Levels of Mercury, Methylmercury and Selenium in Fish: Insights into Children Food Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Reagents, Standard, and Reference Material
2.3. Chemical and Instrumental Analysis
2.3.1. Sample Preparation and Equipment
2.3.2. Quality Control and Assurance
2.4. Exposure Assessment and Dietary Reference Intake
2.5. Molar Ratio (Se:Hg) and Selenium Health Benefit Value (HBVSe)
2.6. Daily and Monthly Consumption Rate Limit
2.7. Statistical Analysis
3. Results and Discussion
3.1. Concentrations of Total Mercury (THg), Methylmercury (MeHg), and Selenium (Se)
3.2. Exposure Assessment and Dietary Reference Intake
3.3. Selenium:Mercury Molar Ratio
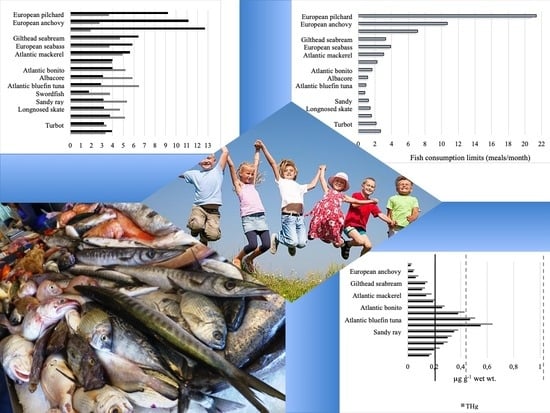
3.4. Daily and Monthly Consumption Rate Limit
3.5. Uncertainties and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Khalili Tilami, S.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Carwile, J.L.; Butler, L.J.; Janulewicz, P.A.; Winter, M.R.; Aschengrau, A. Childhood fish consumption and learning and be-havioral disorders. Int. J. Environ. Res. Publ. Health 2016, 13, 1069. [Google Scholar] [CrossRef] [PubMed]
- Lepping, R.J.; Honea, R.A.; Martin, L.E.; Liao, K.; Choi, I.-Y.; Lee, P.; Papa, V.B.; Brooks, W.M.; Shaddy, D.J.; Carlson, S.E.; et al. Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Dev. Psychobiol. 2019, 61, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Guevel, M.-R.; Sirot, V.; Volatier, J.-L.; Leblanc, J.-C. A Risk-Benefit Analysis of French High Fish Consumption: A QALY Approach. Risk Anal. 2008, 28, 37–48. [Google Scholar] [CrossRef]
- Kumamoto University Study Group. Minamata Disease; Kumamoto University: Kumamoto, Japan, 1966. [Google Scholar]
- Bjørklunda, G.; Dadarb, M.; Mutterc, J.; Aasethd, J. The toxicology of mercury: Current research and emerging trends. Environ. Res. 2017, 159, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Counter, S.; Buchanan, L.H. Mercury exposure in children: A review. Toxicol. Appl. Pharmacol. 2004, 198, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.; Raymond, L.J. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 2010, 278, 112–123. [Google Scholar] [CrossRef]
- Khan, M.A.; Wang, F. Mercury–selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury–selenium antagonism. Environ. Toxicol. Chem. 2009, 28, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.; Ralston, C.R.; Lloyd Blackwell, J., III; Raymond, L.J. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology 2008, 29, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Grgec, A.S.; Kljaković-Gašpić, Z.; Orct, T.; Tičina, V.; Sekovanić, A.; Jurasović, J.; Piasek, M. Mercury and selenium in fish from the eastern part of the Adriatic Sea: A risk-benefit assessment in vulnerable population groups. Chemosphere 2020, 261, 127742. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.M.; Frantzen, S.; Bank, M.S.; Nilsen, B.M.; Duinker, A.; Madsen, L.; Maage, A. Effects of geography and species variation on selenium and mercury molar ratios in Northeast Atlantic marine fish communities. Sci. Total. Environ. 2019, 652, 1482–1496. [Google Scholar] [CrossRef] [PubMed]
- Mirlean, N.; Ferraz, A.H.; Seus-Arrache, E.R.; Andrade, C.F.F.; Costa, L.P.; Johannesson, K.H. Mercury and selenium in the Brazilian subtropical marine products: Food composition and safety. J. Food Compos. Anal. 2019, 84, 103310. [Google Scholar] [CrossRef]
- Plessl, C.; Gilbert, B.M.; Sigmund, M.F.; Theiner, S.; Avenant-Oldewage, A.; Keppler, B.K.; Jirsa, F. Mercury, silver, selenium and other trace elements in three cyprinid fish species from the Vaal Dam, South Africa, including implications for fish consumers. Sci. Total. Environ. 2019, 659, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Sofoulaki, K.; Kalantzi, I.; Machias, A.; Pergantis, S.A.; Tsapakis, M. Metals in sardine and anchovy from Greek coastal areas: Public health risk and nutritional benefits assessment. Food Chem. Toxicol. 2019, 123, 113–124. [Google Scholar] [CrossRef]
- Cardoso, C.; Bernardo, I.; Bandarra, N.M.; Louro Martins, L.; Afonso, A. Portuguese preschool children: Benefit (EPA+DHA ad Se) and risk (MeHg) assessment through the consumption of selected fish species. Food Chem. Toxicol. 2018, 115, 306–314. [Google Scholar] [CrossRef]
- Karimi, R.; Frisk, M.; Fisher, N.S. Contrasting food web factor and body size relationships with Hg and Se concentrations in marine biota. PLoS ONE 2013, 8, e74695. [Google Scholar] [CrossRef]
- Olmedo, P.; Hernández, A.F.; Pla, A.; Femia, P.; Navas-Acien, A.; Gil, F. Determination of essential elements (copper, man-ganese, selenium and zinc) in fish and shellfish samples. Risk and nutritional assessment and mercury–selenium balance. Food Chem. Toxicol. 2013, 62, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle. Molecules 2019, 24, 1273. [Google Scholar] [CrossRef]
- Barone, G.; Storelli, A.; Mallamaci, R.; Storelli, M.M. Comparative Study on Trace Metal Accumulation in Liver of Mediter-ranean Deep-Sea Fish and Their Selenium/Mercury Molar Ratios. Water Air Soil Pollut. 2017, 228, 211. [Google Scholar] [CrossRef]
- Squadrone, S.; Benedetto, A.; Brizio, P.; Prearo, M.; Abete, M. Mercury and selenium in European catfish (Silurus glanis) from Northern Italian Rivers: Can molar ratio be a predictive factor for mercury toxicity in a top predator? Chemosphere 2015, 119, 24–30. [Google Scholar] [CrossRef]
- Storelli, M.M.; Marcotrigiano, G.O. Mercury speciation and relationship between mercury and selenium in liver of Galeus me-lastomus from the Mediterranean Sea. Bull. Environ. Contam. Toxicol. 2002, 69, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Acquavita, A.; Bettoso, N. Mercury and selenium in the grass goby Zosterisessor ophiocephalus (Pisces: Gobiidae) from a mercury contaminated Mediterranean lagoon. Mar. Pollut. Bull. 2018, 135, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Vinceti, M.; D’Agati, M.G.; Arena, G.; Mauceri, V.; Grasso, A.; Fallico, R.; Sciacca, S.; Ferrante, M. Mercury and selenium in take by seafood from the Ionian Sea: A risk evaluation. Ecotoxicol. Environ. Saf. 2014, 100, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Fiches FAO d’intentifcation des Especes pour les Besoins de la peche. In Mediterranee et Mer Noire. Zone de Peche 37; FAO: Rome, Italy, 1987; Volume II: Vertébrés, pp. 761–1530. [Google Scholar]
- FishBase. Available online: https://www.fishbase.org/seach.php (accessed on 25 November 2020).
- Karachle, P.K.; Stergiou, K.I. An update on the feeding habits of fish in the Mediterranean Sea (2002–2015). Mediterr. Mar. Sci. 2017, 18, 43–52. [Google Scholar] [CrossRef]
- Karachle, P.K.; Stergiou, K.I. Trophic levels of north Aegean Sea fishes and comparisons with those from FishBase. Fish. Cent. Res. Rep. 2006, 14, 22–26. [Google Scholar]
- Consoli, P.; Romeo, T.; Battaglia, P.; Castriota, L.; Esposito, V.; Andaloro, F. Feeding habits of the albacore tuna Thunnus alalunga (Perciformes, Scombridae) from central Mediterranean Sea. Mar. Biol. 2008, 155, 113–120. [Google Scholar] [CrossRef]
- Ebert, D.A.; Bizzarro, J.J. Standardized diet compositions and trophic levels of skates (Chondrichthyes: Rajiformes: Rajoidei). Environ. Boil. Fishes 2007, 80, 221–237. [Google Scholar] [CrossRef]
- Fishbase. Scophthalmus maximus (Linnaeus, 1758). Available online: https://www.fishbase.se/summary/Scophthalmus-maximus.html (accessed on 30 November 2020).
- Gazzetta Ufficiale Della Repubblica Italiana (GURI). Metodi di Analisi per la Ricerca di Residui di Metalli Pesanti e Arsenico; GURI: Rome, Italy, 1994. [Google Scholar]
- Ipolyi, I.; Massanisso, P.; Sposato, S.; Fodor, P.; Morabito, R. Concentration levels of total and methylmercury in mussel samples collected along the coasts of Sardinia Island (Italy). Anal. Chim. Acta 2004, 505, 145–151. [Google Scholar] [CrossRef]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C. Aida Aida Turrini on behalf of the INRAN-SCAI 2005–06 Study Group. The Italian National Food Consumption Survey INRAN-SCAI 2005–06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J. 2012, 10, 2985. [Google Scholar]
- Dietary Reference Intake (DRI). The Essential Guide to Nutrient Requirements Institute of Medicine (IOM); The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Ralston, N.V.C.; Ralston, C.R.; Raymond, L.J. Selenium Health Benefit Values: Updated Criteria for Mercury Risk Assessments. Biol. Trace Elem. Res. 2016, 171, 262–269. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (US EPA). Risk Based Concentration Table; United States Environmental Pro-tection Agency: Philadelphia, PA, USA, 2000. [Google Scholar]
- United States Environmental Protection Agency (US EPA). Regional Screening Level (RSL) Summary Table (TR=1E-06, HQ=1); United States Environmental Protection Agency: Philadelphia, PA, USA, 2020. [Google Scholar]
- Gutiérrez-Mosquera, H.; Marrugo-Negrete, J.; Díez, S.; Morales-Mira, G.; Montoya-Jaramillo, L.J.; Jonathan, M. Mercury distribution in different environmental matrices in aquatic systems of abandoned gold mines, Western Colombia: Focus on human health. J. Hazard. Mater. 2021, 404, 124080. [Google Scholar] [CrossRef]
- Nyeste, K.; Dobrocsi, P.; Czeglédi, I.; Czédli, H.; Harangi, S.; Baranyai, E.; Simon, E.; Nagy, S.A.; Antal, L. Age and diet-specific trace element accumulation patterns in different tissues of chub (Squalius cephalus): Juveniles are useful bioindicators of recent pollution. Ecol. Indic. 2019, 101, 1–10. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Regulation (EC) No. 629/2008 of 2 July 2008 Amending Regulation (EC) No. 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2008, 173, 6–7.
- United States Environmental Protection Agency (US EPA). Water Quality Criterion for the Protection of Human Health: Methyl-mercury; U.S. Environmental Protection Agency: Washington, DC, USA, 2001; p. 303. [Google Scholar]
- Hamilton, S.J. Review of selenium toxicity in the aquatic food chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar] [CrossRef]
- Ulusoy, Ş.; Mol, S.; Karakulak, F.; Kahraman, A.E. Selenium-Mercury Balance in Commercial Fish Species from the Turkish Waters. Biol. Trace Elem. Res. 2018, 191, 207–213. [Google Scholar] [CrossRef]
- Gao, S.; Jin, Y.; Hall, K.S.; Liang, C.; Unverzagt, F.W.; Ji, R.; Murrell, J.R.; Cao, J.; Shen, J.; Ma, F.; et al. Selenium Level and Cognitive Function in Rural Elderly Chinese. Am. J. Epidemiol. 2007, 165, 955–965. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to mercury and methylmercury in food. EFSA J. 2004, 34, 1–14. [Google Scholar]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Yang, G.Q.; Wang, S.Z.; Zhou, R.H.; Sun, S.Z. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 1983, 37, 872–881. [Google Scholar] [CrossRef]
- Peterson, S.A.; Ralston, N.V.; Peck, D.V.; Van Sickle, J.; Robertson, J.D.; Spate, V.L.; Morris, J.S. How Might Selenium Moderate the Toxic Effects of Mercury in Stream Fish of the Western U.S.? Environ. Sci. Technol. 2009, 43, 3919–3925. [Google Scholar] [CrossRef] [PubMed]
- Cabañero, A.I.; Madrid, Y.; Cámara, C. Mercury–Selenium Species Ratio in Representative Fish Samples and Their Bioaccessibility by an In Vitro Digestion Method. Biol. Trace Elem. Res. 2007, 119, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, X.; Chan, H.M.; Larssen, T. New insights into traditional health risk assessments of mercury exposure: Im-plications of selenium. Environ. Sci. Technol. 2014, 48, 1206–1212. [Google Scholar] [CrossRef]
- Khan, M.A.K.; Wang, F. Chemical Demethylation of Methylmercury by Selenoamino Acids. Chem. Res. Toxicol. 2010, 23, 1202–1206. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci. Total. Environ. 2011, 409, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.J.; Ralston, N.V.C. Selenium and Mercury in Pelagic Fish in the Central North Pacific Near Hawaii. Biol. Trace Element Res. 2007, 119, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Devesa, V.; Virseda, J.R.; Barberá, R.; Montoro, R.; Velez, D. Mercury and selenium in fish and shellfish: Oc-currence, bioaccessibility and uptake by Caco-2 cells. Food Chem. Toxicol. 2012, 50, 2696–2702. [Google Scholar] [CrossRef]
- Ruelas-Inzunza, J.; Šlejkovec, Z.; Mazej, D.; Fajon, V.; Horvat, M.; Ramos-Osuna, M. Bioaccumulation of As, Hg, and Se in tunas Thunnus albacares and Katsuwonus pelamis from the Eastern Pacific: Tissue distribution and As speciation. Environ. Sci. Pollut. Res. 2018, 25, 19499–19509. [Google Scholar] [CrossRef]
- Ordiano-Flores, A.; Rosíles-Martínez, R.; Galván-Magaña, F. Biomagnification of mercury and its antagonistic interaction with selenium in yellowfin tuna Thunnus albacares in the trophic web of Baja California Sur, Mexico. Ecotoxicol. Environ. Saf. 2012, 86, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Cabañero, A.I.; Carvalho, C.; Madrid, Y.; Batoréu, C.; Cámara, C. Quantification and Speciation of Mercury and Selenium in Fish Samples of High Consumption in Spain and Portugal. Biol. Trace Elem. Res. 2005, 103, 17–36. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency/Food and Drug Administration (US EPA/FDA). Advice about Eating Fish. For Women Who Are or Might Become Pregnant, Breastfeeding Mothers, and Young Children. 2019. Available online: https://www.fda.gov/media/129959/download (accessed on 1 December 2020).
- Mozaffarian, D. Fish, Mercury, Selenium and Cardiovascular Risk: Current Evidence and Unanswered Questions. Int. J. Environ. Res. Public Health 2009, 6, 1894–1916. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.N.; Maulvault, A.L.; Barbosa, V.L.; Fernandez-Tejedor, M.; Tediosi, A.; Kotterman, M.; Heuvel, F.H.V.D.; Robbens, J.; Fernandes, J.O.; Rasmussen, R.R.; et al. Oral bioaccessibility of toxic and essential elements in raw and cooked commercial seafood species available in European markets. Food Chem. 2018, 267, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Dambrosio, A.; Storelli, A.; Garofalo, R.; Busco, V.P.; Storelli, M.M. Estimated Dietary Intake of Trace Metals from Swordfish Consumption: A Human Health Problem. Toxics 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Marcotrigiano, G.O. Total Mercury Levels in Muscle Tissue of Swordfish (Xiphias gladius) and Bluefin Tuna (Thunnus thynnus) from the Mediterranean Sea (Italy). J. Food Prot. 2001, 64, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.; Shannon, M. Technical report: Mercury in the environment: Implications for pediatricians. Pediatrics 2001, 108, 197–205. [Google Scholar] [CrossRef] [PubMed]



| Scientific Name 1 | Common Name | n° Specimens | Length | Trophic Level |
|---|---|---|---|---|
| Demersal-pelagic fish | ||||
| Sardina pilchardus | European pilchard | 20 | 14.6–20.8 17.7 ± 1.9 | 2.76 2 |
| Engraulis encrasicolus | European anchovy | 20 | 12.0–20.0 16.1 ± 2.5 | 3.10 2 |
| Merluccius merluccius | European hake | 15 | 21.5–30.8 25.0 ± 3.2 | 4.09 2 |
| Sparus aurata | Gilthead seabream | 10 | 29.3–35.4 31.9 ± 2.3 | 3.42 2 |
| Dicentrarchus labrax | European seabass | 10 | 26.0–37.0 32.0 ± 3.9 | 3–4.6 3 |
| Scomber scombrus | Atlantic mackerel | 10 | 25.0–36.7 30.4 ± 3.9 | 4.14 2 |
| Scomber japonicus | Chub mackerel | 15 | 12.5–26.8 19.0 ± 4.9 | 3.99 3 |
| Sarda sarda | Atlantic bonito | 8 | 45.0–56.3 50.0 ± 3.7 | 4.48 2 |
| Thunnus alalunga | Albacore | 15 * | - | 4.47 4 |
| Thunnus thynnus | Atlantic bluefin tuna | 15 * | - | 4.30 2 |
| Xiphias gladius | Swordfish | 15 * | - | 4.46 2 |
| Benthic fish | ||||
| Leucoraja circularis | Sandy ray | 8 | 40.0–56.5 47.9 ± 5.8 | 3.80 5 |
| Dipturus oxyrinchus | Longnosed skate | 8 | 55.8–70.2 61.4 ± 5.9 | 3.75 2 |
| Leucoraja fullonica | Shagreen ray | 8 | 43.7–58.8 49.5 ± 5.6 | 3.80 5 |
| Scophthalmus maximus | Turbot | 10 | 35.0–48.0 39.0 ± 4.3 | 4.4 6 |
| Solea solea | Common sole | 10 | 20.0–38.0 26.1 ± 5.5 | 2.95 2 |
| Species | THg | MeHg | Se | THg EWI | MeHg EWI | Se EDI |
|---|---|---|---|---|---|---|
| Pelagic fish | ||||||
| European pilchard | 0.03 | 0.02 | 0.15 | 0.30 | 0.20 | 0.21 |
| European anchovy | 0.05 | 0.04 | 0.22 | 0.50 | 0.40 | 0.31 |
| European hake | 0.08 | 0.06 | 0.29 | 0.80 | 0.60 | 0.41 |
| Gilthead seabream | 0.15 | 0.13 | 0.38 | 1.49 | 1.29 | 0.54 |
| European seabass | 0.13 | 0.11 | 0.30 | 1.29 | 1.09 | 0.43 |
| Atlantic mackerel | 0.18 | 0.14 | 0.40 | 1.79 | 1.39 | 0.57 |
| Chub mackerel | 0.21 | 0.19 | 0.33 | 2.09 | 1.89 | 0.47 |
| Atlantic bonito | 0.28 | 0.26 | 0.44 | 2.78 | 2.58 | 0.62 |
| Albacore | 0.43 | 0.38 | 0.52 | 4.27 | 3.78 | 0.74 |
| Atlantic bluefin tuna | 0.51 | 0.47 | 0.58 | 5.07 | 4.67 | 0.82 |
| Swordfish | 0.64 | 0.55 | 0.44 | 6.36 | 5.47 | 0.62 |
| Benthic fish | ||||||
| Sandy ray | 0.38 | 0.35 | 0.47 | 3.78 | 3.48 | 0.67 |
| Longnose skate | 0.33 | 0.30 | 0.41 | 3.28 | 2.98 | 0.58 |
| Shagreen ray | 0.30 | 0.27 | 0.44 | 2.98 | 2.68 | 0.62 |
| Turbot | 0.24 | 0.20 | 0.30 | 2.39 | 1.99 | 0.43 |
| Common sole | 0.18 | 0.16 | 0.28 | 1.79 | 1.59 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, G.; Storelli, A.; Meleleo, D.; Dambrosio, A.; Garofalo, R.; Busco, A.; Storelli, M.M. Levels of Mercury, Methylmercury and Selenium in Fish: Insights into Children Food Safety. Toxics 2021, 9, 39. https://doi.org/10.3390/toxics9020039
Barone G, Storelli A, Meleleo D, Dambrosio A, Garofalo R, Busco A, Storelli MM. Levels of Mercury, Methylmercury and Selenium in Fish: Insights into Children Food Safety. Toxics. 2021; 9(2):39. https://doi.org/10.3390/toxics9020039
Chicago/Turabian StyleBarone, Grazia, Arianna Storelli, Daniela Meleleo, Angela Dambrosio, Rita Garofalo, Antonio Busco, and Maria Maddalena Storelli. 2021. "Levels of Mercury, Methylmercury and Selenium in Fish: Insights into Children Food Safety" Toxics 9, no. 2: 39. https://doi.org/10.3390/toxics9020039
APA StyleBarone, G., Storelli, A., Meleleo, D., Dambrosio, A., Garofalo, R., Busco, A., & Storelli, M. M. (2021). Levels of Mercury, Methylmercury and Selenium in Fish: Insights into Children Food Safety. Toxics, 9(2), 39. https://doi.org/10.3390/toxics9020039