Ocean Warming May Enhance Biochemical Alterations Induced by an Invasive Seaweed Exudate in the Mussel Mytilus galloprovincialis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Asparagopsis Armata Sampling and Exudate Production
2.2. Mytilus galloprovincialis Sampling and Acclimatization
2.3. Exposure Assay
2.4. Biomarker Analysis
2.4.1. Sample Preparation for Biomarkers Analysis
2.4.2. Oxidative Stress-Related Biomarkers
2.4.3. Cellular Energy Allocation (CEA)
2.5. Statistical Analysis
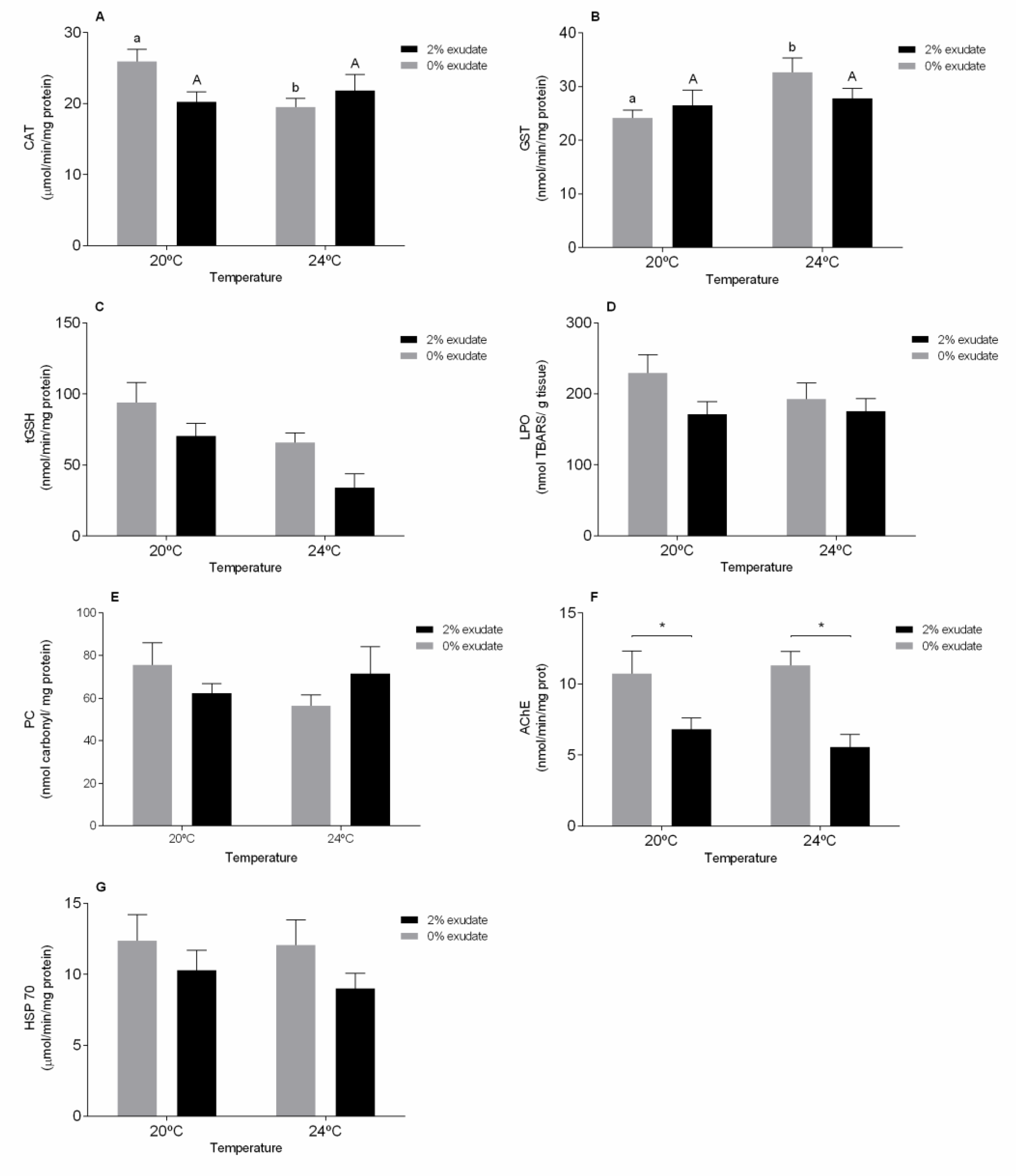
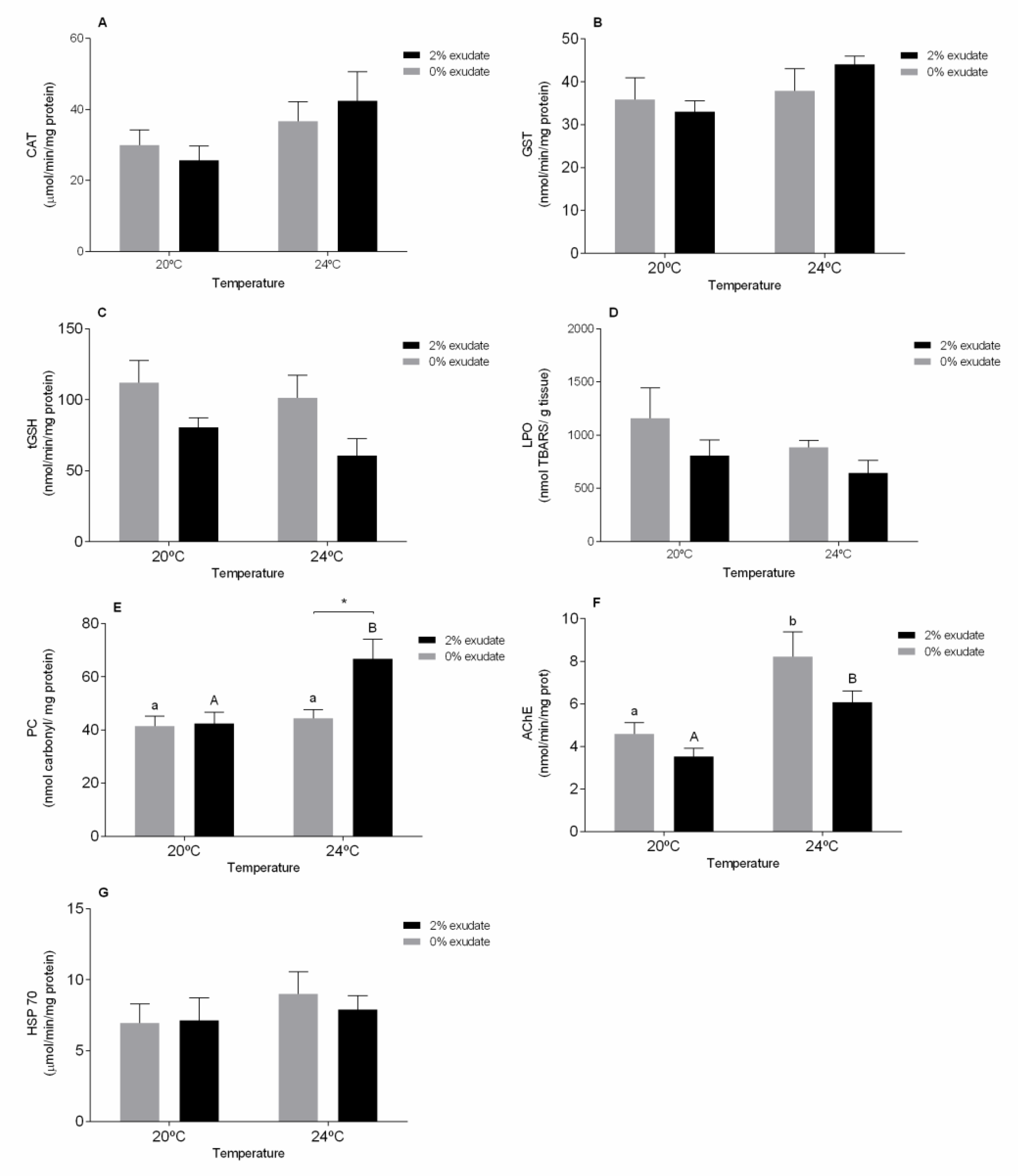
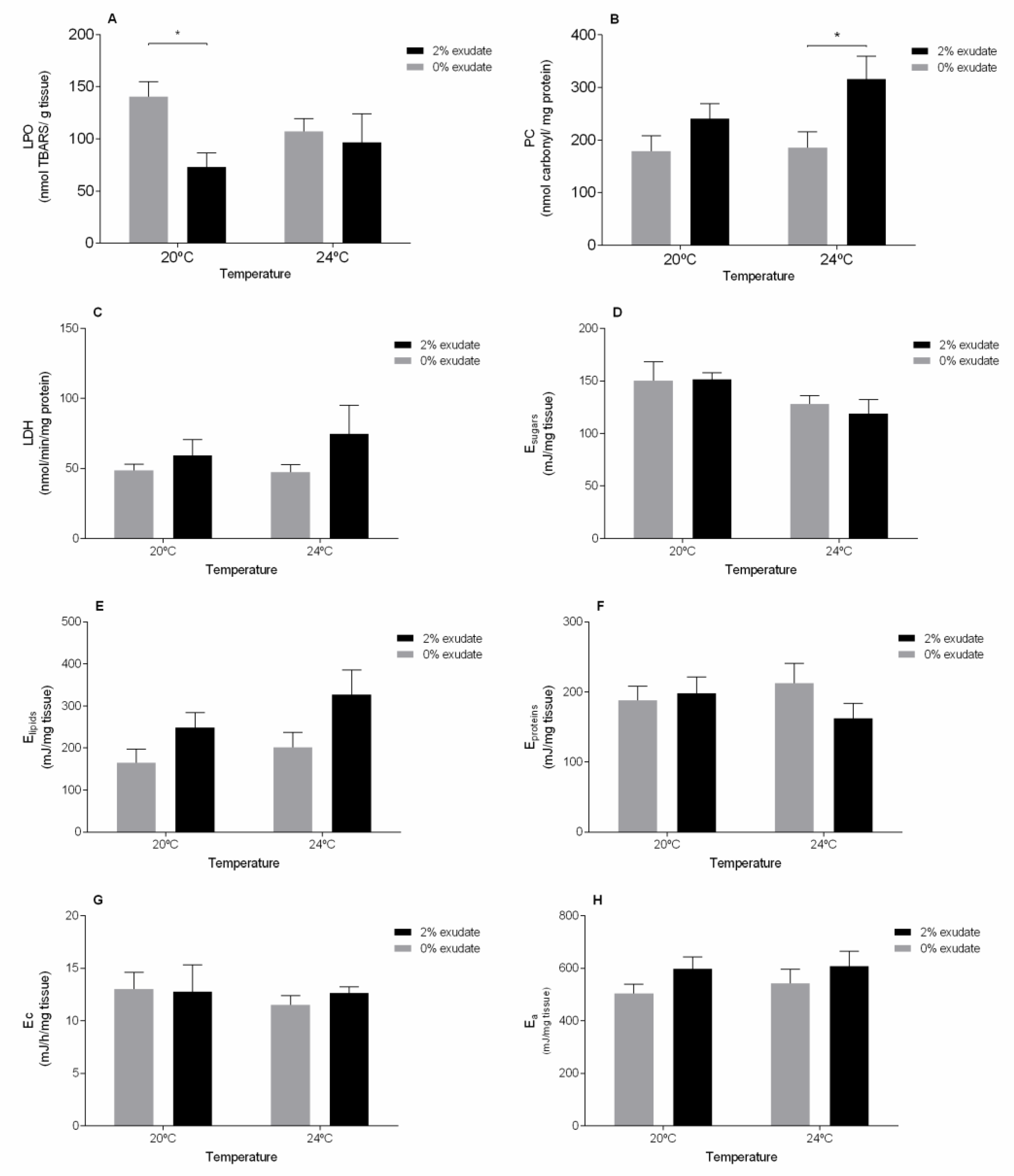
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bindoff, N.L.; Cheung, W.W.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.J.M.; Jiao, N.; Karim, M.S.; Levin, L. Changing ocean, marine ecosystems, and dependent communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; pp. 477–587. [Google Scholar]
- Pörtner, H.O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, C.L.; Burnett, N.P.; Ramsey, M.J.; Wagner, K.; Zippay, M.L. Physiological responses to heat stress in an invasive mussel Mytilus galloprovincialis depend on tidal habitat. Mar. Environ. Res. 2020, 154, 104849. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Henderson, S.; Miller-Ezzy, P.; Li, X.X.; Qin, J.G. Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish Shellfish Immunol. 2019, 86, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; van Kleunen, M.; Kühn, I.; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Chang. Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.A.; Brown, L.; Campbell, M.L.; Canning-Clode, J.; Carlton, J.T.; Castro, N.; Chainho, P.; Chan, F.T.; Creed, J.C.; Curd, A.; et al. Trends in the detection of aquatic non-indigenous species across global marine, estuarine and freshwater ecosystems: A 50-year perspective. Divers. Distrib. 2020, 26, 1780–1797. [Google Scholar] [CrossRef]
- Andreakis, N.; Schaffelke, B. Invasive marine seaweeds: Pest or prize? In Seaweed Biology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 235–262. [Google Scholar]
- Martins, G.M.; Cacabelos, E.; Faria, J.; Álvaro, N.; Prestes, A.C.; Neto, A.I. Patterns of distribution of the invasive alga Asparagopsis armata Harvey: A multi-scaled approach. Aquat. Invasions 2019, 14, 582–593. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Ros, M.; Izquierdo, D.; Soler-Hurtado, M.M. The invasive Asparagopsis armata versus the native Corallina elongata: Differences in associated peracarid assemblages. J. Exp. Mar. Biol. Ecol. 2012, 416–417, 121–128. [Google Scholar] [CrossRef]
- Maggs, C.A.; Stegenga, H. Red algal exotics on North Sea coasts. Helgoländer Meeresunters. 1998, 52, 243–258. [Google Scholar] [CrossRef] [Green Version]
- Dijoux, L.; Viard, F.; Payri, C. The more we search, the more we find: Discovery of a new lineage and a new species complex in the genus Asparagopsis. PLoS ONE 2014, 9, e103826. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.O.; Lemos, M.F.L.; Gaspar, R.; Gonçalves, C.; Neto, J.M. The effects of the invasive seaweed Asparagopsis armata on native rock pool communities: Evidences from experimental exclusion. Ecol. Indic. 2021, 125, 107463. [Google Scholar] [CrossRef]
- McConnell, O.; Fenical, W. Halogen chemistry of the red alga Asparagopsis. Phytochemistry 1977, 16, 367–374. [Google Scholar] [CrossRef]
- Kladi, M.; Vagias, C.; Roussis, V. Volatile halogenated metabolites from marine red algae. Phytochem. Rev. 2004, 3, 337–366. [Google Scholar] [CrossRef]
- Paul, N.A.; de Nys, R.; Steinberg, P. Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mar. Ecol. Prog. Ser. 2006, 306, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Coelho, S.D.; Vieira, H.C.; Oliveira, J.M.M.; Pires, S.F.S.; Rocha, R.J.M.; Rodrigues, A.C.M.; Soares, A.M.V.M.; Bordalo, M.D. How Does Mytilus galloprovincialis Respond When Exposed to the Gametophyte Phase of the Invasive Red Macroalga Asparagopsis armata Exudate? Water 2021, 13, 460. [Google Scholar] [CrossRef]
- Silva, C.O.; Simões, T.; Félix, R.; Soares, A.M.; Barata, C.; Novais, S.C.; Lemos, M.F. Asparagopsis armata exudate cocktail: The quest for the mechanisms of toxic action of an invasive seaweed on marine invertebrates. Biology 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Novais, S.C.; Soares, A.M.; Barata, C.; Lemos, M.F. Impacts of the Invasive Seaweed Asparagopsis armata Exudate on Energetic Metabolism of Rock Pool Invertebrates. Toxins 2021, 13, 15. [Google Scholar] [CrossRef]
- Paul, N.A.; De Nys, R.; Steinberg, P.D. Seaweed–herbivore interactions at a small scale: Direct tests of feeding deterrence by filamentous algae. Mar. Ecol. Prog. Ser. 2006, 323, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, D.; Sen, T.; Dani, V. Effectiveness of Zinc in Modulating the CCl 4—Induced Oxidative Stress in Rat Liver. Toxicol. Mech. Methods 2006, 16, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Pena, A.; Fernandes, J.O. Mussels as bioindicators of diclofenac contamination in coastal environments. Environ. Pollut. 2017, 225, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Andral, B.; Stanisiere, J.Y.; Sauzade, D.; Damier, E.; Thebault, H.; Galgani, F.; Boissery, P. Monitoring chemical contamination levels in the Mediterranean based on the use of mussel caging. Mar. Pollut. Bull. 2004, 49, 704–712. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Shahadat Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Vinagre, C.; Mendonça, V.; Narciso, L.; Madeira, C. Food web of the intertidal rocky shore of the west Portuguese coast–Determined by stable isotope analysis. Mar. Environ. Res. 2015, 110, 53–60. [Google Scholar] [CrossRef]
- Wijsman, J.; Troost, K.; Fang, J.; Roncarati, A. Global production of marine bivalves. Trends and challenges. In Goods and Services of Marine Bivalves; Springer: Cham, Switzerland, 2019; pp. 7–26. [Google Scholar]
- Gazeau, F.; Alliouane, S.; Bock, C.; Bramanti, L.; López Correa, M.; Gentile, M.; Hirse, T.; Pörtner, H.-O.; Ziveri, P. Impact of ocean acidification and warming on the Mediterranean mussel (Mytilus galloprovincialis). Front. Mar. Sci. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Andrade, M.; De Marchi, L.; Soares, A.M.V.M.; Rocha, R.J.M.; Figueira, E.; Freitas, R. Are the effects induced by increased temperature enhanced in Mytilus galloprovincialis submitted to air exposure? Sci. Total Environ. 2019, 647, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.C.; Pereira, V.; Marçal, R.; Marques, A.; Guilherme, S.; Costa, P.R.; Pacheco, M. DNA damage and oxidative stress responses of mussels Mytilus galloprovincialis to paralytic shellfish toxins under warming and acidification conditions–Elucidation on the organ-specificity. Aquat. Toxicol. 2020, 228, 105619. [Google Scholar] [CrossRef] [PubMed]
- Dinh, K.V.; Nguyen, Q.T.T.; Vo, T.-M.-C.; Bui, T.B.; Dao, T.-S.; Tran, D.M.; Doan, N.X.; Truong, T.S.H.; Wisz, M.S.; Nielsen, T.G.; et al. Interactive effects of extreme temperature and a widespread coastal metal contaminant reduce the fitness of a common tropical copepod across generations. Mar. Pollut. Bull. 2020, 159, 111509. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.; Raimundo, J.; Lopes, A.R.; Lopes, C.; Rosa, N.; Brito, P.; Diniz, M.; Caetano, M.; Grilo, T.F. Warming enhances lanthanum accumulation and toxicity promoting cellular damage in glass eels (Anguilla anguilla). Environ. Res. 2020, 191, 110051. [Google Scholar] [CrossRef]
- Hooper, M.J.; Ankley, G.T.; Cristol, D.A.; Maryoung, L.A.; Noyes, P.D.; Pinkerton, K.E. Interactions between chemical and climate stressors: A role for mechanistic toxicology in assessing climate change risks. Environ. Toxicol. Chem. 2013, 32, 32–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.U.; Chen, H.; Gu, H.; Wang, T.; Dupont, S.; Kong, H.; Shang, Y.; Wang, X.; Lu, W.; Hu, M.; et al. Antioxidant responses of the mussel Mytilus coruscus co-exposed to ocean acidification, hypoxia and warming. Mar. Pollut. Bull. 2021, 162, 111869. [Google Scholar] [CrossRef]
- Vieira, H.C.; Bordalo, M.D.; Rodrigues, A.C.M.; Pires, S.F.S.; Rocha, R.J.M.; Soares, A.M.V.M.; Rendón-von Osten, J.; Abreu, S.N.; Morgado, F. Water temperature modulates mercury accumulation and oxidative stress status of common goby (Pomatoschistus microps). Environ. Res. 2021, 193, 110585. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In CRC Handbook of Methods in Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Gravato, C.; Quintaneiro, C.; Bordalo, M.D.; Barata, C.; Soares, A.M.V.M.; Pestana, J.L.T. Energetic costs and biochemical biomarkers associated with esfenvalerate exposure in Sericostoma vittatum. Chemosphere 2017, 189, 445–453. [Google Scholar] [CrossRef]
- Bird, R.P.; Draper, H.H. Comparative studies on different methods of malonaldehyde determination. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 299–305. [Google Scholar]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Vassault, A. Lactate dehydrogenase. In Methods of Enzymatic Analysis—Enzymes: Oxireductases, Transferase; Bergmyer, M.O., Ed.; Academic Press: New York, NY, USA, 1983; pp. 118–126. [Google Scholar]
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recovery 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Gravato, C.; Quintaneiro, C.; Golovko, O.; Žlábek, V.; Barata, C.; Soares, A.M.; Pestana, J.L. Life history and biochemical effects of chlorantraniliprole on Chironomus riparius. Sci. Total Environ. 2015, 508, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Gnaiger, E. Calculation of Energetic and Biochemical Equivalents of Respiratory Oxygen Consumption. In Proceedings of the Polarographic Oxygen Sensors, Berlin/Heidelberg, Germany; 1983; pp. 337–345. [Google Scholar]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Morosetti, B.; Freitas, R.; Pereira, E.; Hamza, H.; Andrade, M.; Coppola, F.; Maggioni, D.; Della Torre, C. Will temperature rise change the biochemical alterations induced in Mytilus galloprovincialis by cerium oxide nanoparticles and mercury? Environ. Res. 2020, 188, 109778. [Google Scholar] [CrossRef]
- Verlecar, X.N.; Jena, K.B.; Chainy, G.B.N. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem. Biol. Interact. 2007, 167, 219–226. [Google Scholar] [CrossRef]
- Del Barga, I.; Frenzilli, G.; Scarcelli, V.; Nigro, M.; Malmvärn, A.; Asplund, L.; Förlin, L.; Sturve, J. Effects of algal extracts (Polysiphonia fucoides) on rainbow trout (Oncorhynchus mykiss): A biomarker approach. Mar. Environ. Res. 2006, 62, S283–S286. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yang, L.; Zhao, Q.; Caen, J.P.; He, H.Y.; Jin, Q.H.; Guo, L.H.; Alemany, M.; Zhang, L.Y.; Shi, Y.F. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ. 2002, 9, 790–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuereb, B.; Lefèvre, E.; Garric, J.; Geffard, O. Acetylcholinesterase activity in Gammarus fossarum (Crustacea Amphipoda): Linking AChE inhibition and behavioural alteration. Aquat. Toxicol. 2009, 94, 114–122. [Google Scholar] [CrossRef]
- Pfeifer, S.; Schiedek, D.; Dippner, J.W. Effect of temperature and salinity on acetylcholinesterase activity, a common pollution biomarker, in Mytilus sp. from the south-western Baltic Sea. J. Exp. Mar. Biol. Ecol. 2005, 320, 93–103. [Google Scholar] [CrossRef]
- Lehtonen, K.K.; Kankaanpää, H.; Leiniö, S.; Sipiä, V.O.; Pflugmacher, S.; Sandberg-Kilpi, E. Accumulation of nodularin-like compounds from the cyanobacterium Nodularia spumigena and changes in acetylcholinesterase activity in the clam Macoma balthica during short-term laboratory exposure. Aquat. Toxicol. 2003, 64, 461–476. [Google Scholar] [CrossRef]
- Matozzo, V.; Fabrello, J.; Masiero, L.; Ferraccioli, F.; Finos, L.; Pastore, P.; Di Gangi, I.M.; Bogialli, S. Ecotoxicological risk assessment for the herbicide glyphosate to non-target aquatic species: A case study with the mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 233, 623–632. [Google Scholar] [CrossRef]
- Ciacci, C.; Barmo, C.; Gallo, G.; Maisano, M.; Cappello, T.; D’Agata, A.; Leonzio, C.; Mauceri, A.; Fasulo, S.; Canesi, L. Effects of sublethal, environmentally relevant concentrations of hexavalent chromium in the gills of Mytilus galloprovincialis. Aquat. Toxicol. 2012, 120–121, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Costa, M.; Leite, C.; Borges, C.; Coppola, F.; Henriques, B.; Monteiro, R.; Russo, T.; Di Cosmo, A.; Soares, A.M.V.M.; et al. Ecotoxicological effects of lanthanum in Mytilus galloprovincialis: Biochemical and histopathological impacts. Aquat. Toxicol. 2019, 211, 181–192. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Attig, H.; Kamel, N.; Sforzini, S.; Dagnino, A.; Jamel, J.; Boussetta, H.; Viarengo, A.; Banni, M. Effects of thermal stress and nickel exposure on biomarkers responses in Mytilus galloprovincialis (Lam). Mar. Environ. Res. 2014, 94, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Magara, G.; Elia, A.C.; Syberg, K.; Khan, F.R. Single contaminant and combined exposures of polyethylene microplastics and fluoranthene: Accumulation and oxidative stress response in the blue mussel, Mytilus edulis. J. Toxicol. Environ. Health Part A 2018, 81, 761–773. [Google Scholar] [CrossRef]
- Patetsini, E.; Dimitriadis, V.K.; Kaloyianni, M. Biomarkers in marine mussels, Mytilus galloprovincialis, exposed to environmentally relevant levels of the pesticides, chlorpyrifos and penoxsulam. Aquat. Toxicol. 2013, 126, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Carini, M.; Butterfield, D.A. Protein Carbonylation. Antioxid. Redox Signal. 2010, 12, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, S.; Ghribi, F.; Chetoui, I.; Aouini, F.; Bouaziz, M.; Houas-Gharsallah, I.; Soudani, N.; El Cafsi, M.h. Effect of storage temperature and time on the fatty acids and nutritional quality of the commercial mussel (Mytilus galloprovincialis). J. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Shahriari, A.; Dawson, N.J.; Bell, R.A.; Storey, K.B. Stable suppression of lactate dehydrogenase activity during anoxia in the foot muscle of Littorina littorea and the potential role of acetylation as a novel posttranslational regulatory mechanism. Enzym. Res. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamantino, T.C.; Almeida, E.; Soares, A.M.V.M.; Guilhermino, L. Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 2001, 45, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Hassoun, E.A.; Stohs, S.J. Cadmium-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J774A.1 cell cultures. Toxicology 1996, 112, 219–226. [Google Scholar] [CrossRef]
- Faggio, C.; Pagano, M.; Alampi, R.; Vazzana, I.; Felice, M.R. Cytotoxicity, haemolymphatic parameters, and oxidative stress following exposures to sub-lethal concentrations of quaternium-15 in Mytilus galloprovincialis. Aquat. Toxicol. 2016, 180, 258–265. [Google Scholar] [CrossRef]
- Oliveira, C.; Almeida, J.; Guilhermino, L.; Soares, A.M.V.M.; Gravato, C. Acute effects of deltamethrin on swimming velocity and biomarkers of the common prawn Palaemon serratus. Aquat. Toxicol. 2012, 124, 209–216. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, H.C.; Rodrigues, A.C.M.; Pires, S.F.S.; Oliveira, J.M.M.; Rocha, R.J.M.; Soares, A.M.V.M.; Bordalo, M.D. Ocean Warming May Enhance Biochemical Alterations Induced by an Invasive Seaweed Exudate in the Mussel Mytilus galloprovincialis. Toxics 2021, 9, 121. https://doi.org/10.3390/toxics9060121
Vieira HC, Rodrigues ACM, Pires SFS, Oliveira JMM, Rocha RJM, Soares AMVM, Bordalo MD. Ocean Warming May Enhance Biochemical Alterations Induced by an Invasive Seaweed Exudate in the Mussel Mytilus galloprovincialis. Toxics. 2021; 9(6):121. https://doi.org/10.3390/toxics9060121
Chicago/Turabian StyleVieira, Hugo C., Andreia C. M. Rodrigues, Sílvia F. S. Pires, Jacinta M. M. Oliveira, Rui J. M. Rocha, Amadeu M. V. M. Soares, and Maria D. Bordalo. 2021. "Ocean Warming May Enhance Biochemical Alterations Induced by an Invasive Seaweed Exudate in the Mussel Mytilus galloprovincialis" Toxics 9, no. 6: 121. https://doi.org/10.3390/toxics9060121
APA StyleVieira, H. C., Rodrigues, A. C. M., Pires, S. F. S., Oliveira, J. M. M., Rocha, R. J. M., Soares, A. M. V. M., & Bordalo, M. D. (2021). Ocean Warming May Enhance Biochemical Alterations Induced by an Invasive Seaweed Exudate in the Mussel Mytilus galloprovincialis. Toxics, 9(6), 121. https://doi.org/10.3390/toxics9060121









