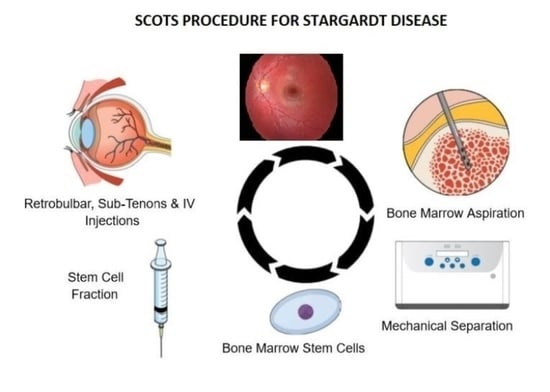
Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease
Abstract
1. Introduction
- Have objective, documented damage to the retina or optic nerve unlikely to improve; OR
- Have objective, documented damage to the retina or optic nerve that is progressive;
- AND have less than or equal to 20/40 best-corrected central visual acuity in one or both eyes AND/OR an abnormal visual field in one or both eyes;
- Be at least 3 months post-surgical treatment intended to treat any ophthalmologic disease and stable;
- If under current medical therapy (pharmacologic treatment) for a retinal or optic nerve disease, be considered stable on that treatment and unlikely to have visual function improvement (for example, glaucoma with intraocular pressure stable on topical medications but visual field damage);
- Have the potential for improvement with BMSC treatment and be at minimal risk of any potential harm from the procedure;
- Be over the age of 18;
- Be medically stable and able to be medically cleared by their primary care physician or a licensed primary care practitioner for the procedure. Medical clearance means that in the estimation of the primary care practitioner, the patient can reasonably be expected to undergo the procedure without significant medical risk to health.
- Patients who are not capable of an adequate ophthalmologic examination or evaluation to document the pathology;
- Patients who are not capable or not willing to undergo follow-up eye exams with the principle investigator or their ophthalmologist or optometrist as outlined in the protocol;
- Patients who are not capable of providing informed consent;
- Patients who may be at significant risk to general health or to the eyes and visual function should they undergo the procedure;
- Procedure: RB (Retrobulbar)Retrobulbar injection of Bone Marrow-Derived Stem Cells (BMSC)Other Name: Retrobulbar injection of stem cells
- Procedure: ST (Subtenon)Subtenon injection of Bone Marrow-Derived Stem Cells (BMSC)Other Name: Subtenon injection of stem cells
- Procedure: IV (Intravenous)Intravenous injection of Bone Marrow-Derived Stem Cells (BMSC)Other Name: Intravenous injection of stem cells
- Procedure: IVIT (Intravitreal)Intravitreal injection of Bone Marrow-Derived Stem Cells (BMSC)Other Name: Intravitreal injection of stem cells
- Procedure: IO (Intraocular)Intraocular injection of Bone Marrow-Derived Stem Cells (BMSC) with vitrectomy prior to intraocular injection. For example, may include a larger amount of stem cells in the intravitreal cavity, intraneuronal injections, or subretinal injections of stem cells.Other Name: Intraocular injection of stem cells with vitrectomy.
- Active Comparator: RB, ST, IVInjections of BMSC retrobulbar (RB), subtenon (ST) and intravenous (IV)Interventions:
- ○
- Procedure: RB (Retrobulbar)
- ○
- Procedure: ST (Subtenon)
- ○
- Procedure: IV (Intravenous)
- Active Comparator: RB, ST, IV, IVITInjections of BMSC retrobulbar, subtenon, intravenous and intravitreal (IVIT)Study Arms:
- ○
- Procedure: RB (Retrobulbar)
- ○
- Procedure: ST (Subtenon)
- ○
- Procedure: IV (Intravenous)
- ○
- Procedure: IVIT (Intravitreal)
- Active Comparator: RB, ST, IV, IOInjection of BMSC retrobulbar, subtenon, intravenous and intraocular (IO) with vitrectomyInterventions:
- ○
- Procedure: RB (Retrobulbar)
- ○
- Procedure: ST (Subtenon)
- ○
- Procedure: IV (Intravenous)
- ○
- Procedure: IO (Intraocular)
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanna, P.; Strauss, R.W.; Gujinami, K.; Michaelides, M. Stargardt Disease: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2017, 101, 25–30. [Google Scholar] [CrossRef]
- Fujinami, K.; Lois, N.; Davidson, A.E.; Mackay, D.S.; Hogg, C.R.; Stone, E.M.; Tsunoda, K.; Tsubota, K.; Bunce, C.; Robson, A.G.; et al. A longitudinal study of Stargardt Disease: Clinical and electrophysiologic assessment, progression, and genotype correlations. Am. J. Ophthalmol. 2013, 155, 1075–1088. [Google Scholar] [CrossRef]
- Fujinami, K.; Zernat, J.; Chana, R.K.; Wright, G.A.; Tsunoda, K.; Ozawa, Y.; Tsubota, K.; Robson, A.G.; Holder, G.E.; Allikmets, R.; et al. Clinical and molecular characteristics of childhood-onset Stargardt Disease. Ophthalmology 2015, 122, 326–334. [Google Scholar] [CrossRef]
- Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT01920867?term=SCOTS&draw=2&rank=3 (accessed on 28 January 2021).
- Kong, X.; West, S.K.; Strauss, R.W.; Munoz, B.; Cideciyan, A.V.; Michaelides, M.; Ho, A.; Ahmed, M.; Schönbach, E.M.; Cheetham, J.K.; et al. Progression of Visual Acuity and Fundus Autofluorescence in Recent-Onset Stargardt Disease: ProgStar Study Report #4. Ophthalmol. Retin. 2017, 1, 514–523. [Google Scholar]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone marrow derived stem cells in the treatment of Age-Related Macular Degeneration. Medicines 2020, 7, 16. [Google Scholar] [CrossRef]
- Travis, G.H.; Golczak, M.; Moise, A.R.; Palczewski, K. Diseases caused by defects in the visual cycle: Retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 469–512. [Google Scholar] [CrossRef]
- Hussain, R.M.; Ciulla, T.A.; Berrocal, A.M.; Gregori, N.Z.; Flynn, H.W., Jr.; Lam, B.L. Stargardt macular dystrophy and evolving therapies. Expert Opin. Biol. Ther. 2018, 18, 1049–1059. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degenerations and Stargardt’s macular dystrophy:follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Dalkara, D.; Goureau, O.; Marazova, K.; Sahel, J.A. Let there be light: Gene and cell therapy for blindness. Hum. Gene Ther. 2016, 27, 134–147. [Google Scholar] [CrossRef]
- Audo, I.; Weleber, R.; Stout, T.; Lauer, A.K.; Pennesi, M.E.; Mohand-Said, S.; Barale, P.-O.; Buggage, R.; Wilson, D.J.; Sahel, J.A. Early findings in a Phase I/IIa clinical program for Stargardt Disease. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3819. [Google Scholar]
- Jimenez-Rolando, B.; Noval, S.; Rosa-Peres, I.; Diaz, E.M.; Del Pozo, A.; Ibañez, C.; Silla, J.C.; Montaño, V.E.F.; Martin-Arenas, R.; Vallespin, E. Next generation sequencing in the diagnosis of Stargardt’s disease. Arch. Soc. Esp. Oftalmol. 2018, 93, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Trapani, I. Dual AAV vectors for Stargardt Disease. Methods Mol. Biol. 2018, 1715, 153–175. [Google Scholar] [PubMed]
- Campa, C.; Gallenga, C.E.; Bolletta, E.; Perri, P. The role of gene therapy in the treatment of retinal diseases: A review. Curr. Gene Ther. 2017, 17, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.W.; Munoz, B.; Ho, A.; Jha, A.; Michaelides, M.; Cideciyan, A.V.; Audo, I.; Birch, D.G.; Hariri, A.H.; Nittala, M.G.; et al. Progression of Stargardt Disease as determined by fundus autofluorescence in the retrospective progression of Stargardt Disease study (ProgStar report no. 9). JAMA Ophthalmol. 2017, 135, 1232–1241. [Google Scholar] [CrossRef]
- Waugh, N.; Loveman, E.; Colquitt, J.; Royle, P. Treatments for dry age-related macular degeneration and Stargardt Disease: A systematic review. Health Technol. Assess. 2018, 22, 1–168. [Google Scholar] [CrossRef]
- Weiss, J.N.; Benes, S.C.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow Derived Stem Cells in the Treatment of Lebers Hereditary Optic Neuropathy. Neural Regen. Res. 2016, 11, 1685–1694. [Google Scholar]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone marrow derived stem cells in the treatment of Dominant Optic Atrophy. Stem Cell Investig. 2019, 6, 41. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study: Bone marrow derived stem cells in the treatment of Retinitis Pigmentosa. Stem Cell Investig. 2018, 5, 18. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone marrow derived stem cells in the treatment of Usher syndrome. Stem Cell Investig. 2019, 6, 31. [Google Scholar] [CrossRef]
- Pesaresi, M.; Bonilla-Pons, S.A.; Simonte, G.; Sanges, D.; Di Vicino, U.; Cosma, M.P. Endogenous Mobilization of Bone-Marrow Cells into the Murine Retina Induces Fusion-Mediated Reprogramming of Muller Glia Cells. EBioMedicine 2018, 30, 38–51. [Google Scholar] [CrossRef]
- Molday, R.S.; Zhong, M.; Quazi, F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim. Biophys. Acta 2009, 1791, 573–583. [Google Scholar] [CrossRef] [PubMed]
| Decimal | Snellen | LogMAR | |||
|---|---|---|---|---|---|
| 0.800 | 20/25 | 0.1 | |||
| 0.625 | 20/32 | 0.2 | |||
| 0.500 | 20/40 | 0.3 | |||
| 0.400 | 20/50 | 0.4 | |||
| 0.317 | 20/63 | 0.5 | |||
| 0.250 | 20/80 | 0.6 | |||
| 0.200 | 20/100 | 0.7 | |||
| 0.160 | 20/125 | 0.8 | |||
| 0.125 | 20/160 | 0.9 | |||
| 0.100 | 20/200 | 1 | |||
| 0.080 | 20/250 | 1.1 | |||
| 0.063 | 20/320 | 1.2 | |||
| 0.050 | 20/400 | 1.3 | |||
| 0.040 | 20/500 | 1.4 | |||
| 0.033 | 20/600 | 1.5 | |||
| 0.025 | 20/800 CF 10 ft. | 1.6 | |||
| 0.020 | 20/1000 CF 8 ft. | 1.7 | |||
| 0.017 | 20/1200 ~CF 7 ft. | 1.8 | 0.014 | 20/1400 CF 6 ft. | 1.85 |
| 0.013 | 20/1600 CF 5 ft. | 1.9 | 0.011 | 20/1800 CF 4 ft. | 1.95 |
| 0.010 | 20/2000 CF 2 ft. | 2 | |||
| 2.1 | |||||
| 2.2 | |||||
| 2.3 | |||||
| 2.4 | |||||
| 2.5 | |||||
| 2.6 | |||||
| 2.7 | |||||
| 2.8 | |||||
| 2.9 | |||||
| 0.001 | 20/20,000 HM | 3 |
| Patient No. | Age (yrs) Gender | Medical History | Ocular History | Family History | PVD OD/OS | Arm # | Pre-Va OD | Post-Va OD | Pre-Va OS | Post-Va OS | Comments | Pre-Va LogMAR OD | Post-Va LogMAR OD | Pre-Va LogMAR OS | Post-Va LogMAROS | Delta LogMAR % Change OD | Delta LogMAR % Change OS | Bilateral Vision |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71/M | DM/S/P prostate surgery(CA) | gene testing confirmed Stargardt | Glaucoma/DM/CA | Y/Y | 2/2 | CF 6′ | CF (?) | CF 6′ | 20/50 + 2 | 1.85 | 1.85? | 1.85 | 0.36 | 0 0% | +1.49 80.5% | improved | |
| 2 | 36/F | - | - gene testing | AMD | N/N | 2/2 | 20/400 | 20/400 | 20/400 | 20/200 + 1 | 1.3 | 1.3 | 1.3 | 0.98 | 0 0% | +0.32 24.6% | improved | |
| 3 | 41/F | Hyperthryoidism | - gene testing | AMD/CA/DM | N/N | 2/2 | 20/400 | 20/400 | CF6′ | CF2′ | 1.3 | 1.3 | 1.85 | 2 | 0 0% | (−0.15) (−10.3%) | no net loss | |
| 4 | 54/F | Cardiac valve abnormality/Asthma | - gene testing | AMD | Y/Y | 2/2 | Cf 6′ | 20/400 | 20/400 | 20/400 | Significant VF improvement OD/OS | 1.85 | 1.3 | 1.3 | 1.3 | +0.55 29.7% | 0 0% | improved |
| 5 | 30/M | - | - gene testing | DM | N/N | 2/2 | 20/50 − 1 | 20/50 + 1 | 20/40 + 2 | 20/40 + 2 | 0.41 | 0.38 | 0.28 | 0.28 | +0.03 7.3% | 0 0% | improved | |
| 6 | 53/M | Atrial Fib. | - gene testing | Stargardts/AMD | N/N | 2/3 | 20/400 | 20/100 | 20/400 | 20/100 | 1.3 | 0.7 | 1.3 | 0.7 | +0.6 46.2% | +0.6 46.2% | improved | |
| 7 | 51/M (brother of #6) | Hypertension | - gene testing | Stargardts/AMD | NN | 2/2 | 20/200 | 20/70 | 20/100 + 1 | 20/60 | 1 | 0.55 | 0.69 | 0.49 | +0.45 45% | +0.2 29% | improved | |
| 8 | 70/M | Hypertension/MI/S/P CABG/ | gene testing confirmed Stargardts | AMD | Y/Y | 1/2 | 20/400 | 20/200 | CF 6′ | 20/250 | 1.3 | 1 | 1.85 | 1.1 | +0.3 23% | +0.75 40.5% | improved | |
| 9 | 56/M | Hypertension | - gene testing | AMD | Y/Y | 1/2 | 20/200 − 1 | 20/200 − 1 | 20/400 | 20/200 − 1 | 1 | 1 | 1.3 | 1 | 0 | +0.3 23% | improved | |
| 10 | 30/M | - | - gene testing | Glaucoma | N/N | 1/1 | 20/400 | 20/200 | 20/200 | 20/400 | 1.3 | 1 | 1 | 1.3 | +0.3 23% | (−0.3) (−30%) | no net change | |
| 11 | 54/M | - | gene testing confirmed Stargardts | Stargardts | N/N | 1/2 | 20/80 + 1 | 20/60 + 1 | 20/200 | 20/200 + 1 | 0.58 | 0.47 | 1 | 0.98 | +0.11 19% | +0.02 2% | improved | |
| 12 | 28/M | - | - gene testing | - | N/N | 2/3 | 20/200 | 20/150 − 1 | 20/200 | 20/150 − 1 | 1 ppd x12yrs | 1 | 0.86 | 1 | 0.86 | +0.14 14% | +0.14 14% | improved |
| 13 | 65/F | - | - gene testing | Stargardts | N/N | 2/3 | 20/400 | 20/400 | 20/400 | 20/400 | 1.3 | 1.3 | 1.3 | 1.3 | 0 0% | 0 0% | no change | |
| 14 | 72/F | COPD | - gene testing | - | Y/N | 3/2 | CF5′ | CF2′ | CF5′ | CF3′ | cataracts OU | 1.9 | 2 | 1.9 | 1.97 | (−0.1) (−5.3%) | (−0.07) (−3.7%) | loss |
| 15 | 26/M | - | - gene testing | - | N/N | 2/2 | 20/400 | 20/200 | 20/400 | 20/200 − 1 | 1.3 | 1 | 1.3 | 1 | +0.3 23% | +0.3 23% | improved | |
| 16 | 29/M | - | - gene testing | AMD/Glaucoma/DM | N/N | 2/2 | CF 6′ | 20/400 + 1 | CF 6′ | 20/200 − 1 | 1.85 | 1.3 | 1.85 | 1 | +0.55 29.7% | +0.85 45.9% | improved | |
| 17 | 53/M | Parkinsons/Cardiac valve abnormality | - gene testing | Stargardts | Y/Y | 2/2 | 20/400 | 20/500 | 20/400 | 20/200 | 1.3 | 1.4 | 1.3 | 1 | (−0.1) (−7.7%) | +0.3 23% | improved | |
| 34 eyes | avg preop | =1.30 | avg delta +0.2344 | change + 17.96% | ||||||||||||||
| p = 0.0004 | ||||||||||||||||||
| 21 eyes improved | improved | change +33.3% |
| Patient No. | Postop Va Change OD | Postop Va Change OS | Postop Total Change in Best Va OU |
|---|---|---|---|
| 1 | No Change | >+12 lines | >+12 lines |
| 2 | No Change | >+3 lines | >+3 lines |
| 3 | No Change | −4 lines | No Change |
| 4 | >5 lines | No Change | >+5 lines |
| 5 | +/− No Change | No Change | +/− No Change |
| 6 | >+5 lines | >+5 lines | >+5 lines |
| 7 | >+3 lines | <+2 lines | <+2 lines |
| 8 | +3 lines | >+8 lines | No Change |
| 9 | No Change | No Change | No Change |
| 10 | +3 lines | −3 lines | No Change |
| 11 | +1 line | No Change | +1 line |
| 12 | +1 line | −1 line | No Change |
| 13 | No Change | No Change | No Change |
| 14 | −3 lines | −2 lines | −2 lines |
| 15 | +3 lines | +3 lines | +3 lines |
| 16 | +5 lines | +8 lines | +8 lines |
| 17 | −1 line | +3 lines | +3 lines |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease. Medicines 2021, 8, 10. https://doi.org/10.3390/medicines8020010
Weiss JN, Levy S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease. Medicines. 2021; 8(2):10. https://doi.org/10.3390/medicines8020010
Chicago/Turabian StyleWeiss, Jeffrey N., and Steven Levy. 2021. "Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease" Medicines 8, no. 2: 10. https://doi.org/10.3390/medicines8020010
APA StyleWeiss, J. N., & Levy, S. (2021). Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease. Medicines, 8(2), 10. https://doi.org/10.3390/medicines8020010