The Prevalence of Inorganic Mercury in Human Kidneys Suggests a Role for Toxic Metals in Essential Hypertension
Abstract
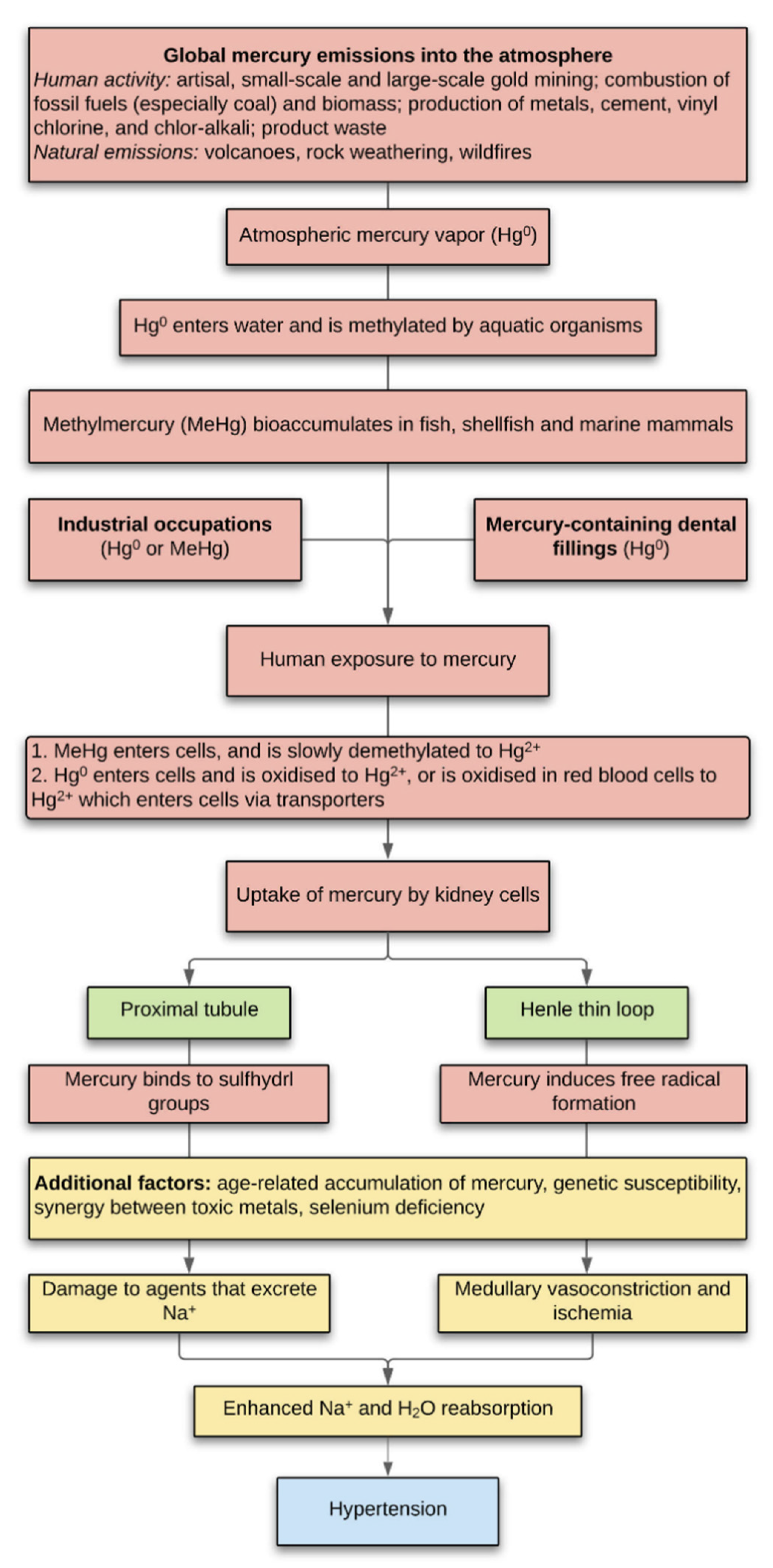
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Sample Collection
2.3. Autometallography
2.4. Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry (LA-ICP-MS)
2.5. Statistical Analyses
3. Results
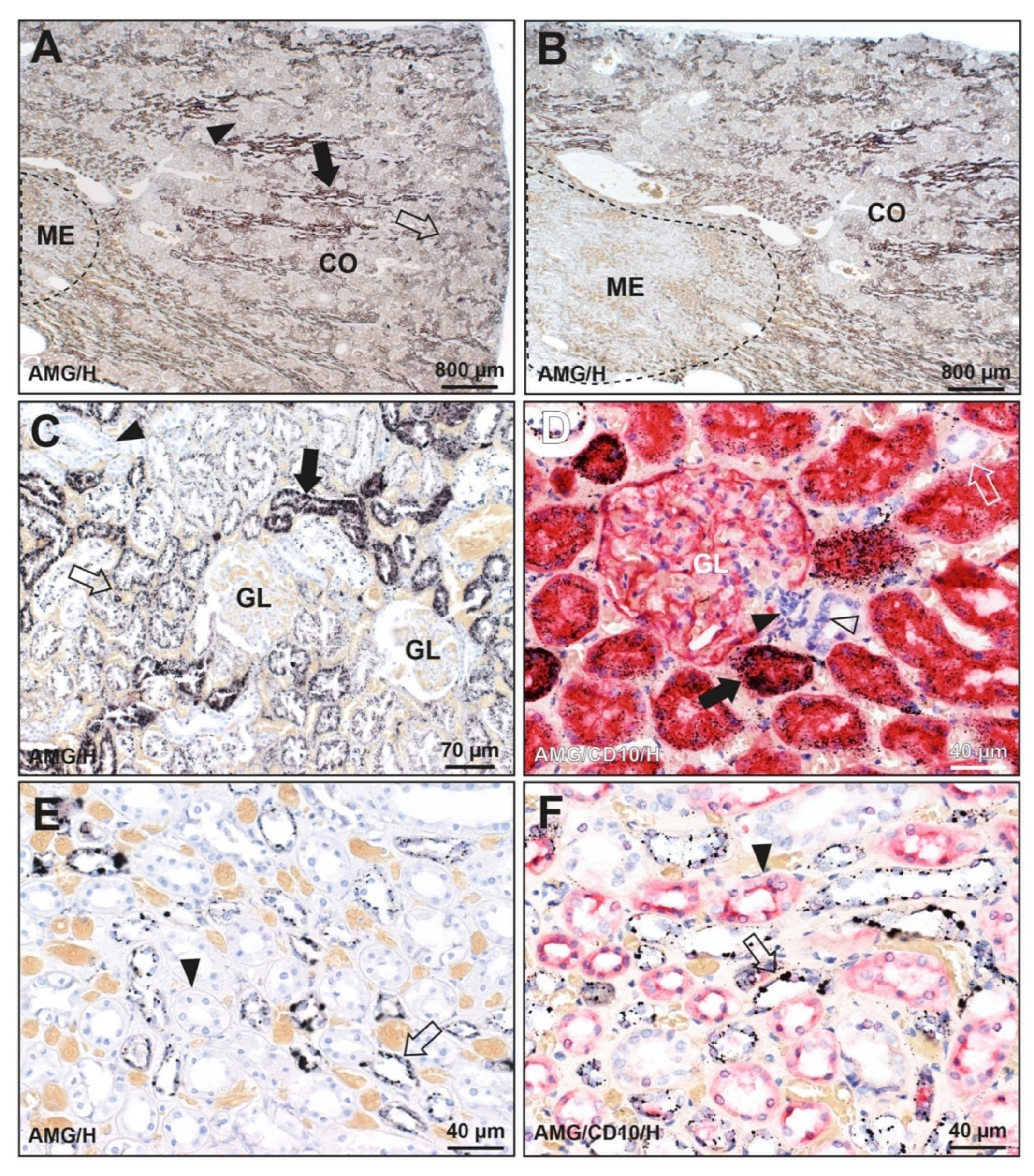
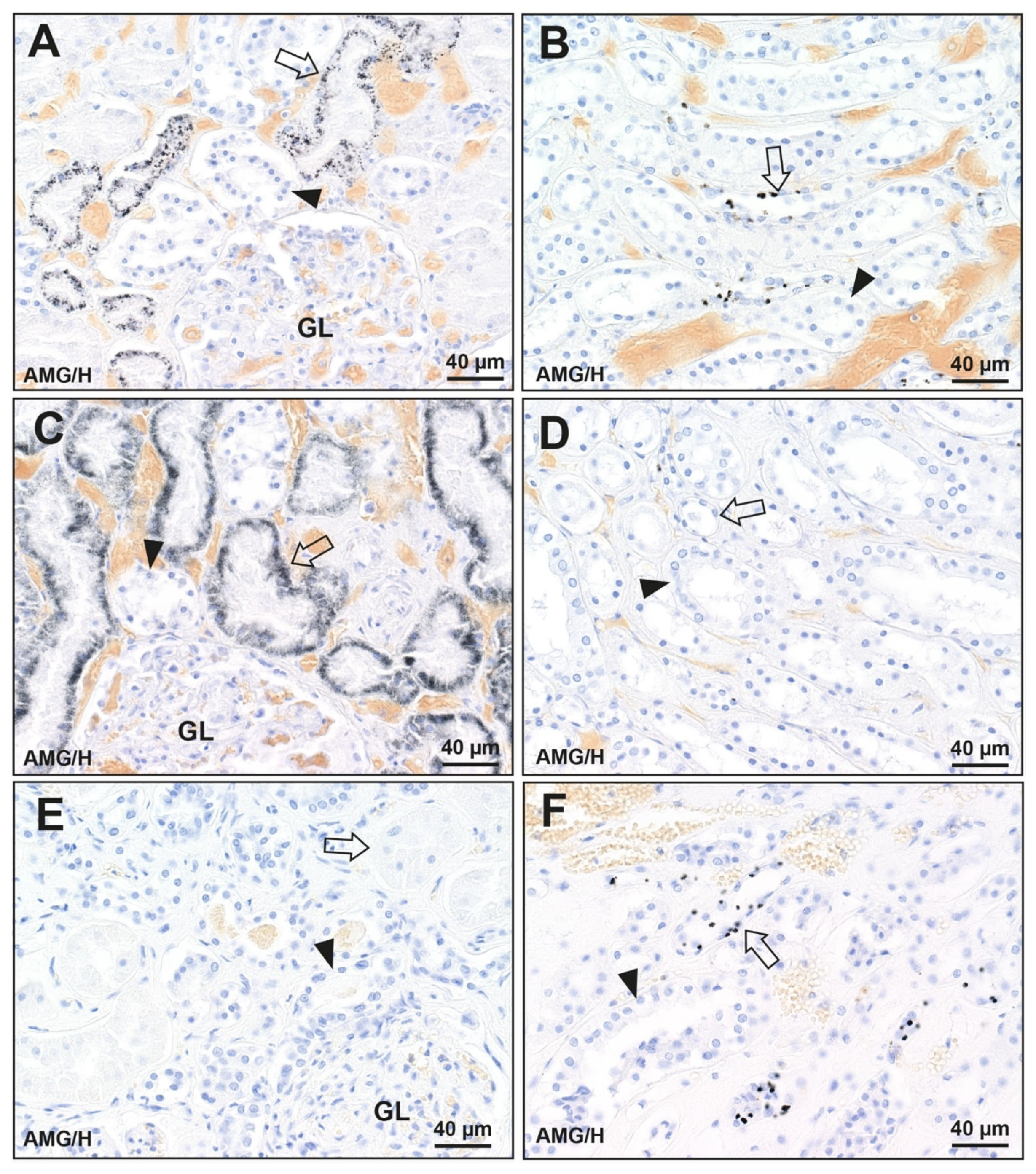
3.1. Distribution of Mercury in the Kidney
3.1.1. Known Mercury Exposure (N = 1)
3.1.2. Unknown Mercury Exposure (N = 128)
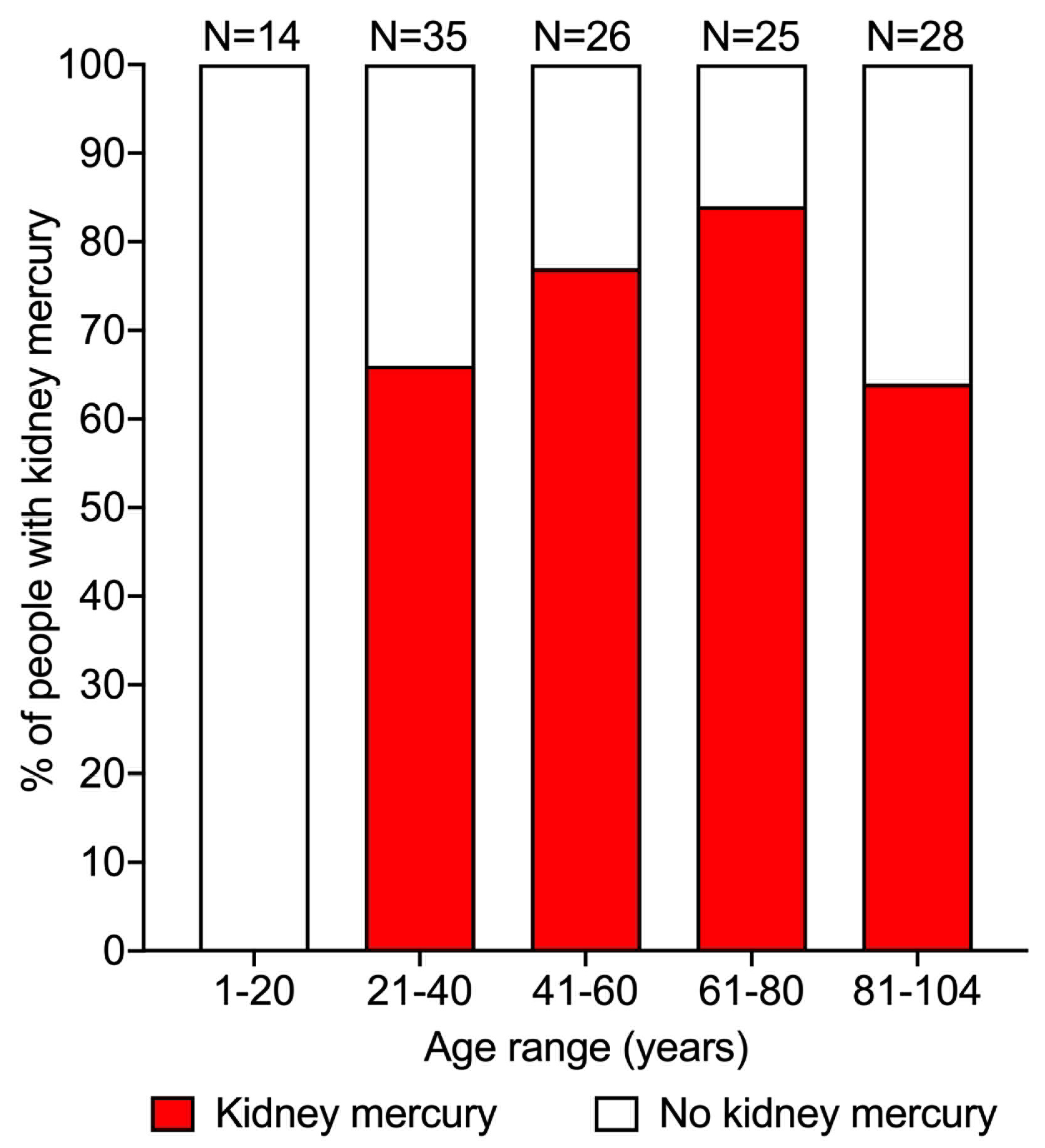
3.2. Prevalence of Mercury in the Kidney
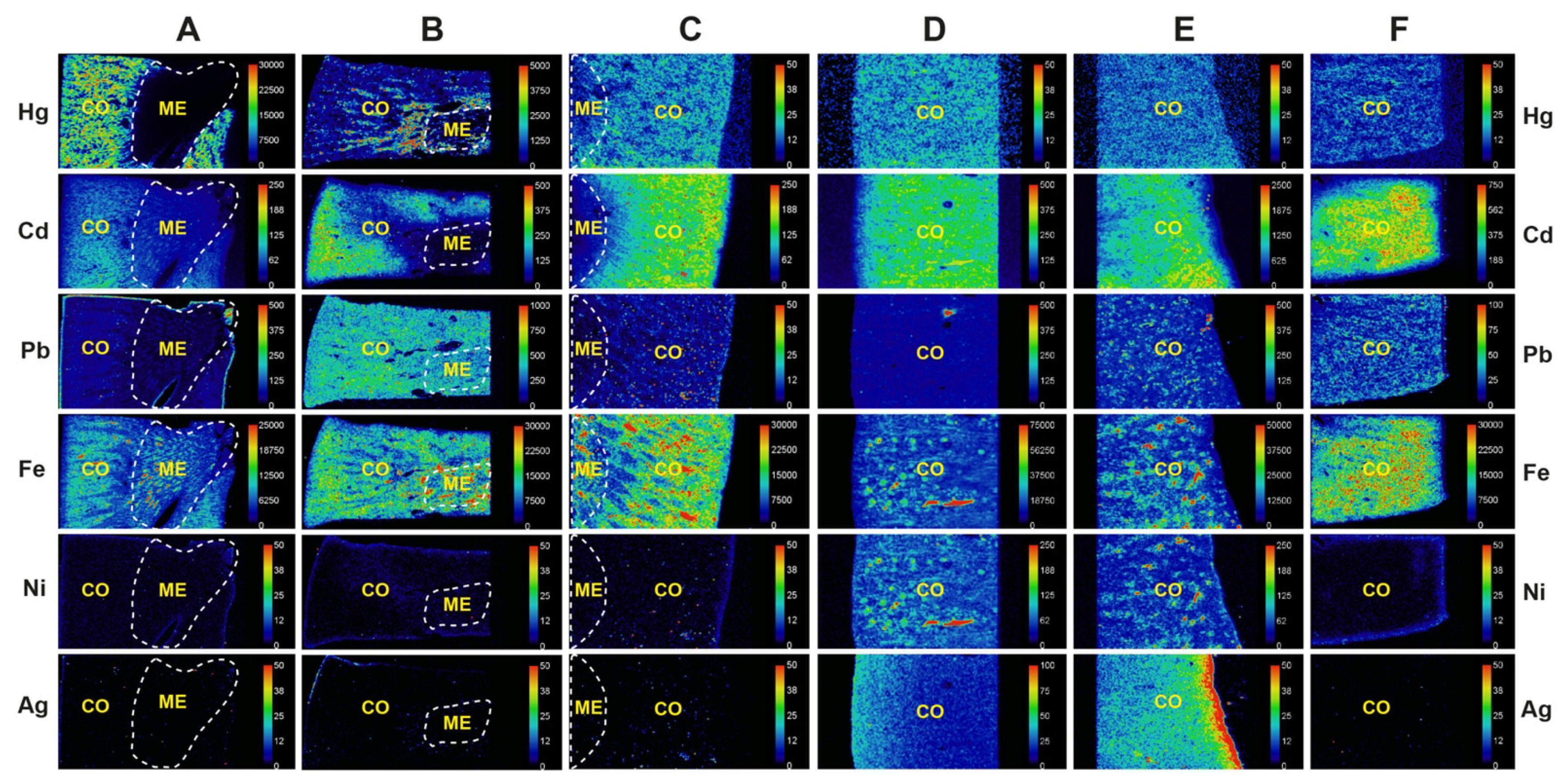
3.3. Metals Detected in the Kidney on LA-ICP-MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Poulter, N.R.; Prabhakaran, D.; Caulfield, M. Hypertension. Lancet 2015, 386, 801–812. [Google Scholar] [CrossRef]
- Guyton, A.C. Dominant role of the kidneys and accessory role of whole-body autoregulation in the pathogenesis of hypertension. Am. J. Hypertens. 1989, 2, 575–585. [Google Scholar] [CrossRef]
- Crowley, S.D.; Coffman, T.M. The inextricable role of the kidney in hypertension. J. Clin. Investig. 2014, 124, 2341–2347. [Google Scholar] [CrossRef]
- Li, X.C.; Zhuo, J.L. Recent Updates on the Proximal Tubule Renin-Angiotensin System in Angiotensin II-Dependent Hypertension. Curr. Hypertens. Rep. 2016, 18, 63. [Google Scholar] [CrossRef]
- Horita, S.; Nakamura, M.; Suzuki, M.; Satoh, N.; Suzuki, A.; Homma, Y.; Nangaku, M. The role of renal proximal tubule transport in the regulation of blood pressure. Kidney Res. Clin. Pract. 2017, 36, 12–21. [Google Scholar] [CrossRef]
- Jung, J.; Basile, D.P.; Pratt, J.H. Sodium reabsorption in the thick ascending limb in relation to blood pressure: A clinical perspective. Hypertension 2011, 57, 873–879. [Google Scholar] [CrossRef][Green Version]
- Cowley, A.W., Jr.; Abe, M.; Mori, T.; O’Connor, P.M.; Ohsaki, Y.; Zheleznova, N.N. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am. J. Physiol. Renal Physiol. 2015, 308, F179–F197. [Google Scholar] [CrossRef]
- Hu, X.F.; Singh, K.; Chan, H.M. Mercury Exposure, Blood Pressure, and Hypertension: A Systematic Review and Dose-response Meta-analysis. Environ. Health Perspect. 2018, 126, 076002. [Google Scholar] [CrossRef]
- Yorifuji, T.; Tsuda, T.; Kashima, S.; Takao, S.; Harada, M. Long-term exposure to methylmercury and its effects on hypertension in Minamata. Environ. Res. 2010, 110, 40–46. [Google Scholar] [CrossRef]
- Inoue, S.; Yorifuji, T.; Tsuda, T.; Doi, H. Short-term effect of severe exposure to methylmercury on atherosclerotic heart disease and hypertension mortality in Minamata. Sci. Total Environ. 2012, 417–418, 291–293. [Google Scholar] [CrossRef]
- Yorifuji, T.; Tsuda, T. Epidemiological studies of neurological signs and symptoms and blood pressure in populations near the industrial methylmercury contamination at Minamata, Japan. Arch. Environ. Occup. Health 2016, 71, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Wakita, Y. Hypertension induced by methyl mercury in rats. Toxicol. Appl. Pharmacol. 1987, 89, 144–147. [Google Scholar] [CrossRef]
- Grotto, D.; de Castro, M.M.; Barcelos, G.R.; Garcia, S.C.; Barbosa, F., Jr. Low level and sub-chronic exposure to methylmercury induces hypertension in rats: Nitric oxide depletion and oxidative damage as possible mechanisms. Arch. Toxicol. 2009, 83, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Wildemann, T.M.; Mirhosseini, N.; Siciliano, S.D.; Weber, L.P. Cardiovascular responses to lead are biphasic, while methylmercury, but not inorganic mercury, monotonically increases blood pressure in rats. Toxicology 2015, 328, 1–11. [Google Scholar] [CrossRef]
- Torres, A.D.; Rai, A.N.; Hardiek, M.L. Mercury intoxication and arterial hypertension: Report of two patients and review of the literature. Pediatrics 2000, 105, E34. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin, Hypertens. 2011, 13, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha Martins, A., Jr.; Carneiro, M.F.H.; Grotto, D.; Adeyemi, J.A.; Barbosa, F., Jr. Arsenic, cadmium, and mercury-induced hypertension: Mechanisms and epidemiological findings. J. Toxicol. Environ. Health B Crit. Rev. 2018, 21, 61–82. [Google Scholar] [CrossRef]
- Danscher, G. Applications of autometallography to heavy metal toxicology. Pharmacol. Toxicol. 1991, 68, 414–423. [Google Scholar] [CrossRef]
- Loumbourdis, N.S.; Danscher, G. Autometallographic tracing of Hg-S quantum dots in frogs exposed to inorganic mercury. Biometals 2008, 21, 311–319. [Google Scholar] [CrossRef]
- Baatrup, E.; Danscher, G. Cytochemical demonstration of mercury deposits in trout liver and kidney following methyl mercury intoxication: Differentiation of two mercury pools by selenium. Ecotoxicol. Environ. Saf. 1987, 14, 129–141. [Google Scholar] [CrossRef]
- Woshner, V.M.; O’Hara, T.M.; Eurell, J.A.; Wallig, M.A.; Bratton, G.R.; Suydam, R.S.; Beasley, V.R. Distribution of inorganic mercury in liver and kidney of beluga and bowhead whales through autometallographic development of light microscopic tissue sections. Toxicol. Pathol. 2002, 30, 209–215. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S.; Cherepanoff, S. Mercury in the retina and optic nerve following prenatal exposure to mercury vapor. PLoS ONE 2019, 14, e0220859. [Google Scholar] [CrossRef] [PubMed]
- Danscher, G.; Zimmer, J. An improved Timm sulphide silver method for light and electron microscopic localization of heavy metals in biological tissues. Histochemistry 1978, 55, 27–40. [Google Scholar] [CrossRef]
- Danscher, G. Autometallography. A new technique for light and electron microscopic visualization of metals in biological tissues (gold, silver, metal sulphides and metal selenides). Histochemistry 1984, 81, 331–335. [Google Scholar] [CrossRef]
- Norgaard, J.O.; Moller-Madsen, B.; Hertel, N.; Danscher, G. Silver enhancement of tissue mercury: Demonstration of mercury in autometallographic silver grains from rat kidneys. J. Histochem. Cytochem. 1989, 37, 1545–1547. [Google Scholar] [CrossRef]
- Hansen, J.C.; Reske-Nielsen, E.; Thorlacius-Ussing, O.; Rungby, J.; Danscher, G. Distribution of dietary mercury in a dog. Quantitation and localization of total mercury in organs and central nervous system. Sci. Total Environ. 1989, 78, 23–43. [Google Scholar] [CrossRef]
- Danscher, G.; Horsted-Bindslev, P.; Rungby, J. Traces of mercury in organs from primates with amalgam fillings. Exp. Mol. Pathol. 1990, 52, 291–299. [Google Scholar] [CrossRef]
- Opitz, H.; Schweinsberg, F.; Grossmann, T.; Wendt-Gallitelli, M.F.; Meyermann, R. Demonstration of mercury in the human brain and other organs 17 years after metallic mercury exposure. Clin. Neuropathol. 1996, 15, 139–144. [Google Scholar]
- Pamphlett, R.; Kum Jew, S. Uptake of inorganic mercury by human locus ceruleus and corticomotor neurons: Implications for amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2013, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K.; Cherian, M.G.; Barfuss, D.W. Mercury-metallothionein and the renal accumulation and handling of mercury. Toxicology 1993, 83, 61–78. [Google Scholar] [CrossRef]
- Zalups, R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000, 52, 113–143. [Google Scholar]
- Zalups, R.K.; Aslamkhan, A.G.; Ahmad, S. Human organic anion transporter 1 mediates cellular uptake of cysteine-S conjugates of inorganic mercury. Kidney Int. 2004, 66, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K.; Joshee, L.; Bridges, C.C. Novel Hg2+-induced nephropathy in rats and mice lacking Mrp2: Evidence of axial heterogeneity in the handling of Hg2+ along the proximal tubule. Toxicol. Sci. 2014, 142, 250–260. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K.; Joshee, L. Toxicological significance of renal Bcrp: Another potential transporter in the elimination of mercuric ions from proximal tubular cells. Toxicol. Appl. Pharmacol. 2015, 285, 110–117. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 55–80. [Google Scholar] [CrossRef]
- Orr, S.E.; Barnes, M.C.; Joshee, L.; Uchakina, O.; McKallip, R.J.; Bridges, C.C. Potential mechanisms of cellular injury following exposure to a physiologically relevant species of inorganic mercury. Toxicol. Lett. 2019, 304, 13–20. [Google Scholar] [CrossRef]
- Pamphlett, R.; Waley, P. Motor neuron uptake of low dose inorganic mercury. J. Neurol. Sci. 1996, 135, 63–67. [Google Scholar] [CrossRef]
- Kedziora, A.; Duflou, J. Attempted suicide by intravenous injection of mercury: A rare cause of cardiac granulomas. A case report. Am. J. Forensic Med. Pathol. 1995, 16, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R.; Waley, P. Uptake of inorganic mercury by the human brain. Acta Neuropathol. 1996, 92, 525–527. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S. Inorganic mercury in human astrocytes, oligodendrocytes, corticomotoneurons and the locus ceruleus: Implications for multiple sclerosis, neurodegenerative disorders and gliomas. Biometals 2018, 31, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Danscher, G.; Moller-Madsen, B. Silver amplification of mercury sulfide and selenide: A histochemical method for light and electron microscopic localization of mercury in tissue. J. Histochem. Cytochem. 1985, 33, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Danscher, G.; Rungby, J. Differentiation of histochemically visualized mercury and silver. Histochem. J. 1986, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R.; Png, F.Y. Shrinkage of motor axons following systemic exposure to inorganic mercury. J. Neuropathol. Exp. Neurol. 1998, 57, 360–366. [Google Scholar] [CrossRef]
- Clapp, W.L.; Croker, B.P. Kidney. In Histology for Pathologists, 4th ed.; Mills, S.E., Ed.; Wolters Klower: Philadelphia, PA, USA, 2012; pp. 891–970. [Google Scholar]
- Faa, G.; Gerosa, C.; Fanni, D.; Nemolato, S.; Marinelli, V.; Locci, A.; Senes, G.; Mais, V.; Van Eyken, P.; Iacovidou, N.; et al. CD10 in the developing human kidney: Immunoreactivity and possible role in renal embryogenesis. J. Matern. Fetal Neonatal Med. 2012, 25, 904–911. [Google Scholar] [CrossRef]
- Danscher, G.; Stoltenberg, M.; Juhl, S. How to detect gold, silver and mercury in human brain and other tissues by autometallographic silver amplification. Neuropathol. Appl. Neurobiol. 1994, 20, 454–467. [Google Scholar] [CrossRef]
- Danscher, G.; Stoltenberg, M.; Kemp, K.; Pamphlett, R. Bismuth autometallography: Protocol, specificity, and differentiation. J. Histochem. Cytochem. 2000, 48, 1503–1510. [Google Scholar] [CrossRef]
- Pamphlett, R.; Satgunaseelan, L.; Kum Jew, S.; Doble, P.A.; Bishop, D.P. Elemental bioimaging shows mercury and other toxic metals in normal breast tissue and in breast cancers. PLoS ONE 2020, 15, e0228226. [Google Scholar] [CrossRef]
- UN Environment Programme. Global Mercury Assessment 2018; UN Environment Programme: Geneva, Switzerland, 2019. [Google Scholar]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Vyas, J.B.; Ballatori, N. Mechanisms of mercury disposition in the body. Am. J. Ind. Med. 2007, 50, 757–764. [Google Scholar] [CrossRef]
- Clarkson, T.W. The toxicology of mercury. Crit. Rev. Clin. Lab. Sci. 1997, 34, 369–403. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Zalups, R.K. Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol. 2017, 91, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Hartmann, H.A. Electron microscopic histochemical study on the localization and distribution of mercury in the nervous system after mercury intoxication. Exp. Neurol. 1972, 35, 122–137. [Google Scholar] [CrossRef]
- Nylander, M.; Friberg, L.; Lind, B. Mercury concentrations in the human brain and kidneys in relation to exposure from dental amalgam fillings. Swed. Dent. J. 1987, 11, 179–187. [Google Scholar]
- Barregard, L.; Svalander, C.; Schutz, A.; Westberg, G.; Sallsten, G.; Blohme, I.; Molne, J.; Attman, P.O.; Haglind, P. Cadmium, mercury, and lead in kidney cortex of the general Swedish population: A study of biopsies from living kidney donors. Environ. Health Perspect. 1999, 107, 867–871. [Google Scholar] [CrossRef][Green Version]
- Barregard, L.; Fabricius-Lagging, E.; Lundh, T.; Molne, J.; Wallin, M.; Olausson, M.; Modigh, C.; Sallsten, G. Cadmium, mercury, and lead in kidney cortex of living kidney donors: Impact of different exposure sources. Environ. Res. 2010, 110, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, G.; Grandi, M.; Cattaneo, C.; Calza, S.; Minoia, C.; Ronchi, A.; Gatti, A.; Severi, G. Dental amalgam and mercury levels in autopsy tissues: Food for thought. Am. J. Forensic Med. Pathol. 2006, 27, 42–45. [Google Scholar] [CrossRef]
- Doris, P.A. Renal proximal tubule sodium transport and genetic mechanisms of essential hypertension. J. Hypertens. 2000, 18, 509–519. [Google Scholar] [CrossRef]
- Quigley, R.; Chakravarty, S.; Zhao, X.; Imig, J.D.; Capdevila, J.H. Increased renal proximal convoluted tubule transport contributes to hypertension in Cyp4a14 knockout mice. Nephron Physiol. 2009, 113, p23–p28. [Google Scholar] [CrossRef]
- Wang, X.; Armando, I.; Upadhyay, K.; Pascua, A.; Jose, P.A. The regulation of proximal tubular salt transport in hypertension: An update. Curr. Opin. Nephrol. Hypertens. 2009, 18, 412–420. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.W., Jr. Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 2008, 52, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Lemos, N.B.; Angeli, J.K.; Faria Tde, O.; Ribeiro, R.F., Jr.; Vassallo, D.V.; Padilha, A.S.; Stefanon, I. Low mercury concentration produces vasoconstriction, decreases nitric oxide bioavailability and increases oxidative stress in rat conductance artery. PLoS ONE 2012, 7, e49005. [Google Scholar] [CrossRef]
- Karimi, R.; Vacchi-Suzzi, C.; Meliker, J.R. Mercury exposure and a shift toward oxidative stress in avid seafood consumers. Environ. Res. 2016, 146, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Andreoli, V.; Sprovieri, F. Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview. Int. J. Environ. Res. Public Health 2017, 14, 93. [Google Scholar] [CrossRef]
- Wildemann, T.M.; Siciliano, S.D.; Weber, L.P. The mechanisms associated with the development of hypertension after exposure to lead, mercury species or their mixtures differs with the metal and the mixture ratio. Toxicology 2016, 339, 1–8. [Google Scholar] [CrossRef]
- Andrade, V.M.; Aschner, M.; Marreilha Dos Santos, A.P. Neurotoxicity of Metal Mixtures. In Neurotoxicity of Metals, 2017/09/11 ed.; Aschner, M., Costa, L.G., Eds.; Springer Nature: Cham, Switzerland, 2017; Volume 18, pp. 227–265. [Google Scholar]
- Orr, S.E.; George, H.S.; Barnes, M.C.; Mathis, T.N.; Joshee, L.; Barkin, J.; Kiefer, A.M.; Seney, C.S.; Bridges, C.C. Co-administration of Selenium with Inorganic Mercury Alters the Disposition of Mercuric Ions in Rats. Biol. Trace Elem. Res. 2020, 195, 187–195. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Sandberg, K.; Ji, H. Sex differences in primary hypertension. Biol. Sex Differ. 2012, 3, 7. [Google Scholar] [CrossRef]
- Doumas, M.; Papademetriou, V.; Faselis, C.; Kokkinos, P. Gender differences in hypertension: Myths and reality. Curr. Hypertens. Rep. 2013, 15, 321–330. [Google Scholar] [CrossRef]
- Hidayat, K.; Du, X.; Zou, S.Y.; Shi, B.M. Blood pressure and kidney cancer risk: Meta-analysis of prospective studies. J. Hypertens. 2017, 35, 1333–1344. [Google Scholar] [CrossRef]
- Kim, C.S.; Han, K.D.; Choi, H.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Association of Hypertension and Blood Pressure with Kidney Cancer Risk: A Nationwide Population-Based Cohort Study. Hypertension 2020, 75, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Semmens, E.O.; Domitrovich, J.; Conway, K.; Noonan, C.W. A cross-sectional survey of occupational history as a wildland firefighter and health. Am. J. Ind. Med. 2016, 59, 330–335. [Google Scholar] [CrossRef]
- Longo, B.M. Adverse Health Effects Associated with Increased Activity at Kilauea Volcano: A Repeated Population-Based Survey. ISNR Public Health 2013, 2013, 475962. [Google Scholar] [CrossRef]
- Brook, R.D.; Brook, J.R.; Tam, E.K. Volcanic smog and cardiometabolic health: Hawaiian hypertension? J. Clin. Hypertens. 2019, 21, 533–535. [Google Scholar] [CrossRef]
- Dong, G.H.; Qian, Z.M.; Xaverius, P.K.; Trevathan, E.; Maalouf, S.; Parker, J.; Yang, L.; Liu, M.M.; Wang, D.; Ren, W.H.; et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension 2013, 61, 578–584. [Google Scholar] [CrossRef]
- Brook, R.D.; Sun, Z.; Brook, J.R.; Zhao, X.; Ruan, Y.; Yan, J.; Mukherjee, B.; Rao, X.; Duan, F.; Sun, L.; et al. Extreme Air Pollution Conditions Adversely Affect Blood Pressure and Insulin Resistance: The Air Pollution and Cardiometabolic Disease Study. Hypertension 2016, 67, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kit, B.K.; Kuklina, E.; Carroll, M.D.; Ostchega, Y.; Freedman, D.S.; Ogden, C.L. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015, 169, 272–279. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S.; Doble, P.A.; Bishop, D.P. Elemental Analysis of Aging Human Pituitary Glands Implicates Mercury as a Contributor to the Somatopause. Front. Endocrinol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R.; Ewan, K.B.; McQuilty, R.; Waley, P. Gender differences in the uptake of inorganic mercury by motor neurons. Neurotoxicol. Teratol. 1997, 19, 287–293. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Macedo, G.L.; Pereira, S.I.; Arrifano, G.P.; Picanco-Diniz, D.L.; do Nascimento, J.L.; Herculano, A.M. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol. Res. 2009, 60, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Nersesyan, A.; Kundi, M.; Waldherr, M.; Setayesh, T.; Misik, M.; Wultsch, G.; Filipic, M.; Mazzaron Barcelos, G.R.; Knasmueller, S. Results of micronucleus assays with individuals who are occupationally and environmentally exposed to mercury, lead and cadmium. Mutat. Res. 2016, 770, 119–139. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S. Mercury Is Taken Up Selectively by Cells Involved in Joint, Bone, and Connective Tissue Disorders. Front. Med. 2019, 6, 168. [Google Scholar] [CrossRef]
- Pamphlett, R.; Colebatch, A.J.; Doble, P.A.; Bishop, D.P. Mercury in Pancreatic Cells of People with and without Pancreatic Cancer. Int. J. Environ. Res. Public Health 2020, 17, 8990. [Google Scholar] [CrossRef] [PubMed]
- Finley, B.D.; Swartzendruber, P.C.; Jaffe, D.A. Particulate mercury emissions in regional wildfire plumes observed at the Mount Bachelor Observatory. Atmos. Environ. 2009, 43, 6074–6083. [Google Scholar] [CrossRef]
- Kristensen, L.J.; Taylor, M.P. Fields and forests in flames: Lead and mercury emissions from wildfire pyrogenic activity. Environ. Health Perspect. 2012, 120, a56–a57. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Dowling, K.; Florentine, S. Effects of prescribed fire and post-fire rainfall on mercury mobilization and subsequent contamination assessment in a legacy mine site in Victoria, Australia. Chemosphere 2018, 190, 144–153. [Google Scholar] [CrossRef]
- Varekamp, J.C.; Buseck, P.R. Global mercury flux from volcanic and geothermal sources. J. Appl. Geochem. 1986, 1, 65–73. [Google Scholar] [CrossRef]
- Streets, D.G.; Devane, M.K.; Lu, Z.; Bond, T.C.; Sunderland, E.M.; Jacob, D.J. All-time releases of mercury to the atmosphere from human activities. Environ. Sci. Technol. 2011, 45, 10485–10491. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Amos, H.M.; Jacob, D.J.; Kocman, D.; Horowitz, H.M.; Zhang, Y.; Dutkiewicz, S.; Horvat, M.; Corbitt, E.S.; Krabbenhoft, D.P.; Sunderland, E.M. Global biogeochemical implications of mercury discharges from rivers and sediment burial. Environ. Sci. Technol. 2014, 48, 9514–9522. [Google Scholar] [CrossRef]
- Louis, W.J.; Doyle, A.E.; Anavekar, S. Plasma norepinephrine levels in essential hypertension. N. Engl. J. Med. 1973, 288, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Plasma catecholamines and essential hypertension. An analytical review. Hypertension 1983, 5, 86–99. [Google Scholar] [CrossRef]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cifkova, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S.; Doble, P.A.; Bishop, D.P. Mercury in the human adrenal medulla could contribute to increased plasma noradrenaline in aging. Sci. Rep. 2021, 11, 2961. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Almeida Lopes, A.C.B.; Urbano, M.R.; Carvalho, M.F.H.; Silva, A.M.R.; Tinkov, A.A.; Aschner, M.; Mesas, A.E.; Silbergeld, E.K.; Paoliello, M.M.B. An updated systematic review on the association between Cd exposure, blood pressure and hypertension. Ecotoxicol. Environ. Saf. 2021, 208, 111636. [Google Scholar] [CrossRef]
- Gambelunghe, A.; Sallsten, G.; Borne, Y.; Forsgard, N.; Hedblad, B.; Nilsson, P.; Fagerberg, B.; Engstrom, G.; Barregard, L. Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ. Res. 2016, 149, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Almeida Lopes, A.C.B.; Silbergeld, E.K.; Navas-Acien, A.; Zamoiski, R.; Martins, A.D.C., Jr.; Camargo, A.E.I.; Urbano, M.R.; Mesas, A.E.; Paoliello, M.M.B. Association between blood lead and blood pressure: A population-based study in Brazilian adults. Environ. Health 2017, 16, 27. [Google Scholar] [CrossRef]
- Han, L.; Wang, X.; Han, R.; Xu, M.; Zhao, Y.; Gao, Q.; Shen, H.; Zhang, H. Association between blood lead level and blood pressure: An occupational population-based study in Jiangsu province, China. PLoS ONE 2018, 13, e0200289. [Google Scholar] [CrossRef]
- Zheutlin, A.R.; Hu, H.; Weisskopf, M.G.; Sparrow, D.; Vokonas, P.S.; Park, S.K. Low-Level Cumulative Lead and Resistant Hypertension: A Prospective Study of Men Participating in the Veterans Affairs Normative Aging Study. J. Am. Heart Assoc. 2018, 7, e010014. [Google Scholar] [CrossRef]
- Drake, P.L.; Hazelwood, K.J. Exposure-related health effects of silver and silver compounds: A review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar] [CrossRef]
- Cobbina, S.J.; Chen, Y.; Zhou, Z.; Wu, X.; Feng, W.; Wang, W.; Mao, G.; Xu, H.; Zhang, Z.; Wu, X.; et al. Low concentration toxic metal mixture interactions: Effects on essential and non-essential metals in brain, liver, and kidneys of mice on sub-chronic exposure. Chemosphere 2015, 132, 79–86. [Google Scholar] [CrossRef]
- Parizek, J.; Ostadalova, I. The protective effect of small amounts of selenite in sublimate intoxication. Experientia 1967, 23, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Ganther, H.E.; Goudie, C.; Sunde, M.L.; Kopecky, M.J.; Wagner, P. Selenium: Relation to decreased toxicity of methylmercury added to diets containing tuna. Science 1972, 175, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Kosta, L.; Byrne, A.R.; Zelenko, V. Correlation between selenium and mercury in man following exposure to inorganic mercury. Nature 1975, 254, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Sumino, K.; Yamamoto, R.; Kitamura, S. A role of selenium against methylmercury toxicity. Nature 1977, 268, 73–74. [Google Scholar] [CrossRef]
- Berlin, M. Interaction between selenium and inorganic mercury. Environ. Health Perspect. 1978, 25, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Ganther, H.E. Modification of methylmercury toxicity and metabolism by selenium and vitamin E: Possible mechanisms. Environ. Health Perspect. 1978, 25, 71–76. [Google Scholar] [CrossRef]
- Drasch, G.; Mailänder, S.; Schlosser, C.; Roider, G. Content of non-mercury-associated selenium in human tissues. Biol. Trace Elem. Res. 2000, 77, 219–230. [Google Scholar] [CrossRef]
- Ralston, N.V.; Blackwell, J.L., 3rd; Raymond, L.J. Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol. Trace Elem. Res. 2007, 119, 255–268. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Raymond, L.J. Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. Biochim. Biophys. Acta Gen. Subj. 2018, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Nylander, M.; Weiner, J. Mercury and selenium concentrations and their interrelations in organs from dental staff and the general population. Br. J. Ind. Med. 1991, 48, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, L.; Palm, B.; Nylander, M.; Nordberg, M. Mercury and selenium distribution in human kidney cortex. Biol. Trace Elem. Res. 1994, 40, 255–265. [Google Scholar] [CrossRef]
- Drasch, G.; Wanghofer, E.; Roider, G.; Strobach, S. Correlation of mercury and selenium in the human kidney. J. Trace Elem. Med. Biol. 1996, 10, 251–254. [Google Scholar] [CrossRef]
- Pamphlett, R.; Mak, R.; Lee, J.; Buckland, M.E.; Harding, A.J.; Kum Jew, S.; Paterson, D.J.; Jones, M.W.M.; Lay, P.A. Concentrations of toxic metals and essential trace elements vary among individual neurons in the human locus ceruleus. PLoS ONE 2020, 15, e0233300. [Google Scholar] [CrossRef]
- Bishop, D.P.; Clases, D.; Fryer, F.; Williams, E.; Wilkins, S.; Hare, D.J.; Cole, N.; Karst, U.; Doble, P.A. Elemental bio-imaging using laser ablation-triple quadrupole-ICP-MS. J. Anal. At. Spectrom. 2016, 31, 197–202. [Google Scholar] [CrossRef]
- Mao, J.; Vanderlelie, J.J.; Perkins, A.V.; Redman, C.W.; Ahmadi, K.R.; Rayman, M.P. Genetic polymorphisms that affect selenium status and response to selenium supplementation in United Kingdom pregnant women. Am. J. Clin. Nutr. 2016, 103, 100–106. [Google Scholar] [CrossRef]
- Hu, X.F.; Sharin, T.; Chan, H.M. Dietary and blood selenium are inversely associated with the prevalence of stroke among Inuit in Canada. J. Trace Elem. Med. Biol. 2017, 44, 322–330. [Google Scholar] [CrossRef]
- Laclaustra, M.; Navas-Acien, A.; Stranges, S.; Ordovas, J.M.; Guallar, E. Serum selenium concentrations and hypertension in the US Population. Circ. Cardiovasc. Qual. Outcomes 2009, 2, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Jin, Y.; Unverzagt, F.W.; Liang, C.; Cheng, Y.; Hake, A.M.; Kuruppu, D.; Ma, F.; Liu, J.; Chen, C.; et al. Longitudinal Association between Selenium Levels and Hypertension in a Rural Elderly Chinese Cohort. J. Nutr. Health Aging 2016, 20, 983–988. [Google Scholar] [CrossRef]
- Kuruppu, D.; Hendrie, H.C.; Yang, L.; Gao, S. Selenium levels and hypertension: A systematic review of the literature. Public Health Nutr. 2014, 17, 1342–1352. [Google Scholar] [CrossRef]
- Parkin Kullmann, J.A.; Pamphlett, R. A Comparison of Mercury Exposure from Seafood Consumption and Dental Amalgam Fillings in People with and without Amyotrophic Lateral Sclerosis (ALS): An International Online Case-Control Study. Int. J. Environ. Res. Public Health 2018, 15, 2874. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C.; Kaneko, J.J.; Raymond, L.J. Selenium health benefit values provide a reliable index of seafood benefits vs. risks. J. Trace Elem. Med. Biol. 2019, 55, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.; Raymond, L.J. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 2010, 278, 112–123. [Google Scholar] [CrossRef] [PubMed]

| ID No. | Age | Sex | Proximal Tubule AMG | Henle Loop AMG | ID No. | Age | Sex | Proximal Tubule AMG | Henle Loop AMG | ID No. | Age | Sex | Proximal Tubule AMG | Henle Loop AMG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 | 1 | F | − | − | K44 | 39 | M | + | + | K87 | 69 | M | − | − |
| K2 | 2 | M | − | − | K45 | 39 | M | − | + | K88 | 70 | M | − | − |
| K3 | 2 | F | − | − | K46 | 39 | M | − | − | K89 | 70 | M | + | + |
| K4 | 2 | F | − | − | K47 | 39 | M | + | + | K90 | 71 | F | − | + |
| K5 | 3 | M | − | − | K48 | 40 | F | − | + | K91 | 72 | F | + | + |
| K6 | 4 | M | − | − | K49 | 40 | F | + | + | K92 | 72 | F | + | + |
| K7 | 9 | M | − | − | K50 | 40 | M | − | + | K93 | 74 | F | − | + |
| K8 | 16 | M | − | − | K51 | 41 | M | − | − | K94 | 75 | M | − | − |
| K9 | 18 | M | − | − | K52 | 41 | F | − | − | K95 | 76 | F | + | + |
| K10 | 18 | F | − | − | K53 | 41 | M | − | + | K96 | 76 | F | + | + |
| K11 | 18 | F | − | − | K54 | 42 | M | + | + | K97 | 77 | F | + | − |
| K12 | 20 | M | − | − | K55 | 43 | M | − | − | K98 | 77 | M | + | − |
| K13 | 20 | F | − | − | K56 | 43 | M | − | − | K99 | 79 | M | − | + |
| K14 | 20 | M | − | − | K57 | 44 | M | − | + | K100 | 80 | F | + | + |
| K15 | 23 | M | − | − | K58 | 44 | M | + | − | K101 | 80 | M | − | + |
| K16 | 24 | M * | + | + | K59 | 45 | M | − | + | K102 | 81 | M | + | + |
| K17 | 24 | M | − | + | K60 | 45 | M | + | − | K103 | 83 | M | + | + |
| K18 | 25 | F | − | − | K61 | 45 | M | − | − | K104 | 85 | M | − | − |
| K19 | 25 | M | + | + | K62 | 46 | M | + | − | K105 | 86 | M | + | + |
| K20 | 26 | F | + | + | K63 | 46 | F | + | + | K106 | 86 | F | + | + |
| K21 | 28 | M | − | − | K64 | 46 | M | + | − | K107 | 87 | M | − | − |
| K22 | 29 | F | − | − | K65 | 47 | M | − | + | K108 | 87 | F | − | + |
| K23 | 29 | M | + | − | K66 | 47 | M | + | + | K109 | 89 | F | − | + |
| K24 | 30 | M | − | − | K67 | 48 | F | + | + | K110 | 95 | F | − | − |
| K25 | 30 | M | − | + | K68 | 49 | F | + | + | K111 | 95 | F | − | + |
| K26 | 30 | M | − | − | K69 | 49 | M | + | + | K112 | 95 | F | − | + |
| K27 | 31 | M | + | − | K70 | 49 | M | + | − | K113 | 95 | M | − | − |
| K28 | 32 | M | − | + | K71 | 49 | M | + | + | K114 | 95 | M | − | + |
| K29 | 32 | M | + | − | K72 | 53 | M | − | + | K115 | 95 | F | + | − |
| K30 | 33 | F | − | − | K73 | 55 | M | − | + | K116 | 95 | M | − | − |
| K31 | 33 | M | − | − | K74 | 58 | M | − | − | K117 | 96 | F | − | − |
| K32 | 34 | M | − | − | K75 | 59 | F | + | + | K118 | 96 | M | + | + |
| K33 | 35 | M | + | + | K76 | 59 | M | + | + | K119 | 96 | M | + | − |
| K34 | 35 | F | + | − | K77 | 61 | M | + | − | K120 | 96 | F | + | − |
| K35 | 35 | F | − | − | K78 | 61 | M | + | + | K121 | 96 | F | − | − |
| K36 | 36 | F | + | + | K79 | 61 | M | + | − | K122 | 97 | F | − | + |
| K37 | 36 | M | − | + | K80 | 61 | M | − | − | K123 | 97 | F | + | + |
| K38 | 37 | M | + | − | K81 | 61 | F | + | + | K124 | 98 | M | − | − |
| K39 | 38 | M | − | − | K82 | 62 | M | − | + | K125 | 98 | M | − | + |
| K40 | 38 | F | + | + | K83 | 63 | F | − | + | K126 | 99 | F | + | − |
| K41 | 38 | M | + | − | K84 | 66 | M | + | − | K127 | 100 | M | − | − |
| K42 | 38 | M | + | − | K85 | 67 | F | + | + | K128 | 104 | F | − | − |
| K43 | 38 | F | − | + | K86 | 67 | M | − | + | K129 | 104 | F | − | + |
| ID | Site | AMG | LA-ICP-MS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hg | Cd | Pb | Fe | Ni | Ag | Cr | Al | Bi | Au | |||
| K16 | Cortex | Positive | + | + | − | + | − | − | − | − | − | − |
| Medulla | Positive | − | − | − | + | − | − | − | − | − | − | |
| K32 | Cortex | Positive | + | + | + | + | + | − | − | − | − | − |
| Medulla | Positive | + | − | + | + | + | − | − | − | − | − | |
| K19 | Cortex | Positive | + | + | − | + | − | − | − | − | − | − |
| Medulla | Positive | − | − | − | + | − | − | − | − | − | − | |
| K69 | Cortex | Positive | + | + | + | + | − | + | − | − | − | − |
| K51 | Cortex | Negative | + | + | + | + | + | + | − | − | − | − |
| K29 | Cortex | Negative | + | + | + | + | − | − | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pamphlett, R.; Doble, P.A.; Bishop, D.P. The Prevalence of Inorganic Mercury in Human Kidneys Suggests a Role for Toxic Metals in Essential Hypertension. Toxics 2021, 9, 67. https://doi.org/10.3390/toxics9030067
Pamphlett R, Doble PA, Bishop DP. The Prevalence of Inorganic Mercury in Human Kidneys Suggests a Role for Toxic Metals in Essential Hypertension. Toxics. 2021; 9(3):67. https://doi.org/10.3390/toxics9030067
Chicago/Turabian StylePamphlett, Roger, Philip A. Doble, and David P. Bishop. 2021. "The Prevalence of Inorganic Mercury in Human Kidneys Suggests a Role for Toxic Metals in Essential Hypertension" Toxics 9, no. 3: 67. https://doi.org/10.3390/toxics9030067
APA StylePamphlett, R., Doble, P. A., & Bishop, D. P. (2021). The Prevalence of Inorganic Mercury in Human Kidneys Suggests a Role for Toxic Metals in Essential Hypertension. Toxics, 9(3), 67. https://doi.org/10.3390/toxics9030067






