Τemporal Variation in Pesticide Residues in Citrus Fruits from Chios, Greece, before and after the Development of an Integrated Pest Management Strategy (IPMS): A Five-Year Study (LIFE13 ENV GR/000414)
Abstract
:1. Introduction
2. Materials and Methods
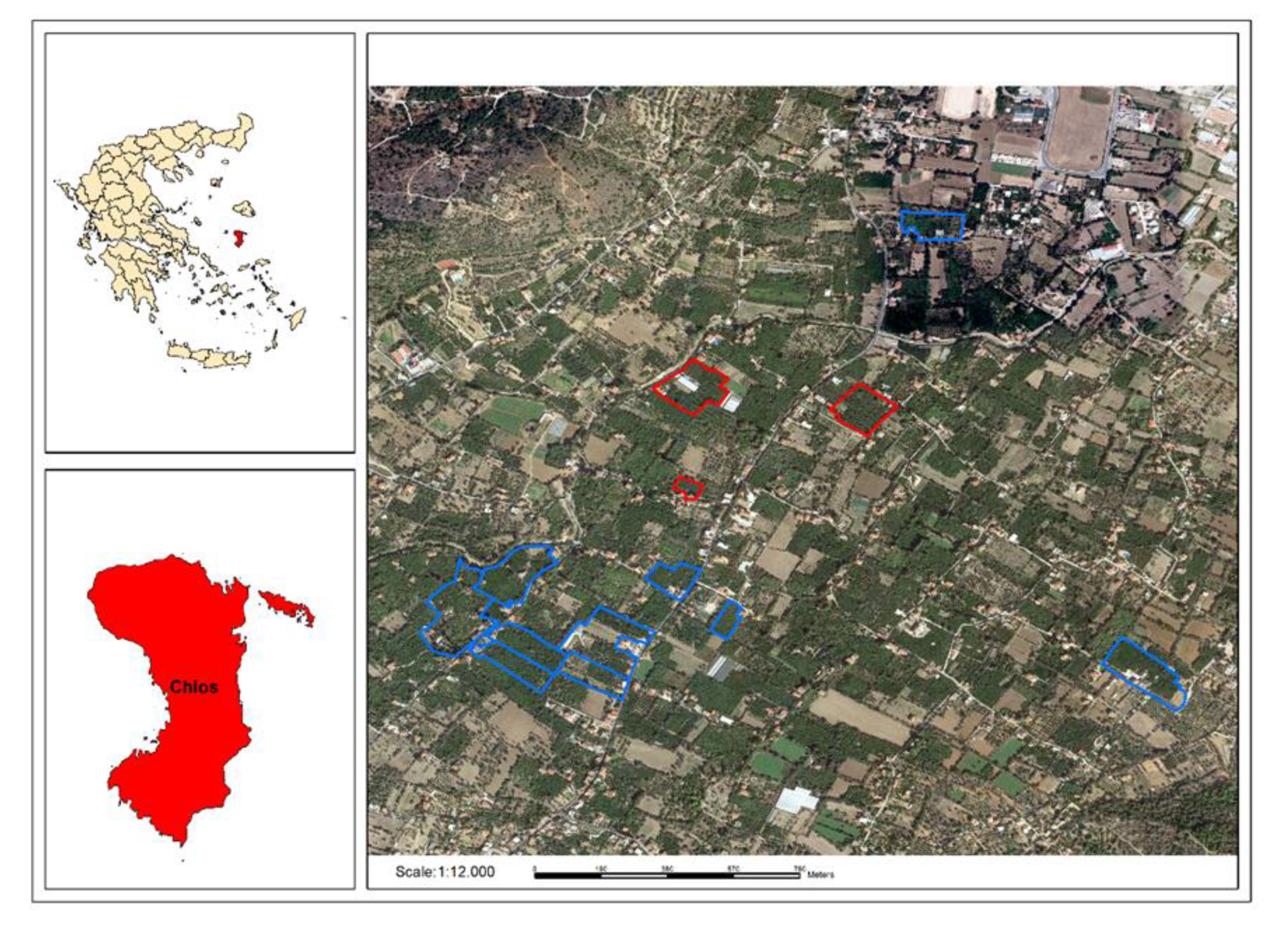
2.1. Studied Area
2.2. Selection of Analyzed Pesticides
2.3. Sampling of Plant Products
2.4. Analytical Methodology
2.4.1. Chemicals and Reagents
2.4.2. Standard Solutions
2.4.3. Sample Preparation
2.4.4. Determination of Compounds—Instrumentation
Analysis of Samples with Gas Chromatography
Analysis of Samples with Liquid Chromatography
2.5. Statistical Analysis
3. Results and Discussion
3.1. Method Performance
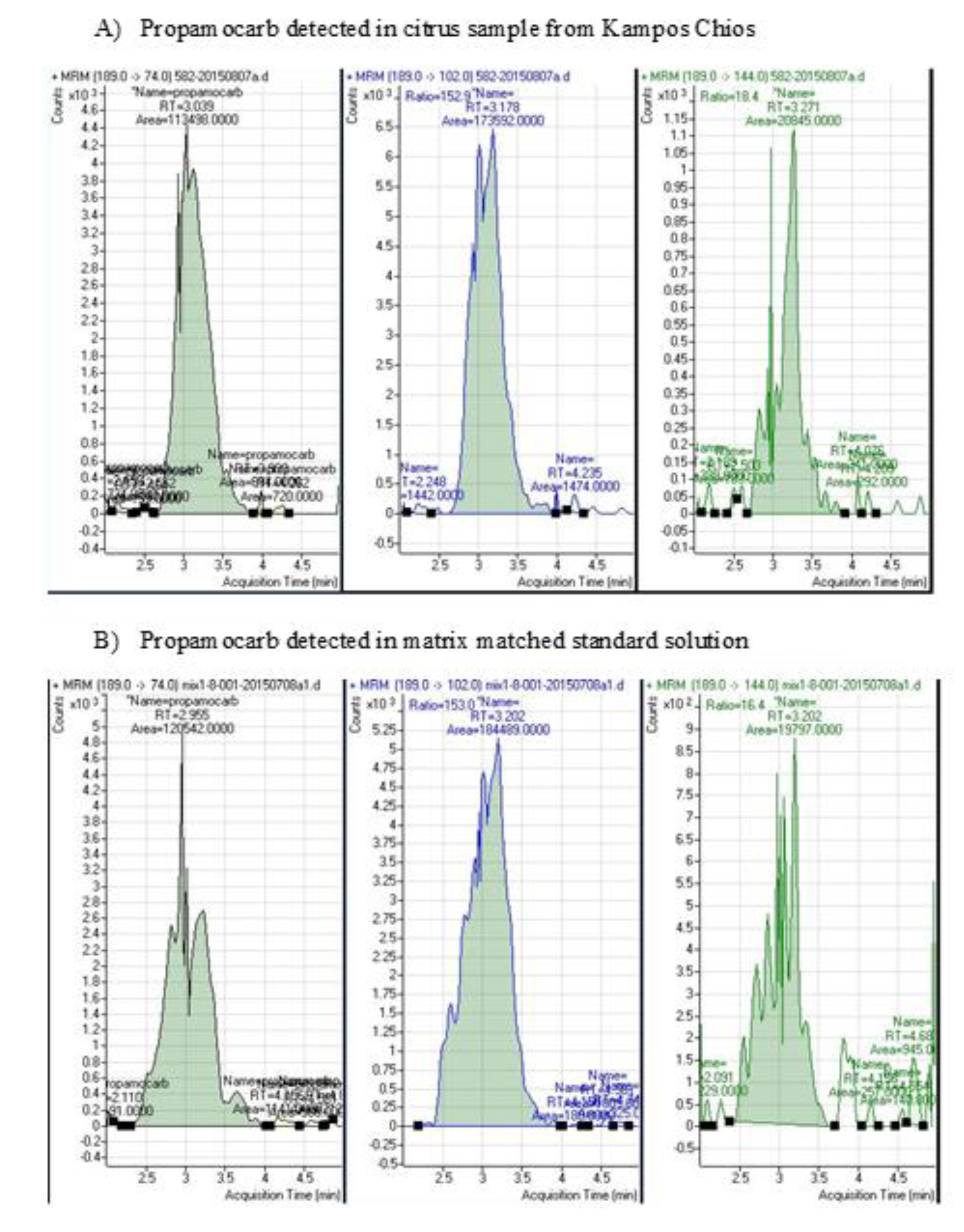
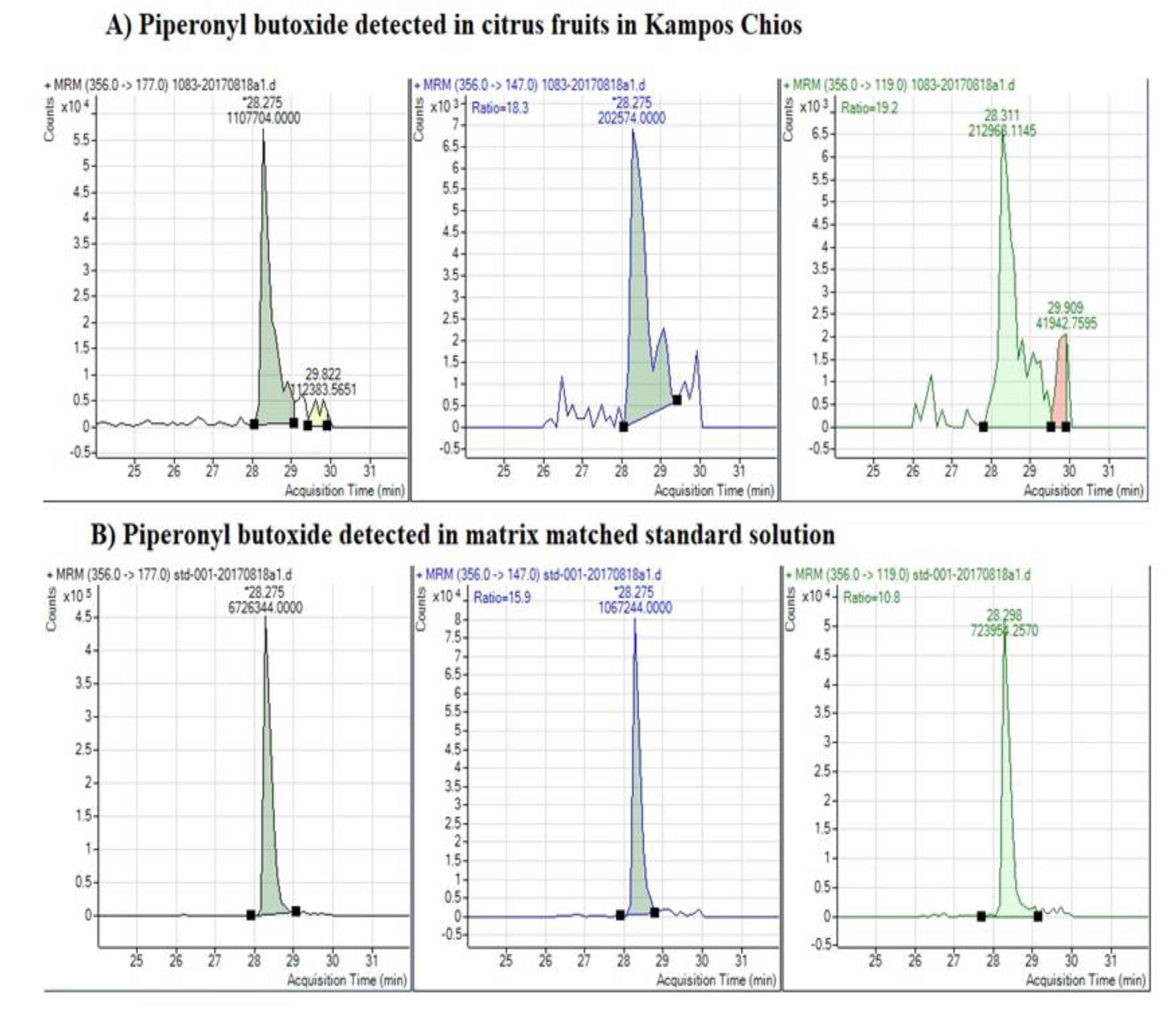
3.2. Detection of Pesticides
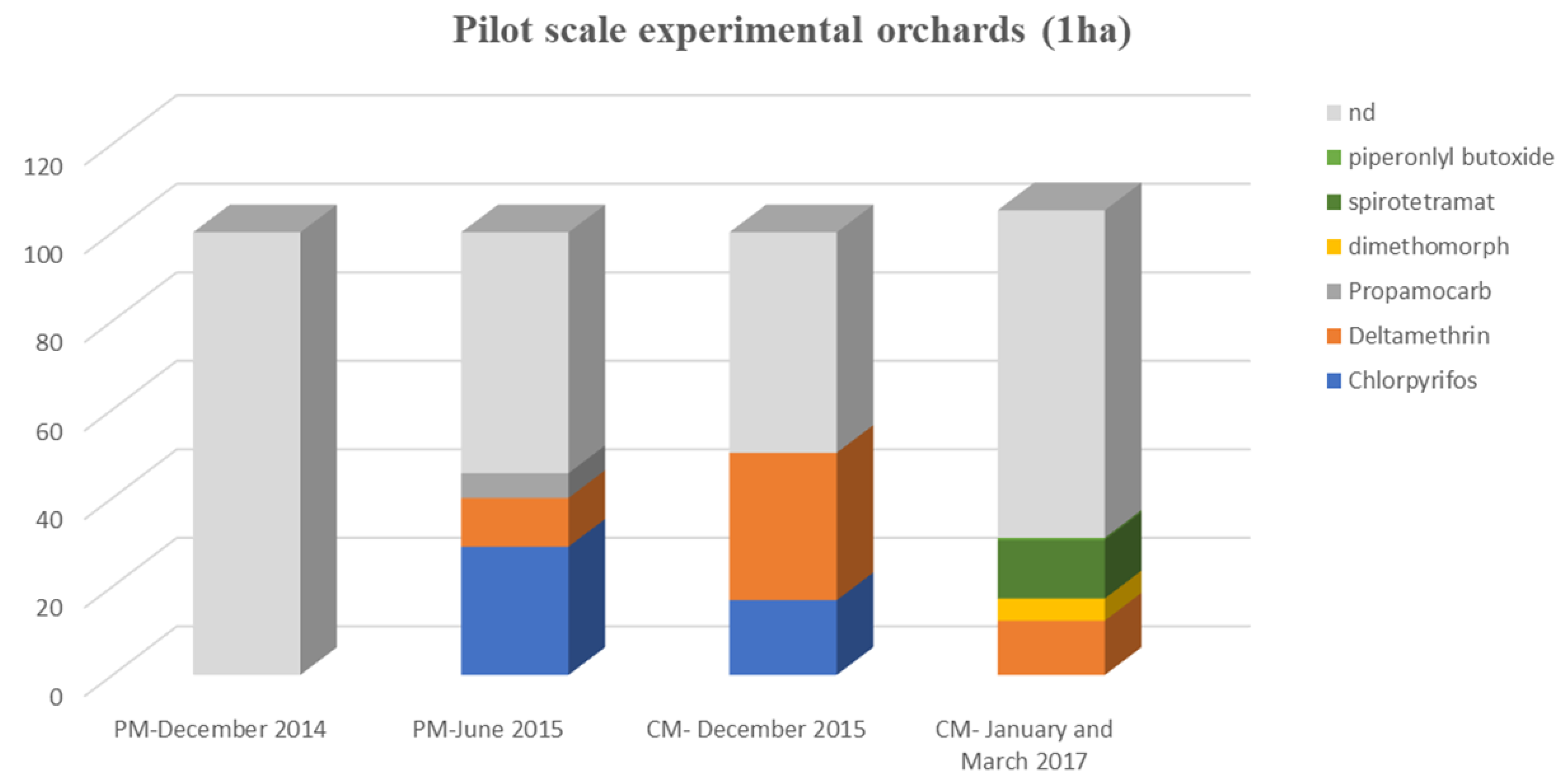
3.2.1. Pesticide Occurrence in Pilot-Scale Experimental Orchards
3.2.2. Pesticide Occurrence in Real-Scale Experimental Orchards
3.2.3. Citrus Samples with Multiple Residues
3.2.4. Citrus Samples with Residues of Non-Authorized Pesticides
3.3. Seasonal Distribution and Variation
3.4. Annual Distribution and Variation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, M.N.; Bint, E.; Naser, S.F.; Khan, M.S. (Eds.) Pesticide food laws and regulations. In Pesticide Residue in Foods: Sources, Management, and Control; Springer International Publishing: Cham, Switzerland, 2017; p. 37. [Google Scholar]
- Livingston, G.; Hack, L.; Steinmann, K.P.; Graftoncardwell, E.E.; Rosenheim, J.A. An ecoinformatics approach to field-scale evaluation of insecticide effects in California citrus: Are citrus thrips and citrus red mite induced pests? J. Econ. Entomol. 2018, 111, 1290. [Google Scholar] [CrossRef]
- Gore, A.C.; Crews, D.; Doan, L.L.; La Merrill, M.A.; Patisaul, H.; Zota, A.; Introduction to Endocrine Disrupting Chemicals (EDCs). A Guide for Public Interest Organizations and Policy-Makers. Endocrine Society. 2014. Available online: https://www.endocrine.org/_/media/endosociety/Files/Advocacy%20and%20Outreach/Important%20Documents/ (accessed on 15 July 2021).
- EPA (Environmental Protection Agency). Exposure Assessment Tools by Chemical Classes—Pesticides. 2018. Available online: https://www.epa.gov/expobox/exposure-assessment-tools-chemical-classes-pesticides (accessed on 20 July 2021).
- WHO (World Health Organization) PESTICIDE Residues in Food. 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/pesticide-residuesin-food (accessed on 20 July 2021).
- Schimmenti, E.; Borsellino, V.; Galati, A. Growth of citrus production among the Euro-Mediterranean countries: Political implications and empirical findings. Span. J. Agric. Res. 2013, 11, 561–577. [Google Scholar] [CrossRef] [Green Version]
- Malcolm, P. History of Citrus. 2006. Available online: http://www.submityourarticle.com/articles/Patrick-Malcolm-1285/lemon-7969.php (accessed on 10 June 2021).
- FAO. FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 28 December 2020).
- Broughton, S. Control of Mediterranean Fruit Fly (Medfly) in Backyards; Gardennote, No. 24 Replaces Bulletin 4565; Government of Western Australia, Department of Agriculture: South Perth, Australia, 2004; ISSN 0817-5969.
- Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC, E. Parliament, Editor. Off. J. Eur. Union 2005, L70, 16. [Google Scholar]
- Regulation (EU) 2017/623 of 30 March 2017 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for acequinocyl, amitraz, coumaphos, diflufenican, flumequine, metribuzin, permethrin, pyraclostrobin and streptomycin in or on certain products. Off. J. Eur. Union 2017, L93, 1–29.
- Regulation (EU) 2020/856 of 9 June 2020 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Cyantraniliprole, Cyazofamid, Cyprodinil, fenpyroximate, Fludioxonil, Fluxapyroxad, Imazalil, Isofetamid, Kresoxim-Methyl, Lufenuron, Mandipropamid, Propamocarb, Pyraclostrobin, Pyriofenone, Pyriproxyfen and Spinetoram in or on Certain Products; European Union: Maastricht, The Netherlands, 2020.
- Minagric-Ministry of Rural Development and Food. 2021. Available online: https://1click.minagric.gr/oneClickUI/frmFytoPro.zul (accessed on 15 August 2021).
- Anastassiades, M.; Schwack, W. Analysis of Carbendazim, benomyl, thiophanate methyl and 2,4-dichlorphenoxyacetic acid in fruits and vegetables after supercritical fluid extraction. J. Chromatogr. A 1998, 825, 45–54. [Google Scholar] [CrossRef]
- Schirra, M.; D’Aquino, S.; Palma, A.; Marceddu, S.; Angioni, A.; Cabras, P.; Scherm, B.; Migheli, Q. Residue Level, persistence, and storage performance of citrus fruit treated with fludioxonil. J. Agric. Food Chem. 2005, 53, 6718. [Google Scholar] [CrossRef]
- Fernandez, M.; Pico, Y.; Manes, J. Pesticide residues in oranges from Valencia (Spain). Food Addit. Contam. 2001, 18, 615–624. [Google Scholar] [CrossRef]
- EN 12393:1998. European Committee for Standardization: Nonfatty Foods—Multiresidue Methods for the Gas Chromatographic Determination of Pesticide Residues. Part 1: General Considerations. Part 2: Methods for Extraction and Clean-Up. Part 3: Determination and Confirmatory Tests. European Standards, CEN/TC 275. Available online: https://standards.iteh.ai/catalog/standards/cen/8eb5a117-6f8c-4473-8416-c7eea98316e1/en-12393-1-1998 (accessed on 5 April 2021).
- Taga, O.; Bilgin, B. Determination of 107 pesticide residues in citrus fruits by gas chromatography/mass spectrometry. Asian J. Chem. 2010, 22, 2367–2374. [Google Scholar]
- Ortelli, D.; Edder, P.; Corvi, C. Pesticide residues survey in citrus fruits. Food Addit. Contam. 2005, 22, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Andraščíková, M.; Hrouzková, S.; Cunha, S.C. Combination of QuEChERS and DLLME for GC-MS determination of pesticide residues in orange samples. Food Addit. Contam. Part A 2012, 30, 286–297. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhao, Q.; Wang, C.; Cui, Y.; Li, J.; Chen, A.; Liang, G.; Jiao, B. Taga, temporal variation, quality and safety assessment of pesticide residues on citrus fruits in China. Chemosphere 2020, 258, 127381. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.I.; Lorenzini, R.; Redondo, M.J.; Font, G. Matrix solid-phase dispersion microextraction and determination by high-performance liquid chromatography with UV detection of pesticide residues in citrus fruit. J. Chromatogr. A 1999, 839, 101–107. [Google Scholar] [CrossRef]
- Ito, Y.; Goto, T.; Oka, H.; Matsumoto, H.; Miyazaki, Y.; Takahashi, N.; Nakasawa, H. Simple and rapid determination of thiabendazole, imazalil, and ophenylphenol in citrus fruits using flow-injection electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2003, 51, 861–866. [Google Scholar] [CrossRef]
- Blasco, C.; Font, G.; Pico’, Y. Multiple-stage spectrometric analysis of six pesticides in oranges by liquid chromatography atmospheric pressure chemical ionization-ion trap mass spectrometry. J. Chromatogr. A 2004, 1043, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Akiyama, Y.; Teranishi, K. Rapid simultaneous determination of o-phenylphenol, diphenyl, thiabendazole, imazalil and its major metabolite in citrus fruits by liquid chromatography-mass spectrometry using atmospheric pressure photoionization. J. Chromatogr. A 2004, 1022, 145–150. [Google Scholar] [CrossRef]
- Zamora, T.; Pozo, O.J.; Lo´pez, F.J.; Herna´ndez, F. Determination of tridemorph and other fungicide residues in fruits samples by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A 2004, 1045, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Soler, C.; Mañes, J.; Picó, Y. Comparison of liquid chromatography using triple quadrupole and quadrupole ion trap mass analyzers to determine pesticide residues in oranges. J. Chromatogr. A. 2005, 1016, 224–233. [Google Scholar] [CrossRef]
- Al-Nasir, F.M.; Jiries, A.G.; Al-Rabadi, G.J.; Alu’datt, M.H.; Tranchant, C.C.; Al-Dalain, S.A.; Alrabadi, N.N.S.; Al-Madanat, O.; Al-Dmour, R.S. Determination of pesticide residues in selected citrus fruits and vegetables cultivated in the Jordan Valley. LWT-Food Sci. Technol. 2020, 123, 109005. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Melis, M.; Pirisi, F.M.; Cabitza, F.; Dedola, F.; Navickiene, S. Determination of buprofezin, pyridaben, and tebufenpyrad residues by gas chromatography mass-selective detection in clementine citrus. J. Agric. Food Chem. 1998, 46, 4255–4259. [Google Scholar] [CrossRef]
- Watanabe, E.; Watanabe, S.; Ito, S.; Hayashi, M.; Watanabe, T.; Yuasa, Y.; Nakasawa, H. Development of an enzymelinked immunosorbent assay for the fungicide imazalil in citrus fruits. J. Agric. Food Chem. 2000, 48, 5124–5130. [Google Scholar] [CrossRef] [PubMed]
- Mirghafouri, M.R.; Abbasi-Moayed, S.; Ghasemi, F.; Hormozi-Nezhad, M.R. Nanoplasmonic sensor array for detection and discrimination of pesticide residues in citrus. Anal. Methods 2020, 12, 5877–5884. [Google Scholar] [CrossRef]
- Greeka. 2020. Available online: https://www.greeka.com/eastern-aegean/chios/villages/kampos/ (accessed on 6 November 2020).
- Citrus-Chios. Available online: http://www.citrus-chios.gr/ (accessed on 6 November 2020).
- Hellenic Statistical Authority. Annual Agricultural Statistical Survey: 2018. 05a. Tree crops. Areas of Compact Plantations, by Region and Regional Unity. Number of Trees and Tree Production for Principal Tree Cultivations, by Region and Regional Unities, 2018. 2020. Available online: https://www.statistics.gr/en/statistics/-/publication/SPG06/- (accessed on 10 April 2021).
- Commission Directive 2002/63/EC. Establishing community methods of sampling for the official control of pesticide residues in and on products of plant and animal origin and repealing directive 79/700/EEC. Off. J. Communities L 2002, 187, 30–43. [Google Scholar]
- Anastassiades, M.; Tasdelen, B.; Sherbaum, E.; Stajnbaher, D. Recent developments in QueEChERS methodology for pesticide multiresidue analysis. In Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety; Ohkawa, H., Miyagawa, H., Lee, P.W., Eds.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- M15. In House Multiresidue LC-MS/MS and GC-MS/MS Method Based on QuΕChERS Method; Working Procedure; Laboratory of Pesticide Residues, Benaki Phytopathological Institute: Kifissia, Greece, unpublished validation data.
- CRL for Single Residue Methods. Analysis of Acidic Pesticides in Wheat Flour Samples by LC-MS(/MS) Using the QuEChERS Method (incl. Optional Alkaline Hydrolysis to Release Covalently Bound Compounds). 2007. Available online: https://www.eurl-pesticides.eu/library/docs/cf/acidicpesticides_wheat_quechers.pdf (accessed on 2 March 2021).
- Anagnostopoulos, C.J.; Liapis, K.; Haroutounian, S.; Paspatis, E. Simultaneous determination of different classes of plant growth regulator in high water content agricultural products by liquid chromatography tandem mass spectrometry and time of flight mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 315–335. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.J.; Aplada Sarli, P.; Miliadis, G.E.; Haroutounian, C.A. Validation of the QuEChERS method for the determination of 25 priority pesticide residues in cereal-based baby foods by gas chromatography with electron capture and nitrogen phosphorous detection. Hell. Plant Prot. J. 2010, 3, 71–80. [Google Scholar]
- SANTE. SANTE/12682/2019. Guidance SANTE/12682/2019—Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; European Commission: Brussels, Belgium, 2019; Available online: https://ec.europa.eu/food/system/files/2020-01/pesticides_mrl_guidelines_wrkdoc_2019-12682.pdf (accessed on 5 March 2021).
- Anagnostopoulos, C. Development of Analytical Methods for the Determination of Xenobiotic Compounds in Foods for Infants and Young Children. Ph.D. Thesis, Laboratory of General Chemistry, Department of Science, Agricultiral University of Athens, Athens, Greece, 2012; 246p. Available online: http://dspace.aua.gr/xmlui/bitstream/handle/10329/5090/Anagnostopoulos_C.pdf?sequence=3 (accessed on 5 October 2021).
- Anagnostopoulos, C.; Miliadis, G.E. Development and validation of an easy multiresidue method for the determination of multiclass pesticide residues using GC-MS/MS and LC-MS/MS in olive oil and olives. Talanta 2013, 112, 1–10. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT Guide for Personal Computers, 8th ed.; SAS Institute: Carry, NC, USA, 2008. [Google Scholar]
- ESYD. Scope of Accreditation of the Pesticide Residues Laboratory of the Benaki Phytopathological Institute. 2021. Available online: http://esydops.gr/portal/p/esyd/en/showOrgInfo.jsp?id=18215 (accessed on 6 October 2021).
- Tomlin, C. The Pesticide Manual, 13th ed.; British Crop Protection Council: Alton, UK, 2003. [Google Scholar]
- NCPI, National Pesticide Information Center. Piperonyl Butoxide General Fact Sheet. 2020. Available online: http://npic.orst.edu/factsheets/pbogen.pdf (accessed on 22 January 2021).
- Metcalf, R.L. Insect control. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). The 2015 European Union report on pesticide residues in food. EFSA J. 2017, 15, 479. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). The 2016 European Union report on pesticide residues in food. EFSA J. 2018, 16, 5348. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). The 2017 European Union Report on pesticide residues in food. EFSA J. 2019, 17, 5743. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). The 2018 European Union Report on pesticide residues in food. EFSA J. 2020, 18, 6057. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). The 2019 European Union Report on pesticide residues in food. EFSA J. 2021, 19, 6491. [Google Scholar] [CrossRef]
- Regulation (EU) 2020/18 of 10 January 2020 Concerning the Non-Renewal of the Approval of the Active Substance Chlorpyrifos, in Accordance with REGULATION (EC) No 1107/2009 of the European Parliament and of The Council Concerning the Placing of Plant Protection Products on the Market and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011; European Union: Maastricht, The Netherlands, 2020; Volume L 7, pp. 14–16.
- Regulation (EU) 2018/832 of 5 June 2018 Amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Cyantraniliprole, Cymoxanil, Deltamethrin, Difenoconazole, Fenamidone, Flubendiamide, Fluopicolide, Folpet, Fosetyl, Mandestrobin, Mepiquat, Metazachlor, Propamocarb, Propargite, Pyrimethanil, Sulfoxaflor and Trifloxystrobin in or on Certain Products; European Union: Maastricht, The Netherlands, 2018; Volume L 140, pp. 38–86.
- Regulation (EU) 2016/1902 of 27 October 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Acetamiprid, Ametoctradin, Azoxystrobin, Cyfluthrin, Difluoroacetic Acid, Dimethomorph, Fenpyrazamine, Flonicamid, Fluazinam, Fludioxonil, Flupyradifurone, Flutriafol, Fluxapyroxad, Metconazole, Proquinazid, Prothioconazole, Pyriproxyfen, Spirodiclofen and Trifloxystrobin in or on Certain Products; European Union: Maastricht, The Netherlands, 2020; Volume L 298, pp. 1–60.
- Regulation (EU) 2019/1015 of 20 June 2019 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels For aminopyralid, Captan, Cyazofamid, Flutianil, Kresoxim-Methyl, Lambda-Cyhalothrin, Mandipropamid, Pyraclostrobin, Spiromesifen, Spirotetramat, Teflubenzuron and Tetraconazole in or on Certain Products; European Union: Maastricht, The Netherlands, 2019; Volume L 165, pp. 23–64.
- Regulation (EU) 2019/552 of 4 April 2019 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Azoxystrobin, Bicyclopyrone, Chlormequat, Cyprodinil, Difenoconazole, Fenpropimorph, Fenpyroximate, Fluopyram, Fosetyl, Isoprothiolane, Isopyrazam, Oxamyl, Prothioconazole, Spinetoram, Trifloxystrobin and Triflumezopyrim in or on Certain Products; European Union: Maastricht, The Netherlands, 2019; Volume L 96, pp. 6–49.
- Regulation (EU) No 1126/2014 of 17 October 2014 Amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Asulam, Cyanamide, Dicloran, Flumioxazin, Flupyrsulfuron-Methyl, Picolinafen and Propisochlor in or on Certain Products; European Union: Maastricht, The Netherlands, 2014; Volume L 30, pp. 46–53.
- Regulation (EU) No 2016/486 of 29 March 2016 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Cyazofamid, Cycloxydim, Difluoroacetic Acid, Fenoxycarb, Flumetralin, Fluopicolide, Flupyradifurone, Fluxapyroxad, Kresoxim-Methyl, Mandestrobin, Mepanipyrim, Metalaxyl-M, Pendimethalin and Tefluthrin in or on Certain Products; European Union: Maastricht, The Netherlands, 2016; Volume L 90, pp. 1–66.
- Golge, O.; Kabak, B. Pesticide residues in table grapes and exposure assessment. J. Agric. Food Chem. 2018, 66, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Basa, C.H.; Velikonja, B.S.; Bavcar, D.; Radeka, S.; Lisjak, K. Plant protection product residues in white grapes and wines of “malvasia istriana” produced in Istria. Food Addit. Contam. Part B Surveill. 2016, 9, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Mebdoua, S.; Lazali, M.; Ounane, S.M.; Tellah, S.; Nabi, F.; Ounane, G. Evaluation of pesticide residues in fruits and vegetables from Algeria. Food Addit. Contam. Part B Surveill. 2017, 10, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jin, S.; Lee, J.; Kim, S.; Kim, Y.; Park, J.; Ko, K.-H.; Ha, W.-W.; Kim, D. Monitoring of Pesticide Residues in Immature Citrus Fruits and the Characteristics by Processing Methods. Korean J. Pestic. Sci. 2018, 22, 300–315. [Google Scholar] [CrossRef]
- Gardner, R.; PES-Pesticide Environmental Stewardship 2021. Understanding the Fate of Pesticides after Application. 2021. Available online: https://pesticidestewardship.org/water/pesticide-fate/ (accessed on 29 September 2021).
| Sampling/Experimental Area | Pesticide | No of Positive Samples (%) | Concentration Range (mg/kg) | Mean Value (mg/kg) | No. (%) of Exceedance |
|---|---|---|---|---|---|
| PM a (December 2014, June 2015) | Chlorpyrifos | 58 (29%) | 0.0041–0.35 | 0.073 | 86.2% |
| Deltamethrin | 22 (11%) | 0.0052–0.12 | 0.0702 | 36.4% | |
| Propamocarb | 11 (5.55%) | 0.0047–0.007 | 0.073 | - | |
| CM b (December 2015, January–March 2017) | Dimethomorph | 11 (5%) | 0.0058–0.1004 | 0.029 | - |
| Chlorpyrifos | 37 (16.9%) | 0.0021–0.098 | 0.025 | 81.1% | |
| Deltamethrin | 57 (26.2%) | 0.002–0.026 | 0.024 | - | |
| Spirotetramat | 29 (13.2%) | 0.0074–0.083 | 0.03 | - | |
| Piperonyl butoxide | 1 (0.45%) | 0.0049 | - | - |
| Sampling/Experimental Area | Pesticide | No of Positive Samples (%) | Concentration Range (mg/kg) | Mean Value (mg/kg) | No. (%) of Exceedance |
|---|---|---|---|---|---|
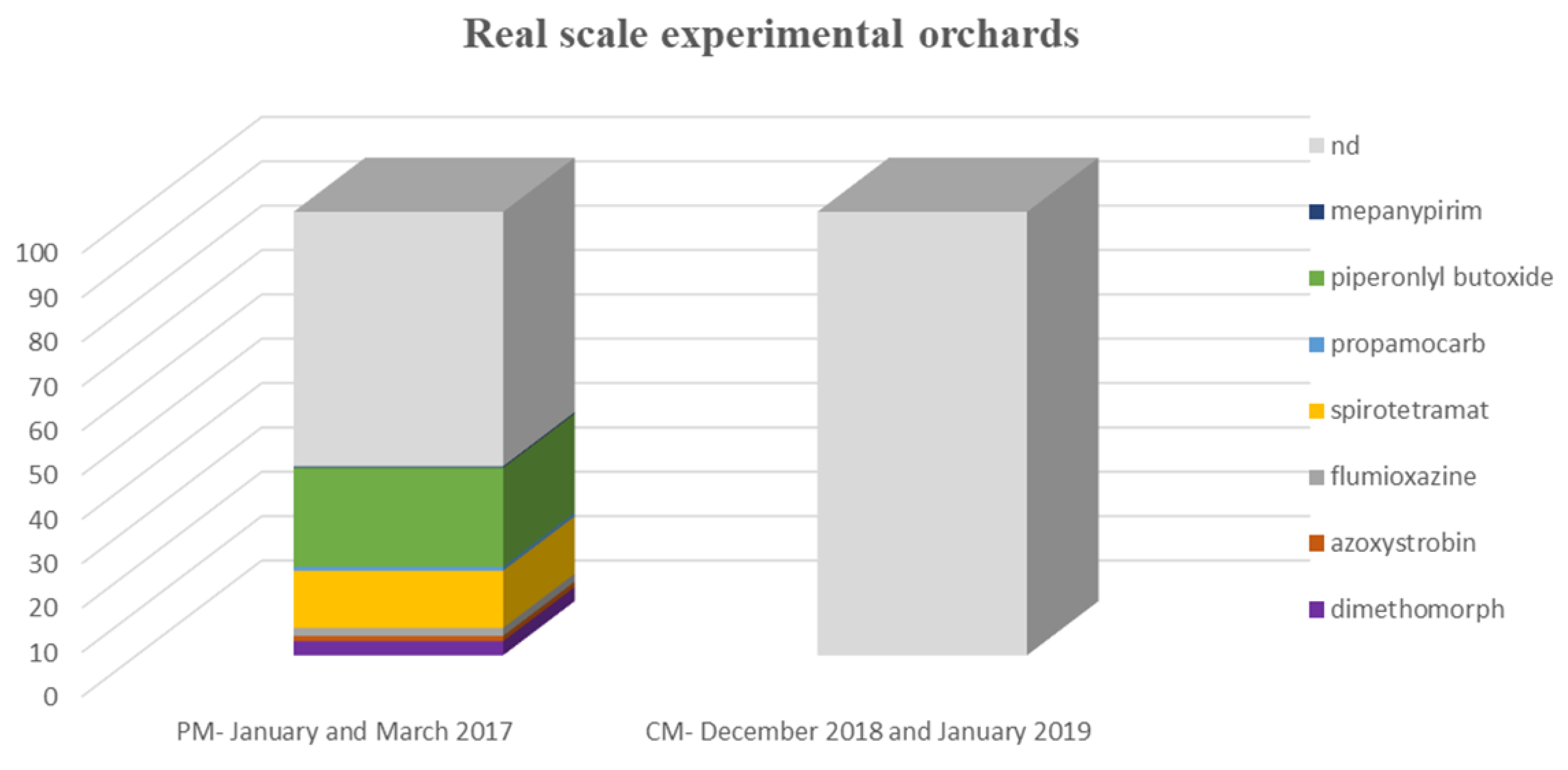
| PM a (January–March 2017) | Dimethomorph | 11 (3.2%) | 0.0026–0.0061 | 0.032 | - |
| Azoxystrobin | 4 (1.2%) | 0.0021–0.0043 | 0.0033 | - | |
| Flumioxazine | 6 (1.8%) | 0.086–0.12 | 0.1 | 100% | |
| Spirotetramat | 44 (12.9%) | 0.0048–0.049 | 0.05 | - | |
| Propamocarb | 3 (0.9%) | 0.0132–0.033 | 0.026 | 100% | |
| Piperonyl butoxide | 76 (22.3%) | 0.0033–0.8013 | 0.048 | - | |
| Mepanypirim | 1 (0.3%) | 0.0075 | - | - | |
| CM b (December 2018–January 2019) | nd | - | - | - | - |
| Pesticide | Type | MRL (mg/kg) | Legislation of MRLs | Authorization in Citrus Crops |
|---|---|---|---|---|
| Chlorpyrifos | Insecticide | 0.01 | Reg. (EU) 2020/1085 [54] | No |
| Deltamethrin | Insecticide | 0.04 | Reg. (EU) 2018/832 [55] | Yes |
| Propamocarb | Fungicide | 0.01 | Reg. (EU) 2020/856 [12] | No |
| Dimethomorph | Fungicide | 0.8 | Reg. (EU) 2016/1902 [56] | Yes |
| Spirotetramat | Insecticide | 1 | Reg. (EU) 2019/1015 [57] | Yes |
| Piperonyl butoxide | Synergist | - | - | - |
| Azoxystrobin | Fungicide | 15 | Reg. (EU) 2019/552 [58] | No |
| Flumioxazine | Herbicide | 0.02 | Reg. (EU) No 2014/1126 [59] | Yes |
| Mepanypirim | Fungicide | 0.01 | Reg. (EU) 2016/486 [60] | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bempelou, E.; Anagnostopoulos, C.; Kiousi, M.; Malatou, P.; Liapis, K.; Kouloussis, N.; Mavraganis, V.; Papadopoulos, N.T. Τemporal Variation in Pesticide Residues in Citrus Fruits from Chios, Greece, before and after the Development of an Integrated Pest Management Strategy (IPMS): A Five-Year Study (LIFE13 ENV GR/000414). Toxics 2021, 9, 323. https://doi.org/10.3390/toxics9120323
Bempelou E, Anagnostopoulos C, Kiousi M, Malatou P, Liapis K, Kouloussis N, Mavraganis V, Papadopoulos NT. Τemporal Variation in Pesticide Residues in Citrus Fruits from Chios, Greece, before and after the Development of an Integrated Pest Management Strategy (IPMS): A Five-Year Study (LIFE13 ENV GR/000414). Toxics. 2021; 9(12):323. https://doi.org/10.3390/toxics9120323
Chicago/Turabian StyleBempelou, Eleftheria, Christos Anagnostopoulos, Maroula Kiousi, Panagiota Malatou, Konstantinos Liapis, Nikos Kouloussis, Vassilis Mavraganis, and Nikolaos T. Papadopoulos. 2021. "Τemporal Variation in Pesticide Residues in Citrus Fruits from Chios, Greece, before and after the Development of an Integrated Pest Management Strategy (IPMS): A Five-Year Study (LIFE13 ENV GR/000414)" Toxics 9, no. 12: 323. https://doi.org/10.3390/toxics9120323
APA StyleBempelou, E., Anagnostopoulos, C., Kiousi, M., Malatou, P., Liapis, K., Kouloussis, N., Mavraganis, V., & Papadopoulos, N. T. (2021). Τemporal Variation in Pesticide Residues in Citrus Fruits from Chios, Greece, before and after the Development of an Integrated Pest Management Strategy (IPMS): A Five-Year Study (LIFE13 ENV GR/000414). Toxics, 9(12), 323. https://doi.org/10.3390/toxics9120323








