Prediction of Excess Enthalpy Using Volume-Translated Peng–Robinson Equation of State
Abstract
1. Introduction
- One weakness of SRK EOS is that often inaccurate results are obtained for liquid densities. Therefore, the same phenomenon can be stated for PSRK EOS, too.
- Unsatisfactory results can be observed in many cases for activity coefficients at infinite dilution, heats of mixing, and for strongly asymmetric systems. This can be explained by the fact that the UNIFAC model, used in the mixing rule, is not able to predict those properties accurately.
2. Calculation of Excess Enthalpy Using VTPR EOS
- For the calculation of parameter a, which accounts for attractive forces, a modified function (so-called Twu- function) is used (pure component i; see Equations (2) and (3). A proper description of the sub- and supercritical range is possible with the help of adjustable parameters L, M, N, and the usage of critical temperature () and pressure (). Compared with Mathias–Copeman- function, used in PSRK EOS, results are more reliable at high reduced temperatures [2,3]. The good applicability is also shown in recent publications; the authors of [15] could successfully model catalytic hydrogenation processes by modification of original Peng–Robinson EOS with Twu- function. For binary mixtures a new mixing rule leads to improved prediction accuracies (Formula (4)) [1,5,10]. The parameter z stands for the mole ratio of component i.
- In order to get a better description for asymmetric systems parameter b, which accounts for repulsive forces, is not calculated by a linear mixing rule (binary mixture). Instead, a quadratic mixing rule as shown in Equation (5) is used. For pure components the equation is similar to original PR EOS (Formula (6)) [5,10].
- With the introduction of parameter c a volume translation is implemented. Together with the usage of PR instead of SRK EOS, this leads to improved results for liquid densities. The volume translation parameter c for pure component i can be calculated on the basis of the difference between experimental density and the density calculated with PR EOS () at the reference temperature as shown in Equation (7) (temperature-independent volume translation) [1,7]. For all the compounds mentioned in this article, c values published by [1] were used. If binary mixtures are regarded, a simple linear mixing rule can be used (Equation (8)) [10].
3. Results
- The main m-file that executes the program contains the basic information needed for the calculations (name of compounds, temperature, pressure) as well as experimental data of the system. The first mentioned information is passed to the excess enthalpy function.
- Calculations of excess enthalpy are performed in a function stored as separate file. It interacts not only with the main m-file but also with other functions described in the next point.
- In order to improve the clarity of the Matlab program, additional functions were written to read necessary data out of an Excel file, to calculate mixing parameters for pure components and the mixture, to carry out calculations of the part, and to differentiate the part with respect to temperature.
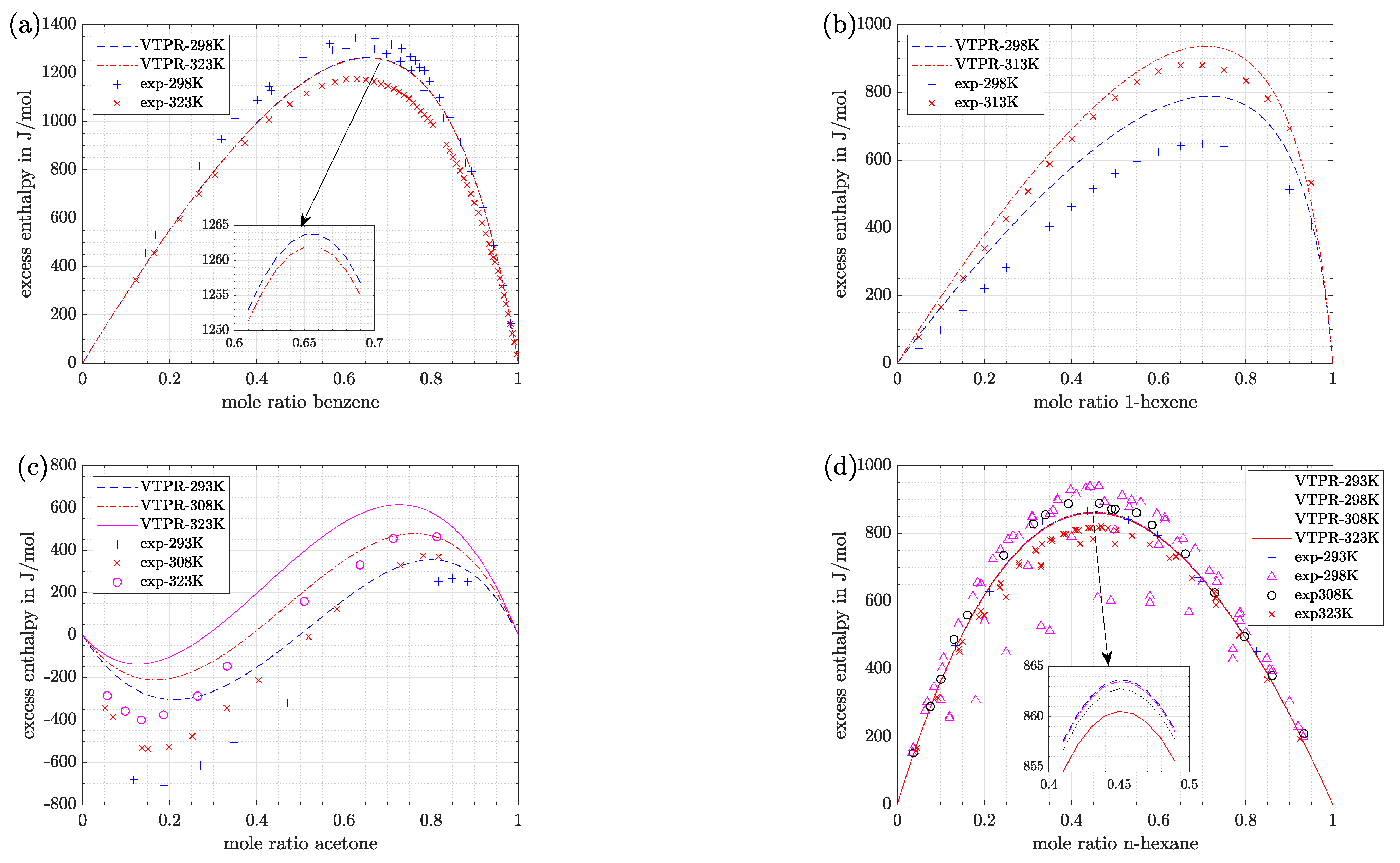
- For the model system n-hexadecane/benzene (Figure 1a) predictions with VTPR EOS are nearly independent of temperature, although experimental data clearly differ at the selected temperatures. At low molar ratios of benzene a good fit can be stated when experimental data at 323.15 K are regarded, while at higher molar ratios the fit is better for a temperature of 298.15 K.
- For the model system 1-hexene/n-BuOH (Figure 1b) predictions are better for a temperature of 313.15 K. Although this time the predicted results show a temperature dependence at 298.15 K, a large gap between predicted and experimental data has to be stated.
- For the model system acetone/water (Figure 1c) deviations between experimental data and predicted values are rather big at all temperatures. Again, a temperature dependence can be stated for experimental as well as predicted values.
- For the model system n-hexane/benzene (Figure 1d) predicted results are nearly independent of temperature. Predictions are best for a temperature of 293.15 K. However, it is visible that experimental data itself show big fluctuations, especially at 298.15 K. This circumstance will be further discussed in the next section.
4. Discussion
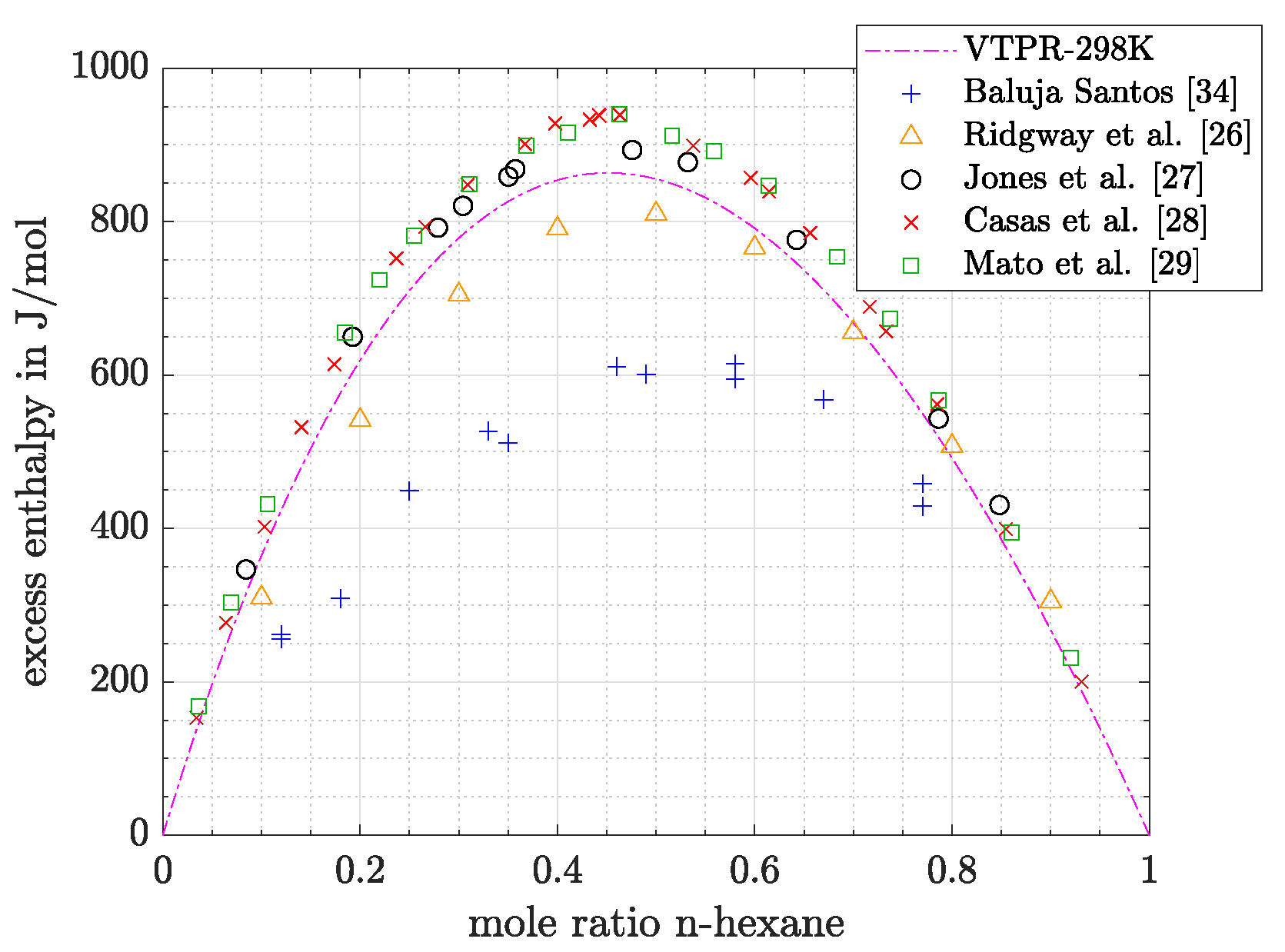
- Slightly lower values for excess enthalpy are measured by Jones et al. [27]. A Brass calorimeter was used. Errors of this apparature were estimated to be below 2% for aqueous/alcohol solutions [30]. Jones et al. [27] compared their results graphically with those obtained by Prigogine et al. [31] and Schnaible et al. [32]. Good agreement between the three publications could be stated at molar ratios of benzene above 0.5, while at lower molar ratios results of Schnaible et al. [32] and Progogine et al. [31] were higher. Finally, excess enthalpies measured by Romani et al. [33] were in the same range as the results of Jones et al. [27], following descriptions of Mato et al. [29].
- Significantly lower values were measured by Ridgway et al. [26] and Baluja Santos [34]. The authors of the first mentioned publication measured their data in a Dewar vessel and state an accuracy around 1% [26]. According to the graphical presentation of Mato et al. [29], Andrade et al. [35] also obtained maximum values for excess enthalpy, which are comparable to those of Ridgway et al. [26].
- Again, it can be seen that the accuracy of predictions was temperature-dependent. Especially for the system 1-hexene/n-BuOH (absolute error 2.5 times higher at 298.15 K compared with the value at 313.15 K) the difference was pronounced for the temperatures which were investigated. However, it has to be noted that the deviations at 313.15 K were low (8.2% or 38 J/mol).
- For the system n-hexane/benzene the same temperature dependence can be stated, although differences were low at all temperatures shown in Figure 5 (<10% or <25 J/mol). For measurements at 298.15 K literature data were already discussed with the help of Figure 4. If the dataset of Baluja Santos [34] is not regarded, relative as well as absolute mean errors are close to those obtained at 323.15 K or slightly higher.
- The other alkane/benzene system (n-hexadecane/benzene) shows slightly higher error values (12–20% or 52–73 J/mol). For both systems it is striking that predictions of VTPR EOS were nearly temperature-independent (Figure 1a,d). An explanation can be given when the main group interaction parameters are regarded (see Table 2; main group 1-CH2 and 3-ACH are relevant). For those mixtures no energy parameter c was determined, which causes the temperature dependence of the derivative of to be missing (see also derivation of temperature derivation described in Section 2 and especially Formulas (24) and (25)).
- Predictions exhibit the greatest deviations for the most polar system acetone/water, which is in agreement with the conclusion drawn based on Figure 1. The high relative and absolute errors (>50% and >200 J/mol) confirm that care must be taken if such a system is regarded. However, it can be said that with VTPR EOS still a tremendous improvement could be achieved compared to previous models. To underline this statement, the results for excess enthalpy of acetone/water system are shown for PSRK EOS in Figure 6.
- Schmid [1] gives relative deviations for different group combinations using VTPR, PSRK, and the modified UNIFAC (Dortmund) model. For the combination HO/CHCO a value of ∼20% is given for the modified UNIFAC (Dortmund) and a value of ∼25% is given for VTPR EOS. However, no information on how these deviations were calculated or what data were used is described.
- Hayashi et al. [36] evaluated predictions of excess enthalpy data using the ASOG GC model. For 51% of 871 binary data sets the maximum absolute deviation is below 100 J/mol, but also for 21.8% a maximum deviation >300 J/mol was stated. For the predictions presented in this article, maximum absolute deviations were for 5 of 11 datasets (system at one temperature taken as dataset) below 100 J/mol, for 3 datasets deviations were between 100 and 150 J/mol, and 3 datasets showed even higher deviations.
- Gmehling et al. [37] gives a mean deviation of 30% or 103.8 J/mol from investigations of 6000 datasets using the modified UNIFAC model. Several authors describe that accuracy of predictions with VTPR EOS is comparable with the modified UNIFAC (Dortmund) model [1,2,3,12]. In the present work only mean deviations of acetone/water system were higher than 30%.
- Vigh et al. [38] gives deviations for excess enthalpy data predicted with the modified UNIFAC models (Lungby and Dortmund). For the system n-hexane/benzene a value 7.6% is given for a temperature range between 25 and 45 C, which is close to the predictions presented in this article if the results of Baluja Santos [34] are neglected. For the system acetone/water a relative deviation of 30.6% (25–35 C) was observed, but maximum experimental excess enthalpy was taken as reference value for all datapoints used for the calculation of relative errors. If this modification is applied to the datasets that were presented beforehand, a good agreement with the literature can be confirmed.
- Chen et al. [39] investigated deviations for the modified UNIFAC (Dortmund) model and PSRK model. For alkane/benzene systems a mean deviation of 66 J/mol or 6.6% is given for the modified UNIFAC (Dortmund) model, while for PSRK EOS clearly higher errors are mentioned (454 J/mol or 43%). If an accuracy close to modified UNIFAC (Dortmund) model is expected, a good agreement can be stated with regard to the results presented in this article (results of Baluja Santos [34] neglected).
5. Conclusions
6. Software
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
| List of Symbols | |
| a | parameter volume-translated Peng–Robinson equation of state, dm bar· mol |
| interaction parameter UNIFAC model, K | |
| b | parameter volume-translated Peng–Robinson equation of state, dm mol |
| interaction parameter UNIFAC model, - | |
| C | constant used for calculation of temperature derivative of parameter a, - |
| c | parameter volume-translated Peng–Robinson equation of state, dm mol |
| interaction parameter UNIFAC model, K | |
| D | constant used for calculation of temperature derivative of parameter a, - |
| g | molar Gibbs energy, J · mol |
| h | molar enthalpy, J · mol |
| L | parameter Twu--function, - |
| M | parameter Twu--function, - |
| N | parameter Twu--function, - |
| P | pressure, bar |
| Q | group surface area UNIFAC model, - |
| R | universal gas constant, J · mol K |
| T | absolute temperature, K |
| / | interaction parameter UNIFAC model, J · mol |
| v | molar volume, dm mol |
| number main group k in component i, - | |
| X | group mole fraction UNIFAC model, - |
| z | compressibility factor, - |
| , | molar ratio of component i/j, - |
| Greek Symbols | |
| temperature-dependent function to calculate parameter a of volume-translated | |
| Peng–Robinson equation of state | |
| activity coefficient | |
| group activity coefficient | |
| surface area fraction UNIFAC model | |
| temperature-dependent interaction parameter UNIFAC model | |
| Abbreviations | |
| AMAD | arithmetic mean absolute deviation |
| AMRD | arithmetic mean relative deviation |
| ASOG | Analytical Solutions of Groups |
| BuOH | butanol |
| EOS | equation of state |
| GC | group contribution |
| mod | modified |
| p | primary |
| PR | Peng–Robinson |
| PSRK | predictive Soave–Redlich–Kwong |
| SRK | Soave–Redlich–Kwong |
| UNIFAC | universial quasichemical theory functional group activity coefficients |
| VTPR | volume-translated Peng–Robinson |
| Superscripts | |
| E | excess |
| id | ideal |
| i | component i |
| Subscripts | |
| crit | critical |
| exp | experimental |
| i,j,k,m,n | component or functional group i/j/k/m/n |
| mix | mixture |
| P | constant pressure |
| PR | Peng–Robinson |
| pred | predicted |
| red | reduced |
| ref | reference |
| res | residual |
| T | constant temperature |
| constant molar ratio of component i |
References
- Schmid, B. Einsatz Einer Modernen Gruppenbeitragszustandsgleichung für die Synthese Thermischer Trennprozesse. Ph.D. Thesis, Carl von Ossietzky University, Oldenburg, Germany, 2011. [Google Scholar]
- Gmehling, J.; Constantinescu, D.; Schmid, B. Group Contribution Methods for Phase Equilibrium Calculations. Annu. Rev. Chem. Biomol. Eng. 2015, 6, 267–292. [Google Scholar] [CrossRef]
- Gmehling, J. Present status and potential of group contribution methods for process development. J. Chem. Thermodyn. 2009, 41, 731–747. [Google Scholar] [CrossRef]
- Fredenslund, A.; Jones, R.L.; Prausnitz, J.M. Group-contribution estimation of activity coefficients in nonideal liquid mixtures. Am. Inst. Chem. Eng. J. 1975, 21, 1086–1099. [Google Scholar] [CrossRef]
- Gmehling, J.; Kleiber, M.; Kolbe, B.; Rarey, J. Chemical Thermodynamics for Process Simulation, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019. [Google Scholar]
- Holderbaum, T.; Gmehling, J. PSRK: A Group Contribution Equation of State Based on UNIFAC. Fluid Phase Equilibria 1991, 70, 251–265. [Google Scholar] [CrossRef]
- Ahlers, J.; Gmehling, J. Development of an universal group contribution equation of state: I. Prediction of liquid densities for pure compounds with a volume translated Peng—Robinson equation of state. Fluid Phase Equilibria 2001, 191, 177–188. [Google Scholar] [CrossRef]
- Atkins, P.W.; de Paula, J. Physikalische Chemie, 5th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 175–177. [Google Scholar]
- Stephan, P.; Schaber, K.; Stephan, K.; Mayinger, F. Thermodynamik- Grundlagen und Technische Anwendungen Band 2: Mehrstoffsysteme und Chemische Reaktionen, 15th ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 137–147. [Google Scholar]
- Ahlers, J.; Gmehling, J. Development of a Universal Group Contribution Equation of State. 2. Prediction of Vapor-Liquid Equilibria for Asymmetric Systems. Ind. Eng. Chem. Res. 2002, 41, 3489–3498. [Google Scholar] [CrossRef]
- Gmehling, J. Potential of group contribution methods for the prediction of phase equilibria and excess properties of complex mixtures. Pure Appl. Chem. 2003, 75, 875–888. [Google Scholar] [CrossRef]
- Schmid, B.; Schedemann, A.; Gmehling, J. Extension of the VTPR Group Contribution Equation of State: Group Interaction Parameters for Additional 192 Group Combinations and Typical Results. Ind. Eng. Chem. Res. 2014, 53, 3393–3405. [Google Scholar] [CrossRef]
- Schmid, B.; Gmehling, J. Present status of the group contribution equation of state VTPR and typical applications for process development. Fluid Phase Equilibria 2016, 425, 443–450. [Google Scholar] [CrossRef]
- Ahlers, J.; Gmehling, J. Development of a Universal Group Contribution Equation of State III. Prediction of Vapor-Liquid Equilibria, Excess Enthalpies, and Activity Coefficients at Infinite Dilution with the VTPR Model. Ind. Eng. Chem. Res. 2002, 41, 5890–5899. [Google Scholar] [CrossRef]
- Nikolaos, C.K. Modeling and simulation of biphasic catalytic hydrogenation of a hydroformylated fuel. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Schmid, B.; Gmehling, J. Revised parameters and typical results of the VTPR group contribution equation of state. Fluid Phase Equilibria 2012, 317, 110–126. [Google Scholar] [CrossRef]
- Fermeglia, M.; Kikic, I. Excess enthalpy calculations by means of equations of state. J. Therm. Anal. 1984, 29, 687–695. [Google Scholar] [CrossRef]
- Weidlich, U.; Gmehling, J. A modified UNIFAC model. 1. Prediction of VLE, hE, and γ∞. Ind. Eng. Chem. Res. 1987, 26, 1372–1381. [Google Scholar] [CrossRef]
- Horstmann, S.; Jabłoniec, A.; Krafczyk, J.; Fischer, K.; Gmehling, J. PSRK group contribution equation of state: Comprehensive revision and extension IV, including critical constants. Fluid Phase Equilibria 2005, 227, 157–164. [Google Scholar] [CrossRef]
- Peña, M.D.; Menduiña, C. Excess enthalpies at 298.15 K of binary mixtures of benzene with n-alkanes. J. Chem. Thermodyn. 1974, 6, 387–393. [Google Scholar]
- Peña, M.D.; Menduiña, C. Excess enthalpies at 323.15 K of binary mixtures of benzene with n-alkanes. J. Chem. Thermodyn. 1974, 6, 1097–1102. [Google Scholar]
- Lundberg, G.W. Thermodynamics of Solutions XI. Heats of Mixing of Hydrocarbons. J. Chem. Eng. Data 1964, 9, 193–198. [Google Scholar] [CrossRef]
- Aguilar, F.; Alaoui, F.E.M.; Segovia, J.J.; Villamañán, M.A.; Montero, E.A. Ether + alcohol + hydrocarbon mixtures in fuels and bio-fuels: Excess enthalpies of binary mixtures containing dibutyl ether (DBE) or 1-butanol and 1-hexene or methylcyclohexane or toluene or cyclohexane or 2,2,4-trimethylpentane at 298.15 K and 313.15 K. Fluid Phase Equilibria 2012, 315, 1–8. [Google Scholar] [CrossRef]
- Excess Enthalpy Data-Enthalpy of Mixing Data Set 1407. Available online: http://www.ddbst.com/en/EED/HE/HE%20Acetone%3BWater.php (accessed on 8 December 2020).
- Excess Enthalpy Data-Enthalpy of Mixing Data Set 2761. Available online: ttp://www.ddbst.com/en/EED/HE/HE%20Benzene%3BHexane.php (accessed on 8 December 2020).
- Ridgway, K.; Butler, P.A. Some Physical Properties of the Ternary System Benzene-Cyclohexane-n-Hexane. J. Chem. Eng. Data 1967, 12, 509–515. [Google Scholar] [CrossRef]
- Jones, H.K.; De, Q.; Lu, B.C.Y. Heats of Mixing of Liquids for the System Ethanol-Benzene-n-Hexane. J. Chem. Eng. Data 1966, 11, 488–492. [Google Scholar] [CrossRef]
- Casas, H.; Segade, L.; Franjo, C.; Jiménez, E.; Paz Andrade, M.I. Excess Molar Enthalpies of Propyl Propanoate + Hexane + Benzene at 298.15 K and 308.15 K. J. Chem. Eng. Data 2000, 45, 445–449. [Google Scholar] [CrossRef]
- Mato, M.M.; Balseiro, J.; Jiménez, E.; Legido, J.L.; Galiñanes, A.V.; Paz Andrade, M.I. Excess Molar Enthalpies and Excess Molar Volumes of the Ternary System 1,2-Dichlorobenzene + Benzene + Hexane at 298.15 K. J. Chem. Eng. Data 2002, 47, 1436–1441. [Google Scholar] [CrossRef]
- Lama, R.F.; Lu, B.C.-Y. Excess Thermodynamic Properties of Aqueous Alcohol Solutions. J. Chem. Eng. Data 1965, 10, 216–219. [Google Scholar] [CrossRef]
- Prigogine, I.; Mathot, V. Application of the Cell Method to the Statistical Thermodynamics of Solutions. J. Chem. Phys. 1952, 20, 49–57. [Google Scholar] [CrossRef]
- Schnaible, H.W.; Van Ness, H.C.; Smith, J.M. Heats of mixing of liquids. AIChE J. 1957, 3, 147–152. [Google Scholar] [CrossRef]
- Romani, L.; Paz-Andrade, M.I. Funciones termodinamicas de exceso a 25. C. III. Benceno + isoomeros del hexano. An. Quim. 1974, 70, 422–425. [Google Scholar]
- Baluja Santos, M.D.C. Aplicacion al estudio de sistemas binarios y ternarios. Acta Cient. Compostel. 1970, 70, 3–15. [Google Scholar]
- Paz-Andrade, M.I.; Regueiro, M. Entalpias de mezcla a temperaturas mediassistema hexanobenceno. Acta Cient. Compostel. 1970, 3–4, 147–152. [Google Scholar]
- Hayashi, H.; Tochigi, K.; Kojima, K. Prediction of excess enthalpy by using thirty-one ASOG groups. Ind. Eng. Chem. Res. 1992, 31, 2795–2804. [Google Scholar] [CrossRef]
- Gmehling, J. From UNIFAC to modified UNIFAC to PSRK with the help of DDB. Fluid Phase Equilibria 1995, 107, 1–29. [Google Scholar] [CrossRef]
- Vigh, L.; Kojima, K. Prediction of Excess Enthalpy and Excess Entropy Using Modified UNIFAC Group Contribution Methods. J. Chem. Eng. Jpn. 1996, 29, 881–884. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Fischer, K.; Gmehling, J. Modification of PSRK mixing rules and results for vapor–liquid equilibria, enthalpy of mixing and activity coefficients at infinite dilution. Fluid Phase Equilibria 2002, 200, 411–429. [Google Scholar] [CrossRef]




| Source | Model System for Which Values Are Presented at Different Temperatures | Evaluation of the Quality of Predictions Based on Presented Graphical Results |
|---|---|---|
| [1] | CO/cyclohexane | small deviations at highest temperature (553.15 K), bigger deviations at lowest temperature (358.15 K) |
| [3] | pentane/acetone | smallest deviations at medium temperature (363 K); bigger deviations at lowest and highest temperature (253/413 K) |
| [10,11] | CO/ethane | smaller deviations at lowest temperature (272 K) and higher molar ratios of ethane |
| [11] | acetone/n-hexane | excellent agreement at higher temperatures (293, 413 K), but bad prediction at lowest temperature (243 K) |
| [12] | ethane/propane | smallest deviations at highest temperature (363.15 K); biggest deviations at lowest temperature (323.15 K) |
| [13] | n-butylmercaptane/toluene | good agreement at all temperatures (283.15, 298.15, 333.15 K); smallest deviations at lowest temperature |
| [14] | cyclohexane/benzene | biggest deviations at lowest temperature (280 K); only small deviations at higher temperatures (323, 363 413 K) |
| [14] | acetone/n-heptane | small deviations at lowest temperature (283 K); bigger deviations at highest temperature (343 K) |
| Maingroup n | Maingroup m | ||||||
|---|---|---|---|---|---|---|---|
| 1(CH2) | 2(C=C) | 171 | −0.04322 | 0 | −88 | −0.05441 | 0 |
| 3(ACH) | 54.2589 | 0.2882 | 0 | 35.4832 | −0.36933 | 0 | |
| 5(OH,p) | 1809.53 | −0.48557 | −0.00232 | 726 | −0.90505 | 0.003154 | |
| 7(H2O) | 2096.92 | −1.65651 | 0.590013 | 56.5882 | 0.588307 | 0.000447 | |
| 9(CH2CO) | 425.312 | 0.68787 | −0.00031 | 284.25 | −1.77309 | 0.001636 | |
| 2(C=C) | 5(OH,p) | 756 | 1 | −0.00193 | 2049.89 | 6 | −0.00133 |
| 7(H2O) | 9(CH2CO) | −314.913 | 0.569307 | 0.002669 | 577.455 | −1.11835 | −0.00062 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köhn, C.; Kanzler, U.; Reichert, C.; Seyfang, B.C. Prediction of Excess Enthalpy Using Volume-Translated Peng–Robinson Equation of State. ChemEngineering 2021, 5, 8. https://doi.org/10.3390/chemengineering5010008
Köhn C, Kanzler U, Reichert C, Seyfang BC. Prediction of Excess Enthalpy Using Volume-Translated Peng–Robinson Equation of State. ChemEngineering. 2021; 5(1):8. https://doi.org/10.3390/chemengineering5010008
Chicago/Turabian StyleKöhn, Christian, Ulrike Kanzler, Christian Reichert, and Bernhard Christian Seyfang. 2021. "Prediction of Excess Enthalpy Using Volume-Translated Peng–Robinson Equation of State" ChemEngineering 5, no. 1: 8. https://doi.org/10.3390/chemengineering5010008
APA StyleKöhn, C., Kanzler, U., Reichert, C., & Seyfang, B. C. (2021). Prediction of Excess Enthalpy Using Volume-Translated Peng–Robinson Equation of State. ChemEngineering, 5(1), 8. https://doi.org/10.3390/chemengineering5010008





