Biokinetic Evaluation of Contrast Media Loaded Carbon Nanotubes Using a Radiographic Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pt-Peapod
2.2. Cytotoxicity Test
2.3. Cellular Uptake Assay
2.4. Cytokine Secretion Assay
2.5. Radiography of the Lung Removed after Intratracheal Administration of Pt-Peapods
3. Results
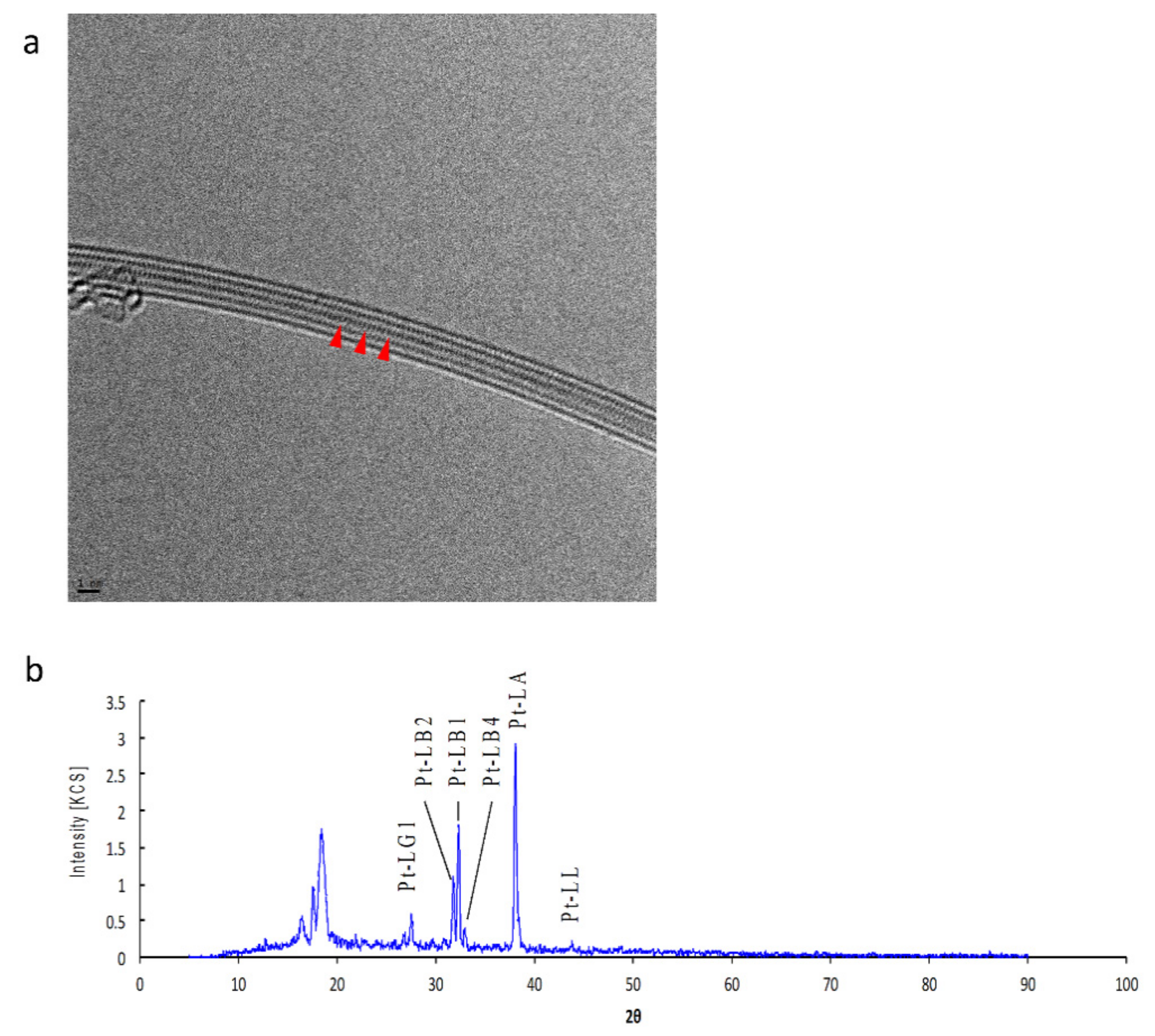
3.1. Pt-Peapods
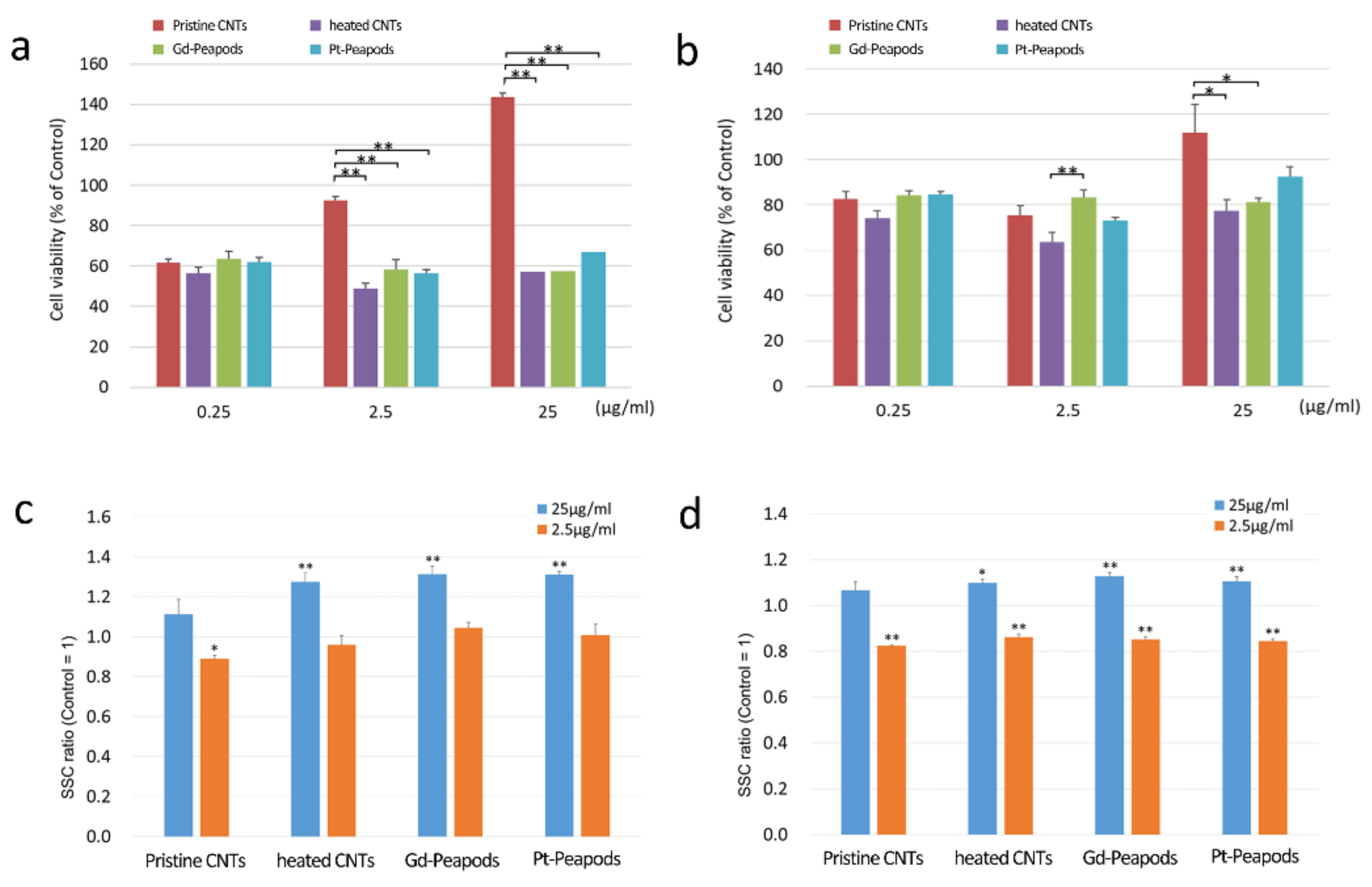
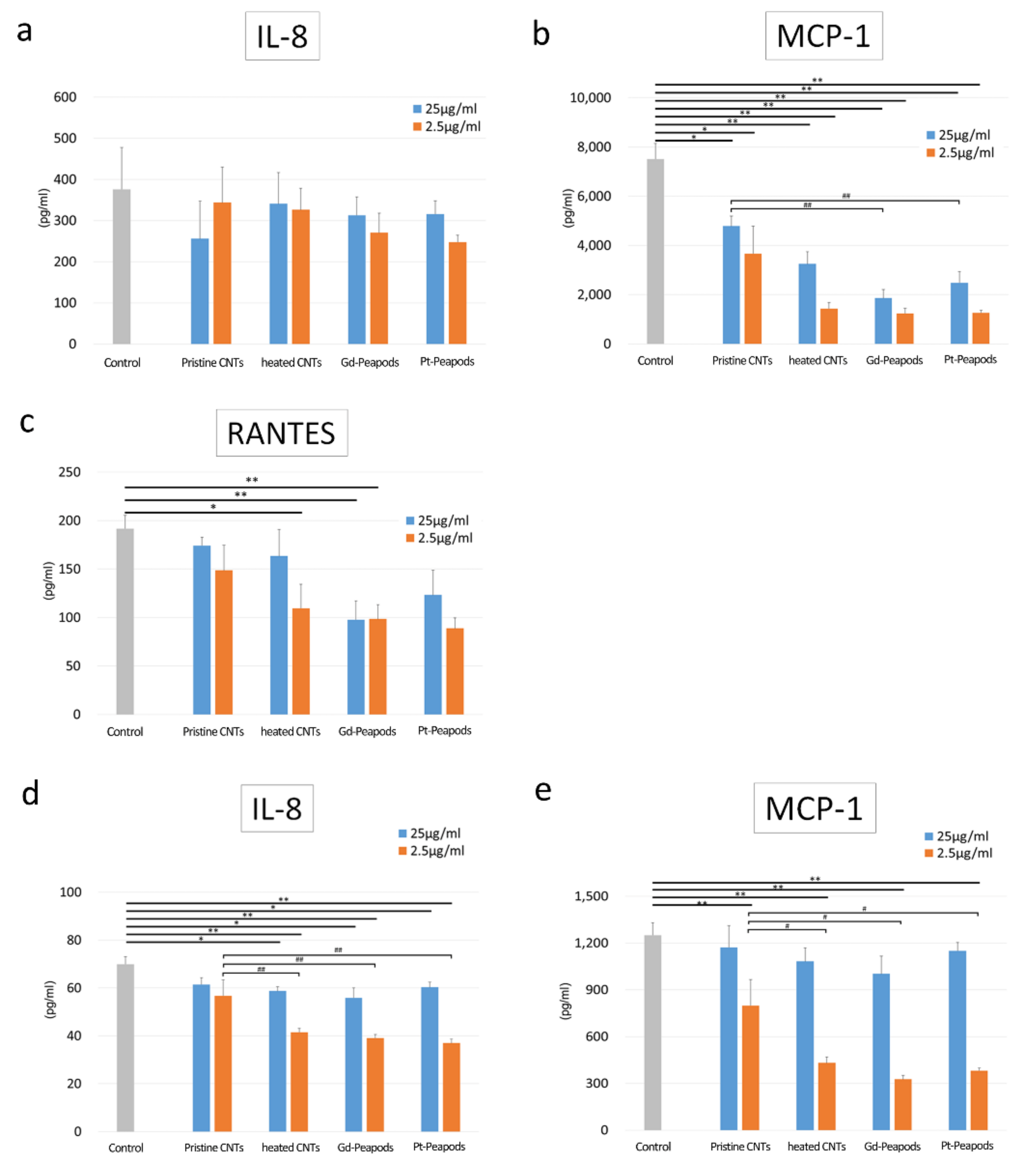
3.2. Cell Assay
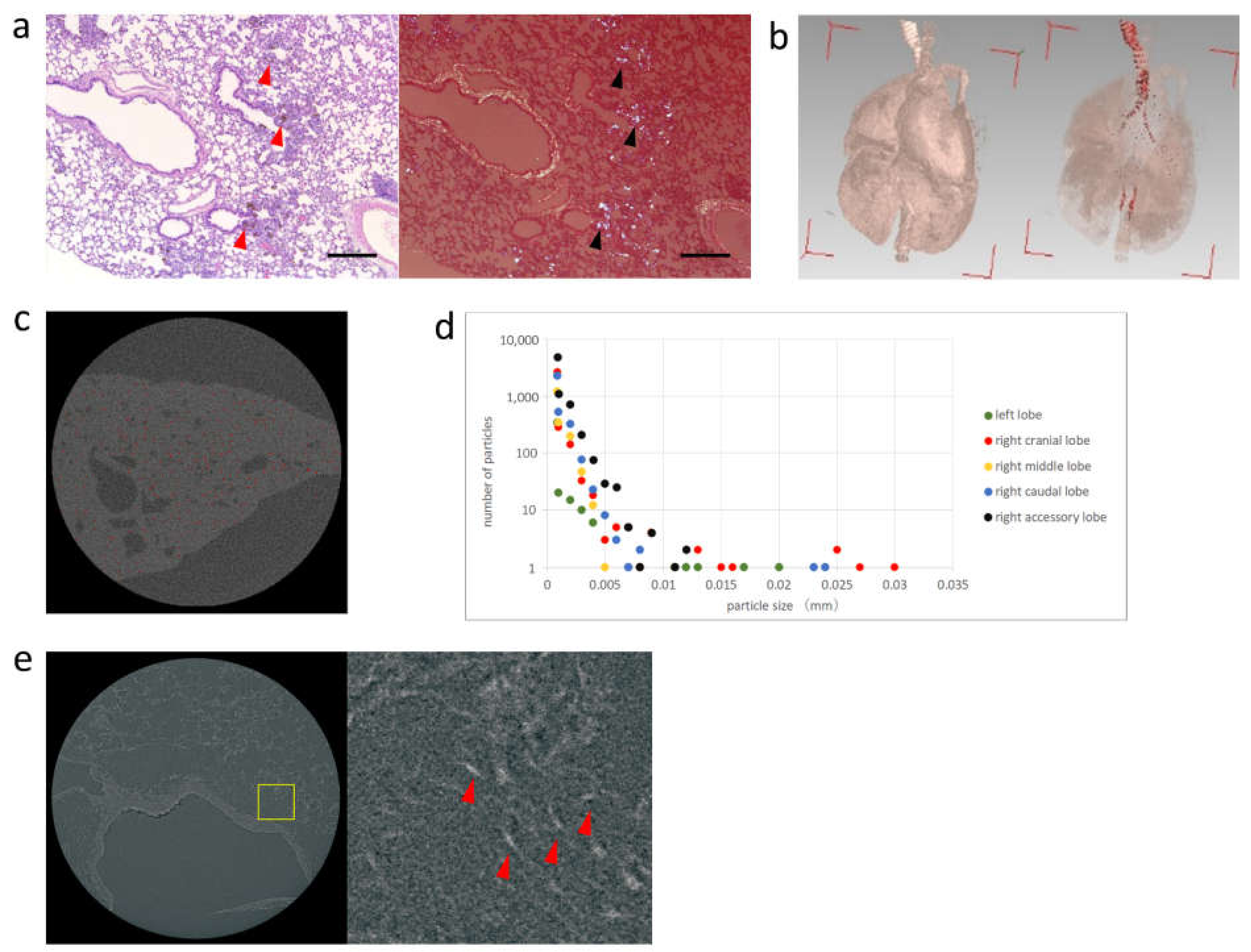
3.3. Radiography of the Lung Removed after Intratracheal Administration of Pt-Peapods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Endo, M.; Strano, M.S.; Ajayan, P.M. Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications; Chapter 2; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Gurel, V.; Morris, D.; Murray, D.W.; Zhitkovich, A.; Kane, A.B.; Hurt, R.H. Bioavailability of nickel in single-wall carbon nanotubes. Adv. Mater. 2007, 19, 2790–2796. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K. Carbon nanotubes: Potential medical applications and safety concerns. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef]
- Ong, L.C.; Chung, F.F.; Tan, Y.; Leong, C.O. Toxicity of single-walled carbon nanotubes. Arch. Toxicol. 2016, 90, 103–118. [Google Scholar] [CrossRef]
- Ema, M.; Gamo, M.; Honda, K. A review of toxicity studies of single-walled carbon nanotubes in laboratory animals. Regul. Toxicol. Pharmacol. 2016, 74, 42–63. [Google Scholar] [CrossRef]
- Mercer, R.R.; Scabilloni, J.F.; Hubbs, A.F.; Wang, L.; Battelli, L.A.; McKinney, W.; Castranova, V.; Porter, D.W. Extrapulmonary transport of MWCNT following inhalation exposure. Part. Fibre Toxicol. 2013, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Czarny, B.; Georgin, D.; Berthon, F.; Plastow, G.; Pinault, M.; Patriarche, G.; Thuleau, A.; L’Hermite, M.M.; Taran, F.; Dive, V. Carbon nanotube translocation to distant organs after pulmonary exposure: Insights from in situ (14)C-radiolabeling and tissue radioimaging. ACS Nano 2014, 8, 5715–5724. [Google Scholar] [CrossRef]
- Faraj, A.A.; Cieslar, K.; Lacroix, G.; Gaillard, S.; Canet-Soulas, E.; Crémillieux, Y. In vivo imaging of carbon nanotube biodistribution using magnetic resonance imaging. Nano Lett. 2009, 9, 1023–1027. [Google Scholar] [CrossRef]
- Wang, L.R.; Xue, X.; Hu, X.; Wei, M.; Zhang, C.; Ge, G.; Liang, X. Structure-dependent mitochondrial dysfunction and hypoxia induced with single-walled carbon nanotubes. Small 2014, 10, 2859–2869. [Google Scholar] [CrossRef]
- Sayes, C.M.; Liang, F.; Hudson, J.L.; Mendez, J.; Guo, W.; Beach, J.M.; Moore, V.C.; Doyle, C.D.; West, J.L.; Billups, W.E.; et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol. Lett. 2006, 161, 135–142. [Google Scholar] [CrossRef]
- Zhang, M.; Morimoto, M.T.; Tajima, N.; Ichiraku, K.; Fujita, K.; Iijima, S.; Yudasaka, M.; Okazaki, T. Size-dependent cell uptake of carbon nanotubes by macrophages: A comparative and quantitative study. Carbon 2018, 127, 93–101. [Google Scholar] [CrossRef]
- Poulsen, S.S.; Jackson, P.; Kling, K.; Knudsen, K.B.; Skaug, V.; Kyjovska, Z.O.; Thomsen, B.L.; Clausen, P.A.; Atluri, R.; Berthing, T.; et al. Multi-walled carbon nanotube physicochemical properties predict pulmonary inflammation and genotoxicity. Nanotoxicology 2016, 10, 1263–1275. [Google Scholar] [CrossRef] [Green Version]
- Guven, A.; Rusakova, I.A.; Lewis, M.T.; Wilson, L.J. Cisplatin@US-tube carbon nanocapsules for enhanced chemotherapeutic delivery. Biomaterials 2012, 33, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Raoof, M.B.; Cisneros, T.; Guven, A.; Phounsavath, S.; Corr, S.J.; Wilson, L.J.; Curley, S.A. Remotely triggered cisplatin release from carbon nanocapsules by radiofrequency fields. Biomaterials 2013, 34, 1862–1869. [Google Scholar] [CrossRef] [Green Version]
- Corr, S.J.; Raoof, M.; Cisneros, B.T.; Orbaek, A.W.; Cheney, M.A.; Law, J.J.; Lara, N.C.; Barron, A.R.; Wilson, L.J.; Curley, S.A. Radiofrequency electric-field heating behaviors of highly enriched semiconducting and metallic single-walled carbon nanotubes. Nano Res. 2015, 8, 2859–2870. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Saito, N.; Haniu, H.; Usui, Y.; Aoki, K.; Hara, K.; Takanashi, S.; Shimizu, M.; Narita, N.; Okamoto, M.; Kobayashi, S.; et al. Safe clinical use of carbon nanotubes as innovative biomaterials. Chem. Rev. 2014, 114, 6040–6079. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Nakae, D.; Fukumori, N.; Tayama, K.; Maekawa, A.; Imai, K.; Hirose, A.; Nishimura, T.; Ohashi, N.; Ogata, A. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J. Toxicol. Sci. 2009, 34, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Takagi, A.; Hirose, A.; Futakuchi, M.; Tsuda, H.; Kanno, J. Dose-dependent mesothelioma induction by intraperitoneal administration of multi-wall carbon nanotubes in p53 heterozygous mice. Cancer Sci. 2012, 103, 1440–1444. [Google Scholar] [CrossRef]
- Marangon, I.; Ménard-Moyon, C.; Kolosnjaj-Tabi, J.; Béoutis, M.L.; Lartigue, L.; Alloyeau, D.; Pach, E.; Ballesteros, B.; Autret, G.; Ninjbadgar, T.; et al. Covalent functionalization of multi-walled carbon nanotubes with a Gadolinium chelate for efficient T1-weighted magnetic resonance imaging. Adv. Funct. Mater. 2014, 24, 7173–7186. [Google Scholar]
- Georgin, D.; Czarny, B.; Botquin, M.; Hermite, M.M.; Pinault, M.; Bouchet-Fabre, B.; Carriere, M.; Poncy, J.; Chau, Q.; Maximilien, R.; et al. Preparation of (14)C-labeled multiwalled carbon nanotubes for biodistribution investigations. J. Am. Chem. Soc. 2009, 131, 14658–14659. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Davis, C.; Cai, W.; He, L.; Chen, X.; Dai, H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 1410–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.W.; Nguyen, F.T.; Lue, N.; Dasari, R.R.; Heller, D.A. Measuring uptake dynamics of multiple identifiable carbon nanotube species via high-speed confocal Raman imaging of live cells. Nano. Lett. 2012, 12, 6170–6174. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Cherukuri, P.; Gannon, C.J.; Leeuw, T.K.; Schmidt, H.K.; Smalley, R.E.; Curley, S.A.; Weisman, R.B. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence. Proc. Natl. Acad. Sci. USA 2006, 103, 18882–18886. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging, and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, T.; Iizumi, Y.; Yudasaka, M.; Kizaka-Kondoh, S.; Okazaki, T. Characterization and biodistribution analysis of oxygen-doped single-walled carbon nanotubes used as in vivo fluorescence imaging probes. Bioconjug. Chem. 2019, 30, 1323–1330. [Google Scholar] [CrossRef]
- Smith, B.W.; Monthioux, M.; Luzzi, D.E. Encapsulated C-60 in carbon nanotubes. Nature 1998, 396, 323–324. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsuruoka, S.; Usui, Y.; Haniu, H.; Aoki, K.; Takanashi, S.; Okamoto, M.; Nomura, H.; Tanaka, M.; Aiso, S.; et al. An advanced in situ imaging method using heavy metal-doped hollow tubes to evaluate the biokinetics of carbon nanotubes in vivo. NPG Asia Mater. 2015, 7, e203. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Nomura, H.; Takanashi, S.; Tanaka, M.; Haniu, H.; Aoki, K.; Okamoto, M.; Kobayashi, S.; Takizawa, T.; Usui, Y.; Oishi, A.; et al. Specific biological responses of the synovial membrane to carbon nanotubes. Sci. Rep. 2015, 5, 14314. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Singh, R.; Pantarotto, D.; Lacerda, L.; Pastorin, G.; Klumpp, C.; Prato, M.; Bianco, A.; Kostarelos, K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc. Natl. Acad. Sci. USA 2006, 103, 3357–3362. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Jia, G.; Wang, H.; Sun, H.; Wang, X.; Yang, S.; Wang, T.; Liu, Y. Translocation and fate of multi-walled carbon nanotubes in vivo. Carbon 2007, 45, 1419–1424. [Google Scholar] [CrossRef]
- Kong, N.; Shimpi, M.R.; Park, J.H.; Ramström, O.; Yan, M. Carbohydrate conjugation through microwave-assisted functionalization of single-walled carbon nanotubes using perfluorophenyl azides. Carbohydr. Res. 2015, 405, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Homayoun, A.; Bannister, C.W.; Yum, K. Single-walled carbon nanotubes as near-infrared optical biosensors for life sciences and biomedicine. Biotechnol. J. 2015, 10, 447–459. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, J.; Wu, L.; Peng, Y.; Yang, X.; Li, X.; Ma, A.; Ma, J.; Chen, G. Research progress of near-infrared fluorescence immunoassay. Micromachines 2019, 10, 422. [Google Scholar] [CrossRef] [Green Version]
- Galassi, T.V.; Antman-Passig, M.; Yaari, Z.; Jessurun, J.; Schwartz, R.E.; Heller, D.A. Long-term in vivo biocompatibility of single-walled carbon nanotubes. PLoS ONE 2020, 15, e0226791. [Google Scholar] [CrossRef]
- Kittel, B.; Ruehl-Fehlert, C.; Morawietz, G.; Klapwijk, J.; Elwell, M.R.; Lenz, B.; O’Sullivan, M.G.; Roth, D.R.; Wadsworth, P.F. Revised guides for organ sampling and trimming in rats and mice—Part 2. A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 2004, 55, 413–431. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takasaka, M.; Kobayashi, S.; Usui, Y.; Haniu, H.; Tsuruoka, S.; Aoki, K.; Saito, N. Biokinetic Evaluation of Contrast Media Loaded Carbon Nanotubes Using a Radiographic Device. Toxics 2021, 9, 331. https://doi.org/10.3390/toxics9120331
Takasaka M, Kobayashi S, Usui Y, Haniu H, Tsuruoka S, Aoki K, Saito N. Biokinetic Evaluation of Contrast Media Loaded Carbon Nanotubes Using a Radiographic Device. Toxics. 2021; 9(12):331. https://doi.org/10.3390/toxics9120331
Chicago/Turabian StyleTakasaka, Mieko, Shinsuke Kobayashi, Yuki Usui, Hisao Haniu, Shuji Tsuruoka, Kaoru Aoki, and Naoto Saito. 2021. "Biokinetic Evaluation of Contrast Media Loaded Carbon Nanotubes Using a Radiographic Device" Toxics 9, no. 12: 331. https://doi.org/10.3390/toxics9120331
APA StyleTakasaka, M., Kobayashi, S., Usui, Y., Haniu, H., Tsuruoka, S., Aoki, K., & Saito, N. (2021). Biokinetic Evaluation of Contrast Media Loaded Carbon Nanotubes Using a Radiographic Device. Toxics, 9(12), 331. https://doi.org/10.3390/toxics9120331






