Application of Modified Spent Mushroom Compost Biochar (SMCB/Fe) for Nitrate Removal from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
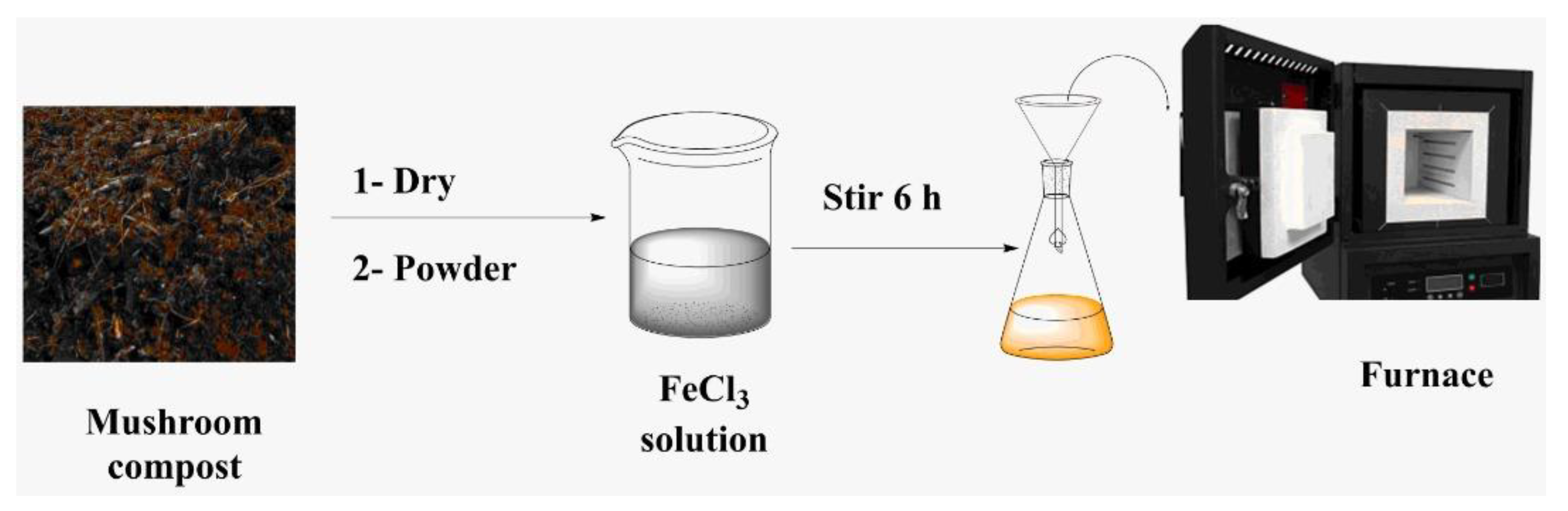
2.1. Preparation of Biochar
2.2. Point of Zero Charge
2.3. Nitrate Desorption Study (Leaching Test)
2.4. Impact of Co-Adsorption (Competing) Anion
2.5. Characterization of Adsorbent
2.6. Adsorption Kinetic
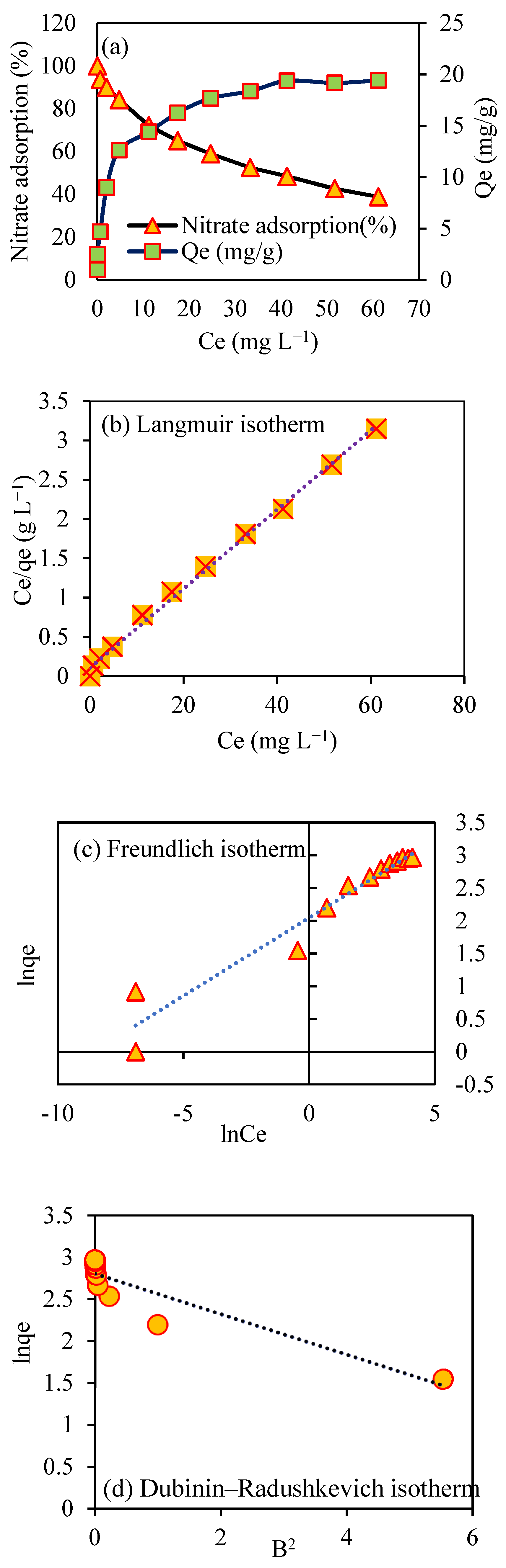
2.7. Sorption Isotherm Models
3. Results
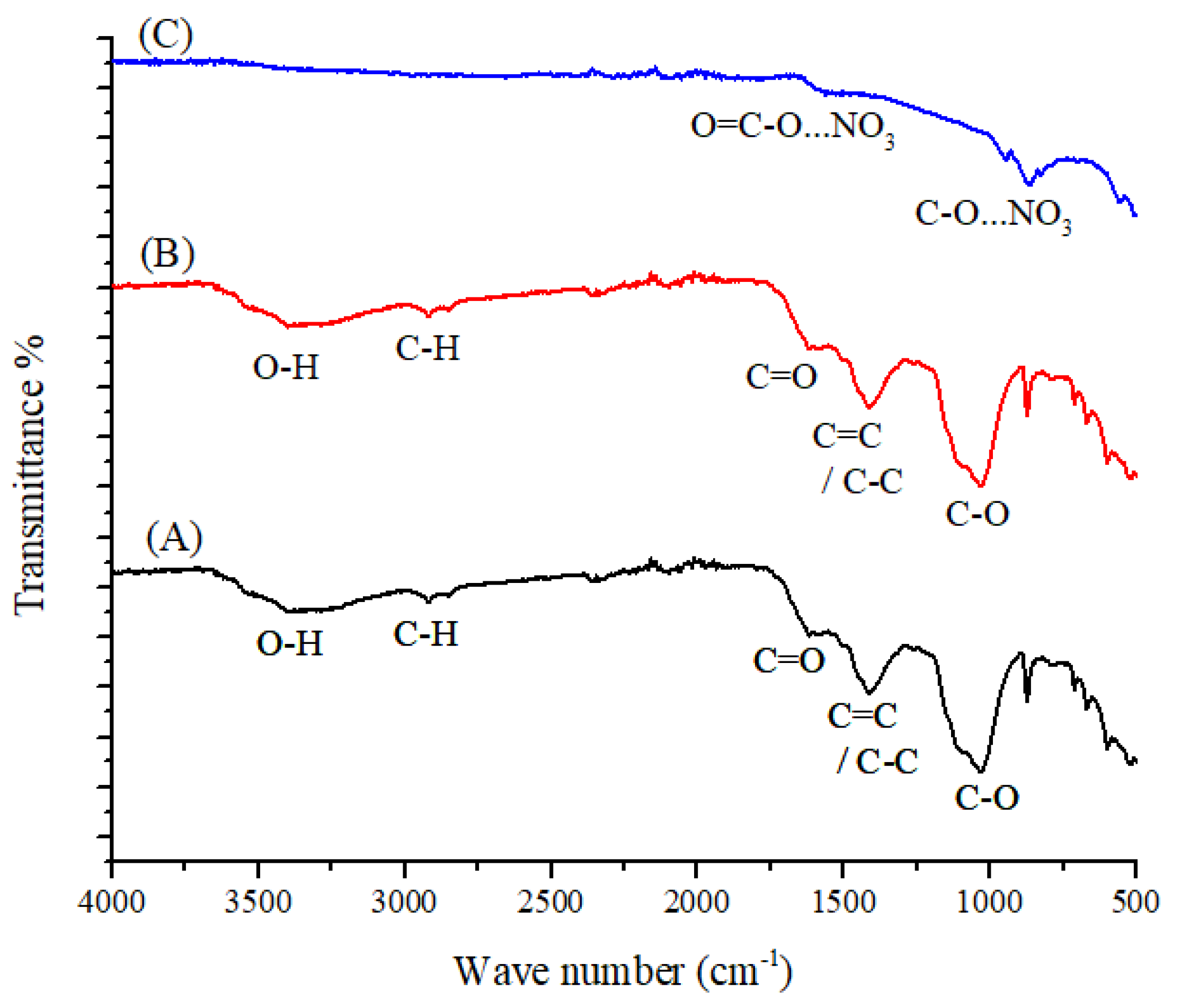
3.1. FT-IR Spectroscopy
3.2. X-ray Diffractometer (XRD)
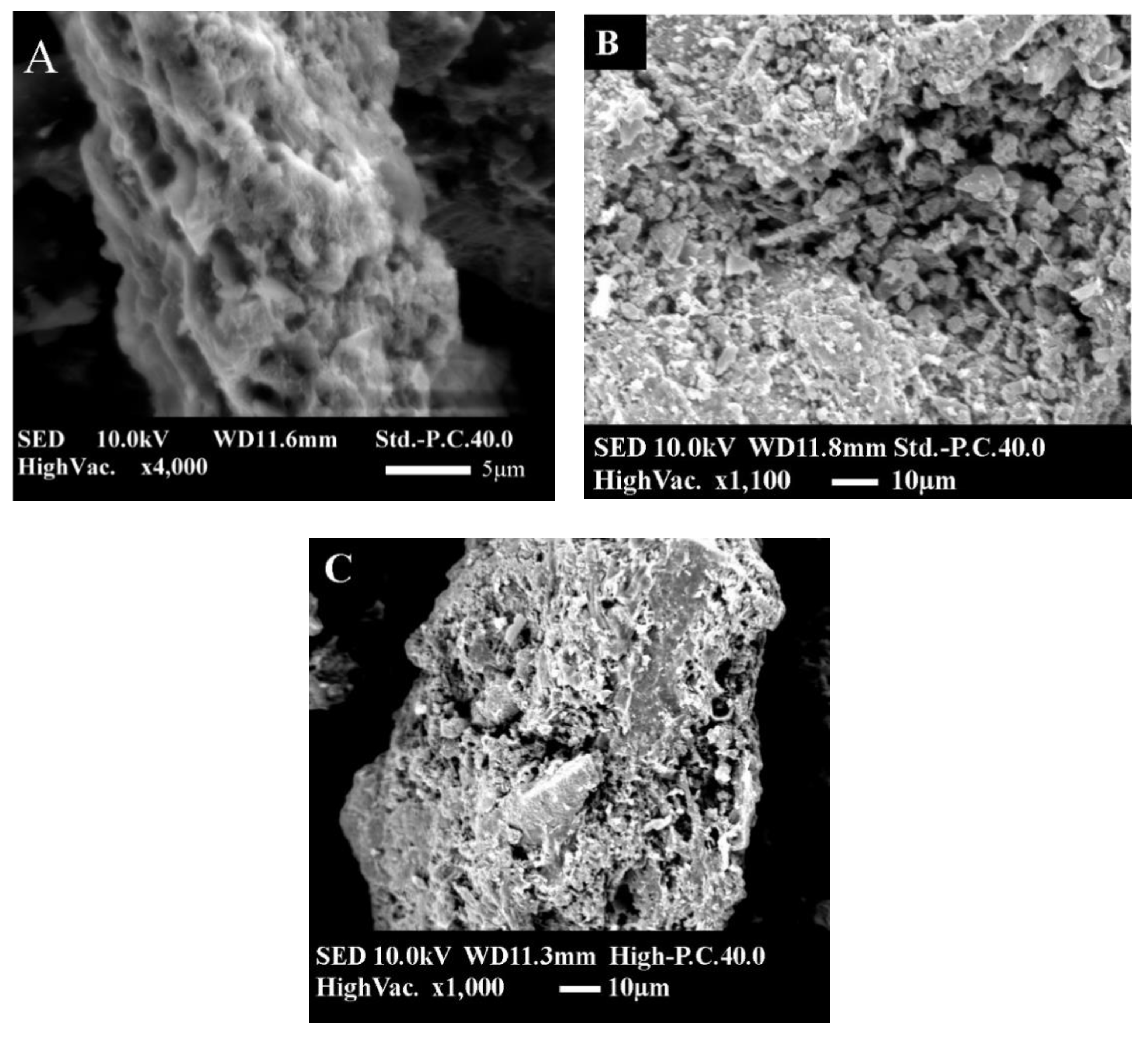
3.3. Surface Morphology of Biochar
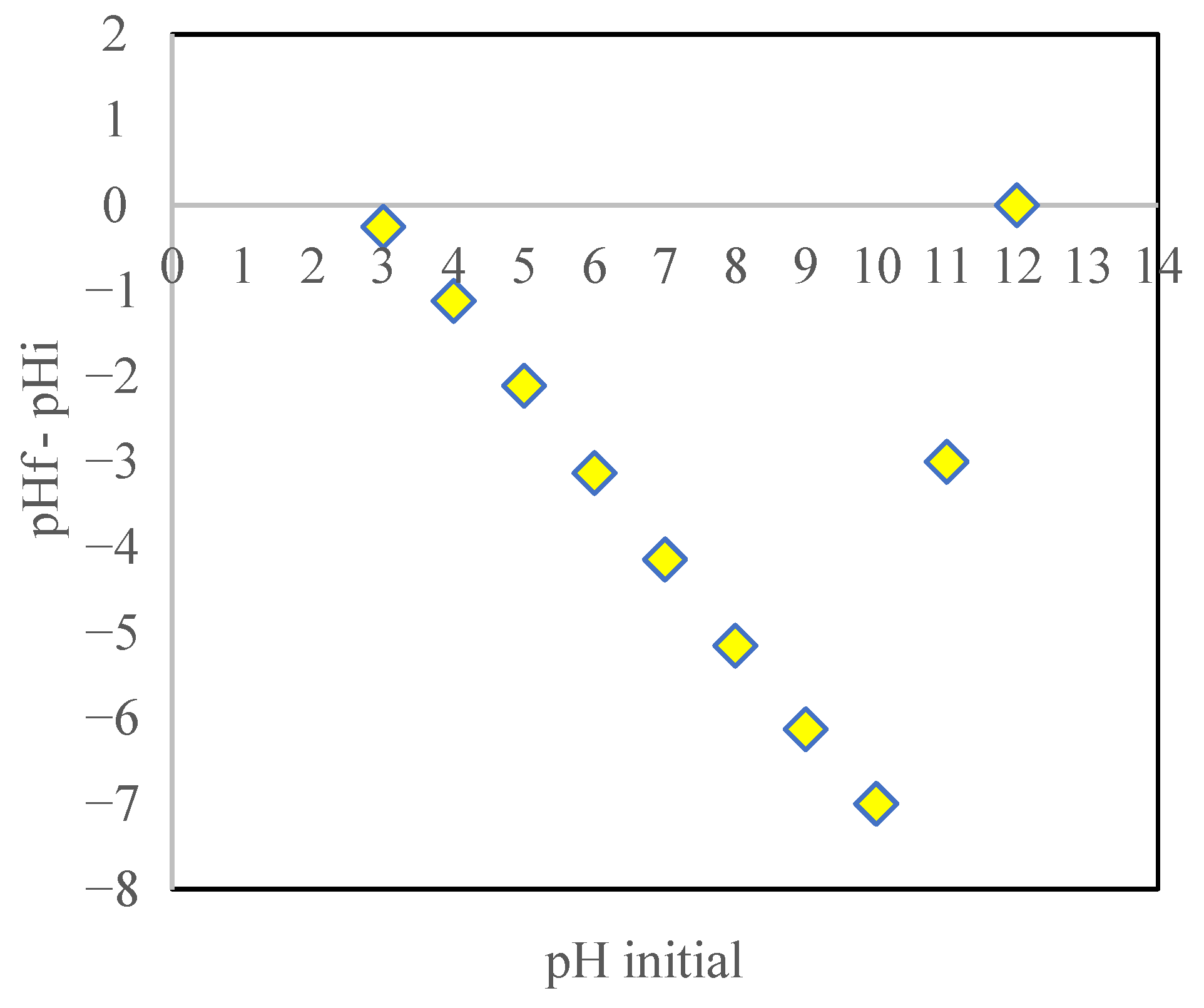
3.4. Point of Zero Charge (pHpzc)
3.5. Iron and Nitrate Leaching Test
3.6. Effect of pH on Adsorption Process
3.7. Effect of Contact Time
3.8. Effect of Initial Nitrate Concentration
3.9. Effect of Co-Existing (Competing) Anions
3.10. Adsorption Kinetic Study
3.11. Adsorption Isotherm Study
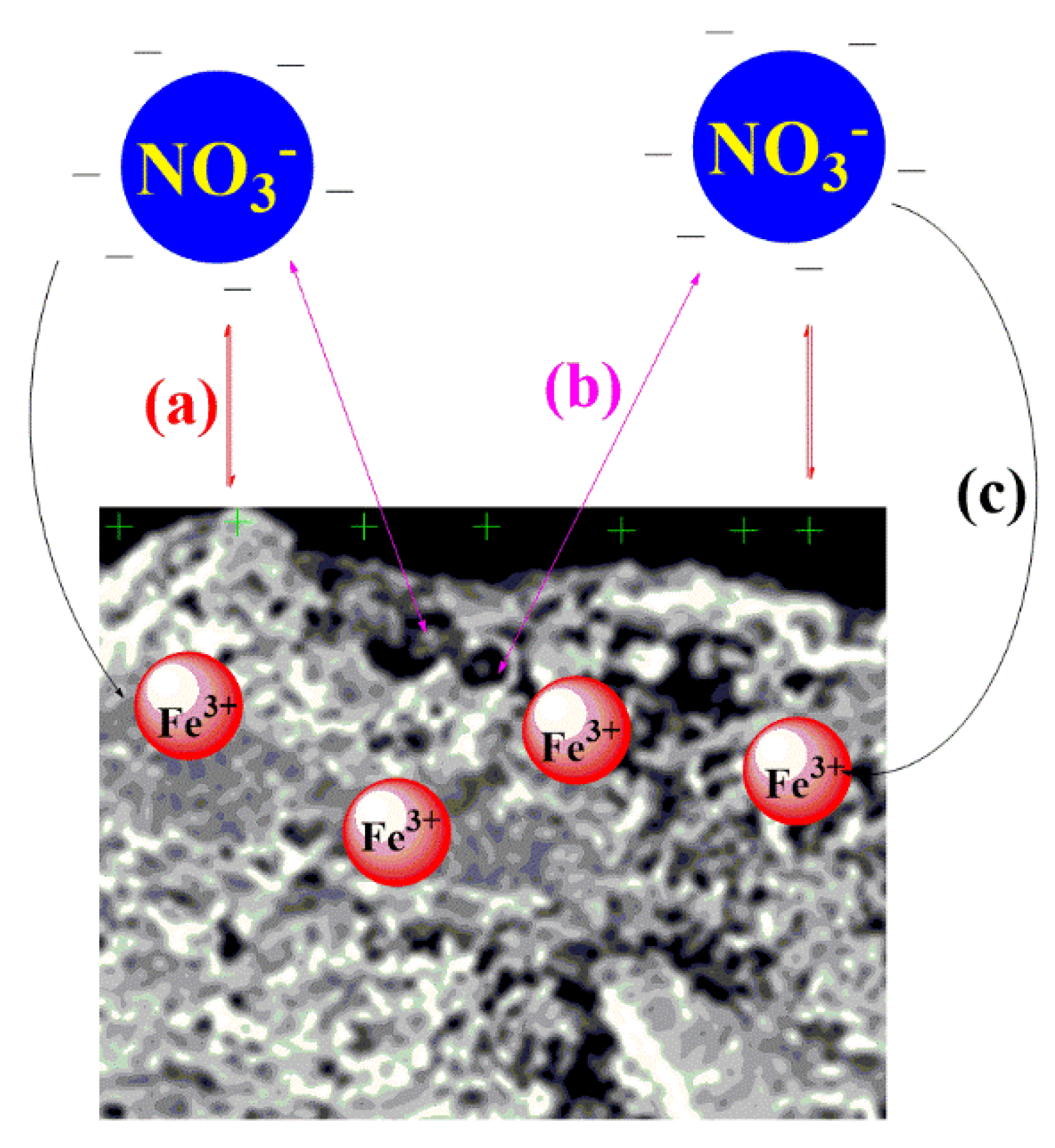
3.12. Adsorption Mechanism of Nitrates on Biochar
3.13. SMCB/Fe Adsorption Efficacy in Comparison to Other Materials
4. Conclusions and Recommendations for Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landon, M.K.; Delin, G.N.; Komor, S.C.; Regan, C.P. Relation of pathways and transit times of recharge water to nitrate concentrations using stable isotopes. Ground Water 2000, 38, 381–395. [Google Scholar] [CrossRef] [Green Version]
- Burow, K.R.; Nolan, B.T.; Rupert, M.G.; Dubrovsky, N.M. Nitrate in groundwater of the United States, 1991–2003. Environ. Sci. Technol. 2010, 44, 4988–4997. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Sobti, R.C. Nitrate removal from ground water: A review. E-J. Chem. 2012, 9, 1667–1675. [Google Scholar] [CrossRef]
- Kim, K.-H.; Yun, S.-T.; Mayer, B.; Lee, J.-H.; Kim, T.-S.; Kim, H.-K. Quantification of nitrate sources in groundwater using hydrochemical and dual isotopic data combined with a bayesian mixing model. Agric. Ecosyst. Environ. 2015, 199, 369–381. [Google Scholar] [CrossRef]
- Xue, D.; Pang, F.; Meng, F.; Wang, Z.; Wu, W. Decision-tree-model identification of nitrate pollution activities in groundwater: A combination of a dual isotope approach and chemical ions. J. Contam. Hydrol. 2015, 180, 25–33. [Google Scholar] [CrossRef]
- Ngatia, L.; Hsieh, Y.; Nemours, D.; Fu, R.; Taylor, R. Potential phosphorus eutrophication mitigation strategy: Biochar carbon composition, thermal stability and pH influence phosphorus sorption. Chemosphere 2017, 180, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- U. S. Food and Drug Administration. EPA, Toxicity and Exposure Assessment for Children’s Health Nitrates and Nitrites-Teach, Chemical Summary; U. S. Food and Drug Administration: White Oak, MA, USA, 2007. [Google Scholar]
- Raboni, M.; Viotti, P.; Rada, E.C.; Conti, F.; Boni, M.R. The sensitivity of a specific denitrification rate under the dissolved oxygen pressure. Int. J. Environ. Res. Public Health 2020, 17, 9366. [Google Scholar] [CrossRef] [PubMed]
- Boni, M.R.; Chiavola, A.; Marzeddu, S. Remediation of lead-contaminated water by virgin coniferous wood biochar adsorbent: Batch and column application. Water Air Soil Pollut. 2020, 231, 1–16. [Google Scholar] [CrossRef]
- Boni, M.; Marzeddu, S.; Tatti, F.; Raboni, M.; Mancini, G.; Luciano, A.; Viotti, P. Experimental and numerical study of biochar fixed bed column for the adsorption of arsenic from aqueous solutions. Water 2021, 13, 915. [Google Scholar] [CrossRef]
- Weyer, P.J.; Cerhan, J.; Kross, B.C.; Hallberg, G.R.; Kantamneni, J.; Breuer, G.; Jones, M.P.; Zheng, W.; Lynch, C.F. Municipal drinking water nitrate level and cancer risk in older women: The Iowa women’s health study. Epidemiology 2001, 12, 327–338. [Google Scholar] [CrossRef]
- Romero-Güiza, M.; Tait, S.; Astals, S.; del Valle-Zermeño, R.; Martínez, M.; Mata-Alvarez, J.; Chimenos, J. Reagent use efficiency with removal of nitrogen from pig slurry via struvite: A study on magnesium oxide and related by-products. Water Res. 2015, 84, 286–294. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Zhou, B.; Zhang, Z.; Liu, S.; Lei, S.; Xiao, R. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Clean. Prod. 2017, 147, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Li, M.; Xiao, R.; Wang, Q.; Zhang, Z.; Huang, H.; Liang, W.; et al. An overview of carbothermal synthesis of metal-biochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon 2018, 129, 674–687. [Google Scholar] [CrossRef]
- Min, L.; Zhongsheng, Z.; Zhe, L.; Haitao, W. Removal of nitrogen and phosphorus pollutants from water by FeCl3- impregnated biochar. Ecol. Eng. 2020, 149, 105792. [Google Scholar] [CrossRef]
- Liu, N.; Charrua, A.B.; Weng, C.-H.; Yuan, X.; Ding, F. Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresour. Technol. 2015, 198, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Naidu, R.; Du, J.; Liu, Y.; Qi, F. Critical review of magnetic biosorbents: Their preparation, application, and regeneration for wastewater treatment. Sci. Total. Environ. 2020, 702, 134893. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Rasit, N.; Ooi, C.K.; Ma, N.L.; Ng, J.-H.; Lam, W.-H.; Chong, C.T.; Chase, H.A. Pyrolysis production of fruit peel biochar for potential use in treatment of palm oil mill effluent. J. Environ. Manag. 2018, 213, 400–408. [Google Scholar] [CrossRef]
- Zhao, H.; Xue, Y.; Long, L.; Hu, X. Adsorption of nitrate onto biochar derived from agricultural residuals. Water Sci. Technol. 2018, 77, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Essandoh, M.; Wolgemuth, D.; Pittman, C.U.; Mohan, D.; Mlsna, T. Adsorption of metribuzin from aqueous solution using magnetic and nonmagnetic sustainable low-cost biochar adsorbents. Environ. Sci. Pollut. Res. 2017, 24, 4577–4590. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor. Chem. Eng. J. 2019, 360, 212–220. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Huang, X.; Wang, W.; Sun, P.; Li, Y.; Han, J. Functionalized biochar-supported magnetic MnFe2O4 nanocomposite for the removal of Pb(II) and Cd(II). RSC Adv. 2019, 9, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, X.; Wang, X.; Song, J.; Wang, H.; Zhang, J.; Zhao, J. Struvite crystallization combined adsorption of phosphate and ammonium from aqueous solutions by mesoporous MgO⿿ loaded diatomite. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 220–227. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N. Removal and recovery of phosphate from water using sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Jasińska, A. Spent mushroom compost (SMC)—retrieved added value product closing loop in agricultural production. Acta Agrar. Debr. 2018, 185–202. [Google Scholar] [CrossRef]
- Chen, G.-J.; Peng, C.-Y.; Fang, J.-Y.; Dong, Y.-Y.; Zhu, X.-H.; Cai, H.-M. Biosorption of fluoride from drinking water using spent mushroom compost biochar coated with aluminum hydroxide. Desalination Water Treat. 2015, 57, 1–11. [Google Scholar] [CrossRef]
- Hanafi, F.H.M.; Rezania, S.; Taib, S.M.; Din, M.F.M.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Wang, C.; Tan, H.; Liu, H.; Wu, B.; Xu, F.; Xu, H. A nanoscale ferroferric oxide coated biochar derived from mushroom waste to rapidly remove Cr(VI) and mechanism study. Bioresour. Technol. Rep. 2019, 7, 100253. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of point of zero charge of natural organic materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef]
- Reck, I.M.; Paixão, R.M.; Bergamasco, R.; Vieira, M.; Vieira, A.M.S. Removal of tartrazine from aqueous solutions using adsorbents based on activated carbon and Moringa oleifera seeds. J. Clean. Prod. 2018, 171, 85–97. [Google Scholar] [CrossRef]
- Hafshejani, L.D.; Hooshmand, A.; Naseri, A.A.; Mohammadi, A.S.; Abbasi, F.; Bhatnagar, A. Removal of nitrate from aqueous solution by modified sugarcane bagasse biochar. Ecol. Eng. 2016, 95, 101–111. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Papiernik, S.K.; Malo, D.D.; Clay, D.E.; Kumar, S.; Gulbrandson, D.W. Nitrate sorption and desorption in biochars from fast pyrolysis. Microporous Mesoporous Mater. 2013, 179, 250–257. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, K.; Jeong, T.-U.; Ahn, K.-H. Influence of pyrolysis temperature on characteristics and phosphate adsorption capability of biochar derived from waste-marine macroalgae (Undaria pinnatifida roots). Bioresour. Technol. 2016, 200, 1024–1028. [Google Scholar] [CrossRef]
- Nodeh, H.R.; Sereshti, H.; Afsharian, E.Z.; Nouri, N. Enhanced removal of phosphate and nitrate ions from aqueous media using nanosized lanthanum hydrous doped on magnetic graphene nanocomposite. J. Environ. Manag. 2017, 197, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Kamaraj, R.; Vasudevan, S. Application of isotherm, kinetic and thermodynamic models for the adsorption of nitrate ions on graphene from aqueous solution. J. Taiwan Inst. Chem. Eng. 2013, 44, 808–814. [Google Scholar] [CrossRef]
- Olgun, A.; Atar, N.; Wang, S. Batch and column studies of phosphate and nitrate adsorption on waste solids containing boron impurity. Chem. Eng. J. 2013, 222, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Y.; Ning, Z.; Shaopeng, Z.; Yayi, D.; Xuntong, Z.; Jiachun, S.; Weiben, Y.; Yuping, W.; Jianqiang, C. A pH- and temperature-responsive magnetic composite adsorbent for targeted removal of nonylphenol. ACS Appl. Mater. Interfaces 2015, 7, 24446–24457. [Google Scholar] [CrossRef] [PubMed]
- Kalantary, R.R.; Dehghanifard, E.; Mohseni-Bandpei, A.; Rezaei, L.; Esrafili, A.; Kakavandi, B.; Azari, A. Nitrate adsorption by synthetic activated carbon magnetic nanoparticles: Kinetics, isotherms and thermodynamic studies. Desalination Water Treat. 2015, 57, 16445–16455. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Xue, L.; Gao, B.; Wan, Y.; Fang, J.; Wang, S.; Li, Y.; Muñoz-Carpena, R.; Yang, L. High efficiency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2016, 63, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Park, Y.-K. Applications of modified biochar-based materials for the removal of environment pollutants: A mini review. Sustainability 2020, 12, 6112. [Google Scholar] [CrossRef]
- Wahab, M.A.; Jellali, S.; Jedidi, N. Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef]
- Keränen, A.; Leiviskä, T.; Hormi, O.; Tanskanen, J. Removal of nitrate by modified pine sawdust: Effects of temperature and co-existing anions. J. Environ. Manag. 2015, 147, 46–54. [Google Scholar] [CrossRef]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Nitrate removal from water by nano-alumina: Characterization and sorption studies. Chem. Eng. J. 2010, 163, 317–323. [Google Scholar] [CrossRef]
- Cengeloglu, Y.; Tor, A.; Ersoz, M.; Arslan, G. Removal of nitrate from aqueous solution by using red mud. Sep. Purif. Technol. 2006, 51, 374–378. [Google Scholar] [CrossRef]
- Demiral, H.; Gündüzoğlu, G. Removal of nitrate from aqueous solutions by activated carbon prepared from sugar beet bagasse. Bioresour. Technol. 2010, 101, 1675–1680. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, W.; Liu, W.; Yu, L.; Qian, Y.; Wang, J.; Wang, J.-J.; Eng, C.; Liu, C.-J.; Jones, K.W.; et al. Synchrotron micro-scale study of trace metal transport and distribution in Spartina alterniflora root system in Yangtze River intertidal zone. Environ. Sci. Pollut. Res. 2015, 22, 18933–18944. [Google Scholar] [CrossRef] [PubMed]
- Alsewaileh, A.S.; Usman, A.; Al-Wabel, M.I. Effects of pyrolysis temperature on nitrate-nitrogen (NO3−-N) and bromate (BrO3−) adsorption onto date palm biochar. J. Environ. Manag. 2019, 237, 289–296. [Google Scholar] [CrossRef]
- Rahdar, S.; Pal, K.; Mohammadi, L.; Rahdar, A.; Goharniya, Y.; Samani, S.; Kyzas, G.Z. Response surface methodology for the removal of nitrate ions by adsorption onto copper oxide nanoparticles. J. Mol. Struct. 2021, 1231, 129686. [Google Scholar] [CrossRef]
- Yu, B.; Xu, J.; Liu, J.-H.; Yang, S.-T.; Luo, J.; Zhou, Q.; Wan, J.; Liao, R.; Wang, H.; Liu, Y. Adsorption behavior of copper ions on graphene oxide-chitosan aerogel. J. Environ. Chem. Eng. 2013, 1, 1044–1050. [Google Scholar] [CrossRef]
- Banu, H.T.; Karthikeyan, P.; Meenakshi, S. Zr4+ ions embedded chitosan-soya bean husk activated bio-char composite beads for the recovery of nitrate and phosphate ions from aqueous solution. Int. J. Biol. Macromol. 2019, 130, 573–583. [Google Scholar] [CrossRef]
- Dewage, N.B.; Liyanage, A.S.; Pittman, C.U.; Mohan, D.; Mlsna, T. Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar. Bioresour. Technol. 2018, 263, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.; Larson, R.; Runge, T. Nitrate sorption to biochar following chemical oxidation. Sci. Total. Environ. 2019, 669, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Xue, Y.; Hu, X.; Zhu, Y. Study on the influence of surface potential on the nitrate adsorption capacity of metal modified biochar. Environ. Sci. Pollut. Res. 2019, 26, 3065–3074. [Google Scholar] [CrossRef] [PubMed]


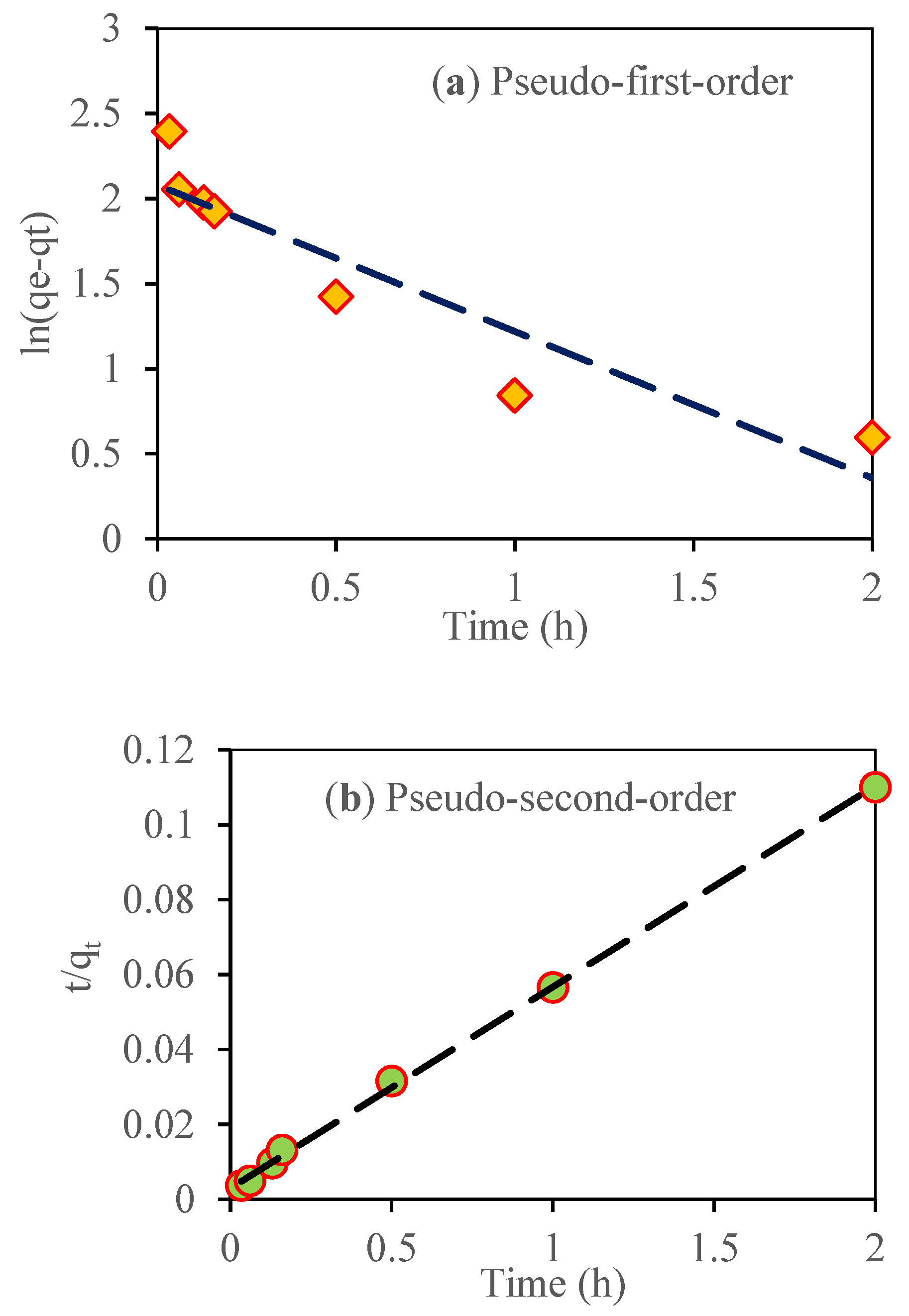
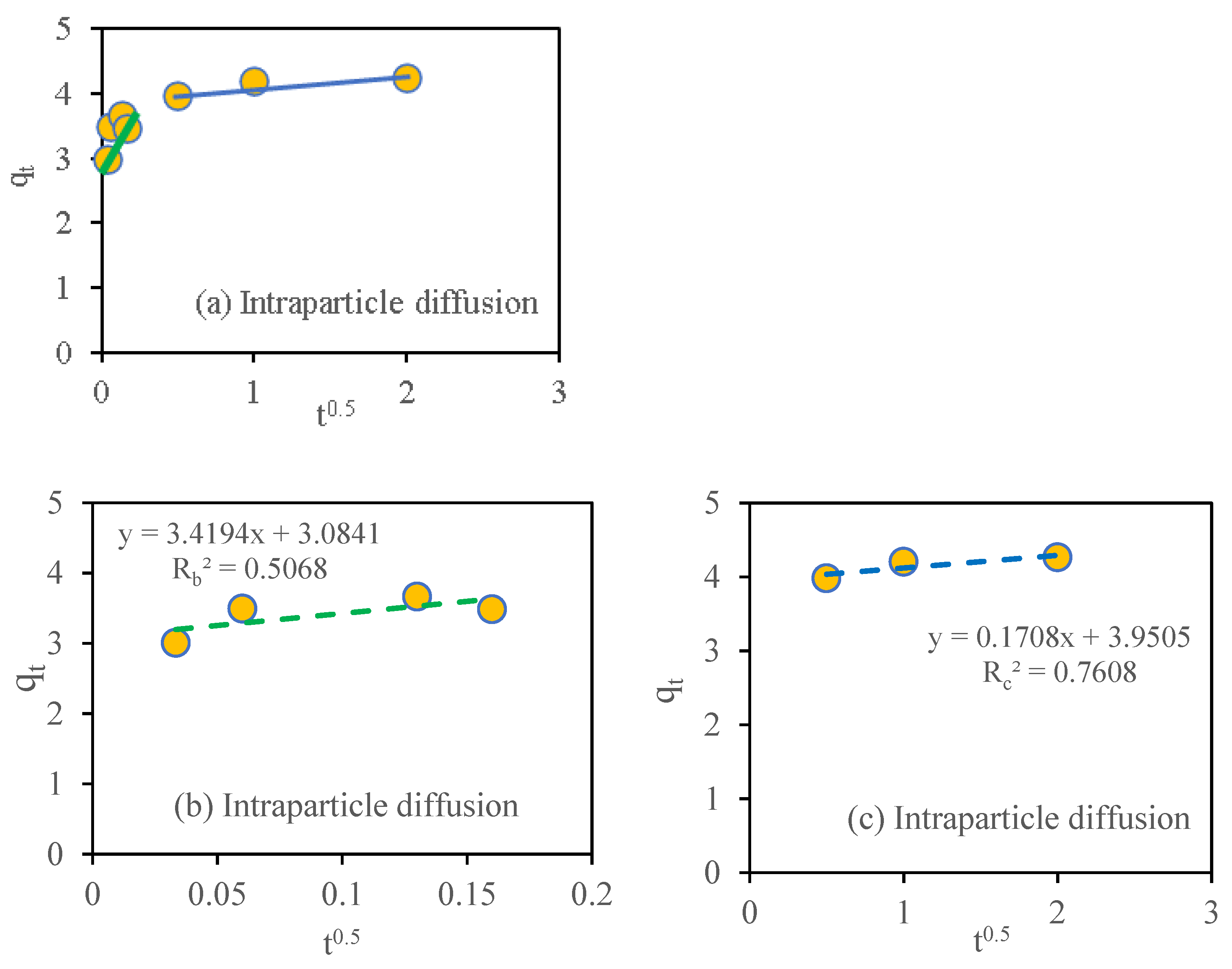
| Model | Reference | Linear Equation | Parameters | Nitrate Adsorption | qe.exp mg g−1 |
|---|---|---|---|---|---|
| Pseudo first order | [31] | 20 | 18.18 | ||
| 0.861 | |||||
| 0.086 | |||||
| Pseudo second order | [44] | 18.62 | 18.18 | ||
| 0.002 | |||||
| 0.999 | |||||
| Intraparticle diffusion | [45] | 3.41 | |||
| 3.08 | |||||
| 0.506 | |||||
| 0.17 | |||||
| 3.9 | |||||
| 0.76 |
| Model | Reference | Linear Equation | Parameters | Nitrate Adsorption |
|---|---|---|---|---|
| Langmuir | [37] | 19.88 | ||
| 0.503 | ||||
| 0.996 | ||||
| 0.032 | ||||
| Freundlich | [7] | 6.7 | ||
| 4.2 | ||||
| 0.94 | ||||
| Dubinin–Radushkevich | [34] | 13.59 | ||
| 0.024 | ||||
| 0.842 | ||||
| Free energy | [37] | 1.44 |
| Biochar Composites | T °C | Nitrate (mg L−1) | Sorption Isotherm | Kinetic | pH | Max Sorption Capacity (mg g−1) | Reference |
|---|---|---|---|---|---|---|---|
| Corn straw-Fe2O3 | 550 | 50 | Langmuir | NA | 15.40 | [16] | |
| Douglas fir-α-Fe2O3/Fe3O4 | 600 | 100 | Langmuir | 2–9 | 15.00 | [56] | |
| Soybean Mg-Al | 500 | 50 | *pso | 7.5 | 45.21 | [7] | |
| Soybean Mg | 500 | 50 | *pso | 8.5 | 77.31 | [7] | |
| Birch wood /H2O2 oxidized | 400 | 500 | Langmuir | NA | 1.49 | [57] | |
| Birch wood /H2O2 oxidized | 600 | 500 | NA | 1.09 | |||
| Corncob /FeCl3 | 600 | 2000 | Langmuir | 3–7 | 14.46 | [20] | |
| Corncob/FeCl3 | 600 | 100 | Langmuir | 3–11 | 32.33 | [58] | |
| Sugarcane bagasse/ ECH | 600 | 100 | Langmuir | *pso | 3 | 28.21 | [34] |
| Wheat-straw/Mg-Fe | 600 | 45 | Langmuir | *pso | 7–8 | 24.8 | [5] |
| Spent mushroom compost/FeCl3·6H2O | 600 | 60 | Langmuir | *pso | 5–7 | 19.88 | this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darajeh, N.; Alizadeh, H.; Leung, D.W.M.; Rashidi Nodeh, H.; Rezania, S.; Farraji, H. Application of Modified Spent Mushroom Compost Biochar (SMCB/Fe) for Nitrate Removal from Aqueous Solution. Toxics 2021, 9, 277. https://doi.org/10.3390/toxics9110277
Darajeh N, Alizadeh H, Leung DWM, Rashidi Nodeh H, Rezania S, Farraji H. Application of Modified Spent Mushroom Compost Biochar (SMCB/Fe) for Nitrate Removal from Aqueous Solution. Toxics. 2021; 9(11):277. https://doi.org/10.3390/toxics9110277
Chicago/Turabian StyleDarajeh, Negisa, Hossein Alizadeh, David W. M. Leung, Hamid Rashidi Nodeh, Shahabaldin Rezania, and Hossein Farraji. 2021. "Application of Modified Spent Mushroom Compost Biochar (SMCB/Fe) for Nitrate Removal from Aqueous Solution" Toxics 9, no. 11: 277. https://doi.org/10.3390/toxics9110277







