Toxicological Effects of Roundup® on Drosophila melanogaster Reproduction
Abstract
:1. Introduction
Specific Hypothesis
2. Materials and Methods
2.1. Stock Maintenance
2.2. Chemicals
2.3. Reproductive Toxicity Experiments
2.4. Statistical Analyses
3. Results
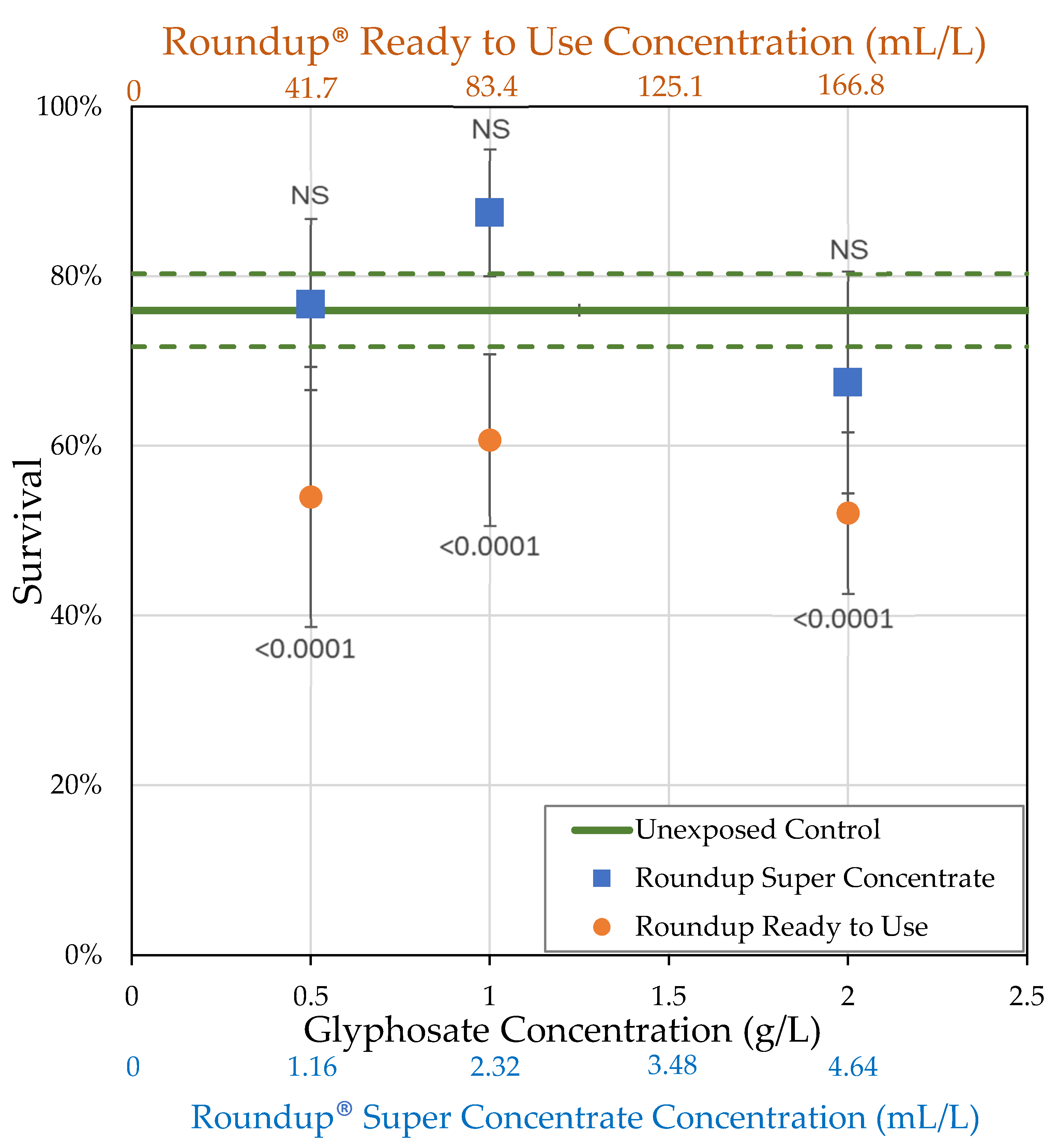
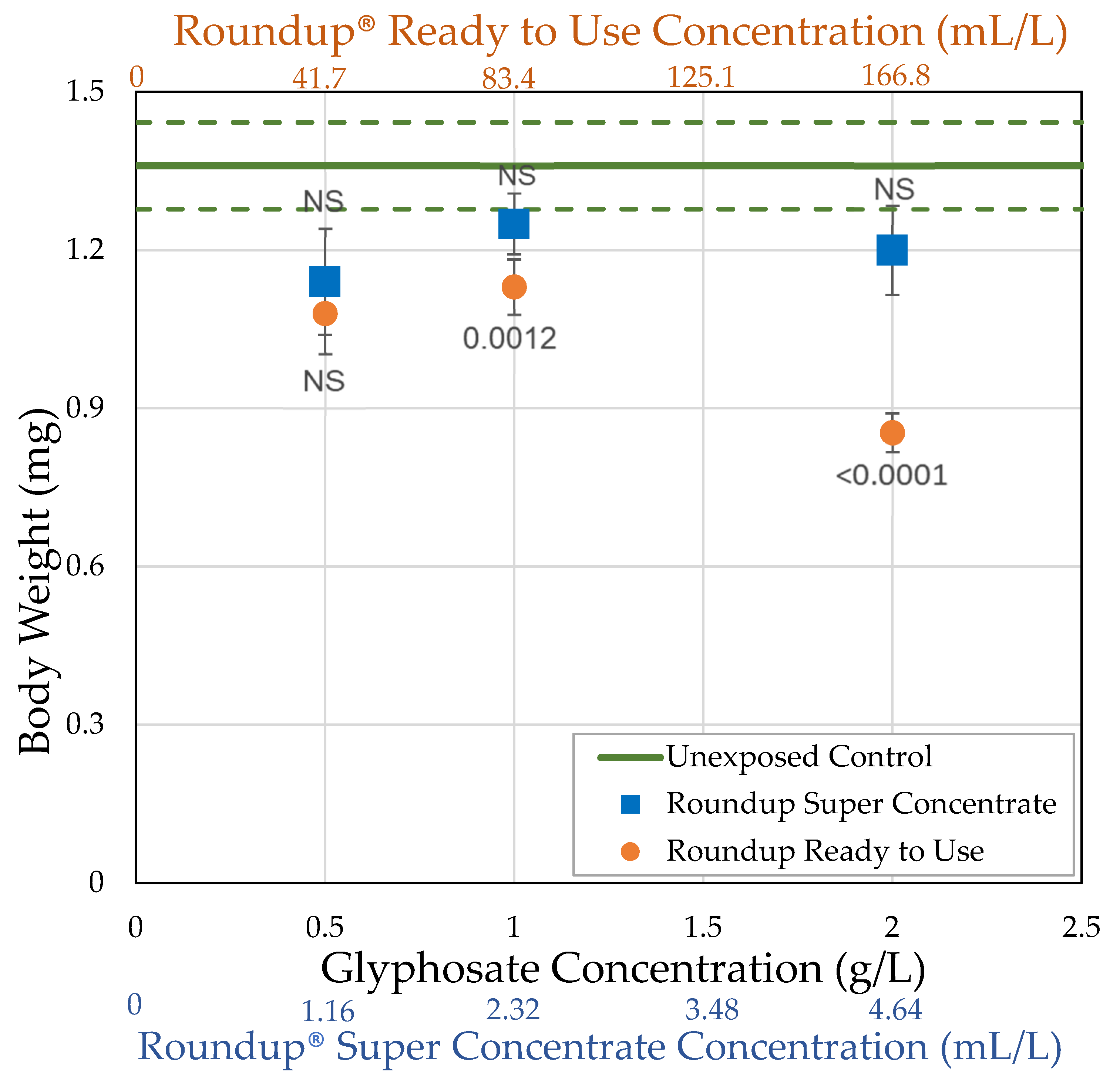
3.1. H0: As Shown Previously, Roundup® Reduces Body Size of Female Drosophila Melanogaster
3.2. H1: Roundup® Interferes with Female Drosophila Melanogaster Reproduction
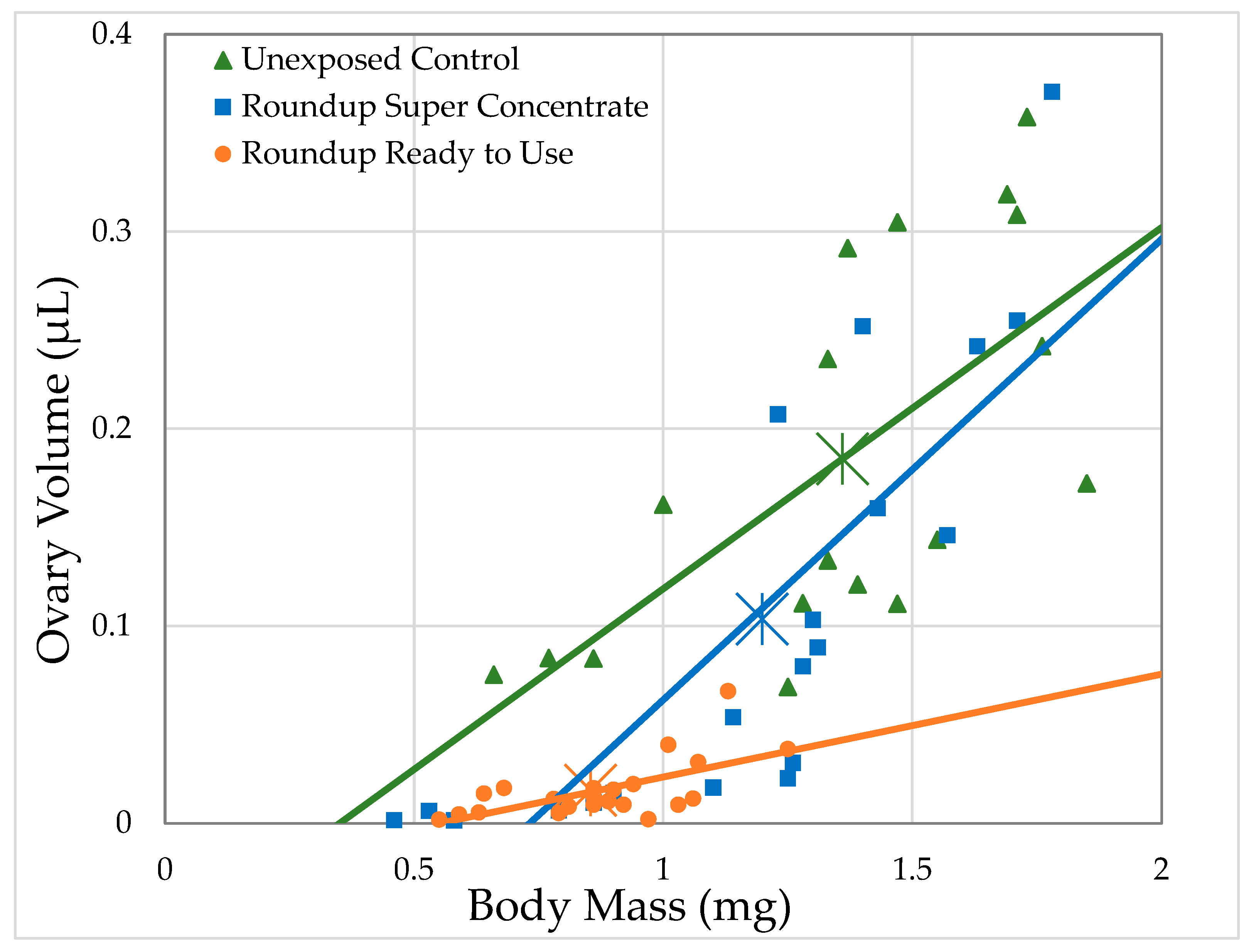
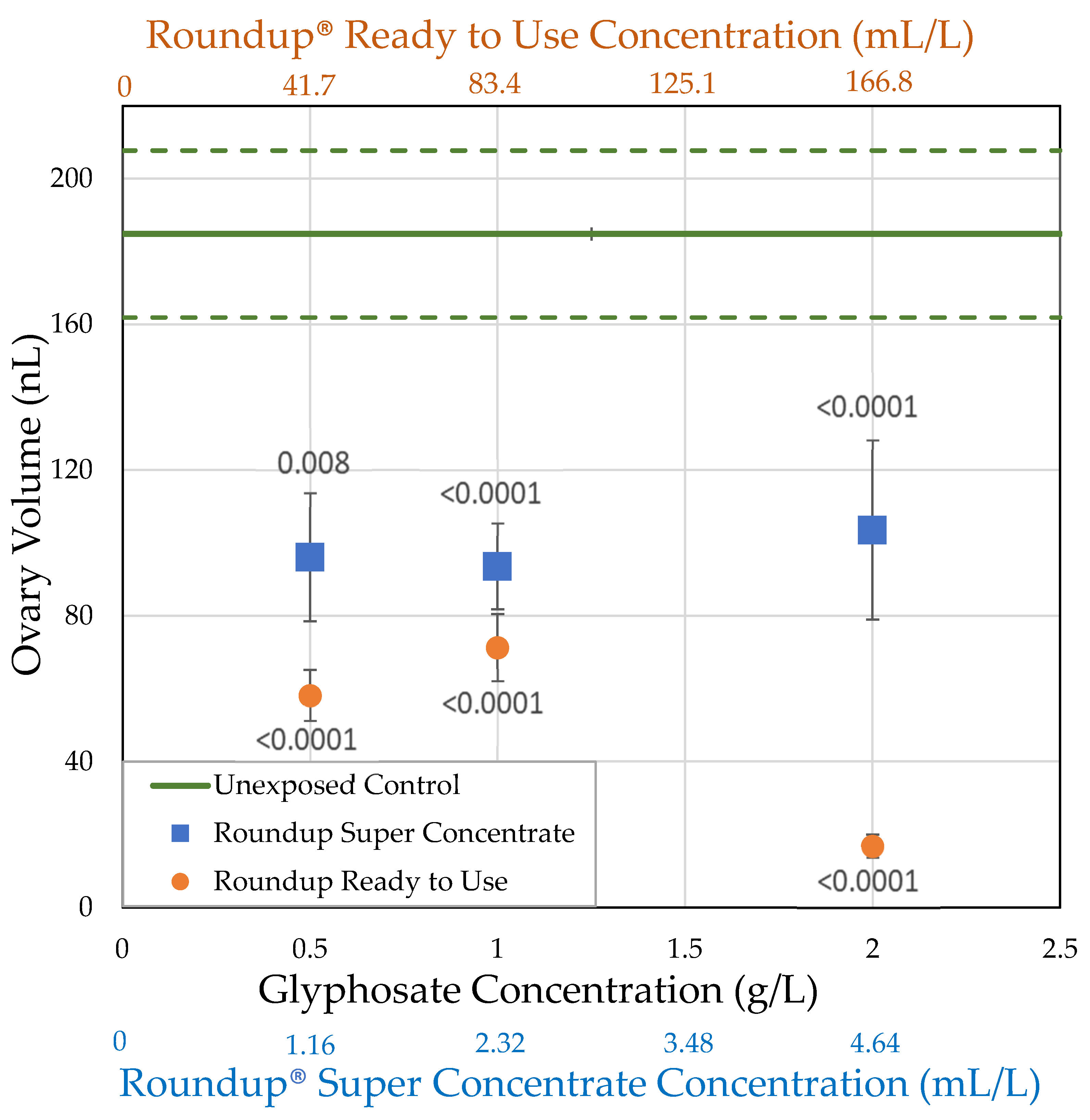
3.2.1. H1-A: Roundup® Reduces Ovary Size in Drosophila Melanogaster
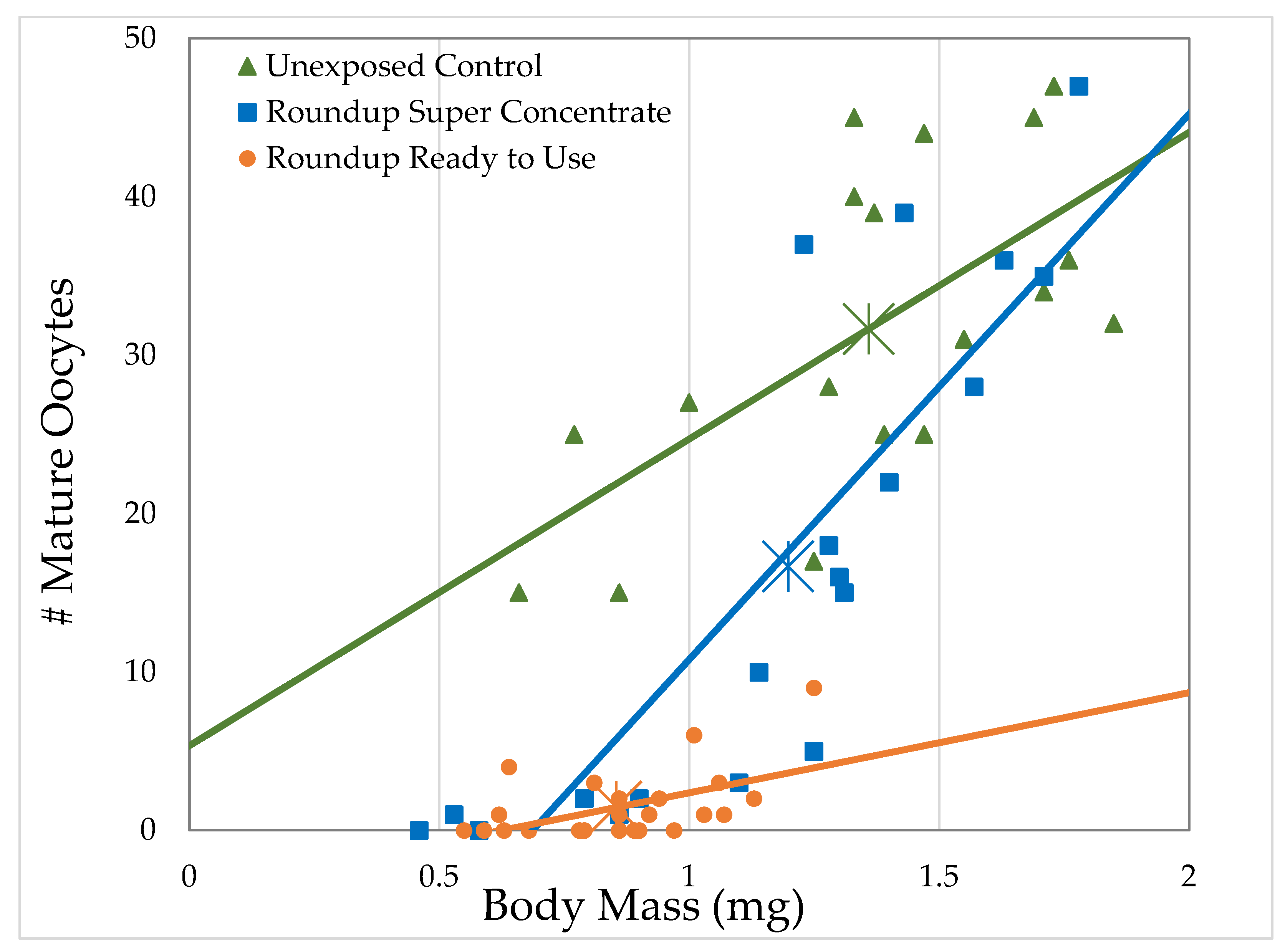
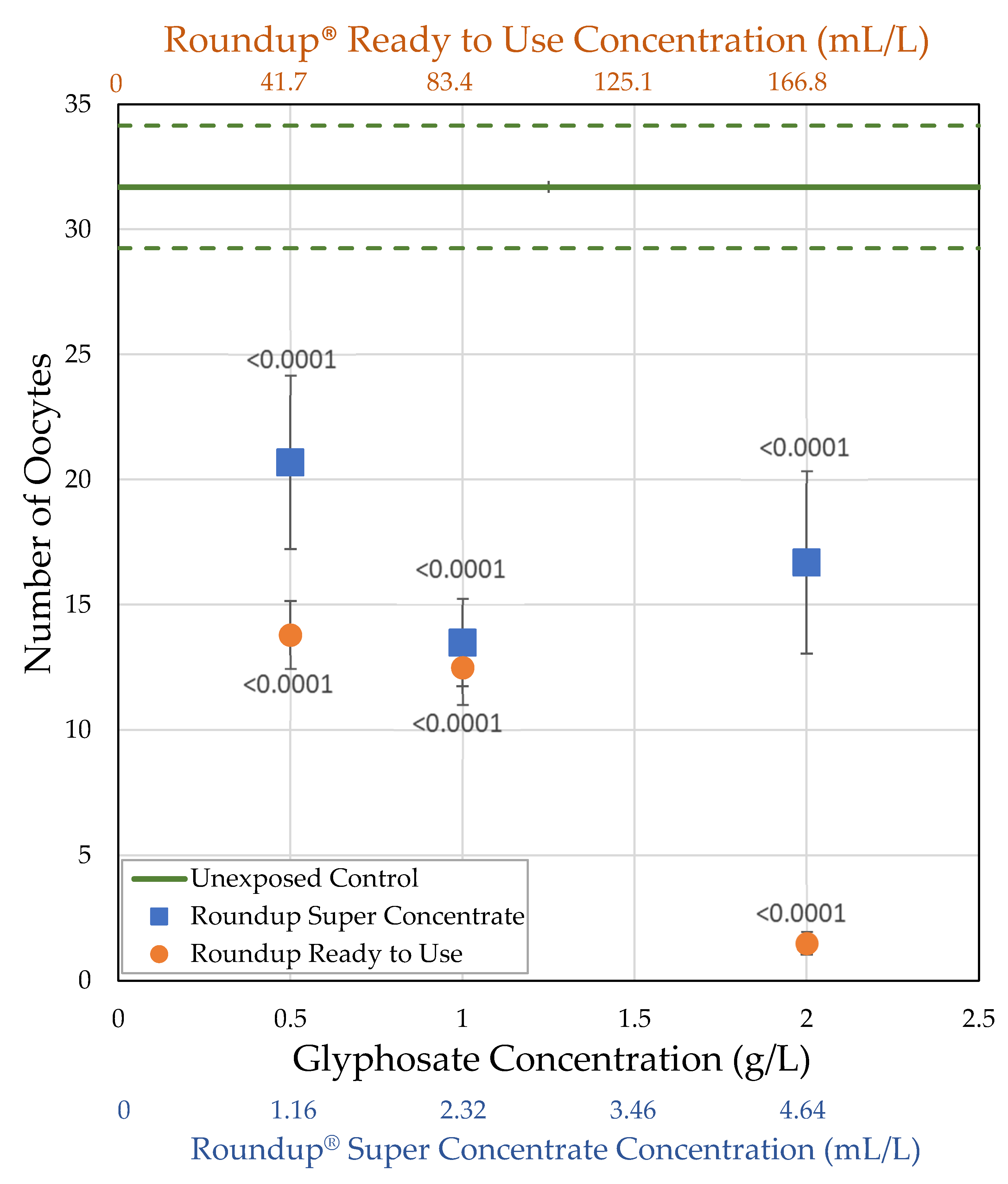
3.2.2. H1-B: Roundup® Interferes with Reproduction by Reducing the Number of Mature Oocytes
3.3. H2: Roundup® Interferes with Reproduction by Reducing Sperm Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Aguiar, L.M.; Figueira, F.H.; Gottschalk, M.S.; da Rosa, C.E. Glyphosate-Based Herbicide Exposure Causes Antioxidant Defence Responses in the Fruit Fly Drosophila Melanogaster. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2016, 185–186, 94–101. [Google Scholar] [CrossRef]
- Pollegioni, L.; Schonbrunn, E.; Siehl, D. Molecular Basis of Glyphosate Resistance-Different Approaches through Protein Engineering: Mechanisms of Glyphosate Resistance. FEBS J. 2011, 278, 2753–2766. [Google Scholar] [CrossRef] [Green Version]
- Cattani, D.; Cesconetto, P.A.; Tavares, M.K.; Parisotto, E.B.; Oliveira, P.V.; Rieg, C.E.H.; Leite, M.C.; Prediger, R.; Wendt, N.; Razzera, G.; et al. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 2017, 387, 67–80. [Google Scholar] [CrossRef]
- Dechartres, J.; Pawluski, J.L.; Gueguen, M.-M.; Jablaoui, A.; Maguin, E.; Rhimi, M.; Charlier, T.D. Glyphosate and Glyphosate-Based Herbicide Exposure during the Peripartum Period Affects Maternal Brain Plasticity, Maternal Behaviour and Microbiome. J. Neuroendocrinol. 2019, 31, e12731. [Google Scholar] [CrossRef]
- Owagboriaye, F.O.; Dedeke, G.A.; Ademolu, K.O.; Olujimi, O.O.; Ashidi, J.S.; Adeyinka, A.A. Reproductive toxicity of Roundup® herbicide exposure in male albino rat. Exp. Toxicol. Pathol. 2017, 69, 461–468. [Google Scholar] [CrossRef]
- Baier, F.; Jedinger, M.; Gruber, E.; Zaller, J.G. Temperature-Dependence of Glyphosate-Based Herbicide’s Effects on Egg and Tadpole Growth of Common Toads. Front. Environ. Sci. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Benamú, M.; Schneider, M.I.; Sánchez, N. Effects of the herbicide glyphosate on biological attributes of Alpaida veniliae (Araneae, Araneidae), in laboratory. Chemosphere 2010, 78, 871–876. [Google Scholar] [CrossRef]
- Schimpf, M.G.; Milesi, M.M.; Ingaramo, P.I.; Luque, E.H.; Varayoud, J. Neonatal exposure to a glyphosate based herbicide alters the development of the rat uterus. Toxicology 2017, 376, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Milesi, M.M.; Lorenz, V.; Pacini, G.; Repetti, M.R.; Demonte, L.D.; Varayoud, J.; Luque, E.H. Perinatal Exposure to a Glyphosate-Based Herbicide Impairs Female Reproductive Outcomes and Induces Second-Generation Adverse Effects in Wistar Rats. Arch. Toxicol. 2018, 92, 2629–2643. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.G.; Telles, L.F.; Hess, R.; Mahecha, G.A.; Oliveira, C.A. Effects of the herbicide Roundup® on the epididymal region of drakes Anas platyrhynchos. Reprod. Toxicol. 2007, 23, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.; Brilisauer, K.; Triebskorn, R.; Forchhammer, K.; Köhler, H.-R. How glyphosate and its associated acidity affect early development in zebrafish (Danio rerio). PeerJ 2019, 7, e7094. [Google Scholar] [CrossRef] [Green Version]
- Brausch, J.M.; Beall, B.; Smith, P.N. Acute and Sub-Lethal Toxicity of Three POEA Surfactant Formulations to Daphnia magna. Bull. Environ. Contam. Toxicol. 2007, 78, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Bednářová, A.; Kropf, M.; Krishnan, N. The surfactant polyethoxylated tallowamine (POEA) reduces lifespan and inhibits fecundity in Drosophila melanogaster- In vivo and in vitro study. Ecotoxicol. Environ. Saf. 2020, 188, 109883. [Google Scholar] [CrossRef]
- Mesnage, R.; Bernay, B.; Séralini, G.-E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 313, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef]
- PubChem. Diquat Dibromide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diquat-dibromide (accessed on 28 February 2021).
- Wehtje, G.; Altland, J.E.; Gilliam, C.H. Interaction of Glyphosate and Pelargonic Acid in Ready-to-Use Weed Control Products. Weed Technol. 2009, 23, 544–549. [Google Scholar] [CrossRef]
- Techer, D.; Milla, S.; Fontaine, P.; Viot, S.; Thomas, M. Influence of Waterborne Gallic and Pelargonic Acid Exposures on Biochemical and Reproductive Parameters in the Zebrafish (Danio Rerio): Influence Of Gallic and Pelargonic Acid Exposure. Environ. Toxicol. 2017, 32, 227–240. [Google Scholar] [CrossRef]
- Talyn, B.; Lemon, R.; Badoella, M.; Melchiorre, D.; Villalobos, M.; Elias, R.; Muller, K.; Santos, M.; Melchiorre, E. Roundup®, but Not Roundup-Ready® Corn, Increases Mortality of Drosophila melanogaster. Toxics 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, M.A.; Romano, R.M.; Santos, L.D.; Wisniewski, P.; Campos, D.A.; de Souza, P.B.; Viau, P.; Bernardi, M.M.; Nunes, M.T.; de Oliveira, C.A. Glyphosate Impairs Male Offspring Reproductive Development by Disrupting Gonadotropin Expression. Arch. Toxicol. 2012, 86, 663–673. [Google Scholar] [CrossRef]
- Druart, C.; Gimbert, F.; Scheifler, R.; De Vaufleury, A. A full life-cycle bioassay with Cantareus aspersus shows reproductive effects of a glyphosate-based herbicide suggesting potential endocrine disruption. Environ. Pollut. 2017, 226, 240–249. [Google Scholar] [CrossRef]
- Schimpf, M.G.; Milesi, M.M.; Luque, E.H.; Varayoud, J. Glyphosate-based herbicide enhances the uterine sensitivity to estradiol in rats. J. Endocrinol. 2018, 239, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Ji, Y.; Song, X.; Guo, H.; Han, L.; Zhang, F.; Liu, X.; Zhang, H.; Zhu, B.; Xu, M. Effects of glyphosate exposure on sperm concentration in rodents: A systematic review and meta-analysis. Environ. Toxicol. Pharmacol. 2017, 55, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Clair, É.; Mesnage, R.; Travert, C.; Séralini, G.-É. A glyphosate-based herbicide induces necrosis and apoptosis in mature rat testicular cells in vitro, and testosterone decrease at lower levels. Toxicol. Vitr. 2012, 26, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.M.; Romano, M.A.; Bernardi, M.M.; Furtado, P.V.; Oliveira, C.A. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Arch. Toxicol. 2009, 84, 309–317. [Google Scholar] [CrossRef]
- Teleken, J.L.; Gomes, E.C.Z.; Marmentini, C.; Moi, M.B.; Ribeiro, R.A.; Balbo, S.L.; Amorim, E.M.P.; Bonfleur, M.L. Glyphosate-Based Herbicide Exposure during Pregnancy and Lactation Malprograms the Male Reproductive Morphofunction in F1 Offspring. J. Dev. Orig. Health Dis. 2020, 11, 146–153. [Google Scholar] [CrossRef]
- Mesnage, R.; Phedonos, A.; Biserni, M.; Arno, M.; Balu, S.; Corton, J.C.; Ugarte, R.; Antoniou, M.N. Evaluation of estrogen receptor alpha activation by glyphosate-based herbicide constituents. Food Chem. Toxicol. 2017, 108, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Rowlands, H. Global Glyphosate Study Pilot Phase Shows Reproductive and Developmental Effects at “Safe” Dose. Available online: https://www.gmoevidence.com/global-glyphosate-study-pilot-phase-shows-reproductive-and-developmental-effects-at-safe-dose/ (accessed on 11 March 2019).
- Larsen, A.E.; Gaines, S.D.; Deschênes, O. Agricultural pesticide use and adverse birth outcomes in the San Joaquin Valley of California. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winchester, P.; Proctor, C.; Ying, J. County-level pesticide use and risk of shortened gestation and preterm birth. Acta Paediatr. 2016, 105, e107–e115. [Google Scholar] [CrossRef] [Green Version]
- Shelton, J.F.; Geraghty, E.M.; Tancredi, D.J.; Delwiche, L.D.; Schmidt, R.J.; Ritz, B.; Hansen, R.L.; Hertz-Picciotto, I. Neurodevelopmental Disorders and Prenatal Residential Proximity to Agricultural Pesticides: The CHARGE Study. Environ. Health Perspect. 2014, 122, 1103–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Ehrenstein, O.S.; Ling, C.; Cui, X.; Cockburn, M.; Park, A.S.; Yu, F.; Wu, J.; Ritz, B. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: Population based case-control study. BMJ 2019, 364, l962. [Google Scholar] [CrossRef] [Green Version]
- Agostini, L.P.; Dettogni, R.S.; dos Reis, R.S.; Stur, E.; dos Santos, E.V.; Ventorim, D.P.; Garcia, F.M.; Cardoso, R.C.; Graceli, J.B.; Louro, I.D. Effects of glyphosate exposure on human health: Insights from epidemiological and in vitro studies. Sci. Total. Environ. 2020, 705, 135808. [Google Scholar] [CrossRef]
- Jennings, B.H. Drosophila–a Versatile Model in Biology & Medicine. Mater. Today 2011, 14, 190–195. [Google Scholar]
- Bier, E.; Bodmer, R. Drosophila, an emerging model for cardiac disease. Gene 2004, 342, 1–11. [Google Scholar] [CrossRef]
- Bilen, J.; Bonini, N.M. Drosophila as a Model for Human Neurodegenerative Disease. Annu. Rev. Genet. 2005, 39, 153–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birse, R.T.; Choi, J.; Reardon, K.; Rodriguez, J.; Graham, S.; Diop, S.; Ocorr, K.; Bodmer, R.; Oldham, S. High-Fat-Diet-Induced Obesity and Heart Dysfunction Are Regulated by the TOR Pathway in Drosophila. Cell Metab. 2010, 12, 533–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grice, S.J.; Liu, J.-L.; Webber, C. Synergistic Interactions between Drosophila Orthologues of Genes Spanned by De Novo Human CNVs Support Multiple-Hit Models of Autism. PLoS Genet. 2015, 11, e1004998. [Google Scholar] [CrossRef]
- De Aguiar, L.M.; Figueira, F.H.; Gottschalk, M.S.; da Rosa, C.E. Corrigendum to “Glyphosate-Based Herbicide Exposure Causes Antioxidant Defence Responses in the Fruit Fly Drosophila Melanogaster Previously Published at CBPC” [Comp. Biochem. Physiol. C 185–186 (2016) 94–101]. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2018, 205, 70–73. [Google Scholar] [CrossRef]
- Burchfield, S.L.; Bailey, D.C.; Todt, C.E.; Denney, R.D.; Negga, R.; Fitsanakis, V.A. Acute Exposure to a Glyphosate-Containing Herbicide Formulation Inhibits Complex II and Increases Hydrogen Peroxide in the Model Organism Caenorhabditis Elegans. Environ. Toxicol. Pharmacol. 2019, 66, 36–42. [Google Scholar] [CrossRef]
- Bali, Y.A.; Ba-M’Hamed, S.; Elhidar, N.; Nafis, A.; Soraa, N.; Bennis, M. Glyphosate based- herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol. Teratol. 2018, 67, 44–49. [Google Scholar] [CrossRef]
- Yu, N.; Tong, Y.; Zhang, D.; Zhao, S.; Fan, X.; Wu, L.; Ji, H. Circular RNA expression profiles in hippocampus from mice with perinatal glyphosate exposure. Biochem. Biophys. Res. Commun. 2018, 501, 838–845. [Google Scholar] [CrossRef]
- Pereira, A.G.; Jaramillo, M.L.; Remor, A.P.; Latini, A.; Davico, C.E.; da Silva, M.L.; Müller, Y.M.R.; Ammar, D.; Nazari, E.M. Low-Concentration Exposure to Glyphosate-Based Herbicide Modulates the Complexes of the Mitochondrial Respiratory Chain and Induces Mitochondrial Hyperpolarization in the Danio Rerio Brain. Chemosphere 2018, 209, 353–362. [Google Scholar] [CrossRef]
- Baier, C.J.; Gallegos, C.E.; Raisman-Vozari, R.; Minetti, A. Behavioral Impairments Following Repeated Intranasal Glyphosate-Based Herbicide Administration in Mice. Neurotoxicol. Teratol. 2017, 64, 63–72. [Google Scholar] [CrossRef]
- Lozano, V.L.; Defarge, N.; Rocque, L.-M.; Mesnage, R.; Hennequin, D.; Cassier, R.; de Vendômois, J.S.; Panoff, J.-M.; Séralini, G.-E.; Amiel, C. Sex-Dependent Impact of Roundup® on the Rat Gut Microbiome. Toxicol. Rep. 2018, 5, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Ringo, J.; Talyn, B.; Brannan, M. Effects of Precocene and Low Protein Diet on Reproductive Behavior in Drosophila Melanogaster (Diptera: Drosophilidae). Ann. Entomol. Soc. Am. 2005, 98, 601–607. [Google Scholar] [CrossRef]
- Avanesyan, A.; Jaffe, B.D.; Guédot, C. Isolating Spermathecae and Determining Mating Status of Drosophila Suzukii: A Protocol for Tissue Dissection and Its Applications. Insects 2017, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Schnakenberg, S.L.; Matias, W.R.; Siegal, M.L. Sperm-Storage Defects and Live Birth in Drosophila Females Lacking Spermathecal Secretory Cells. PLoS Biol. 2011, 9, e1001192. [Google Scholar] [CrossRef] [Green Version]
- Elias, R.; Talyn, B.; Melchiorre, E. Dietary Behavior of Drosophila Melanogaster Given Genetically-Modified Corn Medium, Roundup® in Sucrose, or Roundup® with Corn Medium. J. Xenobiot. in review.
- Mesnage, R.; Benbrook, C.; Antoniou, M.N. Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem. Toxicol. 2019, 128, 137–145. [Google Scholar] [CrossRef]
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of Formulants and Heavy Metals in Glyphosate-Based Herbicides and Other Pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Smith-Spangler, C.; Brandeau, M.L.; Hunter, G.E.; Bavinger, J.C.; Pearson, M.; Eschbach, P.J.; Sundaram, V.; Liu, H.; Schirmer, P.; Stave, C.; et al. Are Organic Foods Safer or Healthier than Conventional Alternatives?: A Systematic Review. Ann. Intern. Med. 2012, 157, 348–366. [Google Scholar] [CrossRef] [Green Version]
- Williamson, C.S. Is Organic Food Better for Our Health? Nutr. Bull. 2007, 32, 104–108. [Google Scholar] [CrossRef]
- Magkos, F.; Arvaniti, F.; Zampelas, A. Organic Food: Buying More Safety or Just Peace of Mind? A Critical Review of the Literature. Crit. Rev. Food Sci. Nutr. 2006, 46, 23–56. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.K.; Davis, S.F. Organic Foods. J. Food Sci. 2006, 71, R117–R124. [Google Scholar] [CrossRef]
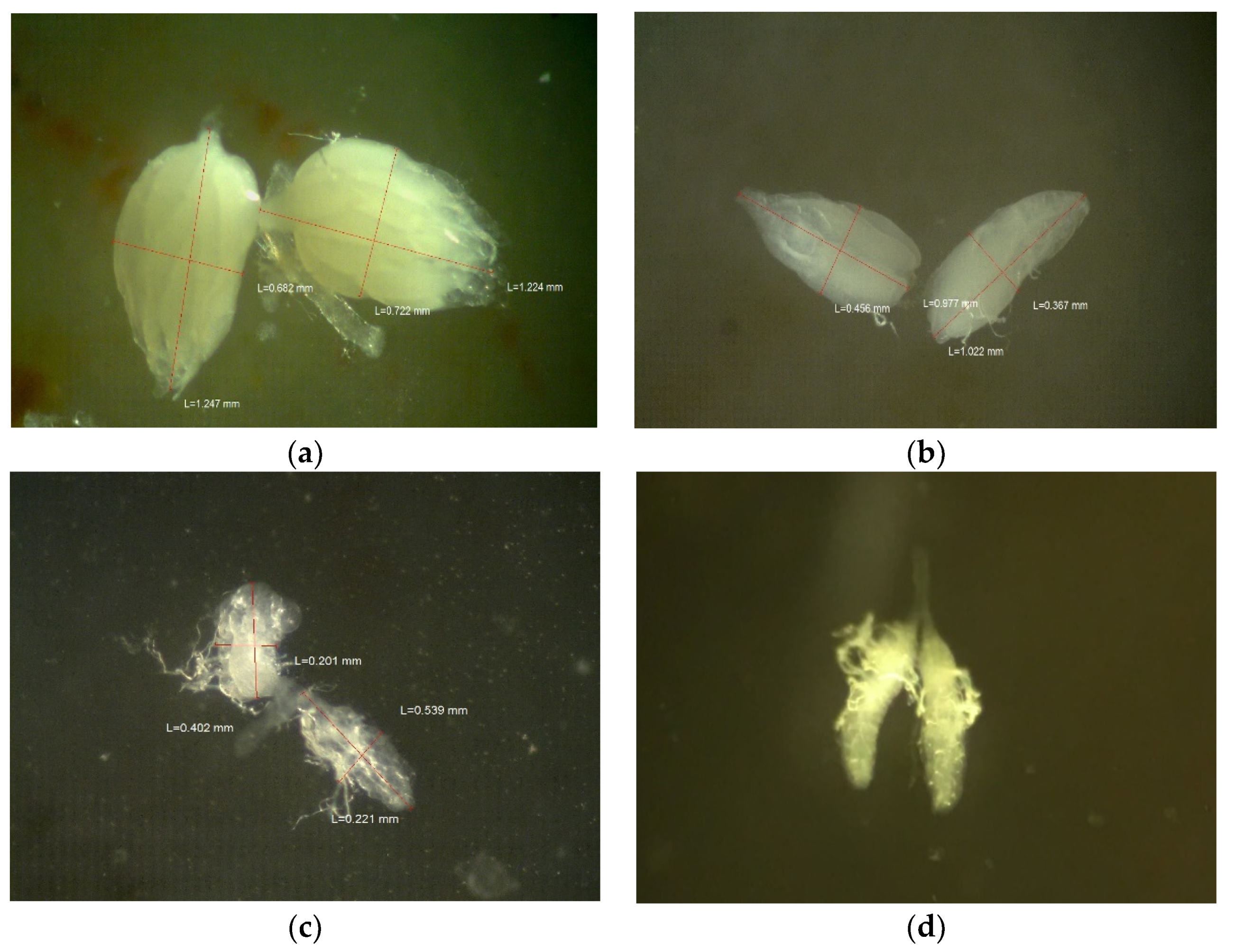
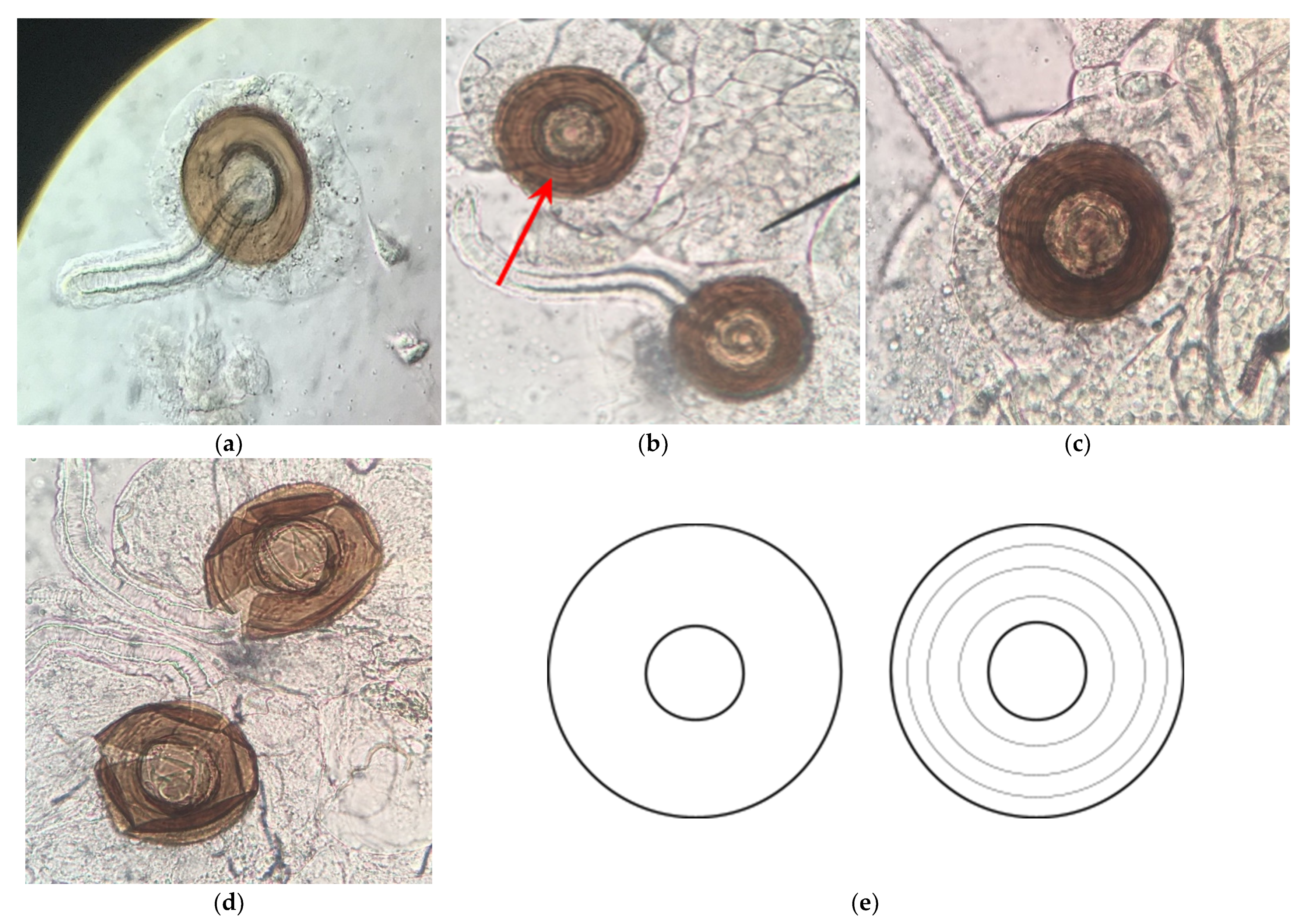
| Formulation | Active Ingredient(s) (Approximate % by Weight) | Other Ingredients (Approximate % by Weight) | ||
|---|---|---|---|---|
| Glyphosate | Pelargonic Acid | POEA (Surfactant) | Other Ingredients and Water | |
| Roundup® Super Concentrate Grass and Weed Control | 41% | 0% | 14.5% | 44.5% |
| Roundup® Ready to Use Weed & Grass Killer III | 2% | 2% | 0% | 96% |
| Formulation (Active Ingredient) (Other Known Ingredients) | Formulation Concentration (mL/L) Glyphosate Concentration (g/L) | Survival (%) | Body Size (mg) | Ovary Volume (nL) | Number of Oocytes |
|---|---|---|---|---|---|
| Control | 0.0 0.0 | = 76.0 ± 4.30 n = 5 | = 1.36 ± 0.0822 n = 18 | = 184.8 ± 22.9 n = 18 | = 31.7 ± 2.45 n = 18 |
| Roundup® Ready to Use (glyphosate, pelargonic acid) | 41.7 0.5 | = 54.0 ± 15.3 n = 5 t = 5.114 p < 0.0001 ** | = 1.08 ± 0.0771 n = 10 t = 2.519 p = 0.0059 * | = 58.2 ± 6.98 n = 10 t = 4.1171 p < 0.0001 ** | = 13.8 ± 1.35 n = 10 t = 5.4456 p < 0.0001 ** |
| 83.4 1.0 | = 60.7 ± 10.1 n = 7 t = 4.204 p < 0.0001 ** | = 1.13 ± 0.0524 n = 25 t = 3.2368 p = 0.0012 ** | = 71.3 ± 9.17 n = 21 t = 5.3487 p < 0.0001 ** | = 12.5 ± 1.49 n = 20 t = 8.28331 p < 0.0001 ** | |
| 166.8 2.0 | = 52.1 ± 9.50 n = 7 t = 6.561 p < 0.0001 ** | = 0.854 ± 0.0366 n = 25 t = 7.2448 p < 0.0001 ** | = 16.8 ± 3.10 n = 23 t = 8.2882 p < 0.0001 ** | = 1.50 ± 0.454 n = 24 t = 14.244 p < 0.0001 ** | |
| Roundup® Super Concentrate (glyphosate) (POEA) | 1.16 0.5 | = 76.7 ± 10.1 n = 3 t = 0.120 p = 0.548 | = 1.14 ± 0.101 n = 12 t = 2.179 p = 0.0147 * | = 96.1 ± 17.6 n = 12 t = 3.1595 p = 0.0008 ** | = 20.7 ± 3.47 n = 12 t = 3.6727 p < 0.0001 ** |
| 2.32 1.0 | = 87.5 ± 7.5 n = 2 t = 1.691 p = 0.955 | = 1.25 ± 0.0578 n = 21 t = 1.3878 p = 0.0827 | = 93.6 ± 11.8 n = 20 t = 4.1959 p < 0.0001 ** | = 13.5 ± 1.75 n = 20 t = 7.852 p < 0.0001 ** | |
| 4.64 2.0 | = 67.5 ± 13.1 n = 4 t = 1.767 p = 0.0386 * | = 1.20 ± 0.0848 n = 21 t = 2.1192 p = 0.017 * | = 103.6 ± 24.6 n = 20 t = 3.7342 p < 0.0001 ** | = 16.7 ± 3.65 n = 19 t = 6.2945 p < 0.0001 ** |
| Response | Independent Variable | n | F Ratio | p-Value | R2 (%) | EC50 |
|---|---|---|---|---|---|---|
| Body weight | Glyphosate Concentration * | 132 | 4.87 | 0.0294 | 4.17 | 5.31 |
| Pelargonic Acid Concentration * | 132 | 23.8 | <0.0001 | 17.6 | 3.28 | |
| Total Herbicide Concentration *,1 | 132 | 19.5 | <0.0001 | 14.8 | 6.18 | |
| Super Concentrate [Glyphosate] | 54 | 0.0211 | 0.8852 | 0.0405 | 47.01 | |
| Ready to Use [Total Herbicide] | 60 | 15.7 | 0.0002 | 21.3 | 5.92 | |
| Ovary Volume | Glyphosate Concentration * | 124 | 2.77 | 0.099 | 2.6 | 0.12 |
| Pelargonic Acid Concentration * | 124 | 27.5 | <0.0001 | 20.9 | 0.13 | |
| Total Herbicide Concentration *,1 | 124 | 18.5 | <0.0001 | 15.1 | 1.02 | |
| Super Concentrate [Glyphosate] | 52 | 0.122 | 0.728 | 0.243 | 0.39 | |
| Ready to Use [Total Herbicide] | 54 | 27.1 | <0.0001 | 34.2 | 0.16 | |
| Number of Oocytes | Glyphosate Concentration * | 123 | 11.9 | 0.0008 | 10.3 | 0.63 |
| Pelargonic Acid Concentration * | 123 | 39.6 | <0.0001 | 27.8 | 0.15 | |
| Total Herbicide Concentration *,1 | 123 | 36.6 | <0.0001 | 26.2 | 1.16 | |
| Super Concentrate [Glyphosate] | 51 | 0.131 | 0.719 | 0.267 | 1.73 | |
| Ready to Use [Herbicide] | 54 | 72.7 | <0.0001 | 58.3 | 0.96 |
| Formulation | Formulation Concentration (mL/L)/Glyphosate Concentration (g/L) | Ovary Volume | # Oocytes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | R2 (%) | χ2 | p-Value | n | R2 (%) | χ2 | p-Value | ||
| Unexposed Control | 0/0 | 18 | 43.2 | 12.2 | 0.0005 | 18 | 42.3 | 11.8 | 0.0006 |
| Ready to Use | 41.7/0.5 | 19 | 5.52 | 0.467 | 0.494 | 19 | 2.25 | 0.184 | 0.668 |
| 83.4/1 | 35 | 32.9 | 9.33 | 0.0023 | 35 | 35.9 | 11.65 | 0.0006 | |
| 166.8/2 | 34 | 39 | 13.4 | 0.0003 | 34 | 23.2 | 7.94 | 0.0048 | |
| Super Concentrate | 1.16/0.5 | 22 | 54.6 | 14.2 | 0.0002 | 22 | 55 | 12.2 | 0.0005 |
| 2.32/1 | 31 | 29.4 | 7.48 | 0.0062 | 31 | 25 | 6 | 0.0143 | |
| 4.64/2 | 30 | 66.5 | 35.8 | <0.0001 | 30 | 72.8 | 45.5 | <0.0001 | |
| Formulation | Formulation Concentration (mL/L)/ Glyphosate Concentration (g/L) | Glyphosate Concentration (g/L) | n at 4 h | n at 2 h |
|---|---|---|---|---|
| Unexposed Control | 0/0 | 0 | 3 | 1 |
| Roundup® Super Concentrate | 41.7/0.5 | 0.5 | 3 | 4 |
| 83.4/1 | 1.0 | 3 | 6 | |
| 166.8/2 | 2.0 | 3 | 0 | |
| Roundup® Ready to Use | 1.16/0.5 | 0.5 | 3 | 0 |
| 2.32/1 | 1.0 | 3 | 1 | |
| 4.64/2 | 2.0 | 3 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muller, K.; Herrera, K.; Talyn, B.; Melchiorre, E. Toxicological Effects of Roundup® on Drosophila melanogaster Reproduction. Toxics 2021, 9, 161. https://doi.org/10.3390/toxics9070161
Muller K, Herrera K, Talyn B, Melchiorre E. Toxicological Effects of Roundup® on Drosophila melanogaster Reproduction. Toxics. 2021; 9(7):161. https://doi.org/10.3390/toxics9070161
Chicago/Turabian StyleMuller, Kelly, Karina Herrera, Becky Talyn, and Erik Melchiorre. 2021. "Toxicological Effects of Roundup® on Drosophila melanogaster Reproduction" Toxics 9, no. 7: 161. https://doi.org/10.3390/toxics9070161
APA StyleMuller, K., Herrera, K., Talyn, B., & Melchiorre, E. (2021). Toxicological Effects of Roundup® on Drosophila melanogaster Reproduction. Toxics, 9(7), 161. https://doi.org/10.3390/toxics9070161






