z.; Zhang, P.; Zhao, Y.; Yu, X.; Xue, P.; Shao, Y.; Li, Q.; Jia, X.; Zhang, Q.; et al. Mercury impact on hematopoietic stem cells is regulated by IFNgamma-dependent bone marrow-resident macrophages in mice. Toxicol. Lett. 2018, 295, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, P.; Yu, X.; Zhao, Y.; Li, Q.; Zhang, Y.; Yang, Z.; Xie, Y.; Xue, P.; Sun, S.; et al. Lead Transiently Promotes Granulocyte-Macrophage Progenitor Differentiation and Subsequently Suppresses Common Myeloid Progenitor Differentiation. Toxicol. Sci. 2017, 160, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, X.; Sun, S.; Li, Q.; Xie, Y.; Li, Q.; Zhao, Y.; Pei, J.; Zhang, W.; Xue, P.; et al. Cadmium modulates hematopoietic stem and progenitor cells and skews toward myelopoiesis in mice. Toxicol. Appl. Pharmacol. 2016, 313, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Q.; Yang, Z.; Shao, Y.; Xue, P.; Qu, W.; Jia, X.; Cheng, L.; He, M.; He, R.; et al. Cadmium Activates Noncanonical Wnt Signaling to Impair Hematopoietic Stem Cell Function in Mice. Toxicol. Sci. 2018, 165, 254–266. [Google Scholar] [CrossRef]
- Zhu, T.; Zhao, Y.; Zhang, P.; Shao, Y.; He, J.; Xue, P.; Zheng, W.; Qu, W.; Jia, X.; Zhou, Z.; et al. Lead Impairs the Development of Innate Lymphoid Cells by Impeding the Differentiation of Their Progenitors. Toxicol. Sci. 2020, 176, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, Z.; Zhao, Y.; Jia, X.; Zhou, Z.; Zhang, Y. Phenotypic and Functional Evaluation of Hematopoietic Stem and Progenitor Cells in Toxicology of Heavy Metals. Curr. Protoc. Toxicol. 2018, 75, 22.27.21–27.27.14. [Google Scholar] [CrossRef] [PubMed]
- Vas, J.; Monestier, M. Immunology of mercury. Ann. N. Y. Acad. Sci. 2008, 1143, 240–267. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.M.; Lee, D.K.; Casiano, C.A.; Bluthner, M.; Johnston, M.M.; Tan, E.M. The autoimmunity-inducing xenobiotic mercury interacts with the autoantigen fibrillarin and modifies its molecular and antigenic properties. J. Immunol. 1997, 158, 3521–3528. [Google Scholar]
- Pollard, K.M.; Pearson, D.L.; Bluthner, M.; Tan, E.M. Proteolytic cleavage of a self-antigen following xenobiotic-induced cell death produces a fragment with novel immunogenic properties. J. Immunol. 2000, 165, 2263–2270. [Google Scholar] [CrossRef]
- Kono, D.H.; Balomenos, D.; Pearson, D.L.; Park, M.S.; Hildebrandt, B.; Hultman, P.; Pollard, K.M. The prototypic Th2 autoimmunity induced by mercury is dependent on IFN-gamma and not Th1/Th2 imbalance. J. Immunol. 1998, 161, 234–240. [Google Scholar]
- Yang, Z.; Zhao, Y.; Li, Q.; Shao, Y.; Yu, E.; Cong, W.; Jia, X.; Qu, W.; Cheng, L.; Xue, P.; et al. Developmental exposure to mercury chloride impairs social behavior in male offspring dependent on genetic background and maternal autoimmune environment. Toxicol. Appl. Pharmacol. 2019, 370, 1–13. [Google Scholar] [CrossRef]
- Hu, H.; Moller, G.; Abedi-Valugerdi, M. Non-responsiveness to mercury-induced autoimmunity in resistant DBA/2 mice is not due to immunosuppression or biased Th1-type response. Scand. J. Immunol. 1998, 48, 515–521. [Google Scholar] [CrossRef]
- Hultman, P.; Bell, L.J.; Enestrom, S.; Pollard, K.M. Murine susceptibility to mercury. II. autoantibody profiles and renal immune deposits in hybrid, backcross, and H-2d congenic mice. Clin. Immunol. Immunopathol. 1993, 68, 9–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Bolivar, V.J.; Lawrence, D.A. Developmental exposure to mercury chloride does not impair social behavior of C57BL/6 x BTBR F(1) mice. J. Immunotoxicol. 2012, 9, 401–410. [Google Scholar] [CrossRef]
- Zhang, Y.; Bolivar, V.J.; Lawrence, D.A. Maternal exposure to mercury chloride during pregnancy and lactation affects the immunity and social behavior of offspring. Toxicol. Sci. 2013, 133, 101–111. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Gao, D.; Bolivar, V.J.; Lawrence, D.A. Induction of autoimmunity to brain antigens by developmental mercury exposure. Toxicol. Sci. 2011, 119, 270–280. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; Bernaudat, L.; Siebert, U.; Roider, G.; Nowak, D.; Drasch, G. Signs and symptoms of mercury-exposed gold miners. Int. J. Occup. Med. Environ. Health 2017, 30, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-Y.; Li, M.-M.; Wang, J.; Yan, J.; Zhou, C.-C.; Yan, C.-H. Blood mercury concentration, fish consumption and anthropometry in Chinese children: A national study. Environ. Int. 2018, 110, 14–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Jones, M.; McCabe, A.; Winslow, G.M.; Avram, D.; MacNamara, K.C. MyD88 signaling in CD4 T cells promotes IFN-gamma production and hematopoietic progenitor cell expansion in response to intracellular bacterial infection. J. Immunol. 2013, 190, 4725–4735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Thai, V.; McCabe, A.; Jones, M.; MacNamara, K. Type I interferons promote severe disease in a mouse model of lethal ehrlichiosis. Infect. Immun. 2014, 82, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Gyllenhammar, I.; Glynn, A.; Levi, M.; Lignell, S.; Berglund, M. Total mercury in hair as biomarker for methylmercury exposure among women in central Sweden- a 23 year long temporal trend study. Environ. Pollut. 2020, 268, 115712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, W.; Zhao, J.; Chen, Q.; Wang, W.; Li, B.; Li, Y.F. Selenium decreases methylmercury and increases nutritional elements in rice growing in mercury-contaminated farmland. Ecotoxicol. Environ. Saf. 2019, 182, 109447. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Fang, J.; Sathyanarayana, P.; Pradeep, A.; Emerson, C.; Wojchowski, D.M. During EPO or anemia challenge, erythroid progenitor cells transit through a selectively expandable proerythroblast pool. Blood 2010, 116, 5334–5346. [Google Scholar] [CrossRef][Green Version]
- Yamanegi, K.; Yamada, N.; Nakasho, K.; Nishiura, H. Erythroblast differentiation at spleen in Q137E mutant ribosomal protein S19 gene knock-in C57BL/6J mice. Immunobiology 2018, 223, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Di Giandomenico, S.; Kermani, P.; Mollé, N.; Yabut, M.M.; Abu-Zeinah, G.; Stephens, T.; Messali, N.; Schreiner, R.; Brenet, F.; Rafii, S.; et al. Megakaryocyte TGFbeta1 partitions erythropoiesis into immature progenitor/stem cells and maturing precursors. Blood 2020, 136, 1044–1054. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, X.; Zhang, Y.; Cai, Y.; Chen, J.; Sivaprakasam, S.; Gurav, A.; Pi, W.; Makala, L.; Wu, J.; et al. RCAD/Ufl1, a Ufm1 E3 ligase, is essential for hematopoietic stem cell function and murine hematopoiesis. Cell Death Differ. 2015, 22, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Böiers, C.; Buza-Vidas, N.; Jensen, C.T.; Pronk, C.J.; Kharazi, S.; Wittmann, L.; Sitnicka, E.; Hultquist, A.; Jacobsen, S.E. Expression and role of FLT3 in regulation of the earliest stage of normal granulocyte-monocyte progenitor development. Blood 2010, 115, 5061–5068. [Google Scholar] [CrossRef]
- Chang, C.-F.; Massey, J.; Osherov, A.; da Costa, L.H.A.; Sansing, L. Bexarotene Enhances Macrophage Erythrophagocytosis and Hematoma Clearance in Experimental Intracerebral Hemorrhage. Stroke 2020, 51, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Chohan, T.A.; Qian, H.; Pan, Y.; Chen, J.Z. Cyclin-dependent kinase-2 as a target for cancer therapy: Progress in the development of CDK2 inhibitors as anti-cancer agents. Curr. Med. Chem. 2015, 22, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Fernandez, M.; Malumbres, M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell 2020, 37, 514–529. [Google Scholar] [CrossRef]
- Suresh, S.; Rajvanshi, P.K.; Noguchi, C.T. The Many Facets of Erythropoietin Physiologic and Metabolic Response. Front. Physiol. 2019, 10, 1534. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Estienne, M.; Bessoles, S.; Echchakir, H.; Pederzoli-Ribeil, M.; Chiron, A.; Aldaz-Carroll, L.; Leducq, V.; Zhang, Y.; Souyri, M.; et al. Erythropoietin is a major regulator of thrombopoiesis in thrombopoietin-dependent and -independent contexts. Exp. Hematol. 2020, 88, 15–27. [Google Scholar] [CrossRef]
- Blake, T.J.; Jenkins, B.J.; D’Andrea, R.J.; Gonda, T.J. Functional cross-talk between cytokine receptors revealed by activating mutations in the extracellular domain of the beta-subunit of the GM-CSF receptor. J. Leukoc. Biol. 2002, 72, 1246–1255. [Google Scholar]
- Fisher, J.W. Erythropoietin: Physiology and pharmacology update. Exp. Biol. Med. (Maywood) 2003, 228, 1–14. [Google Scholar] [CrossRef]
- Hultman, P.; Bell, L.J.; Enestrom, S.; Pollard, K.M. Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic, and intra-H-2 recombinant strains. Clin. Immunol. Immunopathol. 1992, 65, 98–109. [Google Scholar] [CrossRef]
- McMullin, M.F. Investigation and Management of Erythrocytosis. Curr. Hematol. Malig. Rep. 2016, 11, 342–347. [Google Scholar] [CrossRef]
- Spivak, J.L. Polycythemia vera: Myths, mechanisms, and management. Blood 2002, 100, 4272–4290. [Google Scholar] [CrossRef]
- Davila-Gonzalez, D.; Barrios-Ruiz, A.; Fountain, E.; Cheng, L.; Masarova, L.; Verstovsek, S.; Rojas-Hernandez, C.M. Diagnostic Performance of Erythropoietin Levels in Polycythemia Vera: Experience at a Comprehensive Cancer Center. Clin. Lymphoma Myeloma Leuk. 2020. [Google Scholar] [CrossRef]
- Hassanin, M.; Kerek, E.; Chiu, M.; Anikovskiy, M.; Prenner, E.J. Binding Affinity of Inorganic Mercury and Cadmium to Biomimetic Erythrocyte Membranes. J. Phys. Chem. B 2016, 120, 12872–12882. [Google Scholar] [CrossRef]

| Cell Type | Abbreviation | Gating Strategy |
|---|---|---|
| Erythrocyte-megakaryocyte progenitors | EMPs | Lin−c-KithiScal-1−CD41−CD150+CD105− |
| Burst-forming unit-erythroid progenitors | BFU-Es | Lin−c-KithiScal-1−CD41−CD150+CD105+ |
| Colony-forming unit-erythroid progenitors | CFU-Es | Lin−c-KithiScal-1−CD41−CD150−CD105+ |
| Megakaryocyte progenitors | MkPs | Lin−c-KithiScal-1−CD41+ |
| Erythroblast 1 | E1 | CD71+Ter119− |
| Erythroblast 2 | E2 | CD71+Ter119+ |
| Erythroblast 3 | E3 | CD71−Ter119+ |
| Genes | Primers | |
|---|---|---|
| Forward | Reverse | |
| cdk2 | 5′-CTCTCACGGGCATTCCTCTTC-3′ | 5′-CCCTCTGCATTGATAAGCAGG-3′ |
| cdk4 | 5′-AAGGTCACCCTAGTGTTTGAGC-3′ | 5′-CCGCTTAGAAACTGACGCATTAG-3′ |
| epo | 5′-CATCTGCGACAGTCGAGTTCTG-3′ | 5′-CACAACCCATCGTGACATTTTC-3′ |
| epor | 5′- GGACCCTCTCATCTTGACGC-3′ | 5′- CTTGGGATGCCAGGCCAGAT-3′ |
| β-actin | 5′-GGACTTCGAGCAAGAGATGG-3′ | 5′-AGCACTGTGTTGGCGTACAG-3′ |
| Treatment | Dose | B10.S | DBA/2 | |||
|---|---|---|---|---|---|---|
| Blood (μg/L, ppb) | BM (μg/kg protein, ppb) | Blood (μg/L, ppb) | BM (μg/kg protein, ppb) | |||
| H2O | ND | ND | ND | ND | ||
| HgCl2 (μM) | 10 | 26.5 ± 4.3 | ND | – | – | |
| 50 | 242.5 ± 34.2 | 2500.0 ± 800.0 | 487.2 ± 65.2 | 2200.0 ± 600.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Zhao, Y.; Zhu, T.; Xue, P.; Zheng, W.; Yao, Y.; Qu, W.; Jia, X.; Lu, R.; He, M.; et al. Mercury Chloride Impacts on the Development of Erythrocytes and Megakaryocytes in Mice. Toxics 2021, 9, 252. https://doi.org/10.3390/toxics9100252
He J, Zhao Y, Zhu T, Xue P, Zheng W, Yao Y, Qu W, Jia X, Lu R, He M, et al. Mercury Chloride Impacts on the Development of Erythrocytes and Megakaryocytes in Mice. Toxics. 2021; 9(10):252. https://doi.org/10.3390/toxics9100252
Chicago/Turabian StyleHe, Jinyi, Yifan Zhao, Tingting Zhu, Peng Xue, Weiwei Zheng, Ye Yao, Weidong Qu, Xiaodong Jia, Rongzhu Lu, Miao He, and et al. 2021. "Mercury Chloride Impacts on the Development of Erythrocytes and Megakaryocytes in Mice" Toxics 9, no. 10: 252. https://doi.org/10.3390/toxics9100252
APA StyleHe, J., Zhao, Y., Zhu, T., Xue, P., Zheng, W., Yao, Y., Qu, W., Jia, X., Lu, R., He, M., & Zhang, Y. (2021). Mercury Chloride Impacts on the Development of Erythrocytes and Megakaryocytes in Mice. Toxics, 9(10), 252. https://doi.org/10.3390/toxics9100252








