Abstract
Nanosized titanium dioxide (TiO2) nanoparticles were used for the photocatalytic reduction of hexavalent chromium in the presence of formic acid. The photoreduction of Cr(VI) in the absence of formic acid was quite slow. When formic acid was added in the chromium solution as the hole scavenger, a rapid photocatalytic reduction of Cr(VI) was observed, owing to the consumption of hole and the acceleration of the oxidation reaction. Furthermore, three commercial TiO2 nanoparticles (AEROXIDE® P25; Ishihara Sangyo ST-01; FUJIFILM Wako Pure Chemical Corp.) were evaluated for the photoactivity of reduction of Cr(VI).
1. Introduction
Chromium (Cr) is a regulated metal in groundwater as a pollutant [1]. The Cr contamination has emanated from tanneries, dyeing, pigments, electroplating, metal finishing and so on [2]. The chromium occurs in the oxidation states +3 and +6 in the environment. The oxidation state and speciation of chromium are responsible for its toxicity in nature [3]. Cr(VI), with its carcinogenic and mutagenic effects on living organisms, is the most toxic, relatively within the chromium species [4]. Therefore, a significant stage in Cr(VI) pollution remediation is the reduction of highly toxic, soluble and easily migrant Cr(VI) to approximately one hundred times less toxic, easier coordinated and precipitated Cr (III).
Numerous chemical and physicochemical processes, such as ion exchange, chemical precipitation, coagulation, membrane process, reduction and adsorption, have been traditionally proposed [5,6,7]. Among these technologies, the heterogeneous photocatalytic reduction process has become one of the promising methods by virtue of cost-effectiveness, high catalytic performance and no secondary pollution [8].
Thus far, in various semiconductor oxides, titanium dioxide (TiO2) has attracted enormous attention for widespread environmental applications, due to its low-cost, stability, nontoxicity, optical and electrical properties [9]. When nanosized TiO2 particles are irradiated with UV light (λ < 387 nm), photo-induced electrons are generated and excited from the valence bond (VB) to the conduction band (CB). While the photo-induced electrons are generally applied into reducing protons in water to evolve H2 gas, in addition, these electrons can be used to remediate harmful contaminants by reducing hexavalent Cr to a trivalent. Normally, the photocatalytic reduction is more positive for the standard reduction potential of hexavalent Cr, compared with the conduction band of the photocatalyst, though several hundred mVs of overpotential are frequently required owing to mass transfer, kinetic and ohmic losses [10].
Since the oxidation of water to oxygen is a kinetically slow process during the photocatalytic reduction of Cr(VI) to Cr(III), the conversion rate of Cr(VI) generally proceeds very slowly [11]. The addition of hole scavengers during the photoreduction of hexavalent chromium could greatly enhance the photocatalytic reduction of Cr [12,13]. First, it was reported by Sun et al. [14] that the addition of formic acid was very effective for the improvement of photocatalytic Cr reduction with P25 TiO2. Next, Wang et al. [11] described that there was little positive effect of a formic acid scavenger on the reduction of hexavalent chromium in an aqueous solution using TiO2, which was supplied from Zhoushan Nano Company (China). Therefore, in the present work, the photocatalytic reduction of hexavalent chromium with various commercial nanosized TiO2 in the presence of formic acid was evaluated, and the photocatalytic activity of Cr reduction was discussed on the photocatalyst properties, such as the specific surface area and particle diameter.
2. Materials and Methods
2.1. Photocatalysts and Chemicals
Three commercial TiO2 (AEROXIDE® P25; Ishihara Sangyo ST-01; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) was used as received, without further purification. Basic information is as follows, for AEROXIDE® P25: Anatase 75%, rutile 25%, specific surface area 50 m2·g−1, mean particle size 25 nm; for Ishihara Sangyo Kaisha, LTD, ST-01: Specific surface area 300 m2·g−1, mean particle size 7 nm; for FUJIFILM Wako Pure Chemical Corp., anatase form: Specific surface area 8.7 m2·g−1, mean particle size 230 nm [15]. Potassium dichromate, formic acid, sulfuric acid, acetone and 1,5-diphenylcarbazide were purchased from FUJIFILM Wako Pure Chemical Corp., and were of analytical reagent grade. A standard stock solution of Cr(VI) 1000 μg·mL−1 as K2Cr2O7 was obtained from FUJIFILM Wako Pure Chemical Corp.
2.2. Photocatalytic Reduction of Cr(VI)
The Pyrex vessel reactor (inner capacity: 50 cm3) was used for the photocatalytic reduction of hexavalent chromium ions in an aqueous solution. Typically, 20 mg of TiO2 photocatalysts were added to 30 mL of 30 μg·mL−1 Cr(VI) aqueous solution in the reactor. Formic acid (0.30%) was added as the hole scavenger into the solution. The pH was set to 3. Before the illumination, the suspension was allowed to reach adsorption–desorption equilibrium with continuous and vigorous stirring for 30 min in the dark. During the irradiation, the suspensions were still under continuous stirring. A black light (Toshiba Lighting & Technology Corp., Tokyo, Japan, 15 W) was applied with a maximum emission of about 352 nm as the light source, which was positioned on the side of the reactor. The light intensity was measured by a digital UV intensity meter (USHIO, UIT-201) with a sensor (UV-365PD, 330~390 nm), and a value of 0.25 mW·cm−2. The samples, withdrawn at each time interval, were centrifuged at 10,000 rpm for 10 min and their supernatant was subjected to the analysis of Cr(VI).
2.3. Analysis of Hexavalent Chromium
The residual concentration of Cr(VI) was measured with the nesslerization method using a UV-visible spectrometry (AS ONE Corp., ASV11D) at λmax of 540 nm, according to the standard method for the examination of water. First, a 1 mL portion of the solution was taken from the sample and was subjected to centrifugation (12,000 rpm) for 5 min. The supernatant (300 μL) was sampled. The solution (300 μL), 500 μL of sulfuric acid (2 mol·L−1) and 200 μL of 1,5-diphenyl carbazide (10 g·L−1) were transferred into a 25 mL volumetric flask and diluted with pure water.
3. Results and Discussion
3.1. Chromium(VI) Species with pH
Chromium (VI) species may be present in aqueous solution as chromate (CrO42−), dichromate (Cr2O72−), hydrogen chromate (HCrO4−), dihydrogen chromate (chromic acid, H2CrO4), hydrogen dichromate (HCr2O7−), trichromate (Cr3O102−) and tetrachromate (Cr4O132−). The last three ions (HCr2O7−, Cr3O102− and Cr4O132−) have been observed only in solutions of pH < 0 or at a chromium (VI) concentration greater than 1 mol·L−1 [16]. Tandon et al. [17] have presented the influence of pH on chromium (VI) species in solution and used the following equilibrium constant for describing chromium speciation equilibria.
H2CrO4 ⇄ H+ + HCrO4−, k1 = 0.18
HCrO4− ⇄ H+ + CrO42−, k2 = 3.2 × 10−7
2HCrO4− ⇄ Cr2O72− + H2O, k3 = 98
The total chromium (VI) concentration C can be expressed as follows:
The concentration of H2CrO4, that is, [H2CrO4], in a solution of pH and the total chromium (VI) concentration C, was estimated by soling the quadratic equation.
The concentrations of Cr(VI) species can be derived from the following equations.
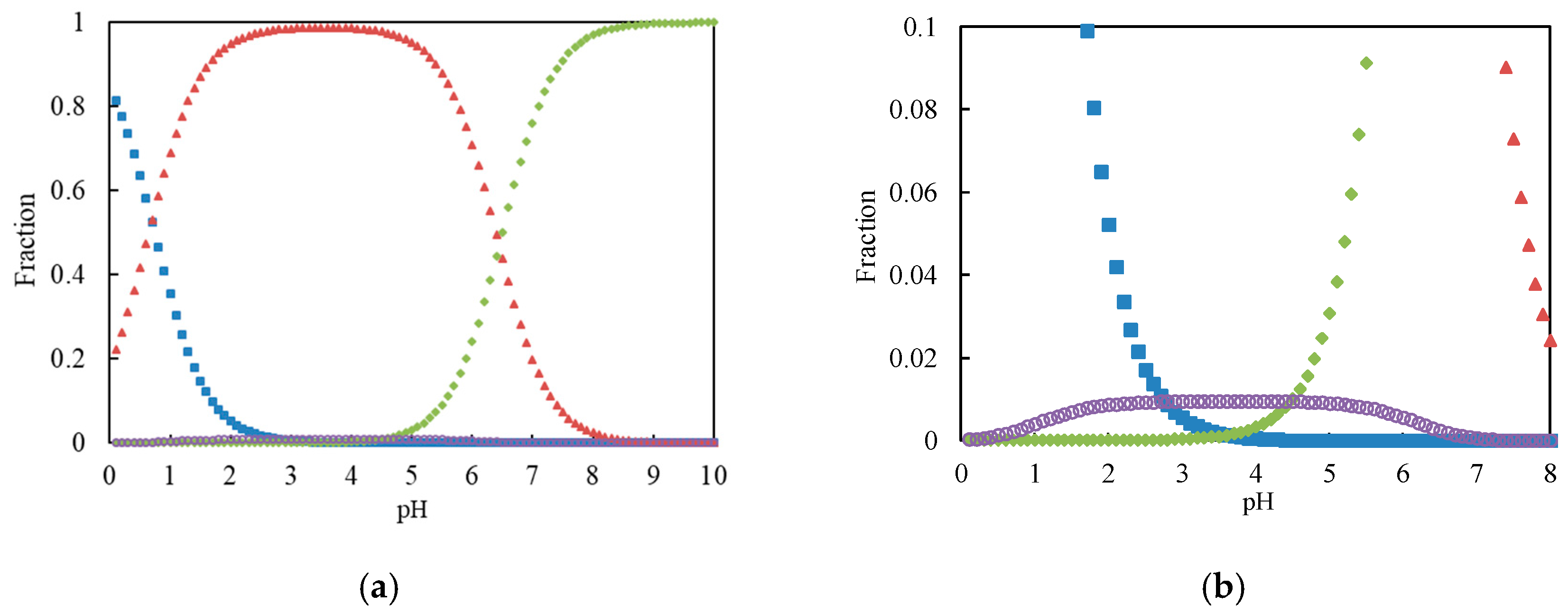
Because the initial concentration of Cr(VI) was 30 μg·mL−1 (0.483 mmol·L−1) in the experiment, the total chromium (VI) concentration C was set to 0.1 mmol·L−1 for the estimation of the chromium (VI) species. The fractions of Cr(VI) species after the calculation with a computer are illustrated in Figure 1.
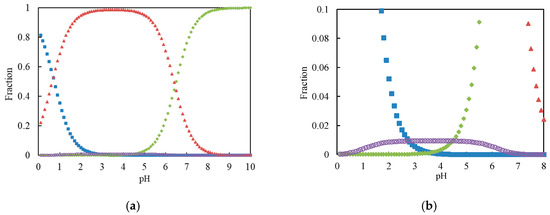
Figure 1.
(a) Distribution of Cr(VI) species as functions of pH. Square (blue): H2CrO4; triangle (red): HCrO4−; diamond (green): CrO42−; circle (purple): Cr2O72−; (b) Magnification for Cr2O72− species.
Diphenylcarbazide appears as a sensitive and specific color reaction with hexavalent chromium in mineral acid solution [18]. The pink colored chromophore is a chelate of chromium (III) and diphenylcarbazone. Diphenylcarbazone is produced and simultaneously combines with chromium when diphenylcarbazide is oxidized by hexavalent chromium [19]. The reaction may be speculated as:
where H4L is diphenylcarbazide:
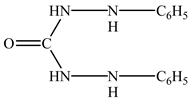 and H2L is diphenylcarbazone:
and H2L is diphenylcarbazone:
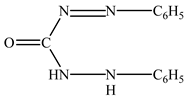
CrO42− + 3H4L + 8H+ = [Cr(III)(HL)2]+ + Cr3+ + H2L + 8H2O


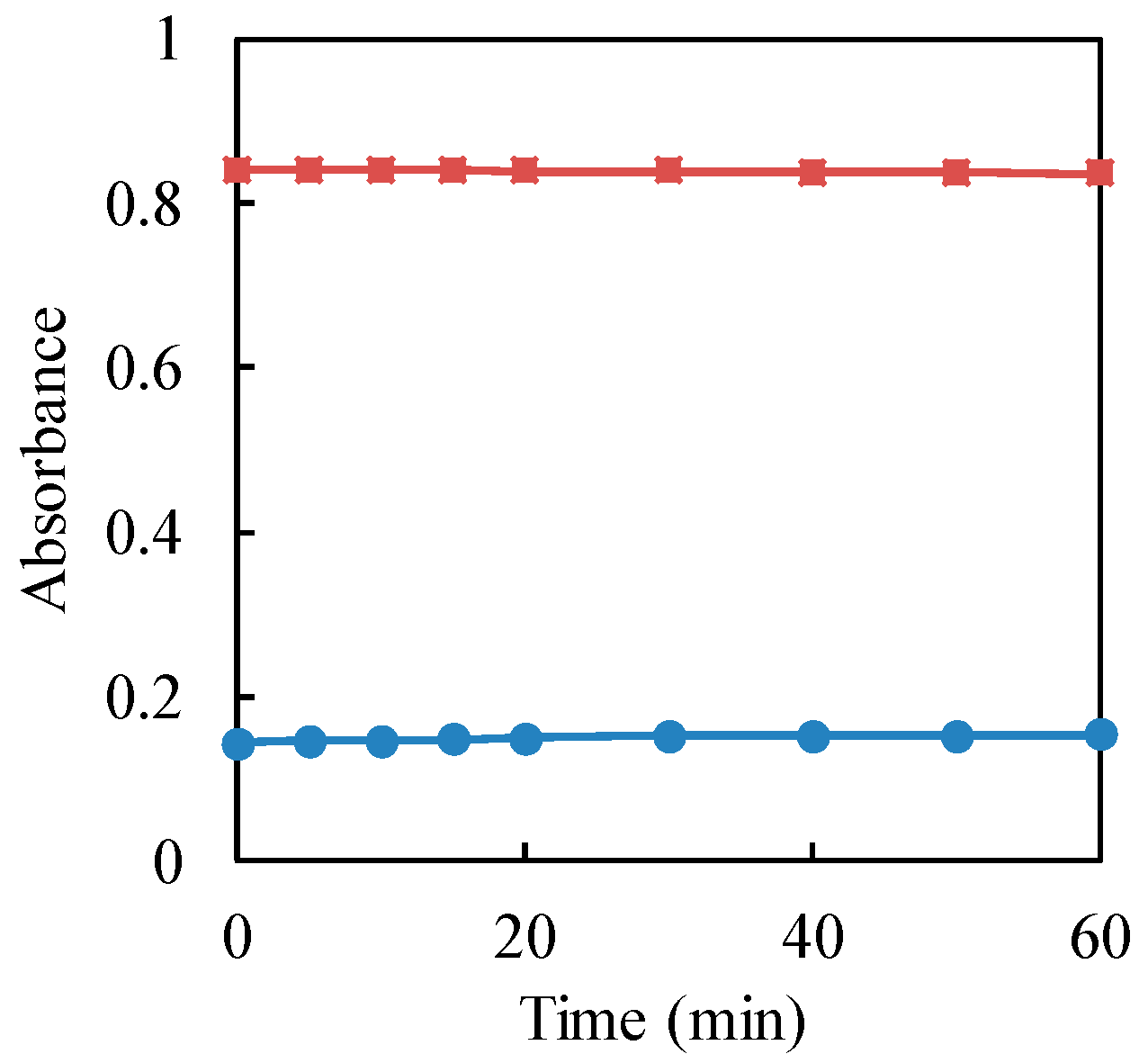
The stability of the complex formation was evaluated for the determination of residual hexavalent chromium in the sample. The effect of the standing time on the absorbance of the resulted complex between 1,5-diphenylcarbazide and Cr(VI) was studied, as shown in Figure 2. From the graph, the absorbance of the complex was almost constant for 60 min. Therefore, the absorbance of the complex was measured after 5 min of standing time, since it was stable from 5 min.
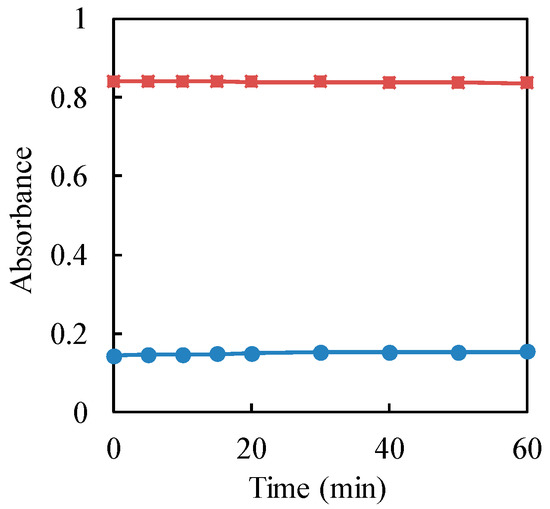
Figure 2.
Effect of standing time on the absorbance of the complex between Cr(VI) and 1,5-diphenylcarbazide. Cr(VI) concentration: Circle (blue) 10 μg·mL−1; square (red): 50 μg·mL−1.
3.2. Effect of Hole Scavengers
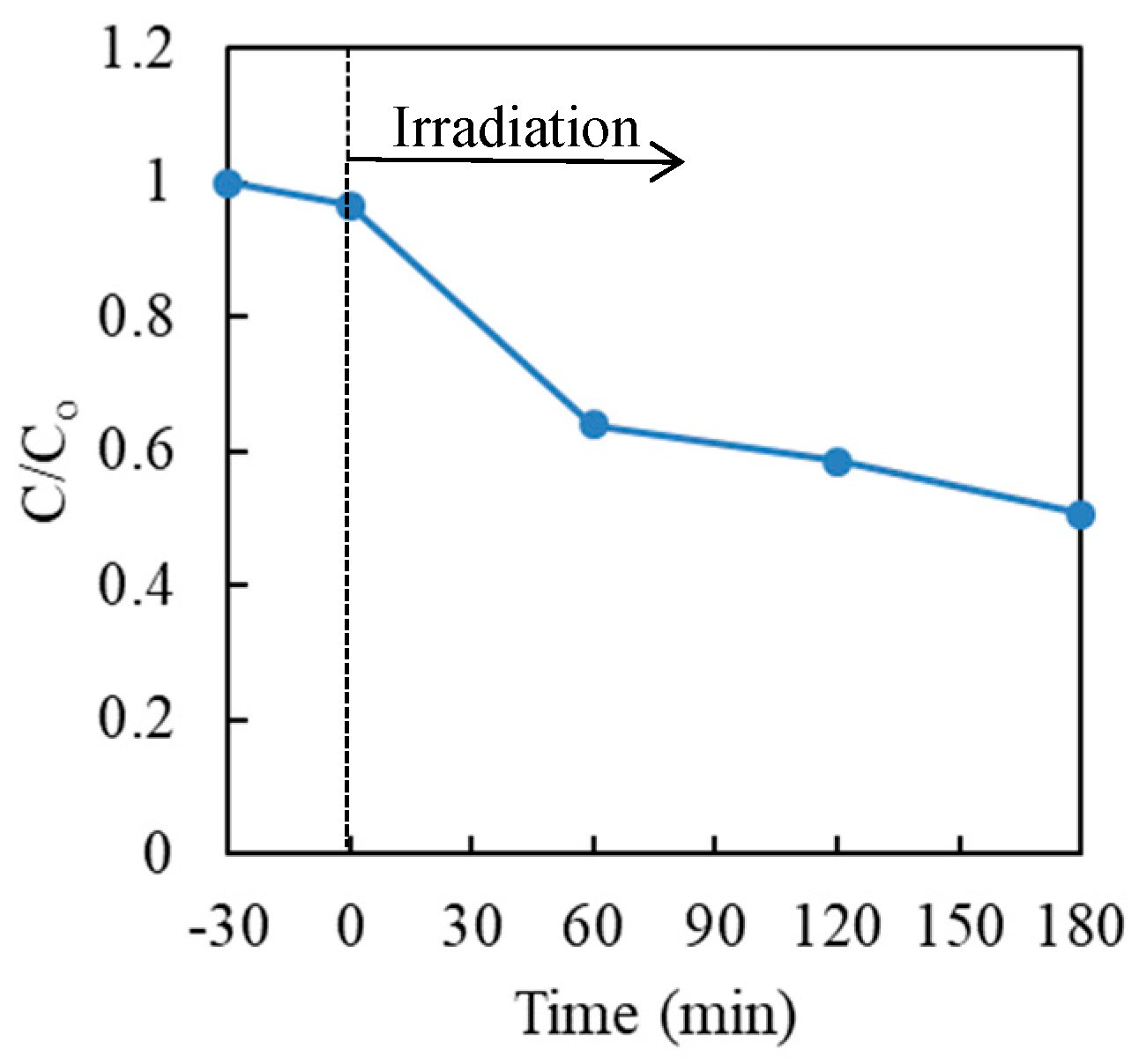
First, the photocatalytic reduction of hexavalent chromium with TiO2 in the aqueous solution was investigated in the absence of a hole scavenger. The results are shown in Figure 3. It was noticed that the photocatalytic reduction efficiency of Cr(VI) with TiO2 without a hole scavenger was quite poor and approximately 50% of Cr(VI) remained in the solution after the photocatalytic treatment, for 3 h.
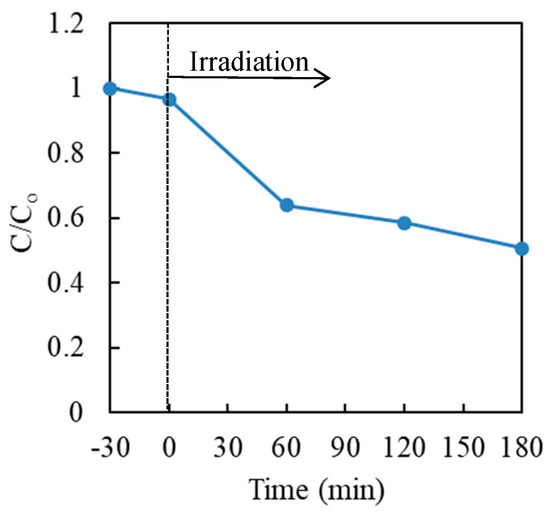
Figure 3.
Effect of time on the photocatalytic reduction of Cr(VI) in aqueous solution with P25 TiO2. Cr(VI) sample: 30 μg·mL−1 (30 mL); TiO2: 20 mg (0.67 mg·mL−1).
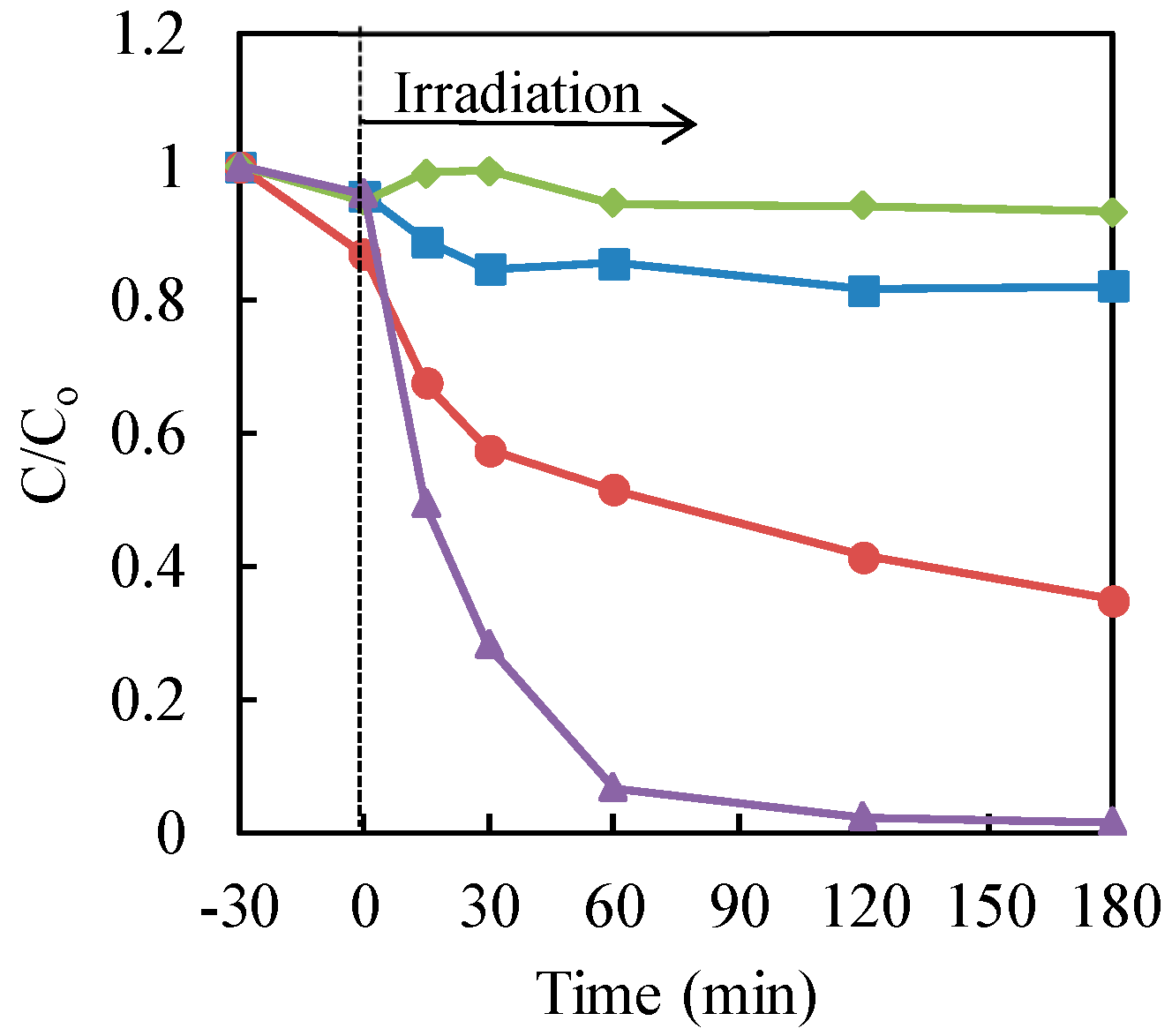
Next, the influence of hole scavengers on the photocatalytic treatment of chromium (VI) with nanosized TiO2 powders in the solution was investigated [20]. Ammonium formate and formic acid were checked as the hole scavengers. These chemical substances could not act as reducing agents. The results are illustrated in Figure 4. From the data, the addition of formic acid was very effective for the photocatalytic reduction of Cr(VI) with nanosized TiO2 powders in an aqueous solution. On the other hand, the occurrence of ammonium ions may disturb the consumption of the hole in the valence band in TiO2 with the formate. Consequently, formic acid could be applied as the hole scavenger for the photocatalytic reduction of chromium (VI) with nanosized TiO2 powders in water.
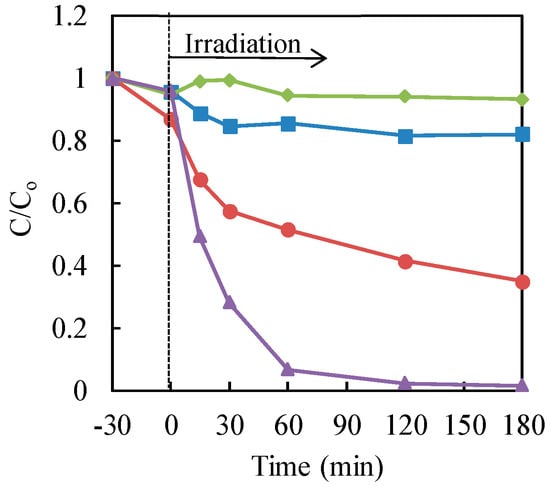
Figure 4.
Effect of the hole scavengers on the photocatalytic reduction of Cr(VI) in aqueous solution with P25 TiO2. Cr(VI) sample: 30 μg·mL−1 (30 mL); TiO2: 20 mg (0.67 mg·mL−1). Triangle (purple): TiO2 with formic acid (3000 μg·mL−1); circle (red): TiO2 with ammonium formate (3000 μg·mL−1); square (blue): formic acid (3000 μg·mL−1) only; diamond (green): ammonium formate (3000 μg·mL−1) only.
3.3. Effect of Commercial TiO2 Type
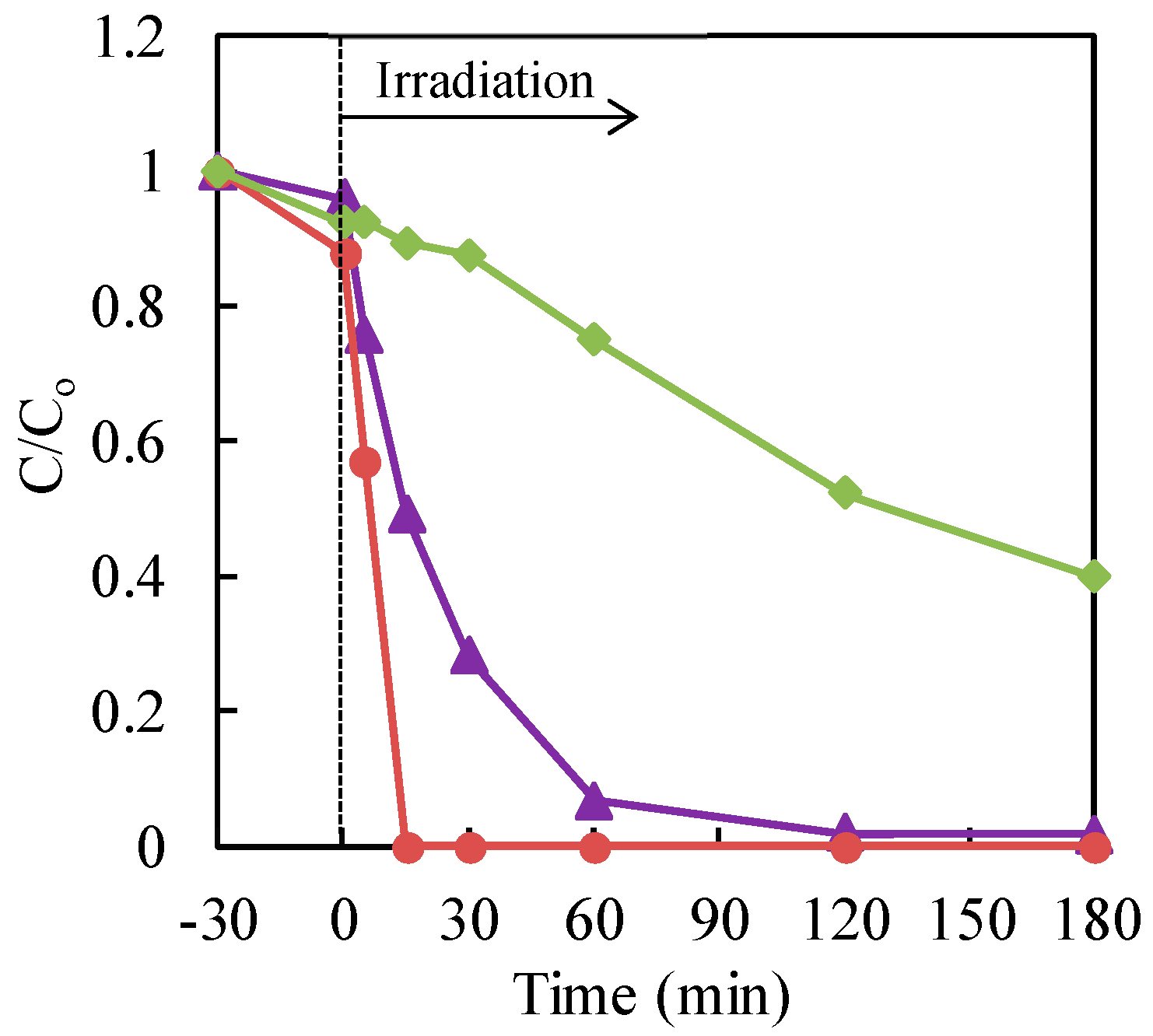
The effect of different commercial TiO2 on the photocatalytic treatment of Cr(VI) with TiO2 nanoparticles in an aqueous solution, in the presence of a formic acid hole scavenger, was studied. The commercial TiO2, AEROXIDE® P25, Ishihara Sangyo ST-01 and FUJIFILM Wako Pure Chemical Corp. were used for the evaluation of the photocatalytic activity. The data are shown in Figure 5. The maximum reduction rate of chromium (VI) was obtained with P25 TiO2.
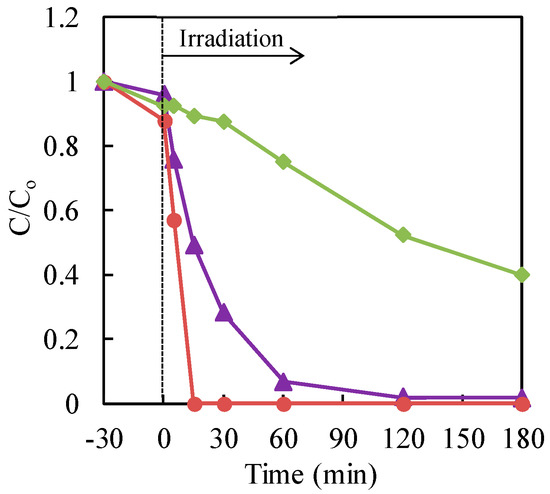
Figure 5.
Effect of different commercial TiO2 on the photocatalytic treatment of Cr(VI) with nanoparticles of TiO2 in an aqueous solution, in the presence of formic acid hole scavenger. Cr(VI) sample: 30 μg·mL−1 (30 mL); TiO2: 20 mg (0.67 mg·mL−1). Circle (red): Ishihara Sangyo ST-01; triangle (purple): AEROXIDE® P25TiO2; diamond (green): FUJIFILM Wako Pure Chemical Corp.
The relationship between the hexavalent chromium (VI) initial concentration Cr(VI) and initial reduction rate (r) can be explained by Langmuir-Hinshelwood model for the heterogeneous photocatalytic reduction process [21].
where k and K are the kinetic rate constant of the surface reaction and the Langmuir-Hinshelwood adsorption equilibrium constant, respectively. If 1 >> K[Cr(VI)], that is, the Cr(VI) concentration is very low, Equation (10) can simplify to the pseudo-first-order kinetic law [22].
where kobs is the pseudo-first-order rate constant (min−1).
The primary reduction reaction can be considered to follow pseudo-first-order kinetics, according to Equation (11). Integrating both sides in Equation (11) gives the following.
where [Cr(VI)]0 is the initial Cr(VI) concentration and t is the irradiation time.
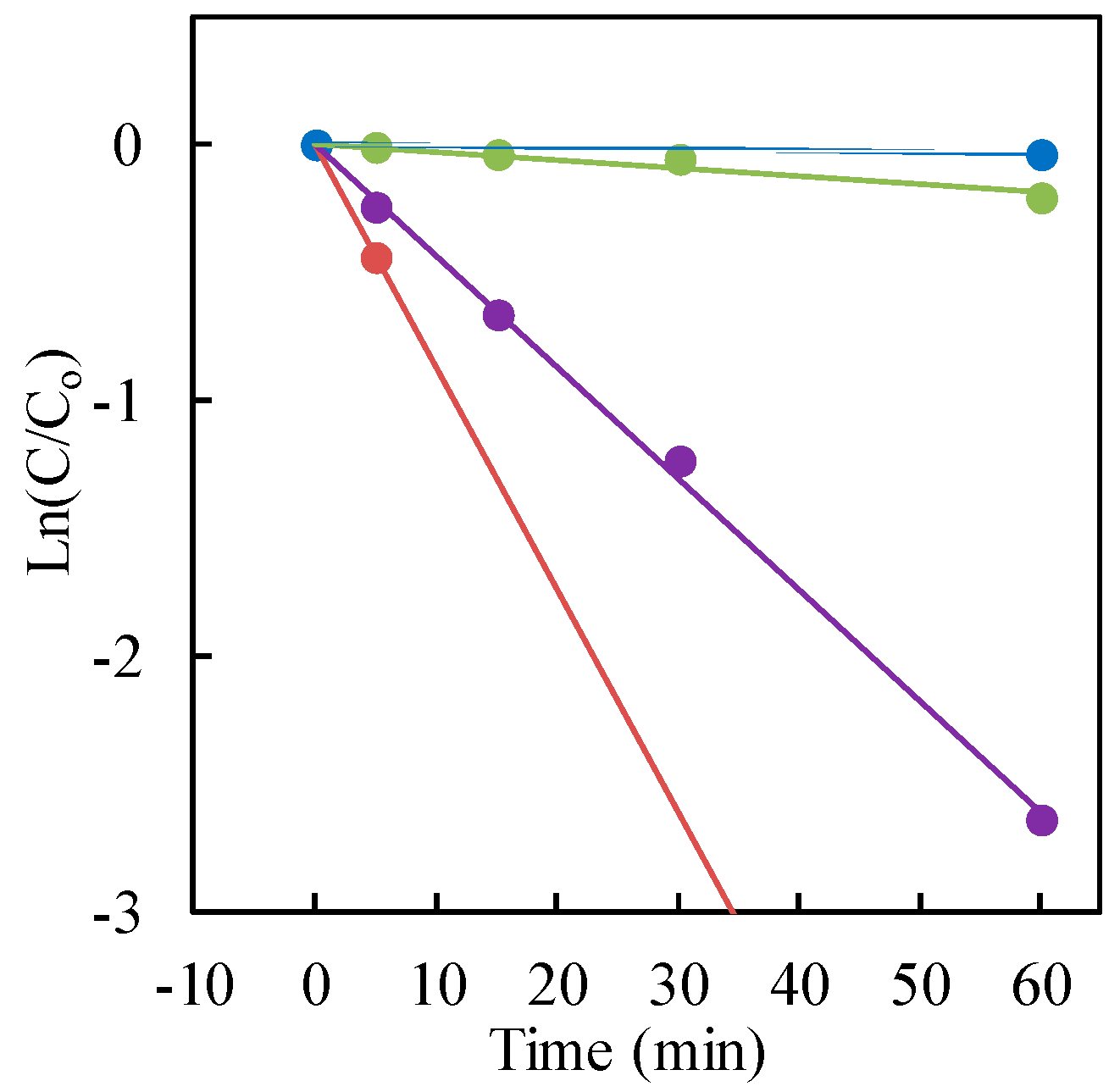
So as to confirm the speculation, −ln(C/C0) was plotted as a function of the treatment time (irradiation time). Because the liner relations were obtained in Figure 6 as expected, the reduction kinetics of Cr(VI) solution could follow pseudo-first-order kinetics, which was consistent with the Langmuir-Hinshelwood model, resulting from the low coverage in the experimental concentration range (30 μg·mL−1). The kinetic parameters containing the rate constant, surface area-normalized rate constant, correlation coefficient and substrate half-life are presented in Table 1.
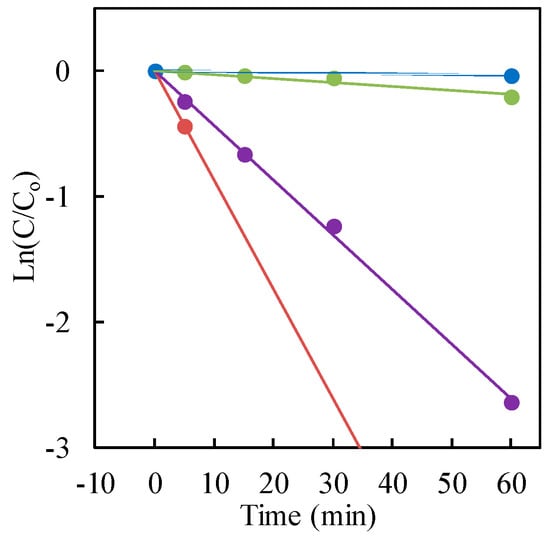
Figure 6.
−ln(C/C0) versus irradiation time. Cr(VI) sample: 30 μg·mL−1 (30 mL); TiO2: 20 mg (0.67 mg·mL−1). With formic acid hole scavenger: red for Ishihara Sangyo ST-01; purple for AEROXIDE® P25TiO2; green for FUJIFILM Wako Pure Chemical Corp. Without hole scavenger: blue for AEROXIDE® P25TiO2.

Table 1.
Kinetic parameters for the photocatalytic reduction of Cr(VI).
The maximum photocatalytic reduction rate for hexavalent chromium was observed with Ishihara Sangyo ST-01 nanosized TiO2 (mean particle size 7 nm). However, the highest rate constant, based on the specific surface area normalization, was obtained with AEROXIDE® P25 TiO2. Therefore, it was concluded from the surface area-normalized rate constant that the surface area of TiO2 can play a significant role in the photocatalytic activity for Cr(VI) reduction in the presence of a formic acid hole scavenger.
3.4. Reaction Mechanism
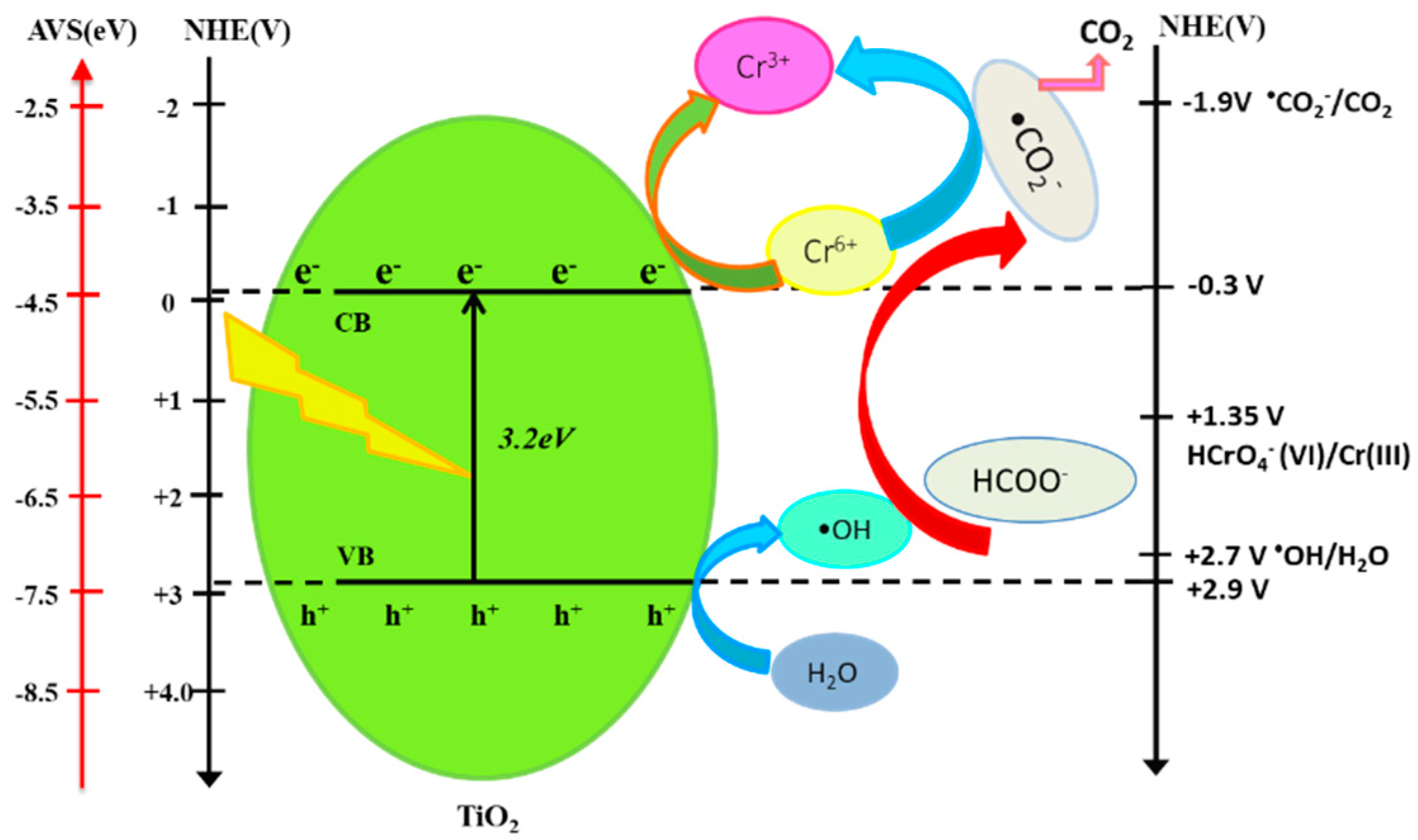
The proposed mechanism for the photocatalytic reduction of hexavalent chromium on nanosized TiO2 in the presence of formic acid is illustrated in Figure 7. The nanosized TiO2 with a bandgap of 3.2 eV can absorb the photons efficiently and be excited to form electrons in the conduction band (CB) and holes in the valance band (VB) under the UV irradiation.
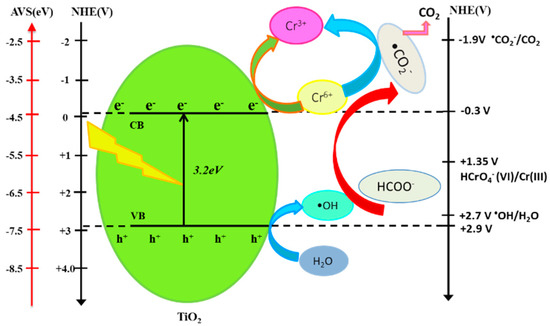
Figure 7.
Reaction mechanism for the photocatalytic reduction of Cr(VI) with TiO2 in the presence of formic acid.
Because the point of zero charge (pzc) of the TiO2 particle is approximately equal to six as TiIV–OH [23]. This means that, when the pH is lower than this value, the TiO2 surface becomes positively charged as TiIV–OH2+. From the estimation of Cr(VI) species as the function of pH, the main chemical species for hexavalent chromium at pH 3 is HCrO4−. Owing to the electrostatic attraction between HCrO4− species and nanosized TiO2 with a relatively large surface area, as well as the facilitation of the proton under acidic conditions, adsorbed Cr(VI) on the surface of TiO2 can be immediately reacted with electrons and be reduced to Cr(III). Because of the presence of formic acid, the hydroxyl radical (•OH), which is produced from the holes and OH−, is able to be captured by formic acid. This will produce reactive •CO2−, which has a relatively negative redox potential, E0(•CO2−/CO2) = −1.9 V vs. NHE [24,25], compared with the redox potential for Cr(VI), E0(HCrO4−/Cr3+) = 1.35 V vs. NHE [26].
TiO2 + hν → TiO2 (eCB− + hVB+)
H2O + hVB+ → •OH + H+
HCrO4− + 7H+ + 3eCB− → Cr3+ + 4H2O
HCOOH + •OH → •CO2− + H3O+
HCrO4− + •CO2− + 5H+ → Cr3+ + CO2 + 3H2O
In the present case, formic acid provided a multifunctional role in accelerating the separation of the holes and electrons, which was advantageous to the photocatalytic activity of nanosized TiO2 and produced reactive radicals (•CO2−). Hence, Cr(VI) can be reduced to trivalent chromium with nanosized TiO2 effectively. In the present work, the surface area of TiO2 may become an important factor in the photocatalytic activity for reducing hexavalent chromium in the presence of a formic acid hole scavenger.
4. Conclusions
The photocatalytic reduction of hexavalent chromium with nanosized TiO2 nanoparticles in the presence of formic acid was investigated. The addition of the hole scavenger, formic acid, was very effective for the enhanced photocatalytic reduction of Cr(VI) on nanosized TiO2 in an aqueous solution. Furthermore, the influence of different commercial TiO2 on the photocatalytic treatment of Cr(VI) with nanosized TiO2 in an aqueous solution, containing formic acid, was checked. As a consequence, the photocatalytic reduction of hexavalent chromium with Ishihara Sangyo ST-01 nanosized TiO2 gave better efficiencies, due to the specific surface area.
Author Contributions
J.B.I. and S.K. conceived and designed the experiments; J.B.I. performed the experiments and wrote the paper; I.T., M.F. and H.K. analyzed the results and advised the project.
Funding
The present research was partly supported by Grant-in-Aid for Scientific Research (C) 15K00602 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Acknowledgments
All experiments were conducted at Mie University. Any opinions, findings, conclusions or recommendations expressed in this paper are those of the authors and do not necessarily reflect the view of the supporting organizations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Korak, J.A.; Huggins, R.; Arias-Paic, P. Regeneration of Pilot-Scale Ion Exchange Columns for Hexavalent Chromium Removal. Water Res. 2017, 118, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Du, X.D.; Yi, X.H.; Wang, P.; Zheng, W.; Deng, J.; Wang, C.C. Robust photocatalytic Reduction of Cr(VI) on UiO-66-NH2(Zr/Hf) Metal-Organic Framework Membrane Under Sunlight Irradiation. Chem. Eng. J. 2019, 356, 393–399. [Google Scholar] [CrossRef]
- Siboni, M.S.; Farroki, M.; Soltani, R.D.C.; Khataee, A.; Tajassosi, S. Photocatalytic Reduction of Hexavalent Chromium over ZnO Nanorods Immobilized on Kaolin. Ind. Eng. Chem. Res. 2014, 53, 1079–1087. [Google Scholar] [CrossRef]
- Shih, Y.J.; Chen, C.W.; Hsia, K.F.; Dong, C.D. Granulation for Extended-Release of Nanoscale Zero-Valent Iron Exemplified by Hexavalent Chromium Reduction in Aqueous Solution. Sep. Purif. Technol. 2015, 156, 1073–1081. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Activated Carbons and Low Cost Adsorbents for Remediation of Tri- and Hexavalent Chromium from Water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U., Jr. Modeling and Evaluation of Chromium Remediation from Water Using Low Cost Bio-Char, a Green Adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef]
- Di Natale, F.; Erto, A.; Lancia, A.; Musmarra, D. Equilibrium and Dynamic Study on Hexavalent Chromium Adsorption onto Activated Carbon. J. Hazard. Mater. 2015, 281, 47–55. [Google Scholar] [CrossRef]
- Qi, H.; Wang, S.; Liu, H.; Gao, Y.; Wang, T.; Huang, Y. Synthesis of an Organic–Inorganic Polypyrrole/Titanium(IV) Biphosphate Hybrid for Cr(VI) Removal. J. Mol. Liq. 2016, 215, 402–409. [Google Scholar] [CrossRef]
- Joubani, M.N.; Siboni, M.S.; Yang, J.K.; Gholami, M.; Farzadkia, M. Photocatalytic Reduction of Hexavalent Chromium with Illuminated ZnO/TiO2 Composite. J. Ind. Eng. Chem. 2015, 22, 317–323. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, C.; Doudrick, K.; Chan, C.K. Hexavalent Chromium Removal Using Metal Oxide Photocatalysts. Appl. Catal. B 2015, 176–177, 740–748. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Deng, K.; She, Y.; Yu, Y.; Tang, H. Visible Light Photocatalytic Reduction of Cr(VI) on TiO2 in Situ Modified with Small Molecular Weight Organic Acids. Appl. Catal. B 2010, 95, 400–407. [Google Scholar] [CrossRef]
- Testa, J.J.; Grela, M.A.; Litter, M.I. Heterogeneous Photocatalytic Reduction of Chromium (VI) over TiO2 Particles in the Presence of Oxalate: Involvement of Cr(V) Species. Environ. Sci. Technol. 2004, 38, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Lee, S.M. Removal of Cr(VI) and Humic Acid by Using TiO2 Photocatalysis. Chemosphere 2006, 63, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. Visible Light Cr(VI) Reduction and Organic Chemical Oxidation by TiO2 Photocatalysis. Environ. Sci. Technol. 2005, 39, 6251–6259. [Google Scholar] [CrossRef]
- Kaneco, S.; Kurimoto, H.; Ohta, K.; Mizuno, T.; Saji, A. Photocatalytic Reduction of CO2 Using TiO2 Powders in Liquid CO2 Medium. J. Photochem. Photobiol. A Chem. 1997, 109, 59–63. [Google Scholar] [CrossRef]
- Szabó, M.; Kalmár, J.; Ditrói, T.; Bellér, G.; Lente, G.; Simic, N.; Fábián, I. Equilibria and Kinetics of Chromium(VI) Speciation in Aqueous Solution—A Comprehensive Study from pH 2 to 11. Inorg. Chim. Acta 2018, 472, 295–301. [Google Scholar] [CrossRef]
- Tandon, R.K.; Crisp, P.T.; Ellis, J.; Baker, R.S. Effect of pH on Chromium(VI) Species in Solution. Talanta 1984, 31, 227–228. [Google Scholar] [CrossRef]
- Gardner, M.; Comber, S. Determination of Trace Concentration of Hexavalent Chromium. Analyst 2002, 127, 153–156. [Google Scholar] [CrossRef]
- Duffy, G.; Maguire, I.; Heery, B.; Gers, P.; Ducrée, J.; Regan, F. ChromiSense: A Colourimetric Lab-on-a-Disc Sensor for Chromium Speciation in Water. Talanta 2018, 178, 392–399. [Google Scholar] [CrossRef]
- Yang, J.K.; Lee, S.M.; Siboni, M.S. Effect of Different Types of Organic Compounds on the Photocatalytic Reduction of Cr(VI). Environ. Technol. 2012, 33, 2027–2032. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.E. Photocatalytic Degradation of Organic Water Contaminants: Mechanisms Involving Hydroxyl Radical Attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Sofi, F.A.; Majid, K.; Mehraj, O. The Visible Light Driven Copper Based Metal-Organic-Framework Heterojunction:HKUST-1@Ag-Ag3PO4 for Plasmon Enhanced Visible Light Photocatalysis. J. Alloys Compd. 2018, 737, 798–808. [Google Scholar] [CrossRef]
- Samad, A.; Ahsan, S.; Tateshi, T.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Indirect Photocatalytic Reduction of Arsenate to Arsenite in Aqueous Solution with TiO2 in the Presence of Hole Scavengers. Chin. J. Chem. Eng. 2018, 26, 529–533. [Google Scholar] [CrossRef]
- Shaban, Y.A.; Maradny, A.A.E.; Farawati, R.K.A. Photocatalytic Reduction of Nitrate in Seawater Using C/TiO2 nanoparticles. J. Photochem. Photobiol. A Chem. 2016, 328, 114–121. [Google Scholar] [CrossRef]
- Xu, Q.; Li, R.; Wang, C.; Yuan, D. Visible-Light Photocatalytic Reduction of Cr(VI) Using Nano-Sized Delafossite (CuFeO2) Synthesized by Hydrothermal Method. J. Alloys Compd. 2017, 723, 441–447. [Google Scholar] [CrossRef]
- Wang, L.; Lia, X.; Tenga, W.; Zhaoa, Q.; Shi, Y.; Yue, R.; Chen, Y. Efficient Photocatalytic Reduction of Aqueous Cr(VI) over Flower-like SnIn4S8 Microspheres under Visible Light Illumination. J. Hazard. Mater. 2013, 244–245, 681–688. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).