Implementation, Design and Cost Assessment of a Membrane-Based Process for Selectively Enriching Desalinated Water with Divalent Seawater Ions
Abstract
:1. Introduction
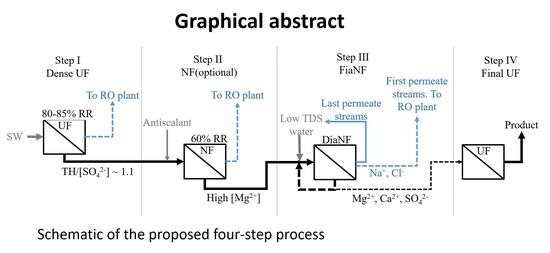
2. Process Description
3. Materials and Methods
4. Results and Discussion
4.1. Dense UF Step
4.2. NF Step (2nd Step in Figure 1)
4.3. DiaNF Step (3rd Step in Figure 1 and the Last Step in the Presented Three-Step Process)
4.4. DiaNF Step (2nd Step of the Two-Step Process)
5. General Design and Layout of a Process for Enriching Desalinated Water with 20 mgMg/L Using the Results from the 3-Step Process
5.1. Process Sequence
5.2. Evaluation of the Number of Membranes Required in the UF–NF–DiaNF Steps
Dense-UF (Membrane: GH) Step
5.3. Cost Assessment
6. Conclusions
- Results from operation of a new three-step (plus UF polish) process are presented for separating divalent ions from seawater with the purpose of elevating the Mg2+ concentration of the product water of seawater desalination plants.
- The first process step is carried out by passing seawater through a dense UF membrane with the purpose of rejecting sulfate and establishing a ratio of 1.1 to 1 between divalent cations and divalent anions in the retentate obtained at ~80% recovery.
- The second and third steps are based on subjecting the retentate of the first step to nanofiltration and diananofiltration, respectively, with the goal of both elevating the Mg2+ concentration and reducing the monovalent ions concentration to low concentrations.
- A general design is presented for a process in which the 1st and 2nd steps are operated continuously and the third (DiaNF) is operated semi-continuously with 12 h cycles.
- The experimental results show that applying 3-step the process results in a product solution characterized by Mg2+ concentration of ~3700 mg/L along with much lower Cl− and Na+ concentrations. As a result, the addition of 20 mg/L of Mg2+ to desalinated water will result in Cl− and Na+ concentration increase of 5.4 and 3.8 mg/L, respectively.
- The overall cost of the three-step process for adding 20 mg/L of Mg2+ to desalinated water was estimated at between $0.0142 and $0.0168 per m3 of desalinated water.
Author Contributions
Funding
Conflicts of Interest
References
- Cotruvo, J.B.J. (Ed.) Calcium and Magnesium in Drinking Water: Public Health Significance, 1st ed.; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Dreizin, Y. Ashkelon seawater desalination project—Off-taker’s self costs, supplied water costs, total costs and benefits. Desalination 2006, 190, 104–116. [Google Scholar] [CrossRef]
- Birnhack, L.; Voutchkov, N.; Lahav, O. Fundamental chemistry and engineering aspects of post-treatment processes for desalinated water—A. Review. Desalination 2011, 273, 2709–2716. [Google Scholar] [CrossRef]
- Isreali Water Authority. Available online: http://www.water.gov.il/HEBREW/PLANNING-AND-DEVELOPMENT/DESALINATION/Pages/default.aspx (accessed on 29 July 2018).
- Birnhack, L.; Fridman, N.; Lahav, O. Potential applications of quarry dolomite for post treatment of desalinated water. Desalin. Water Treat. 2009, 1, 58–67. [Google Scholar] [CrossRef]
- Greiserman, M.; Hasson, D.; Semiat, R.; Shemer, H. Kinetics of dolomite dissolution in a packed bed by acidified desalinated water. Desalination 2016, 39–47. [Google Scholar] [CrossRef]
- Derco, J.; Luptáková, A.; Dudáš, J.; Vrabeľ, M. Recarbonization of drinking water in fluidized-bed reactor. Chem. Pap. 2017, 71, 1771–1779. [Google Scholar] [CrossRef]
- Lahav, O.; Nativ, P.; Birnhack, L. Dolomite dissolution is not an attractive alternative for meeting Ca2+, Mg2+ and alkalinity criteria in desalination plants’ post treatment step. Desalin. Water Treat. 2018, 115, 194–198. [Google Scholar] [CrossRef]
- Birnhack, L.; Lahav, O. A new post-treatment process for attaining Ca2+, Mg2+, SO42− and alkalinity criteria in desalinated water. Water Res. 2007, 41, 3989–3997. [Google Scholar] [CrossRef] [PubMed]
- Telzhensky, M.; Birnhack, L.; Lehmann, O.; Windler, E.; Lahav, O. Selective separation of seawater Mg2+ ions for use in downstream water treatment processes. Chem. Eng. J. 2011, 175, 136–143. [Google Scholar] [CrossRef]
- Birnhack, L.; Nir, O.; Lahav, O. Establishment of the underlying rationale and description of a cheap nanofiltration-based method for supplementing desalinated water with magnesium ions. Water 2014, 6, 1172–1186. [Google Scholar] [CrossRef]
- Lehmann, O.; Eckhaus, O.; Lahav, O.; Birnhack, L. Replenishing Mg(II) to desalinated water by seawater nanofiltration followed by magnetic separation of Mg(OH)2(s) Fe3O4 particles. Desalin. Water Treat. 2016, 57, 19903–19916. [Google Scholar] [CrossRef]
- Nativ, P.; Birnhack, L.; Lahav, O. DiaNanofiltration-based method for inexpensive and selective separation of Mg2+ and Ca2+ ions from seawater, for improving the quality of soft and desalinated waters. Sep. Purif. Technol. 2016, 166, 83–91. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Birnhack, L.; Nativ, P.; Lahav, O. Highly-selective separation of divalent ions from seawater and seawater RO retentate. Sep. Purif. Technol. 2017, 175, 460–468. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Birnhack, L.; Cohen, Y.; Lahav, O. Selective separation of divalent ions from seawater using an integrated ion-exchange/nanofiltration approach. Chem. Eng. Process. Process Intensif. 2018, 126. [Google Scholar] [CrossRef]
- Foley, G. Closed-Loop Cascade Diafiltration. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin, Germany, 2016. [Google Scholar]
- Kovacs, Z. Continuous Diafiltration: Cocurrent and Countercurrent Modes. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin, Germany, 2016. [Google Scholar]
- Appelo, C.A.; Postma, D. Geochemistry, Ground Water and Pollution, 2nd ed.; Appelo, C., Postma, D., Eds.; CRC Press: London, UK, 2005. [Google Scholar]
- Kovács, Z. Batch Diafiltration. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin, Germany, 2016. [Google Scholar]
- U.S. Energy Information Administration, Electric Power Monthly 2017. U.S. Department of Energy. Available online: http://www.eia.gov/electricity/monthly/pdf/epm.pdf (accessed on 3 August 2017).
- Ziolkowska, J.R. Desalination leaders in the global market—Current trends and future perspectives. Water Sci. Technol. Water Supply 2016, 16, 563–578. [Google Scholar] [CrossRef]



| GH Step Brine | SI |
|---|---|
| RR (%) | Gypsum (CaSO4) |
| 0 (raw seawater) | −0.48 |
| 50 | −0.33 |
| 60 | −0.29 |
| 70 | −0.22 |
| 75 | −0.18 |
| 80 | −0.13 |
| 85 | −0.08 |
| RR | Cl− | Ca2+ | K+ | Mg2+ | Na+ | SO42− | TH/SO4 | Na/Mg | SI of Gypsum |
|---|---|---|---|---|---|---|---|---|---|
| g/L | mg/L | mg/L | mg/L | mg/L | mgS/L | eq/eq | g/g | ||
| GH 80RR | 27.11 ± 0.11 | 611 ± 9 | 588 ± 8 | 1949 ± 34 | 14,458 ± 93 | 2758 ± 48 | 1.11 ± 0.001 | 7.42 ± 0.102 | −0.16 ± 0.009 |
| 40 | 26.96 ± 0.13 | 925 ± 14 | 636 ± 14 | 3010 ± 29 | 15,408 ± 246 | 4440 ± 49 | 1.06 ± 0.012 | 5.12 ± 0.082 | 0.12 ± 0.005 |
| 50 | 28.63 ± 0.21 | 1076 ± 2 | 660 ± 12 | 3537 ± 41 | 15,800 ± 197 | 5349 ± 85 | 1.03 ± 0.021 | 4.47 ± 0.1.02 | 0.20 ± 0.005 |
| 55 | 29.20 ± 0.11 | 1167 ± 23 | 669 ± 17 | 3879 ± 50 | 16,007 ± 340 | 5895 ± 82 | 1.02 ± 0.009 | 4.13 ± 0.095 | 0.27 ± 0.005 |
| 60 | 29.36 ± 0.15 | 1293 ± 35 | 686 ± 17 | 4303 ± 70 | 16,294 ± 371 | 6525 ± 97 | 1.03 ± 0.006 | 3.79 ± 0.079 | 0.33 ± 0.012 |
| 65 | 29.34 ± 0.18 | 1439 ± 26 | 707 ± 17 | 4822 ± 44 | 16,648 ± 279 | 7366 ± 75 | 1.02 ± 0.005 | 3.45 ± 0.057 | 0.39 ± 0.009 |
| Cycle | Cl− | Ca2+ | K+ | Mg2+ | Na+ | SO42− | TH/SO4 | Cl/Mg | Na/Mg |
|---|---|---|---|---|---|---|---|---|---|
| g/L | mg/L | mg/L | mg/L | mg/L | mgS/L | eq/eq | g/g | g/g | |
| 0 | 26.97 | 1111 | 631 | 4269 | 15,980 | 5769 | 1.13 | 6.32 | 3.74 |
| 1 | 13.30 | 946 | 306 | 3963 | 7435 | 5752 | 1.04 | 3.36 | 1.88 |
| 2 | 7.10 | ||||||||
| 3 | 3.90 | 851 | 96.7 | 3757 | 2269 | 5586 | 1.01 | 1.04 | 0.60 |
| 4 | 2.09 | 807 | 51.4 | 3711 | 1307 | 5622 | 0.98 | 0.56 | 0.35 |
| 5 | 1.22 | 758 | 27.9 | 3561 | 821 | 5461 | 0.97 | 0.34 | 0.23 |
| 6 | 0.68 | 791 | 15.9 | 3847 | 579 | 5935 | 0.96 | 0.18 | 0.15 |
| Cycle | Cl− | Ca2+ | K+ | Mg2+ | Na+ | SO42− | TH/SO4 | Cl/Mg | Na/Mg |
|---|---|---|---|---|---|---|---|---|---|
| g/L | mg/L | mg/L | mg/L | mg/L | mgS/L | eq/eq | g/g | g/g | |
| 0 | 25.68 | 1171 | 587 | 4234 | 14,740 | 6020 | 1.08 | 6.07 | 3.48 |
| 1 | 13.89 | 1073 | 345 | 4098 | 8410 | 5952 | 1.05 | 3.39 | 2.05 |
| 2 | 8.04 | 947 | 200 | 3812 | 4866 | 5619 | 1.03 | 2.11 | 1.28 |
| 3 | 4.93 | 934 | 122 | 3906 | 2963 | 5826 | 1.01 | 1.26 | 0.76 |
| 4 | 2.82 | 846 | 68 | 3743 | 1707 | 5649 | 0.99 | 0.75 | 0.46 |
| 5 | 1.68 | 797 | 39 | 3653 | 1039 | 5592 | 0.97 | 0.46 | 0.28 |
| 6 | 0.97 | 760 | 22 | 3563 | 661 | 5521 | 0.96 | 0.27 | 0.19 |
| Cycle | Cl− | Ca2+ | K+ | Mg2+ | Na+ | SO42− | TH/SO4 | Cl/Mg | Na/Mg |
|---|---|---|---|---|---|---|---|---|---|
| g/L | mg/L | mg/L | mg/L | mg/L | mgS/L | eq/eq | g/g | g/g | |
| 0 | 22.26 | 640 | 568 | 2158 | 14,080 | 2894 | 1.16 | 10.32 | 6.52 |
| 1 | 11.56 | 548 | 298 | 1919 | 7250 | 2764 | 1.07 | 6.02 | 3.78 |
| 2 | 6.23 | 503 | 168 | 1840 | 3977 | 2724 | 1.04 | 3.39 | 2.16 |
| 3 | 3.54 | 483 | 101 | 1771 | 2272 | 2679 | 1.01 | 2.00 | 1.28 |
| 4 | 2.07 | 443 | 62 | 1713 | 1397 | 2646 | 0.99 | 1.21 | 0.82 |
| 5 | 1.26 | 426 | 40 | 1693 | 876 | 2609 | 0.98 | 0.74 | 0.52 |
| 6 | 0.72 | 411 | 28 | 1683 | 586 | 2660 | 0.96 | 0.43 | 0.35 |
| Cycle | Cl− | Ca2+ | K+ | Mg2+ | Na+ | SO42− | TH/SO4 | Cl/Mg | Na/Mg |
|---|---|---|---|---|---|---|---|---|---|
| g/L | mg/L | mg/L | mg/L | mg/L | mgS/L | eq/eq | g/g | g/g | |
| 0 | 20.74 | 625 | 553 | 2073 | 12,215 | 3100 | 1.04 | 10.01 | 5.89 |
| 1 | 11.15 | 592 | 362 | 2036 | 7270 | 3057 | 1.03 | 5.47 | 3.57 |
| 2 | 7.00 | 551 | 243 | 1953 | 4529 | 2954 | 1.02 | 3.59 | 2.32 |
| 3 | 4.23 | 538 | 165 | 1974 | 2830 | 3031 | 1.00 | 2.14 | 1.43 |
| 4 | 2.44 | 497 | 106 | 1871 | 1674 | 2924 | 0.98 | 1.30 | 0.89 |
| 5 | 1.45 | 467 | 76 | 1812 | 1050 | 2869 | 0.96 | 0.80 | 0.58 |
| 6 | 0.87 | 454 | 56 | 1800 | 731 | 2863 | 0.95 | 0.48 | 0.41 |
| Time (h) | NF Step | DiaNF Sub-Stage #1 Feed Streams | DiaNF Sub-Stage #2 | Product Dosage to RO Permeate (after UF) from Tank | ||
|---|---|---|---|---|---|---|
| Brine Goes to | Brine | Diluent | Feed * | Permeate Goes to | ||
| 0–6 | T1 at Q | from T2 at 6Q | from T3 at 6Q | T4 at Q | ||
| 6–12 | T1 at Q | from T2 at 6Q | T3 at 6Q | T4 at Q | ||
| 12–18 | T2 at Q | from T1 at 6Q | from T3 at 6Q | T4 at Q | ||
| 18–24 | T2 at Q | from T1 at 6Q | T3 at 6Q | T4 at Q | ||
| Parameter | Units | Dense UF Step (GH) | NF Step (DL) | DiaNF Step (DL) |
|---|---|---|---|---|
| Required feed | m3/day | 30,440 | 6088 | 29,223 |
| Recovery ratio | % | 80 | 60 | |
| Produced permeate | m3/day | 24,352 | 3653 | 14,611 ** |
| Produced brine | m3/day | 6088 | 2435 | 2435 |
| membrane permeate flux * | m3/day | 21.9 | 18.7 | 18.7 |
| Number of membranes | 1116 | 196 | 784 | |
| Number of membranes in a train | 5 | 6 | 5 | |
| Plant length | m | 8.3 | 9.9 | 8.3 |
| Plant width | m | 5.3 | 0.83 | 3.9 |
| Plant size | m3 | 144.5 | 27.4 | 106.5 |
| Parameter | Cost Component | Process Step | Cost |
|---|---|---|---|
| 10−2 $ m−3 Desalinated Water | |||
| OPEX | energy | UF (GH) | 0.225 |
| NF (DL) | 0.075 | ||
| DiaNF | 0.187 | ||
| sub-total | 0.486 | ||
| diluent | DiaNF | 0.538 | |
| UF steps | Pre treatment | 0.018 | |
| antiscalant | NF | 0.005 | |
| HCl | NF | 0.005 | |
| membrane replacement | All steps | 1.8 × 10−5 | |
| Total | 1.053 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birnhack, L.; Tang, S.C.N.; Lahav, O. Implementation, Design and Cost Assessment of a Membrane-Based Process for Selectively Enriching Desalinated Water with Divalent Seawater Ions. ChemEngineering 2018, 2, 41. https://doi.org/10.3390/chemengineering2030041
Birnhack L, Tang SCN, Lahav O. Implementation, Design and Cost Assessment of a Membrane-Based Process for Selectively Enriching Desalinated Water with Divalent Seawater Ions. ChemEngineering. 2018; 2(3):41. https://doi.org/10.3390/chemengineering2030041
Chicago/Turabian StyleBirnhack, Liat, Samuel C. N. Tang, and Ori Lahav. 2018. "Implementation, Design and Cost Assessment of a Membrane-Based Process for Selectively Enriching Desalinated Water with Divalent Seawater Ions" ChemEngineering 2, no. 3: 41. https://doi.org/10.3390/chemengineering2030041
APA StyleBirnhack, L., Tang, S. C. N., & Lahav, O. (2018). Implementation, Design and Cost Assessment of a Membrane-Based Process for Selectively Enriching Desalinated Water with Divalent Seawater Ions. ChemEngineering, 2(3), 41. https://doi.org/10.3390/chemengineering2030041