A Co-Culture Model of the Human Respiratory Tract to Discriminate the Toxicological Profile of Cationic Nanoparticles According to Their Surface Charge Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CDs
2.2. Characterization of CDs
2.3. Cell Culture
2.4. Mono- and Co-Culture Models
2.5. Characterization of the Co-Culture Models by Fluorescence Activated Cell Sorting (FACS)
2.6. Cell Exposure to CDs
2.7. Cell Viability Assay
2.8. Assessment of Cell Death Mechanisms
2.9. Oxidative Stress Assessment
2.10. Assessment of CD Cell Uptake
2.11. Cytokine Assay
2.12. Presentation and Statistical Analysis of the Data
3. Results and Discussion
3.1. Physicochemical Characterization of CDs
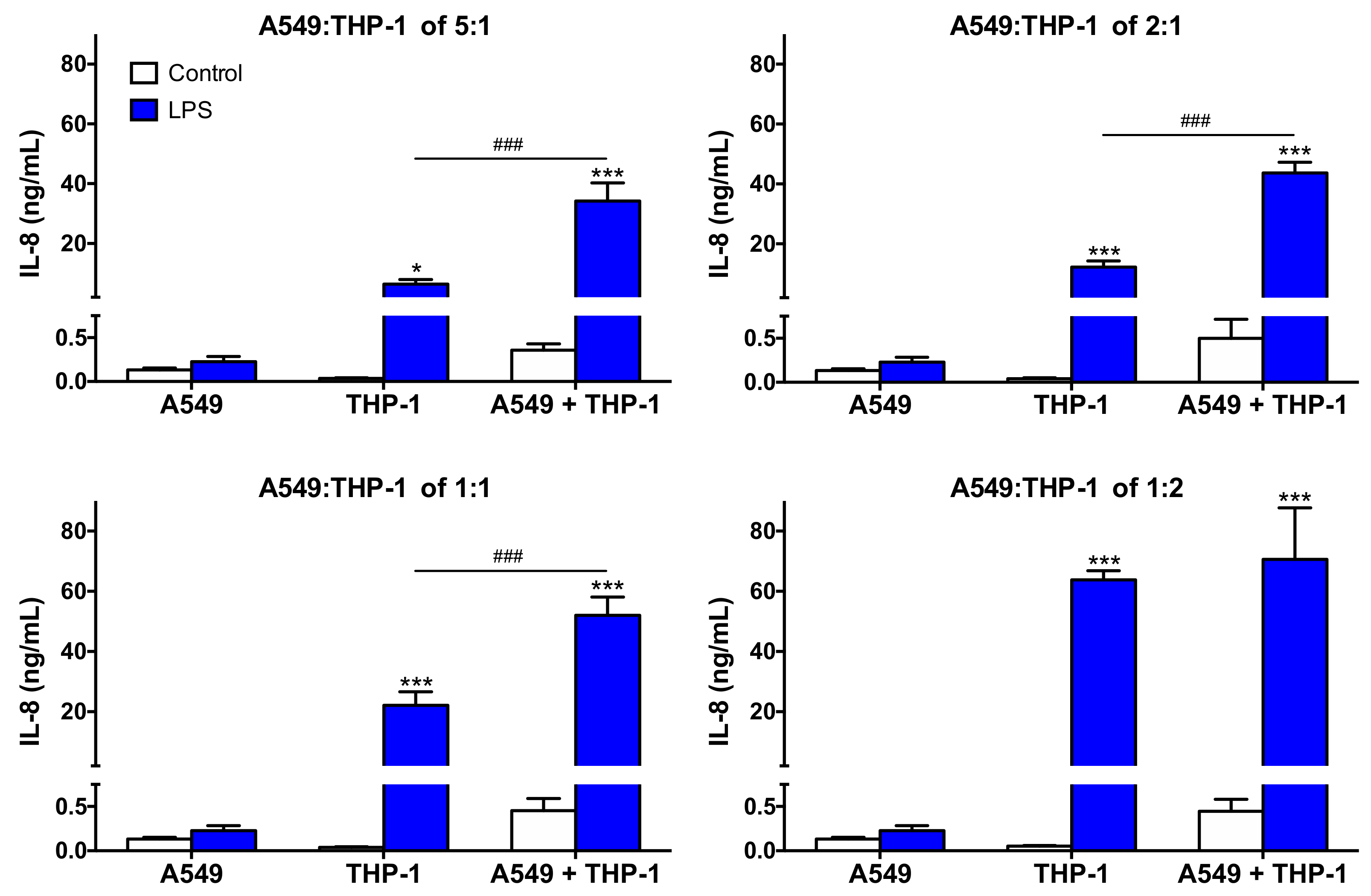
3.2. Characteristics of the Co-Culture According to the A549:THP-1 Seeding Ratio
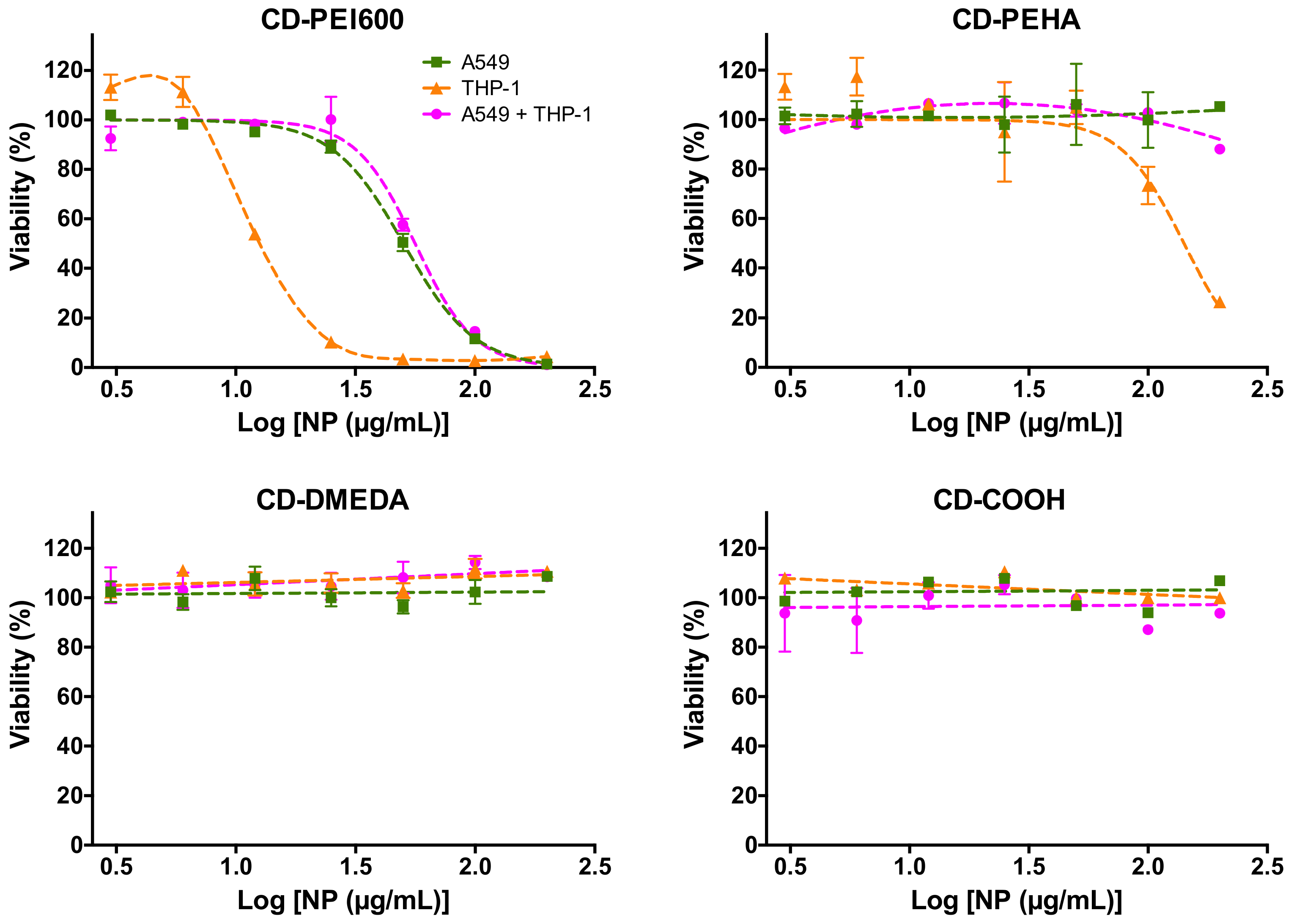
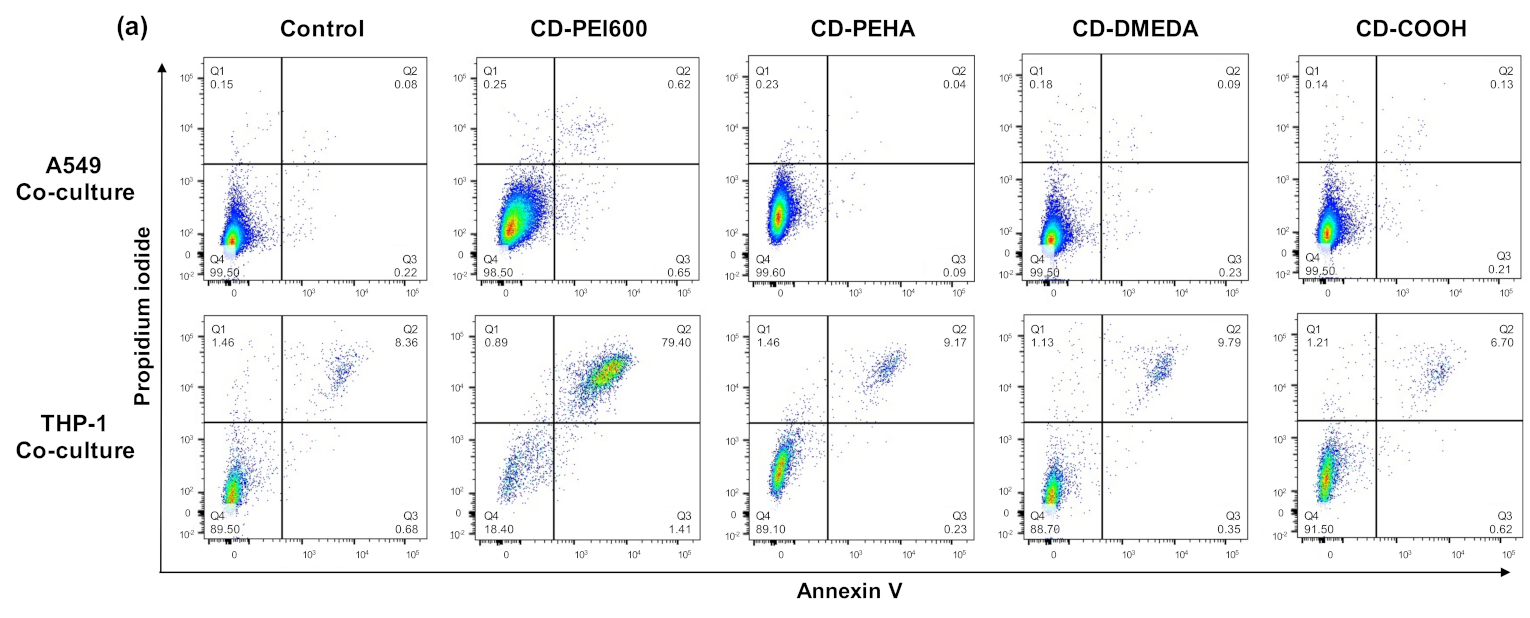
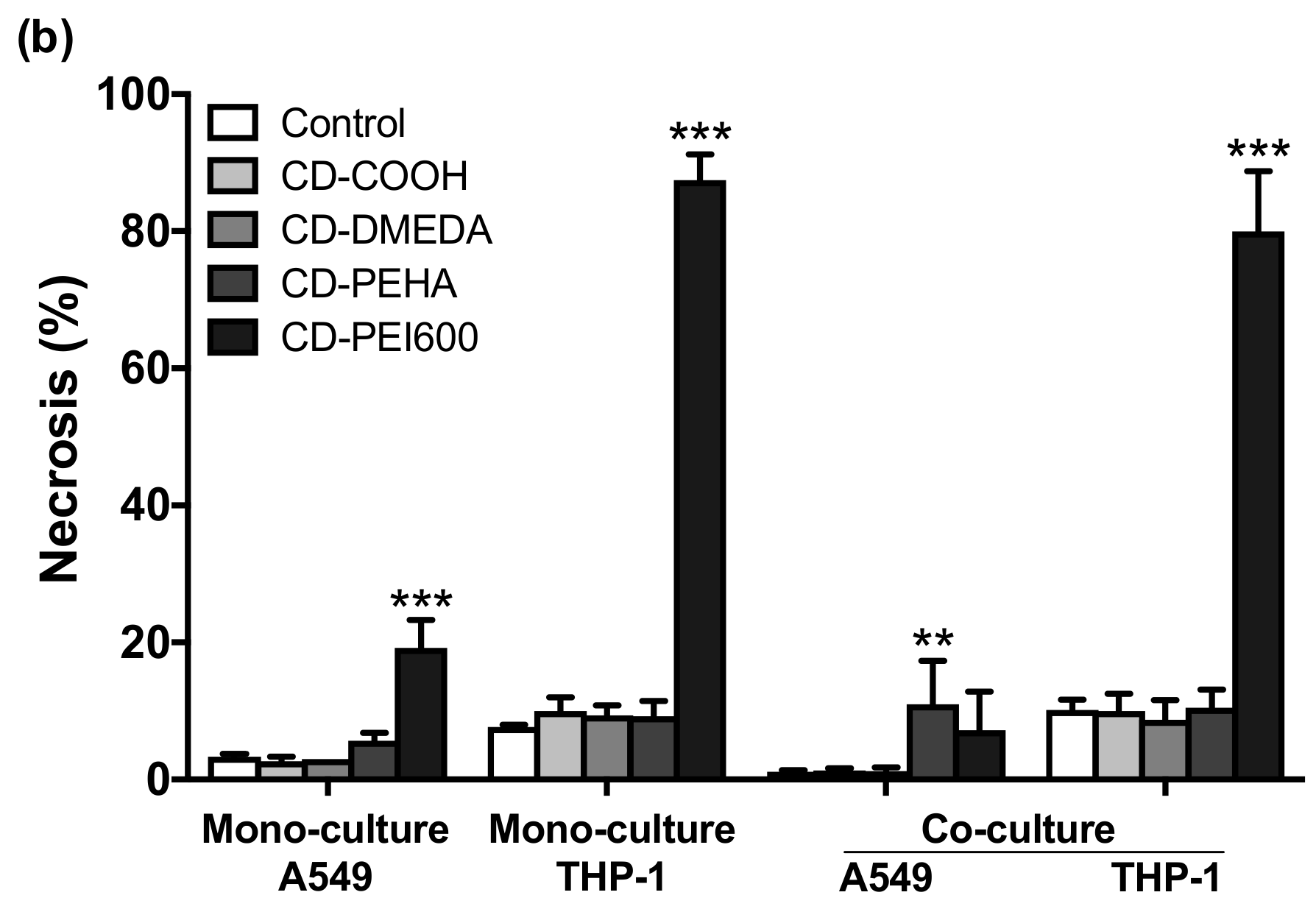
3.3. Cytotoxicity of CDs and Cell Death Mechanisms in Mono- and Co-Cultures
3.4. Internalization of CDs in the Different Culture Models and Cell Types
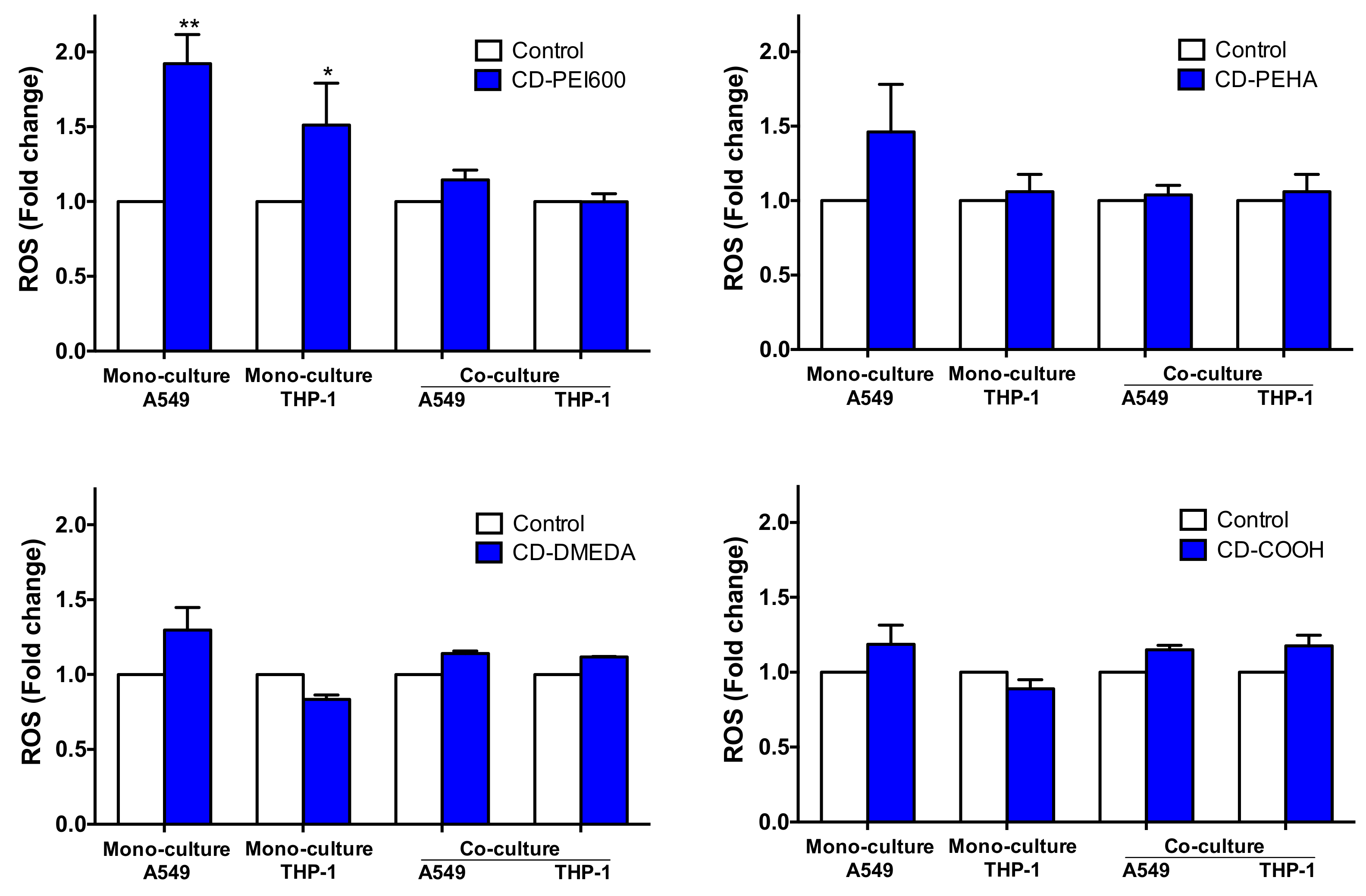
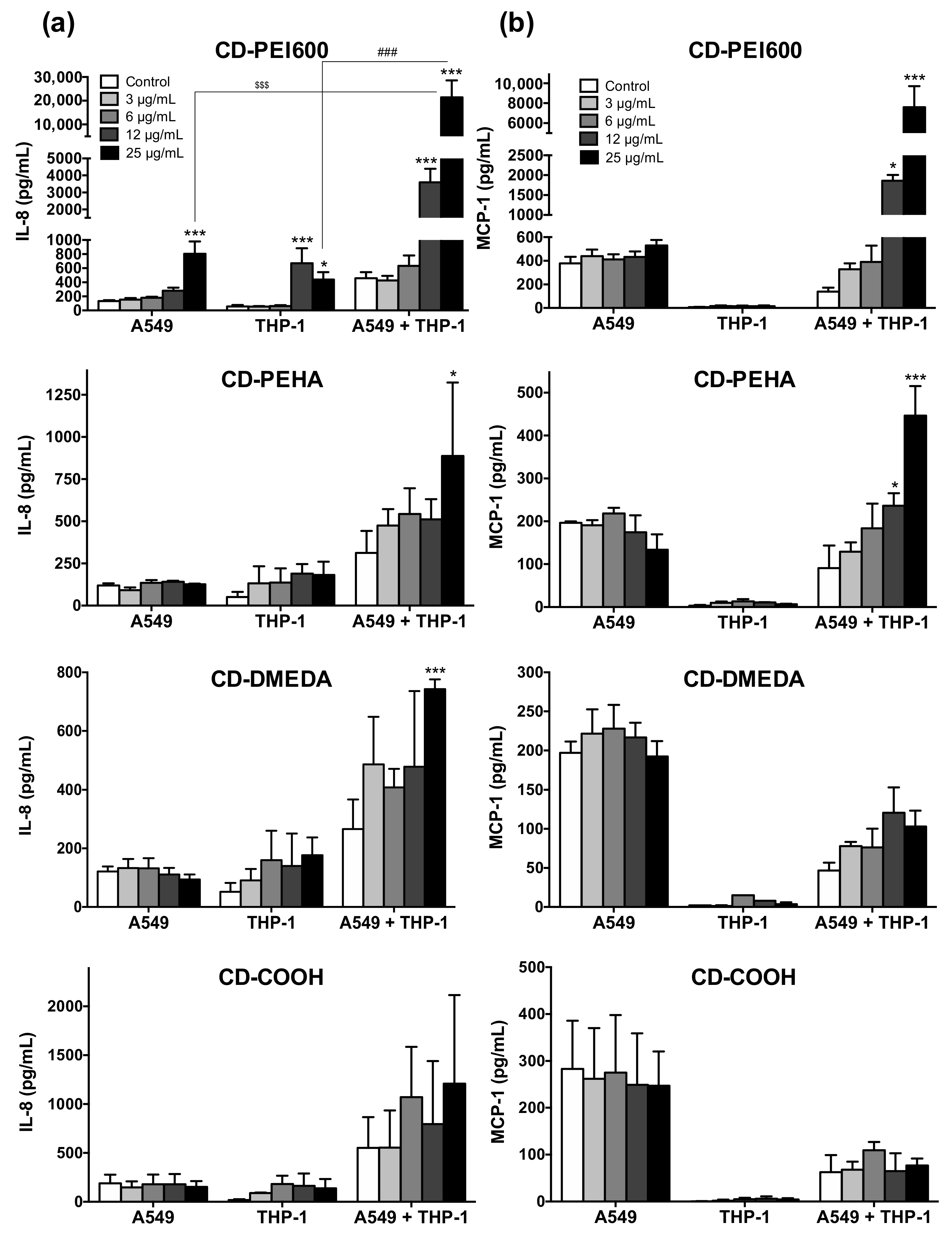
3.5. Oxidative Stress and Inflammatory Response Evoked by CDs in the Mono- and Co-Culture Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Maynard, A.D. Nanotechnology: The next big thing, or much ado about nothing? Ann. Occup. Hyg. 2007, 51, 1–12. [Google Scholar]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Persp. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Semmler-Behnke, M.; Takenaka, S.; Moller, W. Differences in the biokinetics of inhaled nano- versus micrometer-sized particles. Acc. Chem. Res. 2013, 46, 714–722. [Google Scholar] [CrossRef] [Green Version]
- Ronzani, C.; Spiegelhalter, C.; Vonesch, J.L.; Lebeau, L.; Pons, F. Lung deposition and toxicological responses evoked by multi-walled carbon nanotubes dispersed in a synthetic lung surfactant in the mouse. Arch. Toxicol. 2012, 86, 137–149. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef]
- Elgrabli, D.; Floriani, M.; Abella-Gallart, S.; Meunier, L.; Gamez, C.; Delalain, P.; Rogerieux, F.; Boczkowski, J.; Lacroix, G. Biodistribution and clearance of instilled carbon nanotubes in rat lung. Part. Fibre Toxicol. 2008, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.R.; Leite, P.E.; Falagan-Lotsch, P.; Benetti, F.; Micheletti, C.; Budtz, H.C.; Jacobsen, N.R.; Lisboa, P.N.; Rocha, L.A.; Kuhnel, D.; et al. Challenges on the toxicological predictions of engineered nanoparticles. Nanoimpact 2017, 8, 59–72. [Google Scholar] [CrossRef]
- Klein, S.G.; Hennen, J.; Serchi, T.; Blomeke, B.; Gutleb, A.C. Potential of coculture in vitro models to study inflammatory and sensitizing effects of particles on the lung. Toxicol. In Vitro 2011, 25, 1516–1534. [Google Scholar] [CrossRef]
- Drasler, B.; Sayre, P.; Steinhauser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. Nanoimpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Hirsch, C.; Roesslein, M.; Krug, H.F.; Wick, P. Nanomaterial cell interactions: Are current in vitro tests reliable? Nanomedicine 2011, 6, 837–847. [Google Scholar] [CrossRef]
- Bhowmick, R.; Gappa-Fahlenkamp, H. Cells and culture systems used to model the small airway epithelium. Lung 2016, 194, 419–428. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.J.; Ji, Z.X.; Zhang, H.Y. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef]
- Stone, V.; Johnston, H.; Schins, R.P.F. Development of in vitro systems for nanotoxicology: Methodological considerations. Crit. Rev. Toxicol. 2009, 39, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Buchel, C.; et al. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicol. In Vitro 2019, 61, e104610. [Google Scholar] [CrossRef]
- Clift, M.J.; Gehr, P.; Rothen-Rutishauser, B. Nanotoxicology: A perspective and discussion of whether or not in vitro testing is a valid alternative. Arch. Toxicol. 2011, 85, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Frohlich, E. Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles. Artif. Cell. Nanomed. Biotechnol. 2018, 46, 1091–1107. [Google Scholar] [CrossRef] [Green Version]
- Fytianos, K.; Drasler, B.; Blank, F.; von Garnier, C.; Seydoux, E.; Rodriguez-Lorenzo, L.; Petri-Fink, A.; Rothen-Rutishauser, B. Current in vitro approaches to assess nanoparticle interactions with lung cells. Nanomedicine 2016, 11, 2457–2469. [Google Scholar] [CrossRef] [Green Version]
- Alfaro-Moreno, E.; Nawrot, T.S.; Vanaudenaerde, B.M.; Hoylaerts, M.F.; Vanoirbeek, J.A.; Nemery, B.; Hoet, P.H. Co-cultures of multiple cell types mimic pulmonary cell communication in response to urban PM10. Eur. Respir. J. 2008, 32, 1184–1194. [Google Scholar] [CrossRef] [Green Version]
- Muller, L.; Riediker, M.; Wick, P.; Mohr, M.; Gehr, P.; Rothen-Rutishauser, B. Oxidative stress and inflammation response after nanoparticle exposure: Differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J. R. Soc. Interface 2010, 7, S27–S40. [Google Scholar] [CrossRef] [Green Version]
- Dekali, S.; Divetain, A.; Kortulewski, T.; Vanbaelinghem, J.; Gamez, C.; Rogerieux, F.; Lacroix, G.; Rat, P. Cell cooperation and role of the P2X(7) receptor in pulmonary inflammation induced by nanoparticles. Nanotoxicology 2013, 7, 1302–1314. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.J.; Vanaudenaerde, B.; Luyts, K.; Lison, D.; Martens, J.A.; Nemery, B.; Hoet, P.H.M. Cytokine production by co-cultures exposed to monodisperse amorphous silica nanoparticles: The role of size and surface area. Toxicol. Lett. 2012, 211, 98–104. [Google Scholar] [CrossRef]
- Riebeling, C.; Piret, J.P.; Trouiller, B.; Nelissen, I.; Saout, C.; Toussaint, O.; Haase, A. A guide to nanosafety testing: Considerations on cytotoxicity testing in different cell models. Nanoimpact 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Grabowski, N.; Hillaireau, H.; Vergnaud-Gauduchon, J.; Nicolas, V.; Tsapis, N.; Kerdine-Romer, S.; Fattal, E. Surface-modified biodegradable nanoparticles impact on cytotoxicity and inflammation response on a co-culture of lung epithelial cells and human-like macrophages. J. Biomed. Nanotechnol. 2016, 12, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Hino, M.; Kohchi, C.; Nishizawa, T.; Yoshida, A.; Nakata, K.; Inagawa, H.; Hori, H.; Makino, K.; Terada, H.; Sona, G.I. Innate-immune therapy for lung carcinoma based on tissue-macrophage activation with lipopolysaccharide. Anticancer Res. 2005, 25, 3747–3754. [Google Scholar] [PubMed]
- Grotz, B.; Geppert, M.; Mills-Goodlet, R.; Hofer, S.; Hofstatter, N.; Asam, C.; Feinle, A.; Kocsis, K.; Berger, T.; Diwald, O.; et al. Biologic effects of nanoparticle-allergen conjugates: Time-resolved uptake using an in vitro lung epithelial co-culture model of A549 and THP-1 cells. Environ. Sci. Nano 2018, 5, 2184–2197. [Google Scholar] [CrossRef]
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Himaja, A.L.; Karthik, P.S.; Singh, S.P. Carbon Dots: The newest member of the carbon nanomaterials family. Chem. Rec. 2015, 15, 595–615. [Google Scholar] [CrossRef]
- Pierrat, P.; Wang, R.; Kereselidze, D.; Lux, M.; Didier, P.; Kichler, A.; Pons, F.; Lebeau, L. Efficient in vitro and in vivo pulmonary delivery of nucleic acid by carbon dot-based nanocarriers. Biomaterials 2015, 51, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Claudel, M.; Fan, J.H.; Rapp, M.; Pons, F.; Lebeau, L. Influence of carbonization conditions on luminescence and gene delivery properties of nitrogen-doped carbon dots. RSC Adv. 2019, 9, 3493–3502. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, K.; Ghosh, A. Carbon dots: The next generation platform for biomedical applications. Mat. Sci. Eng. C Mater. 2019, 96, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Xu, N.; Fan, J.L.; Sun, W.; Peng, X.J. Carbon dots for in vivo bioimaging and theranostics. Small 2019, 15, e1805087. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Patil, A.; Thakur, S.; Kesharwani, P. Carbon dots: Emerging theranostic nanoarchitectures. Drug Discov. Today 2018, 23, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Luyts, K.; Napierska, D.; Nemery, B.; Hoet, P.H.M. How physico-chemical characteristics of nanoparticles cause their toxicity: Complex and unresolved interrelations. Environ. Sci. Process. Impacts 2013, 15, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, H.M.; Park, M.V.D.Z.; Gosens, I.; De Jong, W.H.; Cassee, F.R. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol. 2014, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Mailander, V.; Landfester, K. Interaction of nanoparticles with cells. Biomacromolecules 2009, 10, 2379–2400. [Google Scholar] [CrossRef]
- Huhn, D.; Kantner, K.; Geidel, C.; Brandholt, S.; De Cock, I.; Soenen, S.J.H.; Gil, P.R.; Montenegro, J.M.; Braeckmans, K.; Mullen, K.; et al. Polymer-coated nanoparticles interacting with proteins and cells: Focusing on the sign of the net charge. ACS Nano 2013, 7, 3253–3263. [Google Scholar] [CrossRef]
- Fan, J.H.; Claudel, M.; Ronzani, C.; Arezki, Y.; Lebeau, L.; Pons, F. Physicochemical characteristics that affect carbon dot safety: Lessons from a comprehensive study on a nanoparticle library. Int. J. Pharm. 2019, 569, e118521. [Google Scholar] [CrossRef]
- Weiss, M.; Fan, J.H.; Claudel, M.; Sonntag, T.; Didier, P.; Ronzani, C.; Lebeau, L.; Pons, F. Density of surface charge is a more predictive factor of the toxicity of cationic carbon nanoparticles than zeta potential. J. Nanobiotechnol. 2021, 19, 5. [Google Scholar] [CrossRef]
- Collot, M.; Kreder, R.; Tatarets, A.L.; Patsenker, L.D.; Mely, Y.; Klymchenko, A.S. Bright fluorogenic squaraines with tuned cell entry for selective imaging of plasma membrane vs. endoplasmic reticulum. Chem. Commun. 2015, 51, 17136–17139. [Google Scholar] [CrossRef] [Green Version]
- Pinkerton, K.E.; Gehr, P.; Castaneda, A.; Crapo, J.D. Architecture and cellular composition of the air-blood barrier. In Comparative Biology of the Normal Lung, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 105–117. [Google Scholar]
- Crapo, J.D.; Barry, B.E.; Gehr, P.; Bachofen, M.; Weibel, E.R. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 1982, 126, 332–337. [Google Scholar]
- Loret, T.; Peyret, E.; Dubreuil, M.; Aguerre-Chariol, O.; Bressot, C.; le Bihan, O.; Amodeo, T.; Trouiller, B.; Braun, A.; Egles, C.; et al. Air-liquid interface exposure to aerosols of poorly soluble nanomaterials induces different biological activation levels compared to exposure to suspensions. Part. Fibre Toxicol. 2016, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Marriott, H.M.; Gascoyne, K.A.; Gowda, R.; Geary, I.; Nicklin, M.J.H.; Iannelli, F.; Pozzi, G.; Mitchell, T.J.; Whyte, M.K.B.; Sabroe, I.; et al. Interleukin-1β regulates CXCL8 release and influences disease outcome in response to Streptococcus pneumoniae, defining intercellular cooperation between pulmonary epithelial cells and macrophages. Infect. Immun. 2012, 80, 1140–1149. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.H.; Seo, J.Y.; Oh, J.; Kim, J.S.; Kim, E.J.; Kim, J.S. In vitro and in vivo anti-inflammatory activities of mixed fruit and vegetable juice. Food Sci. Biotechnol. 2016, 25, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.H.; Wickremasinghe, M.I.Y.; Sharland, M.; Friedland, J.S. Synergistic upregulation of interleukin-8 secretion from pulmonary epithelial cells by direct and monocyte-dependent effects of respiratory syncytial virus infection. J. Virol. 2000, 74, 8425–8433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Cao, X.; Wang, F.; Jiang, H.; Feng, D.; Guo, H.; Du, L.; Jin, Y.; Chen, Y.; Yin, X.; et al. LFG-500, a novel synthetic flavonoid, suppresses epithelial-mesenchymal transition in human lung adenocarcinoma cells by inhibiting NLRP3 in inflammatory microenvironment. Cancer Lett. 2017, 400, 137–148. [Google Scholar] [CrossRef]
- Morris, G.E.; Parker, L.C.; Ward, J.R.; Jones, E.C.; Whyte, M.K.B.; Brightling, C.E.; Bradding, P.; Dower, S.K.; Sabroe, I. Cooperative molecular and cellular networks regulate Toll-like receptor-dependent inflammatory responses. FASEB J. 2006, 20, 2153–2155. [Google Scholar] [CrossRef] [Green Version]
- Havrdova, M.; Hola, K.; Skopalik, J.; Tomankova, K.; Martin, P.A.; Cepe, K.; Polakova, K.; Tucek, J.; Bourlinos, A.B.; Zboril, R. Toxicity of carbon dots—Effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle. Carbon 2016, 99, 238–248. [Google Scholar] [CrossRef]
- Sima, M.; Vrbova, K.; Zavodna, T.; Honkova, K.; Chvojkova, I.; Ambroz, A.; Klema, J.; Rossnerova, A.; Polakova, K.; Malina, T.; et al. The differential effect of carbon dots on gene expression and DNA methylation of human embryonic lung fibroblasts as a function of surface charge and dose. Int. J. Mol. Sci. 2020, 21, 4763. [Google Scholar] [CrossRef] [PubMed]
- Kletting, S.; Barthold, S.; Repnik, U.; Griffiths, G.; Loretz, B.; Schneider-Daum, N.; de Souza Carvalho-Wodarz, C.; Lehr, C.M. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX 2018, 35, 211–222. [Google Scholar] [CrossRef]
- Wottrich, R.; Diabate, S.; Krug, H.F. Biological effects of ultrafine model particles in human macrophages and epithelial cells in mono- and co-culture. Int. J. Hyg. Environ. Health 2004, 207, 353–361. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 2015, 574, 193–203. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.O.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Shao, B.; He, Z.; Ye, T.; Luo, M.; Sang, Y.; Liang, X.; Wang, W.; Luo, S.; Yang, S.; et al. Cationic nanocarriers induce cell necrosis through impairment of Na(+)/K(+)-ATPase and cause subsequent inflammatory response. Cell Res. 2015, 25, 237–253. [Google Scholar] [CrossRef] [Green Version]
- Ronzani, C.; Van Belle, C.; Didier, P.; Spiegelhalter, C.; Pierrat, P.; Lebeau, L.; Pons, F. Lysosome mediates toxicological effects of polyethyleneimine-based cationic carbon dots. J. Nanopart. Res. 2019, 21, 4. [Google Scholar] [CrossRef]
- Marano, F.; Hussain, S.; Rodrigues-Lima, F.; Baeza-Squiban, A.; Boland, S. Nanoparticles: Molecular targets and cell signalling. Arch. Toxicol. 2011, 85, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein-nanoparticle interactions: Opportunities and challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef] [PubMed]
- Ritz, S.; Schottler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein corona of nanoparticles: Distinct proteins regulate the cellular uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manke, A.; Wang, L.Y.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.F.; Adamcakova-Dodd, A.; Steines, B.R.; Jing, X.F.; Salem, A.K.; Thorne, P.S. Comparison of in vitro toxicity of aerosolized engineered nanomaterials using air-liquid interface mono-culture and co-culture models. Nanoimpact 2020, 18, 100215. [Google Scholar] [CrossRef]
- Jantzen, K.; Roursgaard, M.; Desler, C.; Loft, S.; Rasmussen, L.J.; Moller, P. Oxidative damage to DNA by diesel exhaust particle exposure in co-cultures of human lung epithelial cells and macrophages. Mutagenesis 2012, 27, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Hufnagel, M.; Neuberger, R.; Wall, J.; Link, M.; Friesen, A.; Hartwig, A. Impact of differentiated macrophage-like cells on the transcriptional toxicity profile of CuO nanoparticles in co-cultured lung epithelial cells. Int. J. Mol. Sci. 2021, 22, 5044. [Google Scholar] [CrossRef] [PubMed]
- Kose, O.; Tomatis, M.; Leclerc, L.; Belblidia, N.B.; Hochepied, J.F.; Turci, F.; Pourchez, J.; Forest, V. Impact of the physicochemical features of TiO2 nanoparticles on their in vitro toxicity. Chem. Res. Toxicol. 2020, 33, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Meindl, C.; Ohlinger, K.; Zrim, V.; Steinkogler, T.; Frohlich, E. Screening for effects of inhaled nanoparticles in cell culture models for prolonged exposure. Nanomaterials 2021, 11, 606. [Google Scholar] [CrossRef]




| Characteristics | CD-PEI600 | CD-PEHA | CD-DMEDA | CD-COOH |
|---|---|---|---|---|
| Zeta potential ζ (mV) | +31.8 ± 1.1 | +29.2 ± 2.2 | +11.1 ± 2.2 | −43.3 ± 3.2 |
| Surface charge density Qek (µmol/mg) | 4.70 | 3.25 | 0.01 | - |
| Hydrodynamic diameter D (nm) | 11.0 ± 3.4 | 10.2 ± 3.2 | 28.7 ± 4.1 | 50.7 ± 0.9 |
| Photoluminescence λmax/λex/λem (nm) | 350/365/460 | 350/370/465 | a/315/465 | a/370/445 |
| Viability loss | Necrosis | Uptake | ROS | IL-8 | MCP-1 | ||
|---|---|---|---|---|---|---|---|
| A549 | CD-PEI600 | + | + | + | + | + | - |
| CD-PEHA | - | - | + | - | - | - | |
| CD-DMEDA | - | - | + | - | - | - | |
| CD-COOH | - | - | - | - | - | - | |
| THP-1 | CD-PEI600 | + | + | + | + | + | - |
| CD-PEHA | + | - | + | - | - | - | |
| CD-DMEDA | - | - | - | - | - | - | |
| CD-COOH | - | - | - | - | - | - | |
| A549+THP-1 | CD-PEI600 | + | + | + | - | + | + |
| CD-PEHA | - | - | + | - | + | + | |
| CD-DMEDA | - | - | + | - | + | - | |
| CD-COOH | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arezki, Y.; Cornacchia, J.; Rapp, M.; Lebeau, L.; Pons, F.; Ronzani, C. A Co-Culture Model of the Human Respiratory Tract to Discriminate the Toxicological Profile of Cationic Nanoparticles According to Their Surface Charge Density . Toxics 2021, 9, 210. https://doi.org/10.3390/toxics9090210
Arezki Y, Cornacchia J, Rapp M, Lebeau L, Pons F, Ronzani C. A Co-Culture Model of the Human Respiratory Tract to Discriminate the Toxicological Profile of Cationic Nanoparticles According to Their Surface Charge Density . Toxics. 2021; 9(9):210. https://doi.org/10.3390/toxics9090210
Chicago/Turabian StyleArezki, Yasmin, Juliette Cornacchia, Mickaël Rapp, Luc Lebeau, Françoise Pons, and Carole Ronzani. 2021. "A Co-Culture Model of the Human Respiratory Tract to Discriminate the Toxicological Profile of Cationic Nanoparticles According to Their Surface Charge Density " Toxics 9, no. 9: 210. https://doi.org/10.3390/toxics9090210
APA StyleArezki, Y., Cornacchia, J., Rapp, M., Lebeau, L., Pons, F., & Ronzani, C. (2021). A Co-Culture Model of the Human Respiratory Tract to Discriminate the Toxicological Profile of Cationic Nanoparticles According to Their Surface Charge Density . Toxics, 9(9), 210. https://doi.org/10.3390/toxics9090210






