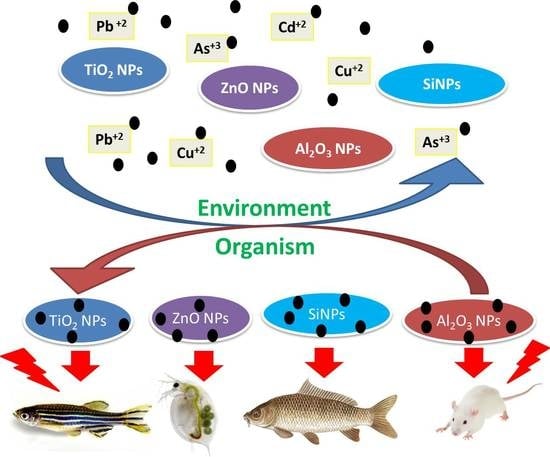
Effects of Co-Exposure of Nanoparticles and Metals on Different Organisms: A Review
Abstract
:1. Introduction
2. Titanium Dioxide Nanoparticles
2.1. TiO2NPs and Cd Co-Exposure
2.2. TiO2NPs and As Co-Exposure
2.3. TiO2NPs and Cu Co-Exposure
2.4. TiO2NPs and Pb Co-Exposure
3. Zinc Oxide Nanoparticles
4. Silica Nanoparticles (SiNPs)
5. Aluminum Oxide Nanoparticles
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Długosz, O.; Sochocka, M.; Ochnik, M.; Banach, M. Metal and bimetallic nanoparticles: Flow synthesis, bioactivity and toxicity. J. Colloid Interface Sci. 2021, 586, 807–818. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kokhaei, P.; Ghorbani, M.; Eslami, M. Application of different nanoparticles in the diagnosis of colorectal cancer. Gene Rep. 2020, 21, 100896. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- El-Hakim, A.; Yasmina, M.; Abdel-Rahman Mohamed, A.; Khater, S.I.; Hamed Arisha, A.; Metwally, M.M.; Nassan, M.A.; Hassan, M.E. Chitosan-Stabilized Selenium Nanoparticles and Metformin Synergistically Rescue Testicular Oxidative Damage and Steroidogenesis-Related Genes Dysregulation in High-Fat Diet/Streptozotocin-Induced Diabetic Rats. Antioxidants 2021, 10, 17. [Google Scholar] [CrossRef]
- Jones, C.F.; Grainger, D.W. In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 2009, 61, 438–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunhikrishnan, A.; Shon, H.K.; Bolan, N.S.; El Saliby, I.; Vigneswaran, S. Sources, distribution, environmental fate, and ecological effects of nanomaterials in wastewater streams. Crit. Rev. Environ. Sci. Technol. 2015, 45, 277–318. [Google Scholar] [CrossRef]
- Chernyshev, V.; Zakharenko, A.; Ugay, S.; Hien, T.; Hai, L.; Kholodov, A.; Burykina, T.; Stratidakis, A.; Mezhuev, Y.O.; Tsatsakis, A. Morphologic and chemical composition of particulate matter in motorcycle engine exhaust. Toxicol. Rep. 2018, 5, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Kettler, K.; Veltman, K.; van de Meent, D.; van Wezel, A.; Hendriks, A.J. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ. Toxicol. Chem. 2014, 33, 481–492. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Y.; Zhang, J.; Mu, Q.; Zhang, W.; Butch, E.R.; Snyder, S.E.; Yan, B. Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility. Nat. Nanotechnol. 2010, 5, 683–689. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, Y.; Jia, J.; Gao, N.; Li, Y.; Zhang, R.; Jiang, G.; Yan, B. Perturbation of physiological systems by nanoparticles. Chem. Soc. Rev. 2014, 43, 3762–3809. [Google Scholar] [CrossRef]
- Armstrong, D.; Bharali, D.J.; Armstrong, D.; Bharali, D. Oxidative Biomarkers to Assess the Nanoparticle-Induced Oxidative Stress. In Oxidative Stress and Nanotechnology. Methods and Protocols; Humana Press: Totowa, NJ, USA, 2013; pp. 205–219. [Google Scholar]
- Bystrzejewska-Piotrowska, G.; Golimowski, J.; Urban, P.L. Nanoparticles: Their potential toxicity, waste and environmental management. Waste Manag. 2009, 29, 2587–2595. [Google Scholar] [CrossRef]
- Ma, H.; Wallis, L.K.; Diamond, S.; Li, S.; Canas-Carrell, J.; Parra, A. Impact of solar UV radiation on toxicity of ZnO nanoparticles through photocatalytic reactive oxygen species (ROS) generation and photo-induced dissolution. Environ. Pollut. 2014, 193, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zang, X.; Wu, Z.; Liu, J.; Wang, D. Altered protein S-glutathionylation depicts redox imbalance triggered by transition metal oxide nanoparticles in a breastfeeding system. NanoImpact 2021, 22, 100305. [Google Scholar] [CrossRef]
- García-Alonso, J.; Khan, F.R.; Misra, S.K.; Turmaine, M.; Smith, B.D.; Rainbow, P.S.; Luoma, S.N.; Valsami-Jones, E. Cellular internalization of silver nanoparticles in gut epithelia of the estuarine polychaete Nereis diversicolor. Environ. Sci. Technol. 2011, 45, 4630–4636. [Google Scholar] [CrossRef]
- Rodriguez-Yanez, Y.; Munoz, B.; Albores, A. Mechanisms of toxicity by carbon nanotubes. Toxicol. Mech. Methods 2013, 23, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Ji, J.; Tian, X.; Liu, N.; Yang, K.; Wu, F.; Wang, Z.; Xing, B. Environmental behavior and toxicity of engineered nanomaterials. Chin. Sci. Bull. 2009, 54, 3590–3604. [Google Scholar]
- Rotoli, B.M.; Bussolati, O.; Bianchi, M.G.; Barilli, A.; Balasubramanian, C.; Bellucci, S.; Bergamaschi, E. Non-functionalized multi-walled carbon nanotubes alter the paracellular permeability of human airway epithelial cells. Toxicol. Lett. 2008, 178, 95–102. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Leite, P.E.; Falagan-Lotsch, P.; Benetti, F.; Micheletti, C.; Budtz, H.C.; Jacobsen, N.R.; Lisboa-Filho, P.N.; Rocha, L.A.; Kühnel, D.; et al. Challenges on the toxicological predictions of engineered nanoparticles. NanoImpact 2017, 8, 59–72. [Google Scholar]
- Wang, J.; Nie, Y.; Dai, H.; Wang, M.; Cheng, L.; Yang, Z.; Chen, S.; Zhao, G.; Wu, L.; Guang, S. Parental exposure to TiO2 NPs promotes the multigenerational reproductive toxicity of Cd in Caenorhabditis elegans via bioaccumulation of Cd in germ cells. Environ. Sci. Nano 2019, 6, 1332–1342. [Google Scholar] [CrossRef]
- Tomno, R.M.; Nzeve, J.K.; Mailu, S.N.; Shitanda, D.; Waswa, F. Heavy metal contamination of water, soil and vegetables in urban streams in Machakos municipality, Kenya. Sci. Afr. 2020, 9, e00539. [Google Scholar] [CrossRef]
- Alam, R.; Ahmed, Z.; Howladar, M.F. Evaluation of heavy metal contamination in water, soil and plant around the open landfill site Mogla Bazar in Sylhet, Bangladesh. Groundw. Sustain. Dev. 2020, 10, 100311. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; ElHady, M.; Hassanin, M.E.; Mohamed, A.A.-R. Alleviative effects of dietary Indian lotus leaves on heavy metals-induced hepato-renal toxicity, oxidative stress, and histopathological alterations in Nile tilapia, Oreochromis niloticus (L.). Aquaculture 2019, 509, 198–208. [Google Scholar] [CrossRef]
- Mohamed, A.A.-R.; El-Houseiny, W.; Abd Elhakeem, E.-M.; Ebraheim, L.L.; Ahmed, A.I.; Abd El-Hakim, Y.M. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890. [Google Scholar] [CrossRef]
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A. Occurrence and removal of titanium at full scale wastewater treatment plants: Implications for TiO2 nanomaterials. J. Environ. Monit. 2011, 13, 1195–1203. [Google Scholar] [PubMed]
- Kägi, R.; Ulrich, A.; Sinnet, B.; Vonbank, R.; Wichser, A.; Zuleeg, S.; Simmler, H.; Brunner, S.; Vonmont, H.; Burkhardt, M. Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environ. Pollut. 2008, 156, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Q.; Asif, M.; Shaheen, S.; Hayat, M.T.; Ali, S. Chapter 6—Cadmium Contamination in Water and Soil. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 141–161. [Google Scholar]
- Sastry, K.; Rashmi, H.; Rao, N. Nanotechnology patents as R&D indicators for disease management strategies in agriculture. J. Intellect. Prop. Rights 2010, 15, 197–205. [Google Scholar]
- Simonin, M.; Richaume, A.; Guyonnet, J.P.; Dubost, A.; Martins, J.M.F.; Pommier, T. Titanium dioxide nanoparticles strongly impact soil microbial function by affecting archaeal nitrifiers. Sci. Rep. 2016, 6, 33643. [Google Scholar] [CrossRef]
- Wang, L.; Cui, X.; Cheng, H.; Chen, F.; Wang, J.; Zhao, X.; Lin, C.; Pu, X. A review of soil cadmium contamination in China including a health risk assessment. Environ. Sci. Pollut. Res. 2015, 22, 16441–16452. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef]
- Khan, S.; Rehman, S.; Khan, A.Z.; Khan, M.A.; Shah, M.T. Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol. Environ. Saf. 2010, 73, 1820–1827. [Google Scholar] [CrossRef]
- Roy, M.; McDonald, L.M. Metal Uptake in Plants and Health Risk Assessments in Metal-Contaminated Smelter Soils. Land Degrad. Dev. 2015, 26, 785–792. [Google Scholar] [CrossRef]
- Yu, Z.; Hao, R.; Zhang, L.; Zhu, Y. Effects of TiO2, SiO2, Ag and CdTe/CdS quantum dots nanoparticles on toxicity of cadmium towards Chlamydomonas reinhardtii. Ecotoxicol. Environ. Saf. 2018, 156, 75–86. [Google Scholar] [CrossRef]
- Xia, B.; Chen, J.; Zhou, Y. Cellular oxidative damage of HEK293T cells induced by combination of CdCl(2) and nano-TiO(2). J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 290–294. [Google Scholar] [CrossRef]
- Du, H.; Zhu, X.; Fan, C.; Xu, S.; Wang, Y.; Zhou, Y. Oxidative damage and OGG1 expression induced by a combined effect of titanium dioxide nanoparticles and lead acetate in human hepatocytes. Environ. Toxicol. 2012, 27, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, H.; Zhang, Z.; Niu, Q.; Chen, Y.; Crittenden, J.C. Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere 2007, 67, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Balbi, T.; Smerilli, A.; Fabbri, R.; Ciacci, C.; Montagna, M.; Grasselli, E.; Brunelli, A.; Pojana, G.; Marcomini, A.; Gallo, G.; et al. Co-exposure to n-TiO2 and Cd2+ results in interactive effects on biomarker responses but not in increased toxicity in the marine bivalve M. galloprovincialis. Sci. Total Environ. 2014, 493, 355–364. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Q.; Jiang, L.; Yu, Z.; Jiang, D.; Yin, D. Combined effects of titanium dioxide and humic acid on the bioaccumulation of cadmium in Zebrafish. Environ. Pollut. 2011, 159, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Fan, W.-H.; Wang, W.-X. Role of titanium dioxide nanoparticles in the elevated uptake and retention of cadmium and zinc in Daphnia magna. Environ. Sci. Technol. 2012, 46, 469–476. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Legros, S.; Von der Kammer, F.; Hofmann, T.; Baun, A. The potential of TiO2 nanoparticles as carriers for cadmium uptake in Lumbriculus variegatus and Daphnia magna. Aquat. Toxicol. 2012, 118–119, 1–8. [Google Scholar] [CrossRef]
- Yang, W.W.; Miao, A.J.; Yang, L.Y. Cd2+ Toxicity to a green alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO2 engineered nanoparticles. PLoS ONE 2012, 7, e32300. [Google Scholar]
- Tan, C.; Wang, W.-X. Modification of metal bioaccumulation and toxicity in Daphnia magna by titanium dioxide nanoparticles. Environ. Pollut. 2014, 186, 36–42. [Google Scholar] [CrossRef]
- Yang, W.-W.; Wang, Y.; Huang, B.; Wang, N.-X.; Wei, Z.-B.; Luo, J.; Miao, A.-J.; Yang, L.-Y. TiO2 nanoparticles act as a carrier of Cd bioaccumulation in the ciliate Tetrahymena thermophila. Environ. Sci. Technol. 2014, 48, 7568–7575. [Google Scholar] [CrossRef]
- Vale, G.; Franco, C.; Diniz, M.S.; Santos, M.M.C.d.; Domingos, R.F. Bioavailability of cadmium and biochemical responses on the freshwater bivalve Corbicula fluminea—the role of TiO2 nanoparticles. Ecotoxicol. Environ. Saf. 2014, 109, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, X.; Niu, Q.; Chen, Y.; Crittenden, J.C. Enhanced accumulation of arsenate in carp in the presence of titanium dioxide nanoparticles. Water Air Soil Pollut. 2007, 178, 245–254. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, X.; Zhang, Z.; Chen, Y.; Crittenden, J.C. Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite. Environ. Pollut. 2009, 157, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, J.; Irons, D.R.; Wang, J. Synergistic toxic effect of nano-TiO2 and As (V) on Ceriodaphnia dubia. Sci. Total. Environ. 2011, 409, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.M.; Josende, M.E.; Ruas, C.P.; Gelesky, M.A.; Júnior, F.M.R.d.S.; Fattorini, D.; Regoli, F.; Monserrat, J.M.; Ventura-Lima, J. Biochemical responses induced by co-exposition to arsenic and titanium dioxide nanoparticles in the estuarine polychaete Laeonereis acuta. Toxicology 2017, 376, 51–58. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Z.; Yan, Y.; Li, J.; Yan, C.; Xing, B. Titanium dioxide nanoparticles enhance inorganic arsenic bioavailability and methylation in two freshwater algae species. Environ. Pollut. 2018, 238, 631–637. [Google Scholar] [CrossRef]
- Fan, W.; Cui, M.; Liu, H.; Wang, C.; Shi, Z.; Tan, C.; Yang, X. Nano-TiO2 enhances the toxicity of copper in natural water to Daphnia magna. Environ. Pollut. 2011, 159, 729–734. [Google Scholar] [CrossRef]
- Fan, W.; Cui, M.; Shi, Z.; Tan, C.; Yang, X. Enhanced oxidative stress and physiological damage in Daphnia magna by copper in the presence of nano-TiO2. J. Nanomater. 2012, 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeldt, R.R.; Seitz, F.; Zubrod, J.P.; Feckler, A.; Merkel, T.; Lüderwald, S.; Bundschuh, R.; Schulz, R.; Bundschuh, M. Does the presence of titanium dioxide nanoparticles reduce copper toxicity? A factorial approach with the benthic amphipod Gammarus fossarum. Aquat. Toxicol. 2015, 165, 154–159. [Google Scholar] [CrossRef]
- Zhang, R.; Niu, Y.; Li, Y.; Zhao, C.; Song, B.; Li, Y.; Zhou, Y. Acute toxicity study of the interaction between titanium dioxide nanoparticles and lead acetate in mice. Environ. Toxicol. Pharmacol. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Miao, W.; Zhu, B.; Xiao, X.; Li, Y.; Dirbaba, N.B.; Zhou, B.; Wu, H. Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat. Toxicol. 2015, 161, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Vicari, T.; Dagostim, A.C.; Klingelfus, T.; Galvan, G.L.; Monteiro, P.S.; da Silva Pereira, L.; Silva de Assis, H.C.; Cestari, M.M. Co-exposure to titanium dioxide nanoparticles (NpTiO2) and lead at environmentally relevant concentrations in the Neotropical fish species Hoplias intermedius. Toxicol. Rep. 2018, 5, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, F.; Zhai, S.; Zhou, H.; Liu, S.; Jiang, G.; Yan, B. Susceptibility of overweight mice to liver injury as a result of the ZnO nanoparticle-enhanced liver deposition of Pb2+. Environ. Sci. Technol. 2017, 51, 1775–1784. [Google Scholar] [CrossRef]
- Khayal, E.E.; Ibrahim, H.M.; Shalaby, A.M.; Alabiad, M.A.; El-Sheikh, A.A. Combined lead and zinc oxide-nanoparticles induced thyroid toxicity through 8-OHdG oxidative stress-mediated inflammation, apoptosis, and Nrf2 activation in rats. Environ. Toxicol. 2021. [Google Scholar] [CrossRef]
- Teng, C.; Jia, J.; Wang, Z.; Yan, B. Oral Co-Exposures to zinc oxide nanoparticles and CdCl2 induced maternal-fetal pollutant transfer and embryotoxicity by damaging placental barriers. Ecotoxicol. Environ. Saf. 2020, 189, 109956. [Google Scholar] [CrossRef]
- Guo, M.; Xu, X.; Yan, X.; Wang, S.; Gao, S.; Zhu, S. In vivo biodistribution and synergistic toxicity of silica nanoparticles and cadmium chloride in mice. J. Hazard. Mater. 2013, 260, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yang, X.; Shi, Y.; Liang, S.; Zhao, T.; Duan, J.; Sun, Z. Co-exposure subacute toxicity of silica nanoparticles and lead acetate on cardiovascular system. Int. J. Nanomed. 2018, 13, 7819. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, J.; Forthaus, B.E.; Wang, J. Synergistic toxic effect of nano-Al2O3 and As (V) on Ceriodaphnia dubia. Environ. Pollut. 2011, 159, 3003–3008. [Google Scholar] [CrossRef]
- Aitken, R.J.; Chaudhry, M.; Boxall, A.; Hull, M. Manufacture and use of nanomaterials: Current status in the UK and global trends. Occup. Med. 2006, 56, 300–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible colloidal suspensions based on magnetic iron oxide nanoparticles: Synthesis, characterization and toxicological profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of health safety aspects of titanium dioxide nanoparticles in food application. NanoImpact 2020, 18, 100224. [Google Scholar] [CrossRef]
- Robichaud, C.O.; Uyar, A.E.; Darby, M.R.; Zucker, L.G.; Wiesner, M.R. Estimates of Upper Bounds and Trends in Nano-TiO2 Production as a Basis for Exposure Assessment. Environ. Sci. Technol. 2009, 43, 4227–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, N.B.; Baun, A. The nano cocktail: Ecotoxicological effects of engineered nanoparticles in chemical mixtures. Integr. Environ. Assess. Manag. Int. J. 2010, 6, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Engates, K.E.; Shipley, H.J. Adsorption of Pb, Cd, Cu, Zn, and Ni to titanium dioxide nanoparticles: Effect of particle size, solid concentration, and exhaustion. Environ. Sci. Pollut. Res. 2011, 18, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Luo, Z.; Yan, Y.; Wang, Z.; Chi, Q.; Yan, C.; Xing, B. Arsenate accumulation, distribution, and toxicity associated with titanium dioxide nanoparticles in Daphnia magna. Environ. Sci. Technol. 2016, 50, 9636–9643. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-W.; Li, Y.; Miao, A.-J.; Yang, L.-Y. Cd2+ toxicity as affected by bare TiO2 nanoparticles and their bulk counterpart. Ecotoxicol. Environ. Saf. 2012, 85, 44–51. [Google Scholar] [CrossRef]
- Chen, J.; Qian, Y.; Li, H.; Cheng, Y.; Zhao, M. The reduced bioavailability of copper by nano-TiO2 attenuates the toxicity to Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2015, 22, 12407–12414. [Google Scholar] [CrossRef]
- Wang, J.; Dai, H.; Nie, Y.; Wang, M.; Yang, Z.; Cheng, L.; Liu, Y.; Chen, S.; Zhao, G.; Wu, L.; et al. TiO(2) nanoparticles enhance bioaccumulation and toxicity of heavy metals in Caenorhabditis elegans via modification of local concentrations during the sedimentation process. Ecotoxicol. Env. Saf. 2018, 162, 160–169. [Google Scholar] [CrossRef]
- Asztemborska, M.; Jakubiak, M.; Stęborowski, R.; Chajduk, E.; Bystrzejewska-Piotrowska, G. Titanium Dioxide Nanoparticle Circulation in an Aquatic Ecosystem. Water Air Soil Pollut. 2018, 229, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Akeel, K. The pollution of water by trace elements research trends. Adv. Bioremediation Phytoremediation 2017. [Google Scholar] [CrossRef] [Green Version]
- Nigro, M.; Bernardeschi, M.; Costagliola, D.; Della Torre, C.; Frenzilli, G.; Guidi, P.; Lucchesi, P.; Mottola, F.; Santonastaso, M.; Scarcelli, V. n-TiO2 and CdCl2 co-exposure to titanium dioxide nanoparticles and cadmium: Genomic, DNA and chromosomal damage evaluation in the marine fish European sea bass (Dicentrarchus labrax). Aquat. Toxicol. 2015, 168, 72–77. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Megharaj, M.; Naidu, R. Toxicity of tri-and penta-valent arsenic, alone and in combination, to the cladoceran Daphnia carinata: The influence of microbial transformation in natural waters. Environ. Geochem. Health 2009, 31, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.; Meng, X.; Korfiatis, G.P.; Jing, C. Adsorption mechanism of arsenic on nanocrystalline titanium dioxide. Environ. Sci. Technol. 2006, 40, 1257–1262. [Google Scholar] [CrossRef]
- Yan, C.; Yang, F.; Wang, Z.; Wang, Q.; Seitz, F.; Luo, Z. Changes in arsenate bioaccumulation, subcellular distribution, depuration, and toxicity in Artemia salina nauplii in the presence of titanium dioxide nanoparticles. Environ. Sci. Nano 2017, 4, 1365–1376. [Google Scholar] [CrossRef]
- Yang, F.; Zeng, L.; Luo, Z.; Wang, Z.; Huang, F.; Wang, Q.; Drobne, D.; Yan, C. Complex role of titanium dioxide nanoparticles in the trophic transfer of arsenic from Nannochloropsis maritima to Artemia salina nauplii. Aquat. Toxicol. 2018, 198, 231–239. [Google Scholar] [CrossRef]
- Nunes, S.M.; Müller, L.; Simioni, C.; Ouriques, L.C.; Gelesky, M.A.; Fattorini, D.; Regoli, F.; Monserrat, J.M.; Ventura-Lima, J. Impact of different crystalline forms of nTiO2 on metabolism and arsenic toxicity in Limnoperna fortunei. Sci. Total. Environ. 2020, 728, 138318. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, D.; Sun, C. Effect of heavy metals on the sorption of hydrophobic organic compounds to wood charcoal. Environ. Sci. Technol. 2007, 41, 2536–2541. [Google Scholar] [CrossRef]
- Rosenfeldt, R.R.; Seitz, F.; Schulz, R.; Bundschuh, M. Heavy metal uptake and toxicity in the presence of titanium dioxide nanoparticles: A factorial approach using Daphnia magna. Environ. Sci. Technol. 2014, 48, 6965–6972. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.K.; Parvin, E.; Islam, M.M.; Akter, M.S.; Khan, S.; Al-Mamun, M.H. Lead-and cadmium-induced histopathological changes in gill, kidney and liver tissue of freshwater climbing perch Anabas testudineus (Bloch, 1792). Chem. Ecol. 2014, 30, 532–540. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Ji, H. Stabilization process and potential of agro-industrial waste on Pb-Contaminated soil around Pb–Zn mining. Environ. Pollut. 2020, 260, 114069. [Google Scholar] [CrossRef]
- Gonzalez, H.O.; Roling, J.A.; Baldwin, W.S.; Bain, L.J. Physiological changes and differential gene expression in mummichogs (Fundulus heteroclitus) exposed to arsenic. Aquat. Toxicol. 2006, 77, 43–52. [Google Scholar] [CrossRef]
- Hu, S.; Han, J.; Yang, L.; Li, S.; Guo, Y.; Zhou, B.; Wu, H. Bioconcentration, depuration and toxicity of Pb in the presence of titanium dioxide nanoparticles in zebrafish larvae. Aquat. Toxicol. 2019, 214, 105257. [Google Scholar] [CrossRef] [PubMed]
- Matouke, M.M.; Mustapha, M. Bioaccumulation and physiological effects of copepods sp.(Eucyclop sp.) fed Chlorella ellipsoides exposed to titanium dioxide (TiO2) nanoparticles and lead (Pb2+). Aquat. Toxicol. 2018, 198, 30–39. [Google Scholar] [CrossRef]
- Oya-Silva, L.F.; Vicari, T.; Disner, G.R.; Lirola, J.R.; Klingelfus, T.; Gonçalves, H.d.L.S.; Leite, T.P.B.; de Morais Calado, S.L.; Voigt, C.L.; de Assis, H.C.S. Tissue-specific genotoxicity and antioxidant imbalance of titanium dioxide nanoparticles (NPTiO2) and inorganic lead (PbII) in a neotropical fish species. Environ. Toxicol. Pharmacol. 2021, 82, 103551. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; Mccall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef]
- Chalew, T.E.A.; Ajmani, G.S.; Huang, H.; Schwab, K.J. Evaluating nanoparticle breakthrough during drinking water treatment. Environ. Health Perspect. 2013, 121, 1161–1166. [Google Scholar] [CrossRef]
- Majedi, S.M.; Lee, H.K.; Kelly, B.C. Chemometric analytical approach for the cloud point extraction and inductively coupled plasma mass spectrometric determination of zinc oxide nanoparticles in water samples. Anal. Chem. 2012, 84, 6546–6552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Eng, C.Y.; Stuckey, D.C.; Zhou, Y. Effects of ZnO nanoparticle exposure on wastewater treatment and soluble microbial products (SMPs) in an anoxic-aerobic membrane bioreactor. Chemosphere 2017, 171, 446–459. [Google Scholar] [CrossRef]
- Li, Q.L.; Sun, Y.; Sun, Y.L.; Wen, J.; Zhou, Y.; Bing, Q.M.; Isaacs, L.D.; Jin, Y.; Gao, H.; Yang, Y.W. Mesoporous Silica Nanoparticles Coated by Layer-by-Layer Self-assembly Using Cucurbit[7]uril for in Vitro and in Vivo Anticancer Drug Release. Chem. Mater. A Publ. Am. Chem. Society 2014, 26, 6418–6431. [Google Scholar] [CrossRef] [Green Version]
- Mai, W.X.; Meng, H. Mesoporous silica nanoparticles: A multifunctional nano therapeutic system. Integr. Biol. Quant. Biosci. Nano Macro 2013, 5, 19–28. [Google Scholar] [CrossRef]
- Keller, A.A.; Lazareva, A. Predicted releases of engineered nanomaterials: From global to regional to local. Environ. Sci. Technol. Lett. 2014, 1, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Liu, X.; Zhang, A.; Lu, D.; Li, G.; Zhang, Q.; Liu, Q.; Jiang, G. Distinguishing the sources of silica nanoparticles by dual isotopic fingerprinting and machine learning. Nat. Commun. 2019, 10, 1620. [Google Scholar] [CrossRef]
- Yousef, M.I.; Mutar, T.F.; Kamel, M.A.E.L.N. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol. Rep. 2019, 6, 336–346. [Google Scholar] [CrossRef]
- Stanley, J.K.; Coleman, J.G.; Weiss, C.A., Jr.; Steevens, J.A. Sediment toxicity and bioaccumulation of nano and micron—sized aluminum oxide. Environ. Toxicol. Chem. 2010, 29, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yokel, R.A.; Hennig, B.; Toborek, M. Manufactured aluminum oxide nanoparticles decrease expression of tight junction proteins in brain vasculature. J. Neuroimmune Pharmacol. 2008, 3, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanyam, A.; Sailaja, N.; Mahboob, M.; Rahman, M.; Hussain, S.M.; Grover, P. In vivo genotoxicity assessment of aluminium oxide nanomaterials in rat peripheral blood cells using the comet assay and micronucleus test. Mutagenesis 2009, 24, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, M.; Ji, J.; Gao, F.; Bai, R.; Chen, C.; Wang, Z.; Zhang, C.; Niu, Q. In vivo toxicity of nano-alumina on mice neurobehavioral profiles and the potential mechanisms. Int. J. Immunopathol. Pharmacol. 2011, 24, 23S. [Google Scholar]
- Oesterling, E.; Chopra, N.; Gavalas, V.; Arzuaga, X.; Lim, E.J.; Sultana, R.; Butterfield, D.A.; Bachas, L.; Hennig, B. Alumina nanoparticles induce expression of endothelial cell adhesion molecules. Toxicol. Lett. 2008, 178, 160–166. [Google Scholar] [CrossRef]
- Dey, S.; Bakthavatchalu, V.; Tseng, M.T.; Wu, P.; Florence, R.L.; Grulke, E.A.; Yokel, R.A.; Dhar, S.K.; Yang, H.-S.; Chen, Y. Interactions between SIRT1 and AP-1 reveal a mechanistic insight into the growth promoting properties of alumina (Al2O3) nanoparticles in mouse skin epithelial cells. Carcinogenesis 2008, 29, 1920–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.-Y.; Lee, S.-M.; Yang, J.-K. Removal of As (III) and As (V) by natural and synthetic metal oxides. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 202–207. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.; Choi, I.; Rengaraj, S.; Yi, J. Arsenic removal using mesoporous alumina prepared via a templating method. Environ. Sci. Technol. 2004, 38, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Yadanaparthi, S.K.R.; Graybill, D.; von Wandruszka, R. Adsorbents for the removal of arsenic, cadmium, and lead from contaminated waters. J. Hazard. Mater. 2009, 171, 1–15. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, Y.; Lin, K.; Chen, L.; Dong, Q.; Huang, C. Toxic effects of copper ion in zebrafish in the joint presence of CdTe QDs. Environ. Pollut. 2013, 176, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Von der Kammer, F.; Hofmann, T.; Baalousha, M.; Ottofuelling, S.; Baun, A. Algal testing of titanium dioxide nanoparticles—testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 2010, 269, 190–197. [Google Scholar] [CrossRef]
- Dalai, S.; Pakrashi, S.; Bhuvaneshwari, M.; Iswarya, V.; Chandrasekaran, N.; Mukherjee, A. Toxic effect of Cr (VI) in presence of n-TiO2 and n-Al2O3 particles towards freshwater microalgae. Aquat. Toxicol. 2014, 146, 28–37. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Qiao, J.; Wang, H.; Li, L. Synergistic effects of nano-sized titanium dioxide and zinc on the photosynthetic capacity and survival of Anabaena sp. Int. J. Mol. Sci. 2013, 14, 14395–14407. [Google Scholar] [CrossRef]
- Erickson, R.J.; Brooke, L.T.; Kahl, M.D.; Venter, F.V.; Harting, S.L.; Markee, T.P.; Spehar, R.L. Effects of laboratory test conditions on the toxicity of silver to aquatic organisms. Environ. Toxicol. Chem. Int. J. 1998, 17, 572–578. [Google Scholar] [CrossRef]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. Int. J. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Qu, R.-J.; Wang, X.-H.; Feng, M.-B.; Li, Y.; Liu, H.-X.; Wang, L.-S.; Wang, Z.-Y. The toxicity of cadmium to three aquatic organisms (Photobacterium phosphoreum, Daphnia magna and Carassius auratus) under different pH levels. Ecotoxicol. Environ. Saf. 2013, 95, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Seitz, F.; Rosenfeldt, R.R.; Storm, K.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Bundschuh, M. Effects of silver nanoparticle properties, media pH and dissolved organic matter on toxicity to Daphnia magna. Ecotoxicol. Environ. Saf. 2015, 111, 263–270. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Hull, M.S.; Bednar, A.J.; Goss, J.D.; Gunter, J.C.; Bouldin, J.L.; Vikesland, P.J.; Steevens, J.A. Fractionating nanosilver: Importance for determining toxicity to aquatic test organisms. Environ. Sci. Technol. 2010, 44, 9571–9577. [Google Scholar] [CrossRef]
- Playle, R.C. Using multiple metal–gill binding models and the toxic unit concept to help reconcile multiple-metal toxicity results. Aquat. Toxicol. 2004, 67, 359–370. [Google Scholar] [CrossRef]
- Guan, R.; Wang, W.-X. Comparison between two clones of Daphnia magna: Effects of multigenerational cadmium exposure on toxicity, individual fitness, and biokinetics. Aquat. Toxicol. 2006, 76, 217–229. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Gao, Y.; Mu, L.; Zhou, Q. Knowledge gaps between nanotoxicological research and nanomaterial safety. Environ. Int. 2016, 94, 8–23. [Google Scholar] [CrossRef]
- Mendes, L.A.; Maria, V.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Multigenerational exposure of Folsomia candida to silver: Effect of different contamination scenarios (continuous versus pulsed and recovery). Sci. Total Environ. 2018, 631-632, 326–333. [Google Scholar] [CrossRef]
- Fajana, H.O.; Jegede, O.O.; James, K.; Hogan, N.S.; Siciliano, S.D. Uptake, toxicity, and maternal transfer of cadmium in the oribatid soil mite, Oppia nitens: Implication in the risk assessment of cadmium to soil invertebrates. Environ. Pollut. 2020, 259, 113912. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, S.; Long, Y.; Gong, S.; Tian, B.; Ma, T. Impacts of multi-walled carbon nanotubes on ecotoxicity of Cd and BDE-47 in sediments. Acta Sci. Circumstantiae 2015, 35, 4150–4158. [Google Scholar]
- Fan, W.; Liang, D.; Wang, X.; Ren, J.; Xiao, S.; Zhou, T. Two-generational effects and recovery of arsenic and arsenate on Daphnia magna in the presence of nano-TiO2. Ecotoxicol. Environ. Saf. 2019, 172, 136–143. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Hsieh, Y.K.; Wang, C.F.; Chen, I.C.; Huang, Y.J. Trojan-horse mechanism in the cellular uptake of silver nanoparticles verified by direct intra- and extracellular silver speciation analysis. Environ. Sci. Technol. 2015, 49, 3813–3821. [Google Scholar] [CrossRef] [PubMed]
- El-Dib, F.I.; Mohamed, D.E.; El-Shamy, O.A.A.; Mishrif, M.R. Study the adsorption properties of magnetite nanoparticles in the presence of different synthesized surfactants for heavy metal ions removal. Egypt. J. Pet. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Bagbi, Y.; Sarswat, A.; Mohan, D.; Pandey, A.; Solanki, P.R. Lead (Pb2+) adsorption by monodispersed magnetite nanoparticles: Surface analysis and effects of solution chemistry. J. Environ. Chem. Eng. 2016, 4, 4237–4247. [Google Scholar] [CrossRef]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef]
- Kim, I.; Lee, B.-T.; Kim, H.-A.; Kim, K.-W.; Kim, S.D.; Hwang, Y.-S. Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna. Chemosphere 2016, 143, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, W.; Wu, B.; Yu, J.; Yu, H.; Zhang, X.-X.; Torres-Duarte, C.; Cherr, G.N. Low levels of graphene and graphene oxide inhibit cellular xenobiotic defense system mediated by efflux transporters. Nanotoxicology 2016, 10, 597–606. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Wu, B.; Shen, Z.; Cherr, G.N.; Zhang, X.-X.; Li, M. Comparison of cytotoxicity and inhibition of membrane ABC transporters induced by MWCNTs with different length and functional groups. Environ. Sci. Technol. 2016, 50, 3985–3994. [Google Scholar] [CrossRef]


| Tested Conditions | Nanoparticle | Heavy Metal | Tested Organism | Co-Exposure Outcome | Refs. |
|---|---|---|---|---|---|
| I. In vitro models | TiO2NPs | Cd+2 | Human embryo kidney 293T (HEK293T) cells | Cd+2 and TiO2NPs exert synergistic effects on the cellular oxidative damage of HEK293T cells | [35] |
| Pb+2 | Human embryo hepatocytes | TiO2NPs and Pb+2 in combination induced cytotoxicity and oxidative stress in the absence of photoactivation. | [36] | ||
| II. In vivo models | TiO2NPs | Cd+2 | Carp (Cyprinus carpio) | A positive correlation was found between Cd+2 and TiO2NPs concentrations. | [37] |
| The Mediterranean mussel (Mytilus galloprovincialis) | TiO2NPs and Cd+2 co-exposure did not increase adverse effects in M. galloprovincialis. | [38] | |||
| Zebrafish (Danio rerio) | The presence of TiO2 NPs with Cd+2 slightly increased the uptake rate constants of Cd+2 in fish | [39] | |||
| Water flea (Daphnia magna) | TiO2NPs the uptake and retention of Cd+2 | [40] | |||
| Water column crustacean Daphnia magna Sediment oligochaete Lumbriculus variegatus | TiO2NPs increased the total Cd+2 body burden, but no change in toxicity was observed. | [41] | |||
| Chlamydomonas reinhardtii | TiO2 NPs presence alleviated the Cd+2 toxicity | [42] | |||
| Water flea (Daphnia magna) | TiO2NPs transport Cd+2 and Zn+2 into D. magna.TiO2NPs provide potential adsorption binding sites for Cd+2 within the D.magna gut. | [43] | |||
| The ciliate Tetrahymena thermophila | TiO2NPs enhanced Cd+2 accumulation | [44] | |||
| Asian clam (Corbicula fluminea) | The presence of TiO2NPs did not affect Cd+2 uptake by C. fluminea. | [45] | |||
| As | Carp (Cyprinus carpio) | TiO2NPs increased As+5 concentrations and bioavailability | [46,47] | ||
| Water flea (Ceriodaphnia dubia) | As+5 sorption onto the TiO2NPs surface contributes to the toxicity once nanoparticles enter the body. | [48] | |||
| Laeonereis acuta | TiO2NPs and As+3 co-exposure synergistically toxic | [49] | |||
| Freshwater algae (Microcystis aeruginosa and Scenedesmus obliquus) | TiO2NPs boosted As+3 and As+5 accumulation and methylation | [50] | |||
| Cu+2 | Water flea (Daphnia magna) | The coexistence of TiO2NPs with Cu+2 enhances the toxicity of Cu+2 to daphnids even at low concentrations | [51] | ||
| Water flea (Daphnia magna) | Cu+2 in the presence of TiO2NPs induced higher levels of oxidative stress and physiological damage | [52] | |||
| The leaf shredding amphipod Gammarus fossarum | The presence of TiO2NPs largely eliminated Cu+2-induced toxicity. | [53] | |||
| Pb+2 | Mice | No synergistic interaction exists between TiO2NPs and Pb+2. | [54] | ||
| Zebrafish (Danio rerio) larvae | TiO2NPs increase bioconcentration of Pb+2 | [55] | |||
| Neotropical fish species Hoplias intermedius | TiO2NPs induced oxidative stress increase at co-exposure with Pb+2 | [56] | |||
| ZnONPs | Pb+2 | Mice | ZnONPs enhanced the deposition of Pb in all major organs in the overweight mice | [57] | |
| Pb+2 | Rat | The joint exposure of Pb+2 and ZnONPs resulted in an additive toxic effect on the thyroid gland | [58] | ||
| Cd+2 | Mice | Combined ZnONPs and Cd+2 exposures at the organogenesis stage induced higher fetal deformity | [59] | ||
| SiNPs | Cd+2 | Mice | Synergistic effect of SiNPs and Cd+2 | [60] | |
| Pb+2 | Sprague Dawley male rats | Co-exposure to SiNPs and Pb+2 resulted in additive and synergistic effects on the cardiovascular system. | [61] | ||
| Al2O3NPs | As+5 | Ceriodaphnia dubia | Al2O3NPs and inorganic As+5 co-exposure resulted in enhanced toxic effect | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Elhakim, Y.M.; Hashem, M.M.; Abo-EL-Sooud, K.; Hassan, B.A.; Elbohi, K.M.; Al-Sagheer, A.A. Effects of Co-Exposure of Nanoparticles and Metals on Different Organisms: A Review. Toxics 2021, 9, 284. https://doi.org/10.3390/toxics9110284
Abd-Elhakim YM, Hashem MM, Abo-EL-Sooud K, Hassan BA, Elbohi KM, Al-Sagheer AA. Effects of Co-Exposure of Nanoparticles and Metals on Different Organisms: A Review. Toxics. 2021; 9(11):284. https://doi.org/10.3390/toxics9110284
Chicago/Turabian StyleAbd-Elhakim, Yasmina M., Mohamed M. Hashem, Khaled Abo-EL-Sooud, Bayan A. Hassan, Khlood M. Elbohi, and Adham A. Al-Sagheer. 2021. "Effects of Co-Exposure of Nanoparticles and Metals on Different Organisms: A Review" Toxics 9, no. 11: 284. https://doi.org/10.3390/toxics9110284
APA StyleAbd-Elhakim, Y. M., Hashem, M. M., Abo-EL-Sooud, K., Hassan, B. A., Elbohi, K. M., & Al-Sagheer, A. A. (2021). Effects of Co-Exposure of Nanoparticles and Metals on Different Organisms: A Review. Toxics, 9(11), 284. https://doi.org/10.3390/toxics9110284