- Article
From Baker’s Yeast to Skin Rejuvenation: Insights into the Anti-Wrinkle Properties of Chitin–Glucans Extracted from Saccharomyces cerevisiae
- Xiaosong Wang,
- Mojtaba Koosha and
- Vladimir A. Vinokurov
- + 2 authors
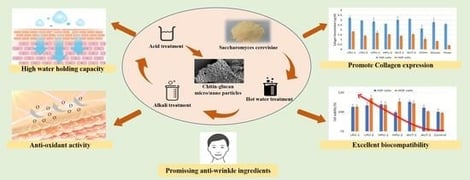
While Saccharomyces cerevisiae (baker’s yeast) offers a safe, non-animal source of chitin-glucan (CG), its potential as a functional cosmetic ingredient has been overshadowed by industrial sources like Aspergillus niger. This study advances the existing literature by establishing a critical structure–function relationship for CG micro/nano particles extracted via three physical disruption methods: ultrasonic bath, ultrasonic probe, and autoclaving. The obtained CG was systematically characterized by physicochemical and biological tests. A significant trade-off was identified: while autoclaving (40 min) resulted in lower mass yield compared to ultrasonication, it produced particles with the highest crystallinity, an enriched chitin/glucan ratio, and the smallest particle size (~70% of particles with mean diameter of 480 ± 33 nm). Structurally, these sub-micron particles demonstrated superior colloidal stability and a physical “barrier effect” for sustained hydration, outperforming the amorphous structures typically associated with mild extraction. The anti-wrinkle efficacy was validated through a specific “triad” mechanism: (1) the insoluble 3D network ensures prolonged water retention, (2) the particles exhibit robust free radical scavenging activity (~67%), and (3) most notably, the specific nano-structure significantly upregulated Collagen Type I-α1 expression in human dermal fibroblasts (HDF) and human skin fibroblasts (HSF), surpassing commercial chitin controls. These findings prove that the extraction-induced nano-structure, rather than mass yield, is the determinant factor for bioactivity, positioning S. cerevisiae CG as a high-performance, multi-target ingredient for anti-aging formulations.
2 March 2026



![(left) Flow facility of the falling liquid film. (right) MPFS probes from inside with 640 pin probes [32].](https://mdpi-res.com/cdn-cgi/image/w=281,h=192/https://mdpi-res.com/ChemEngineering/ChemEngineering-10-00032/article_deploy/html/images/ChemEngineering-10-00032-g001-550.jpg)