Methylmercury-Induced Metabolic Alterations in Caenorhabditis elegans Are Diet-Dependent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. C. elegans Strains and Worm Maintenance
2.3. Dose–Response Survival Curves
2.4. Mercury Quantification
2.5. Triglyceride Quantification
2.6. Nile Red Staining
2.7. RNA Isolation and Real-Time qPCR Gene Expression
2.8. Feeding Behavioral Analysis
2.9. Glutathione Quantification
2.10. Intracellular Reactive Oxygen Species Determination
2.11. Protein Oxidation Quantification
2.12. Oxidative Stress Reporter Assay
2.13. Statistics
3. Results
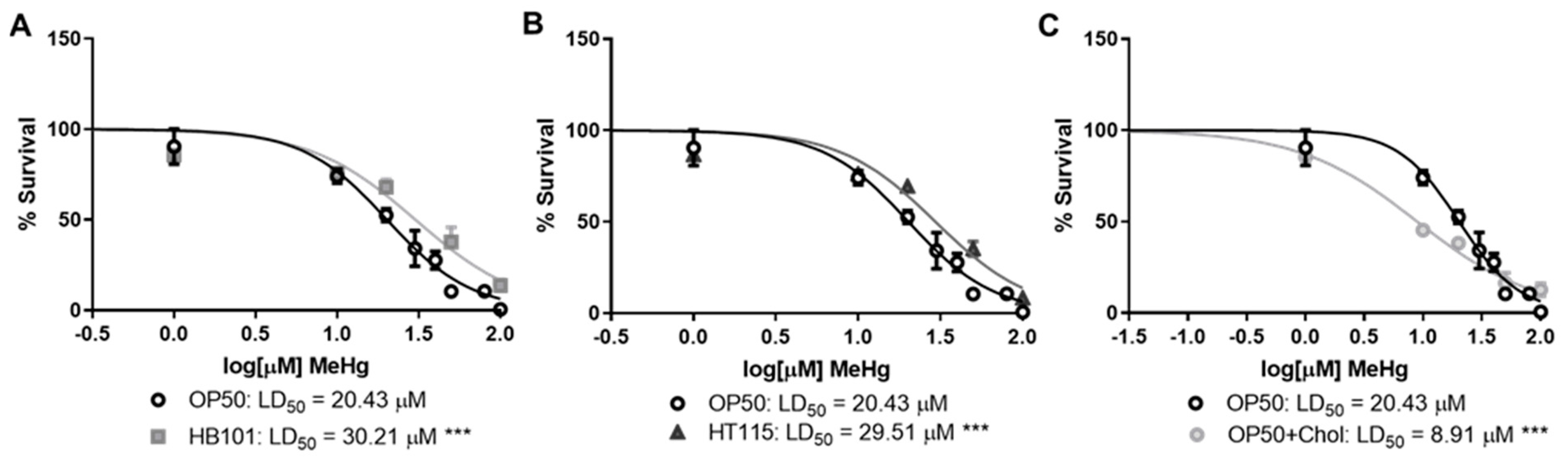
3.1. Bacterial Diet Affects MeHg Toxicity
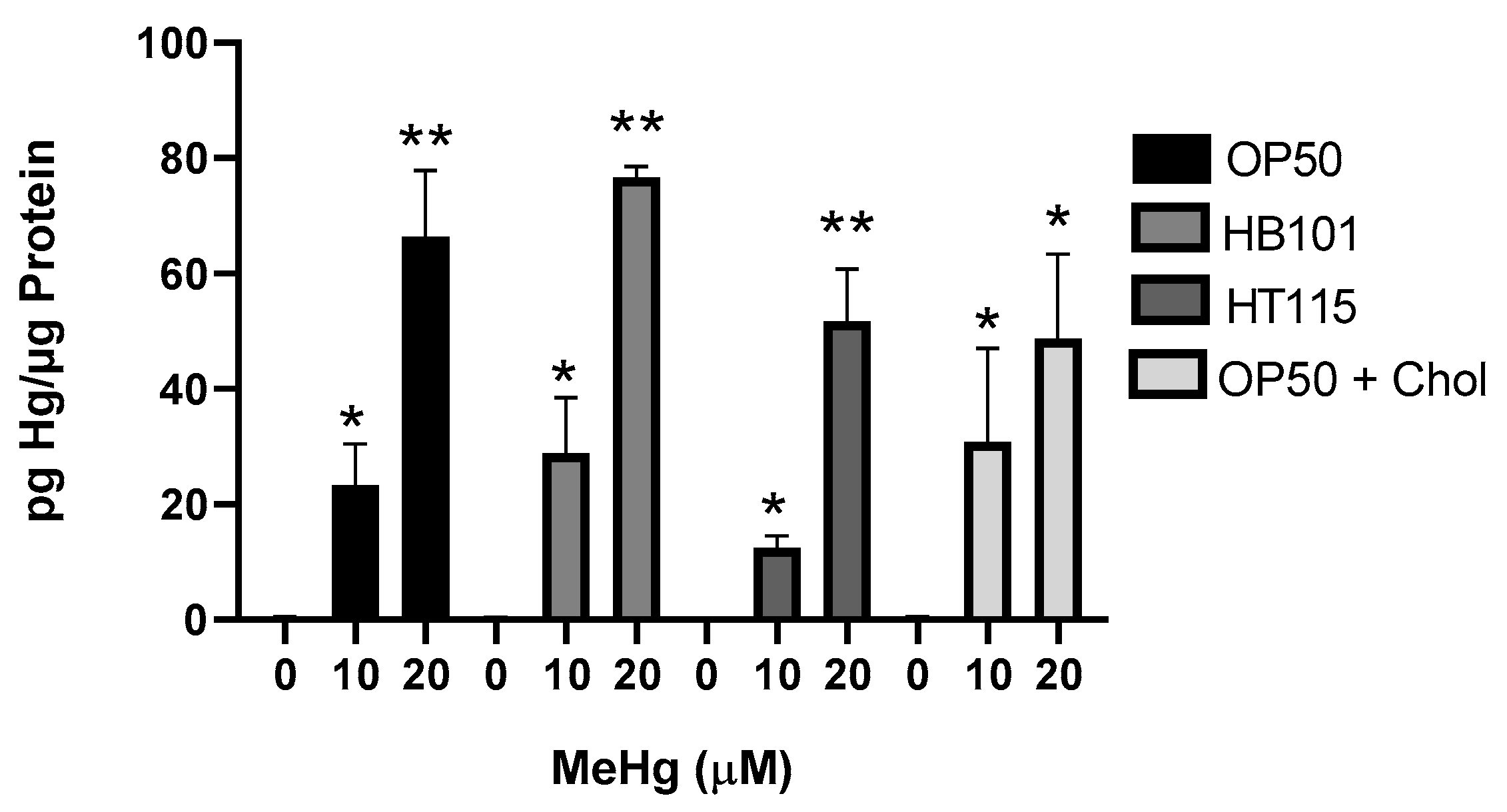
3.2. Diet Did Not Alter Mercury Accumulation
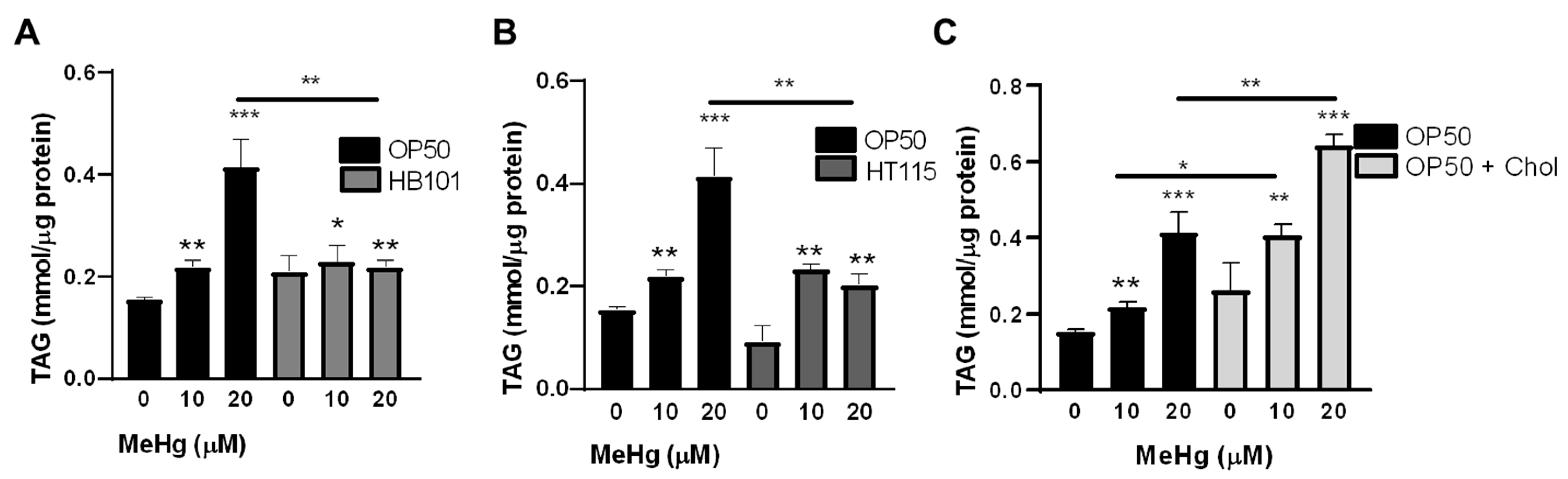
3.3. Bacterial Diet Altered Lipid Accumulation in Response to MeHg
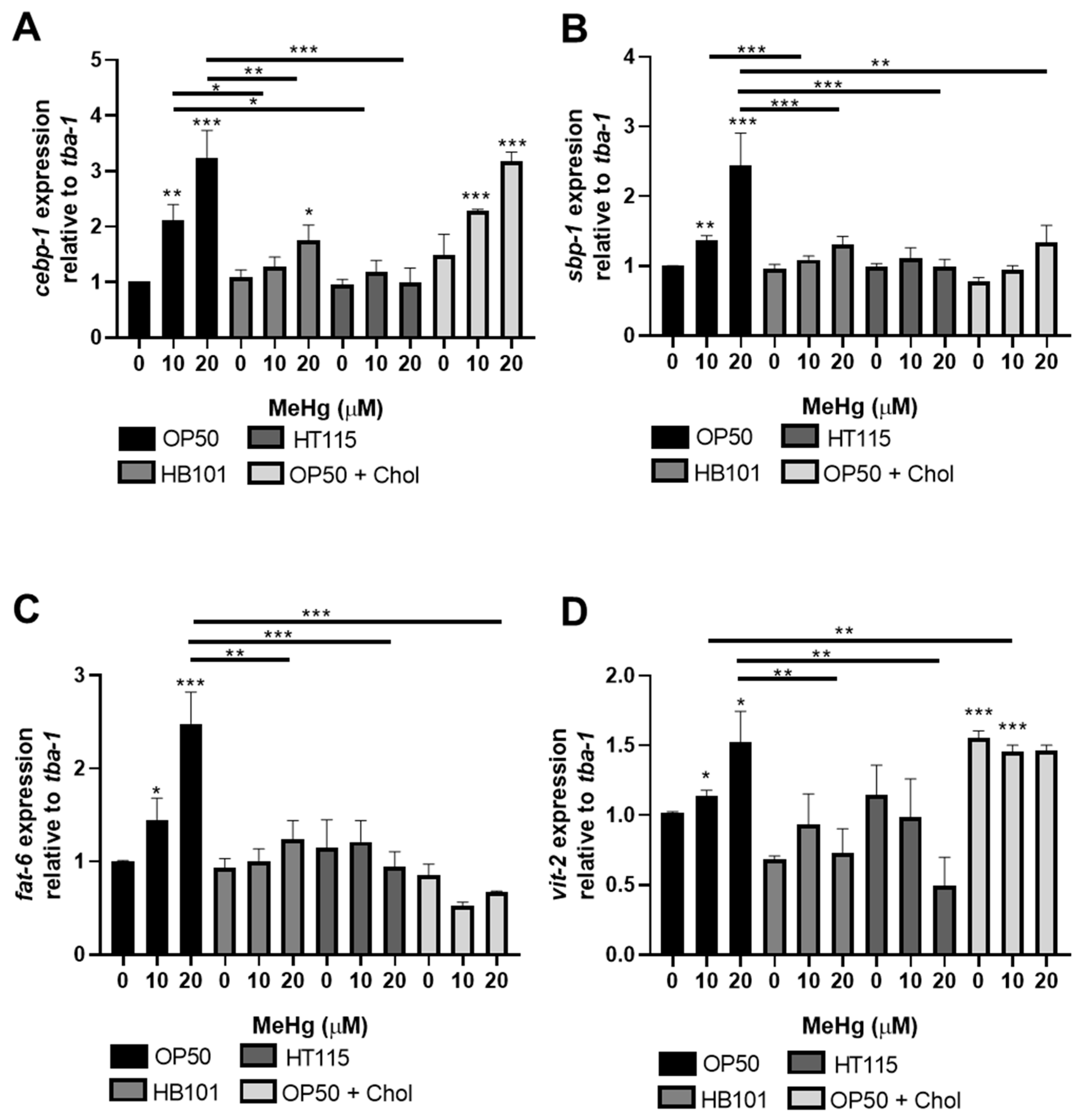
3.4. MeHg-Induced Pro-Adipogenic Gene Transcription Is Diet-Dependent
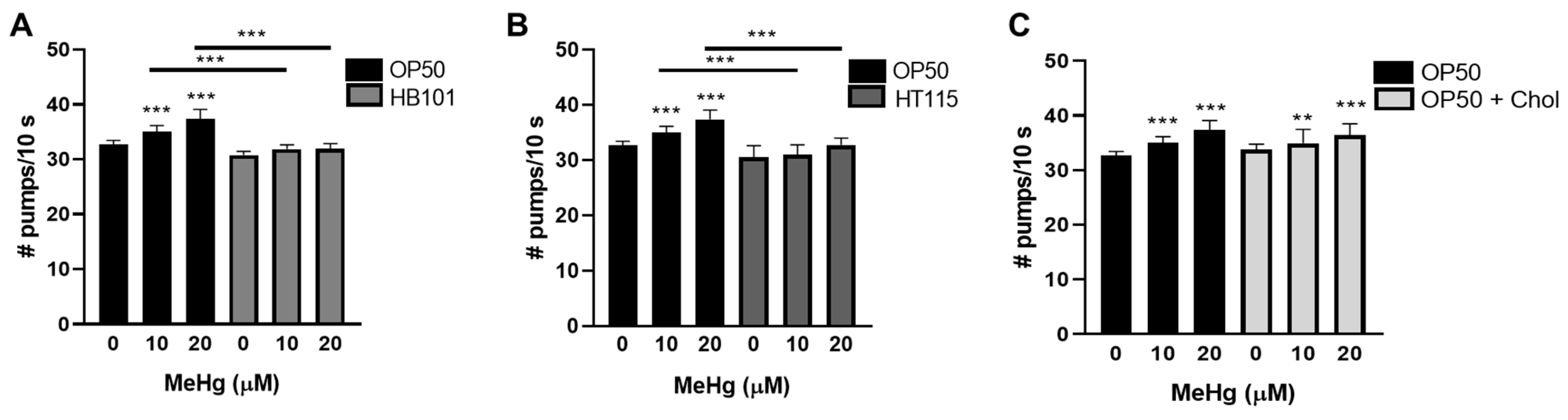
3.5. Feeding Behavior in Response to MeHg Is Dependent on Diet
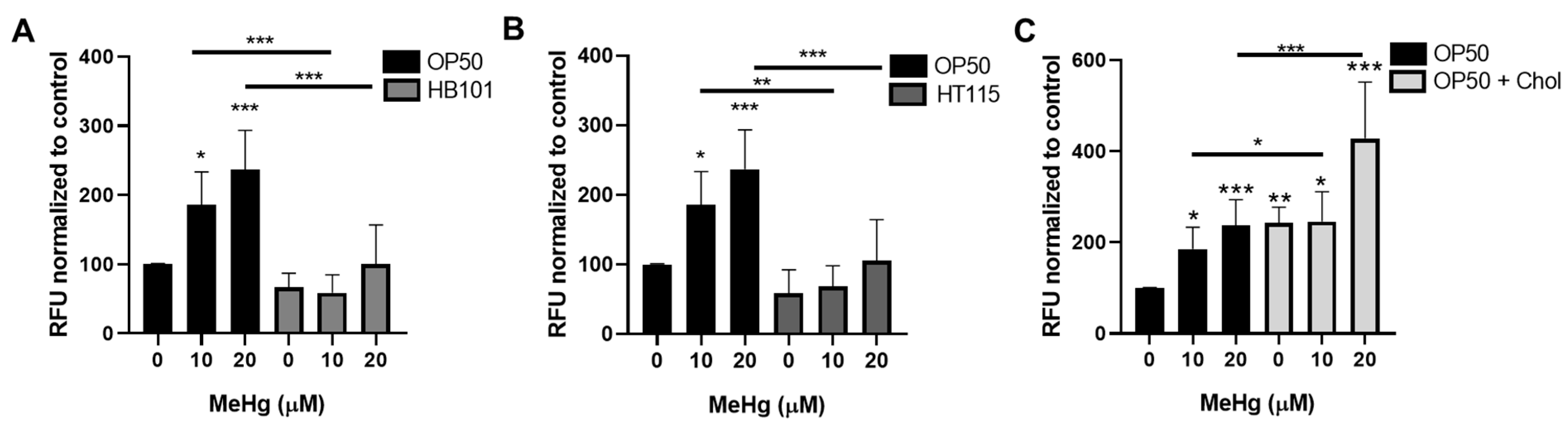
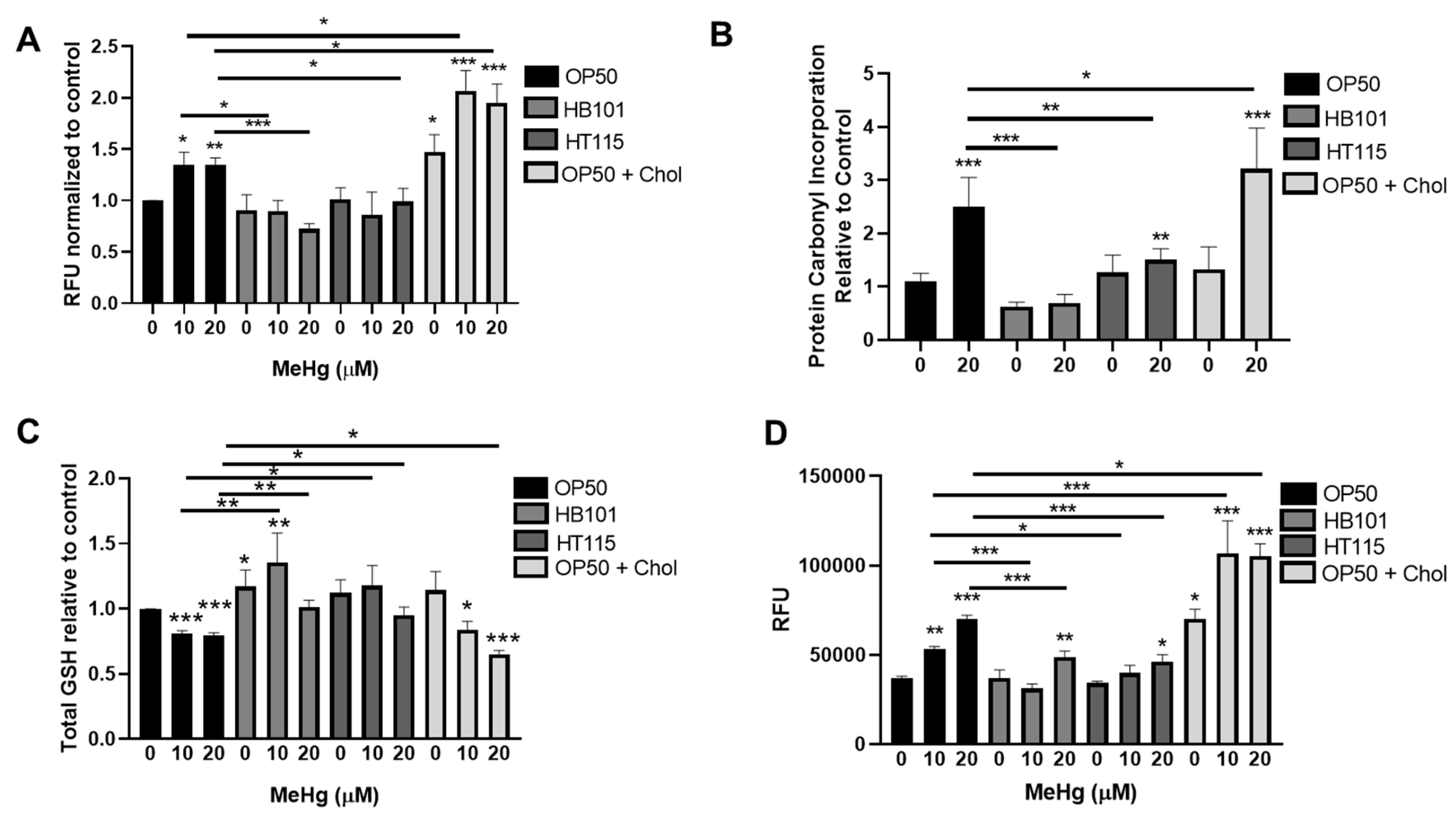
3.6. Bacterial Diet Improves Measures of Oxidative Stress in Response to MeHg
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chamorro-Garcia, R.; Blumberg, B. Current Research Approaches and Challenges in the Obesogen Field. Front. Endocrinol. 2019, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Kassotis, C.D.; Stapleton, H.M. Endocrine-Mediated Mechanisms of Metabolic Disruption and New Approaches to Examine the Public Health Threat. Front. Endocrinol. 2019, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, T.W.; Magos, L. The Toxicology of Mercury and Its Chemical Compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Sonawane, B.; Butler, R.N.; Trasande, L.; Callan, R.; Droller, D. Early Environmental Origins of Neurodegenerative Disease in Later Life. Environ. Health Perspect. 2005, 113, 1230–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulka, C.M.; Persky, V.W.; Daviglus, M.L.; Durazo-Arvizu, R.A.; Argos, M. Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res. 2019, 168, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mukherjee, B.; Park, S.K. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ. Int. 2018, 121, 683–694. [Google Scholar] [CrossRef]
- Lee, K. Blood mercury concentration in relation to metabolic and weight phenotypes using the KNHANES 2011–2013 data. Int. Arch. Occup. Environ. Health 2018, 91, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Ha, K.H.; He, K.; Kim, D.J. Association between Blood Mercury Level and Visceral Adiposity in Adults. Diabetes Metab. J. 2017, 41, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Seo, E. Association between Toenail Mercury and Metabolic Syndrome is Modified by Selenium. Nutrients 2016, 8, 424. [Google Scholar] [CrossRef] [Green Version]
- Caito, S.W.; Newell-Caito, J.; Martell, M.; Crawford, N.; Aschner, M. Methylmercury Induces Metabolic Alterations in Caenorhabditis elegans: Role for C/EBP Transcription Factor. Toxicol. Sci. 2020, 174, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Williams, V.J. Marine fish food in the United States and methylmercury risk. Int. J. Environ. Health Res. 2009, 19, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Simmons-Willis, T.A.; Koh, A.S.; Clarkson, T.W.; Ballatori, N. Transport of a neurotoxicant by molecular mimicry: The methylmercury–l-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem. J. 2002, 367, 239–246. [Google Scholar] [CrossRef]
- Caito, S.W.; Zhang, Y.; Aschner, M. Involvement of AAT transporters in methylmercury toxicity in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2013, 435, 546–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, T.; Yasutake, A.; Hirayama, K. Influence of dietary levels of protein and sulfur amino acids on the fate of methylmercury in mice. Toxicology 1994, 93, 225–234. [Google Scholar] [CrossRef]
- Meydani, M.; Meydani, S.N.; Hathcock, J.N. Effects of dietary methionine, methylmercury, and atrazine on ex-vivo synthesis of prostaglandin E1 and thromboxane B2. Prostaglandins Leukot. Med. 1984, 14, 267–278. [Google Scholar] [CrossRef]
- Cabañero, A.I.; Madrid, A.Y.; Cámara, C. Selenium Long-Term Administration and Its Effect on Mercury Toxicity. J. Agric. Food Chem. 2006, 54, 4461–4468. [Google Scholar] [CrossRef] [PubMed]
- Folven, K.I.; Glover, C.N.; Malde, M.K.; Lundebye, A.-K. Does selenium modify neurobehavioural impacts of developmental methylmercury exposure in mice? Environ. Toxicol. Pharmacol. 2009, 28, 111–119. [Google Scholar] [CrossRef]
- Wu, H.; Xu, L.; Ballantyne, C.M. Dietary and Pharmacological Fatty Acids and Cardiovascular Health. J. Clin. Endocrinol. Metab. 2020, 105, 1030–1045. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Strain, J.J.; Davidson, P.W.; Thurston, S.W.; Harrington, D.; Mulhern, M.S.; McAfee, A.J.; van Wijngaarden, E.; Shamlaye, C.F.; Henderson, J.; Watson, G.; et al. Maternal PUFA Status but Not Prenatal Methylmercury Exposure Is Associated with Children’s Language Functions at Age Five Years in the Seychelles. J. Nutr. 2012, 142, 1943–1949. [Google Scholar] [CrossRef]
- Strain, J.J.; Yeates, A.J.; van Wijngaarden, E.; Thurston, S.W.; Mulhern, M.S.; McSorley, E.M.; Watson, G.E.; Love, T.M.; Smith, T.H.; Yost, K.; et al. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: Associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am. J. Clin. Nutr. 2015, 101, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Strain, J.J.; Love, T.M.; Yeates, A.J.; Weller, D.; Mulhern, M.S.; McSorley, E.M.; Thurston, S.W.; Watson, G.E.; Mruzek, D.; Broberg, K.; et al. Associations of prenatal methylmercury exposure and maternal polyunsaturated fatty acid status with neurodevelopmental outcomes at 7 years of age: Results from the Seychelles Child Development Study Nutrition Cohort 2. Am. J. Clin. Nutr. 2021, 113, 304–313. [Google Scholar] [CrossRef]
- Nøstbakken, O.J.; Bredal, I.L.; Olsvik, P.A.; Huang, T.S.; Torstensen, B.E. Effect of Marine Omega 3 Fatty Acids on Methylmercury-Induced Toxicity in Fish and Mammalian CellsIn Vitro. J. Biomed. Biotechnol. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.-F.; Li, X.; Shi, M.; Li, D. n-3 Polyunsaturated Fatty Acids and Metabolic Syndrome Risk: A Meta-Analysis. Nutrients 2017, 9, 703. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Cheong, M.C.; Lee, H.-J.; Na, K.; Joo, H.-J.; Avery, L.; You, Y.; Paik, Y.-K. NSBP-1 mediates the effects of cholesterol on insulin/IGF-1 signaling in Caenorhabditis elegans. Cell. Mol. Life Sci. 2013, 70, 1623–1636. [Google Scholar] [CrossRef] [Green Version]
- Stiernagle, T. Maintenance of C. elegans. In C. Elegans: A Practical Approach; Hope, I.A., Ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Caito, S.W.; Aschner, M. NAD+Supplementation Attenuates Methylmercury Dopaminergic and Mitochondrial Toxicity in Caenorhabditis Elegans. Toxicol. Sci. 2016, 151, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Finley, E.J.; Chakraborty, S.; Slaughter, J.C.; Aschner, M. Early-Life Exposure to Methylmercury in Wildtype and pdr-1/parkin Knockout, C. elegans. Neurochem. Res. 2013, 38, 1543–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pino, E.C.; Webster, C.M.; Carr, C.E.; Soukas, A.A. Biochemical and High Throughput Microscopic Assessment of Fat Mass in Caenorhabditis Elegans. J. Vis. Exp. 2013, 73, e50180. [Google Scholar] [CrossRef] [Green Version]
- Sawin, E.R.; Ranganathan, R.; Horvitz, H. C. elegans Locomotory Rate Is Modulated by the Environment through a Dopaminergic Pathway and by Experience through a Serotonergic Pathway. Neuron 2000, 26, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Caito, S.W.; Aschner, M. Quantification of Glutathione in Caenorhabditis elegans. Curr. Protoc. Toxicol. 2015, 64, 6.18.1–6.18.6. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.S.; Lee, M.H.; Cha, D.S. Measurement of Intracellular ROS in Caenorhabditis elegans Using 2’,7’-Dichlorodihydrofluorescein Diacetate. Bio-Protocol 2018, 8, e2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, K.; Adachi, H.; Fujiwara, Y.; Ishii, N. Protein carbonyl accumulation in aging dauer formation-defective (daf) mutants of Caenorhabditis elegans. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 1999, 54, B47–B51. [Google Scholar] [CrossRef] [Green Version]
- Stiernagle, T. Maintenance of C. Elegans. WormBook 2006, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, K.K.; Liang, B.; Watts, J.L. The Influence of Bacterial Diet on Fat Storage in C. elegans. PLoS ONE 2009, 4, e7545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruszkiewicz, J.A.; de Macedo, G.T.; Miranda-Vizuete, A.; Bowman, A.B.; Bornhorst, J.; Schwerdtle, T.; Soares, F.A.A.; Aschner, M. Sex-Specific Response of Caenorhabditis elegans to Methylmercury Toxicity. Neurotox. Res. 2019, 35, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Schmidt, H.; Lai, B.; Ge, K. Transcriptional and Epigenomic Regulation of Adipogenesis. Mol. Cell. Biol. 2019, 39, e00601-18. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; DeBose-Boyd, R.A. Regulation of Cholesterol and Fatty Acid Synthesis. Cold Spring Harb. Perspect. Biol. 2011, 3, a004754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath, H.; Ntambi, J.M. The role of stearoyl-CoA desaturase in obesity, insulin resistance, and inflammation. Ann. N. Y. Acad. Sci. 2011, 1243, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Van Gilst, M.R.; Hadjivassiliou, H.; Jolly, A.; Yamamoto, K.R. Nuclear Hormone Receptor NHR-49 Controls Fat Consumption and Fatty Acid Composition in C. elegans. PLoS Biol. 2005, 3, e53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, T.; Horikawa, M.; Shimamura, S.; Hashimoto, T.; Sakamoto, K. Fat accumulation in Caenorhabditis elegans is mediated by SREBP homolog SBP-1. Genes Nutr. 2010, 5, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, M. Is vitellogenin an ancestor of apolipoprotein B-100 of human low-density lipoprotein and human lipoprotein lipase? Biochem. J. 1988, 255, 1057–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreiem, A.; Shan, M.; Okoniewski, R.J.; Sanchez-Morrissey, S.; Seegal, R.F. Methylmercury inhibits dopaminergic function in rat pup synaptosomes in an age-dependent manner. Neurotoxicology Teratol. 2009, 31, 312–317. [Google Scholar] [CrossRef]
- Shao, Y.; Chan, H.M. Effects of methylmercury on dopamine release in MN9D neuronal cells. Toxicol. Mech. Methods 2015, 25, 637–644. [Google Scholar] [CrossRef]
- Kanthe, P.S.; Patil, B.S.; Das, K.K. Terminalia arjuna supplementation ameliorates high fat diet-induced oxidative stress in nephrotoxic rats. J. Basic Clin. Physiol. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.P.; Gurtu, S.; Chakravarthi, S.; Moorthy, M.; Palanisamy, U.D. Geraniin Protects High-Fat Diet-Induced Oxidative Stress in Sprague Dawley Rats. Front. Nutr. 2018, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Lasker, S.; Rahman, M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef]
- Pei, Y.; Otieno, D.; Gu, I.; Lee, S.-O.; Parks, J.S.; Schimmel, K.; Kang, H.W. Effect of quercetin on nonshivering thermogenesis of brown adipose tissue in high-fat diet-induced obese mice. J. Nutr. Biochem. 2021, 88, 108532. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Li, X.; Yin, Z.; Jiang, H.; Sidoryk-Wegrzynowicz, M.; Milatovic, D.; Cai, J.; Aschner, M. Methylmercury Induces Acute Oxidative Stress, Altering Nrf2 Protein Level in Primary Microglial Cells. Toxicol. Sci. 2010, 116, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Finley, E.J.; Caito, S.; Slaughter, J.C.; Aschner, M. The Role of skn-1 in Methylmercury-Induced Latent Dopaminergic Neurodegeneration. Neurochem. Res. 2013, 38, 2650–2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, K.T.; Ashrafi, K. Caenorhabditis elegans as an emerging model for studying the basic biology of obesity. Dis. Model. Mech. 2009, 2, 224–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, T.; Aschner, M. Bacteria affect Caenorhabditis elegans responses to MeHg toxicity. NeuroToxicology 2019, 75, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.; van Wijngaarden, E.; Shamlaye, C.; Strain, J.; Myers, G. Putting findings from the Seychelles Child Development Study into perspective: The importance of a historical special issue of the Seychelles Medical and Dental Journal. NeuroToxicology 2020, 76, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.C. Identification of functional domains affected by developmental exposure to methylmercury: Faroe islands and related studies. NeuroToxicology 2000, 21, 1039–1044. [Google Scholar] [PubMed]
- Bélanger, M.-C.; Dewailly, É.; Berthiaume, L.; Noël, M.; Bergeron, J.; Mirault, M.-É.; Julien, P. Dietary contaminants and oxidative stress in Inuit of Nunavik. Metabolism 2006, 55, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Stuhr, N.L.; Curran, S.P. Bacterial diets differentially alter lifespan and healthspan trajectories in C. elegans. Commun. Biol. 2020, 3, 653. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Motomura, E.; Yanagisawa, R.; Hoang, V.A.T.; Mogi, M.; Mori, T.; Nakamura, M.; Takeya, M.; Eto, K. Evaluation of neurobehavioral impairment in methylmercury-treated KK-Ay mice by dynamic weight-bearing test. J. Appl. Toxicol. 2019, 39, 221–230. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yanagisawa, R.; Motomura, E.; Nakamura, M.; Sakamoto, M.; Takeya, M.; Eto, K. Increased methylmercury toxicity related to obesity in diabetic KK-Ay mice. J. Appl. Toxicol. 2014, 34, 914–923. [Google Scholar] [CrossRef]
- Leocádio, P.C.L.; Dias, R.P.; Pinto, D.V.; Reis, J.M.; Nascimento, J.C.R.; Brito, G.A.D.C.; Valença, J.T.; Foureaux, G.; Ferreira, A.J.; Windmöller, C.C.; et al. Pollutants and nutrition: Are methylmercury effects on blood pressure and lipoprotein profile comparable to high-fat diet in mice? Ecotoxicol. Environ. Saf. 2020, 204, 111036. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R.; Mallett, A.K.; Wise, A. The Effect of Diet on the Mammalian Gut Flora and Its Metabolic Activities. CRC Crit. Rev. Toxicol. 1985, 16, 31–103. [Google Scholar] [CrossRef] [PubMed]
- Ayotte, P.; Carrier, A.; Ouellet, N.; Boiteau, V.; Abdous, B.; Sidi, E.A.L.; Chateau-Degat, M.-L.; Dewailly, É. Relation between Methylmercury Exposure and Plasma Paraoxonase Activity in Inuit Adults from Nunavik. Environ. Health Perspect. 2011, 119, 1077–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, E.; de Oliveira, J.; Dutra, M.F.; Santos, D.B.; Gonçalves, C.A.; Goldfeder, E.M.; de Bem, A.F.; Prediger, R.; Aschner, M.; Farina, M. Does Methylmercury-Induced Hypercholesterolemia Play a Causal Role in Its Neurotoxicity and Cardiovascular Disease? Toxicol. Sci. 2012, 130, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Lin, X.; Lin, S. Neuropeptide Y and Metabolism Syndrome: An Update on Perspectives of Clinical Therapeutic Intervention Strategies. Front. Cell Dev. Biol. 2021, 9, 695623. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, B.; Peres, T.V.; Dos Santos, A.A.; Bornhorst, J.; Morcillo, P.; Gonçalves, C.L.; Aschner, M.; Vieira, T.P. Methylmercury Affects the Expression of Hypothalamic Neuropeptides That Control Body Weight in C57BL/6J Mice. Toxicol. Sci. 2018, 163, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.; Fordahl, S. Obesity and dietary fat influence dopamine neurotransmission: Exploring the convergence of metabolic state, physiological stress, and inflammation on dopaminergic control of food intake. Nutr. Res. Rev. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Haburcak, M.; Avena, N.; Moyer, M.; Hoebel, B.; Pothos, E. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 2009, 159, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Estes, M.K.; Bland, J.J.; Ector, K.K.; Puppa, M.J.; Powell, D.W.; Lester, D.B. A high fat western diet attenuates phasic dopamine release. Neurosci. Lett. 2021, 756, 135952. [Google Scholar] [CrossRef] [PubMed]
- Rojic-Becker, D.; Portero-Tresserra, M.; Martí-Nicolovius, M.; Vale-Martínez, A.; Guillazo-Blanch, G. Effects of caloric restriction on monoaminergic neurotransmission, peripheral hormones, and olfactory memory in aged rats. Behav. Brain Res. 2021, 409, 113328. [Google Scholar] [CrossRef] [PubMed]
- Dallière, N.; Holden-Dye, L.; Dillon, J.; O’Connor, V.; Walker, R.J. Caenorhabditis elegans Feeding Behaviors. In Oxford Research Encyclopedia of Neuroscience; Oxford University Press (OUP): Oxford, UK, 2017. [Google Scholar]
- Hills, T.; Brockie, P.J.; Maricq, A.V. Dopamine and Glutamate Control Area-Restricted Search Behavior in Caenorhabditis elegans. J. Neurosci. 2004, 24, 1217–1225. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Gadea, A.; Topper, S.; Young, L.; Crisp, A.; Kressin, L.; Elbel, E.; Maples, T.; Brauner, M.; Erbguth, K.; Axelrod, A.; et al. Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proc. Natl. Acad. Sci. USA 2011, 108, 17504–17509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvitz, H.R.; Chalfie, M.; Trent, C.; Sulston, J.E.; Evans, P.D. Serotonin and Octopamine in the Nematode Caenorhabditis elegans. Science 1982, 216, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Niacaris, T.; Avery, L. Serotonin regulates repolarization of the C. elegans pharyngeal muscle. J. Exp. Biol. 2003, 206, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Dent, J.A.; Davis, M.; Avery, L. avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 1997, 16, 5867–5879. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.R.; Pérez, C.L.; van Gilst, M.R.; Lee, B.H.; Ashrafi, K. Neural and Molecular Dissection of a C. elegans Sensory Circuit that Regulates Fat and Feeding. Cell Metab. 2008, 8, 118–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culbreth, M.; Aschner, M. Dysregulation of Glutamate Cycling Mediates Methylmercury-Induced Neurotoxicity. Adv. Neurobiol. 2016, 13, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Dahm, K.C.S.; Schwalm, F.D.; Brusque, A.M.; Frizzo, M.E.; Zeni, G.; Souza, D.; da Rocha, J.B.T. Methylmercury Increases Glutamate Release from Brain Synaptosomes and Glutamate Uptake by Cortical Slices from Suckling Rat Pups: Modulatory Effect of Ebselen. Toxicol. Sci. 2003, 73, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Mutkus, L.; Aschner, J.L.; Fitsanakis, V.A.; Aschner, M. The In Vitro Uptake of Glutamate in GLAST and GLT-1 Transfected Mutant CHO-K1 Cells Is Inhibited by Manganese. Biol. Trace Elem. Res. 2005, 107, 221–230. [Google Scholar] [CrossRef]
- Rodríguez-Palero, M.J.; López-Díaz, A.; Marsac, R.; Gomes, J.-E.; Olmedo, M.; Artal-Sanz, M. An automated method for the analysis of food intake behaviour in Caenorhabditis elegans. Sci. Rep. 2018, 8, 221–230. [Google Scholar] [CrossRef] [Green Version]
- You, Y.J.; Kim, J.; Raizen, D.M.; Avery, L. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: A model for satiety. Cell Metab. 2008, 7, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, C.; Avery, L. The FMRFamide Neuropeptide FLP-20 Acts as a Systemic Signal for Starvation Responses in Caenorhabditis elegans. Mol. Cells 2021, 44, 529–537. [Google Scholar] [CrossRef]
- Liu, H.; Qin, L.-W.; Li, R.; Zhang, C.; Al-Sheikh, U.; Wu, Z.-X. Reciprocal modulation of 5-HT and octopamine regulates pumping via feedforward and feedback circuits in C. elegans. Proc. Natl. Acad. Sci. USA 2019, 116, 7107–7112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vial, G.; Dubouchaud, H.; Couturier, K.; Cottet-Rousselle, C.; Taleux, N.; Athias, A.; Galinier, A.; Casteilla, L.; Leverve, X.M. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J. Hepatol. 2011, 54, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [Green Version]
- Fritz, K.S.; Petersen, D.R. Exploring the Biology of Lipid Peroxidation-Derived Protein Carbonylation. Chem. Res. Toxicol. 2011, 24, 1411–1419. [Google Scholar] [CrossRef] [Green Version]
- Farina, M.; Aschner, M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: An intriguing interplay. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 129285. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Usuki, F. Methylmercury-Mediated Oxidative Stress and Activation of the Cellular Protective System. Antioxidants 2020, 9, 1004. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, N.; Martell, M.; Nielsen, T.; Khalil, B.; Imtiaz, F.; Nguidjo, E.; Newell-Caito, J.L.; Bornhorst, J.; Schwerdtle, T.; Caito, S.W. Methylmercury-Induced Metabolic Alterations in Caenorhabditis elegans Are Diet-Dependent. Toxics 2021, 9, 287. https://doi.org/10.3390/toxics9110287
Crawford N, Martell M, Nielsen T, Khalil B, Imtiaz F, Nguidjo E, Newell-Caito JL, Bornhorst J, Schwerdtle T, Caito SW. Methylmercury-Induced Metabolic Alterations in Caenorhabditis elegans Are Diet-Dependent. Toxics. 2021; 9(11):287. https://doi.org/10.3390/toxics9110287
Chicago/Turabian StyleCrawford, Nicole, Megan Martell, Tyson Nielsen, Belal Khalil, Farooq Imtiaz, Etienne Nguidjo, Jennifer L. Newell-Caito, Julia Bornhorst, Tanja Schwerdtle, and Samuel W. Caito. 2021. "Methylmercury-Induced Metabolic Alterations in Caenorhabditis elegans Are Diet-Dependent" Toxics 9, no. 11: 287. https://doi.org/10.3390/toxics9110287
APA StyleCrawford, N., Martell, M., Nielsen, T., Khalil, B., Imtiaz, F., Nguidjo, E., Newell-Caito, J. L., Bornhorst, J., Schwerdtle, T., & Caito, S. W. (2021). Methylmercury-Induced Metabolic Alterations in Caenorhabditis elegans Are Diet-Dependent. Toxics, 9(11), 287. https://doi.org/10.3390/toxics9110287







