Abstract
The main techniques used for organic pollutant removal from water are adsorption, reductive and oxidative processes, phytoremediation, bioremediation, separation by membranes and liquid–liquid extraction. In this review, strengths and weaknesses of the different purification techniques are discussed, with particular attention to the newest results published in the scientific literature. This study highlighted that adsorption is the most frequently used method for water purification, since it can balance high organic pollutants removal efficiency, it has the possibility to treat a large quantity of water in semi-continuous way and has acceptable costs.
1. Introduction
A large concern is growing in the world because of the environmental pollution. This phenomenon affects every dimension of the biosphere. In particular, water pollution is extremely worrying, since hydric resources are at the basis of life and of all human activities.
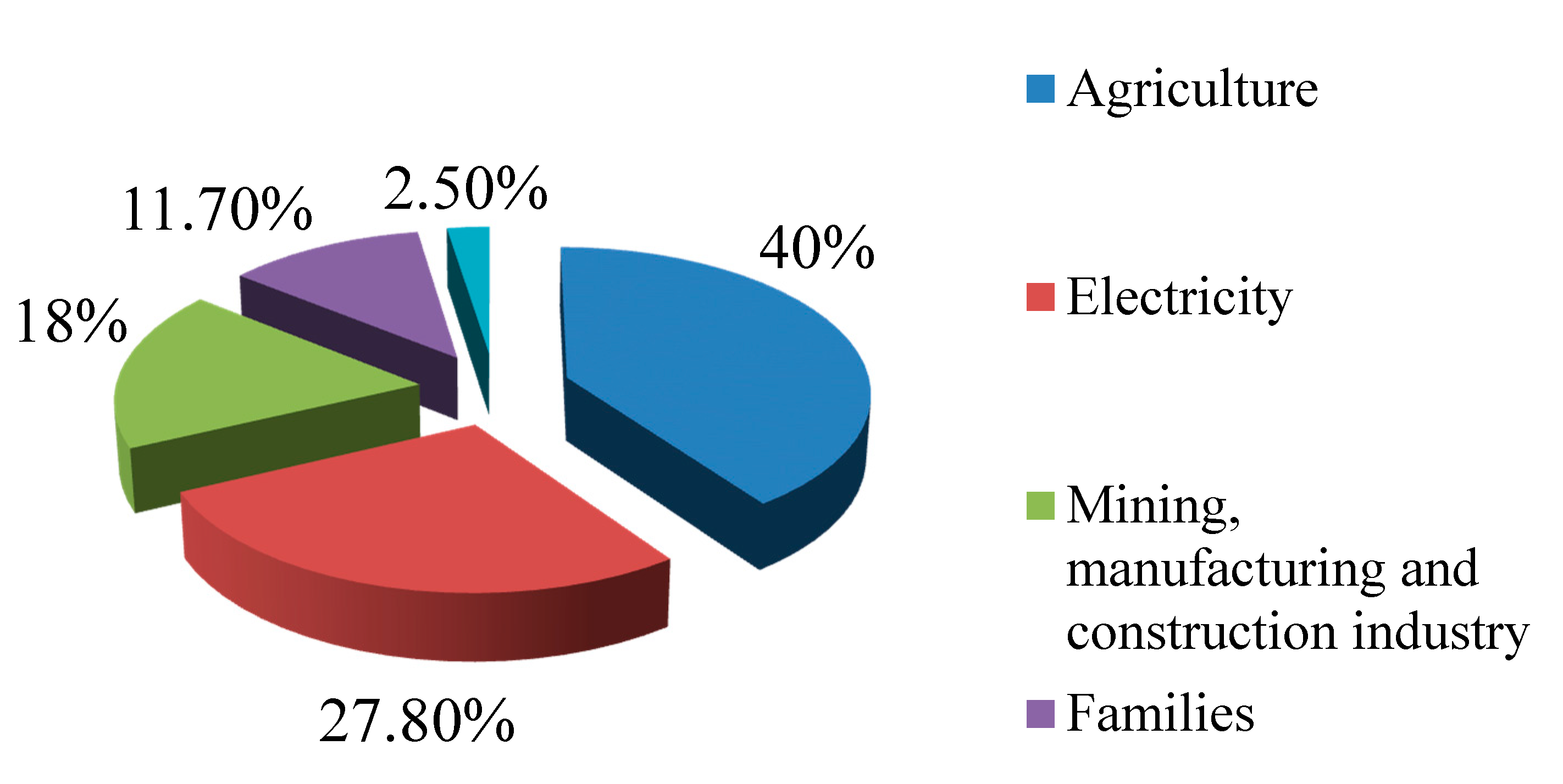
Data about the European water consumption of the main economic sectors in 2015, released by the European Environment Agency [1], are reported in the diagram in Figure 1.
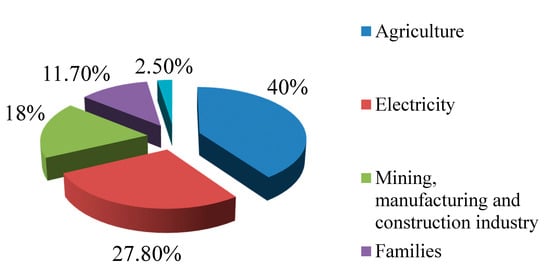
Figure 1.
European water consumption in 2015.
Figure 1 shows that the sector with the highest demand for water is agriculture (40% of the total consumption), followed by electricity generation (27.80%), mining, manufacturing and construction industry (18%), and then domestic consumption (11.70%) and the services sector (2.50%).
According to the European Environment Agency’s Water Exploitation Index (WEI), economic activities in Europe use around 243,000 cubic hectoliters of water per year [1]. Most of this water is then returned to the environment (over 140,000 cubic hectometers); however, it is often accompanied by impurities or pollutants, including dangerous chemicals, that can lead to serious consequences on ecosystems and human health [1]. Therefore, in recent years, a growing interest in the removal of pollutants from water has been detected.
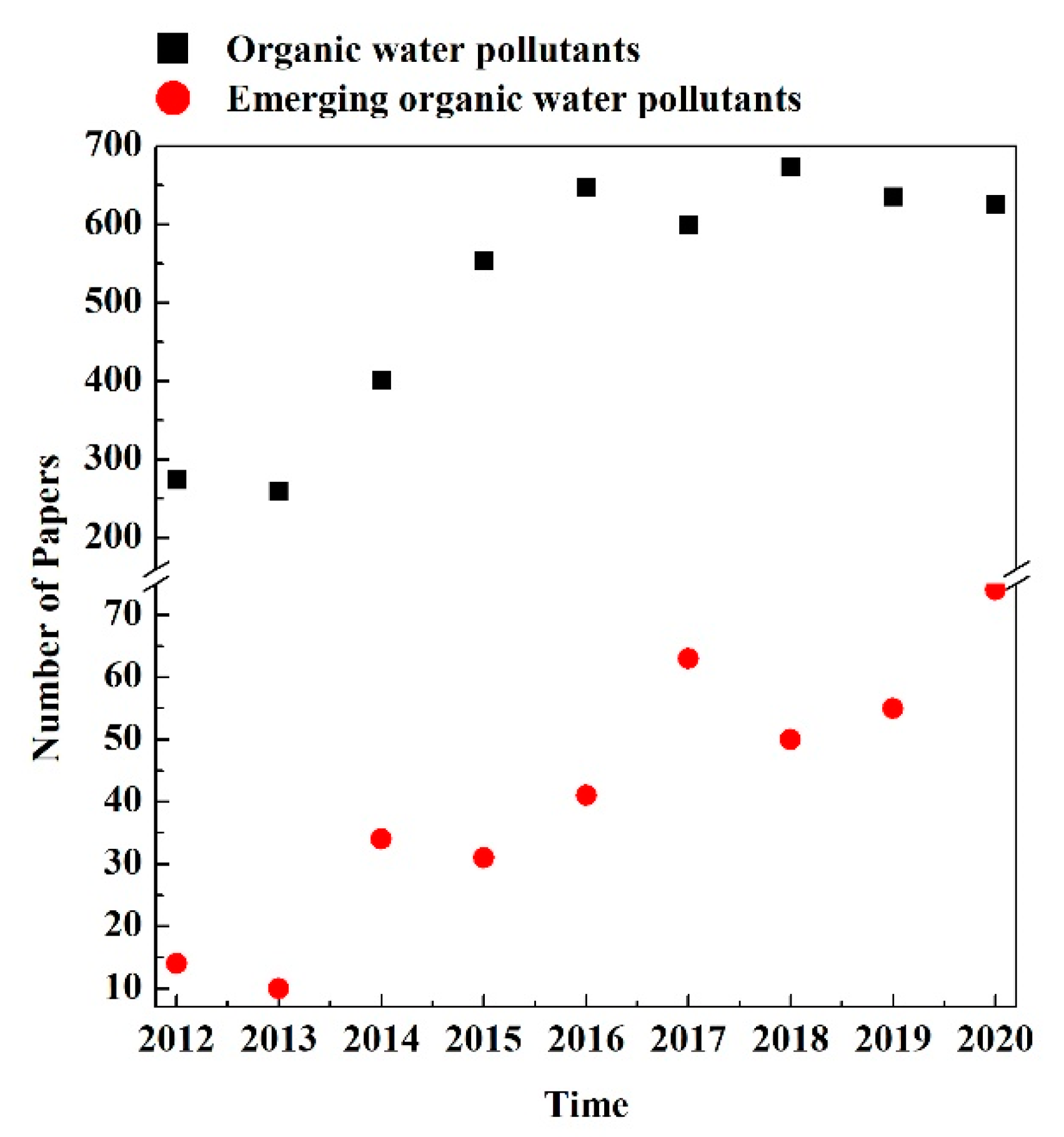
Figure 2 reports the number of papers published from 2012 to 2020 concerning the removal of classical and emerging organic pollutants from water. The reported values were collected from the database SCOPUS; in particular, the following keywords were used for the research:
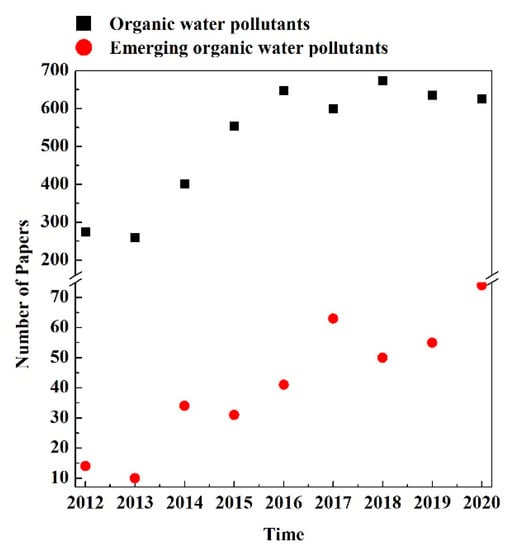
Figure 2.
Number of papers found in SCOPUS, published in the period of 2012–2020, concerning the removal of classical and emerging organic pollutants from water.
- Classical pollutants: “water purification”, “organic pollutants”
- Emerging pollutants: “water purification”, “emerging pollutants”.
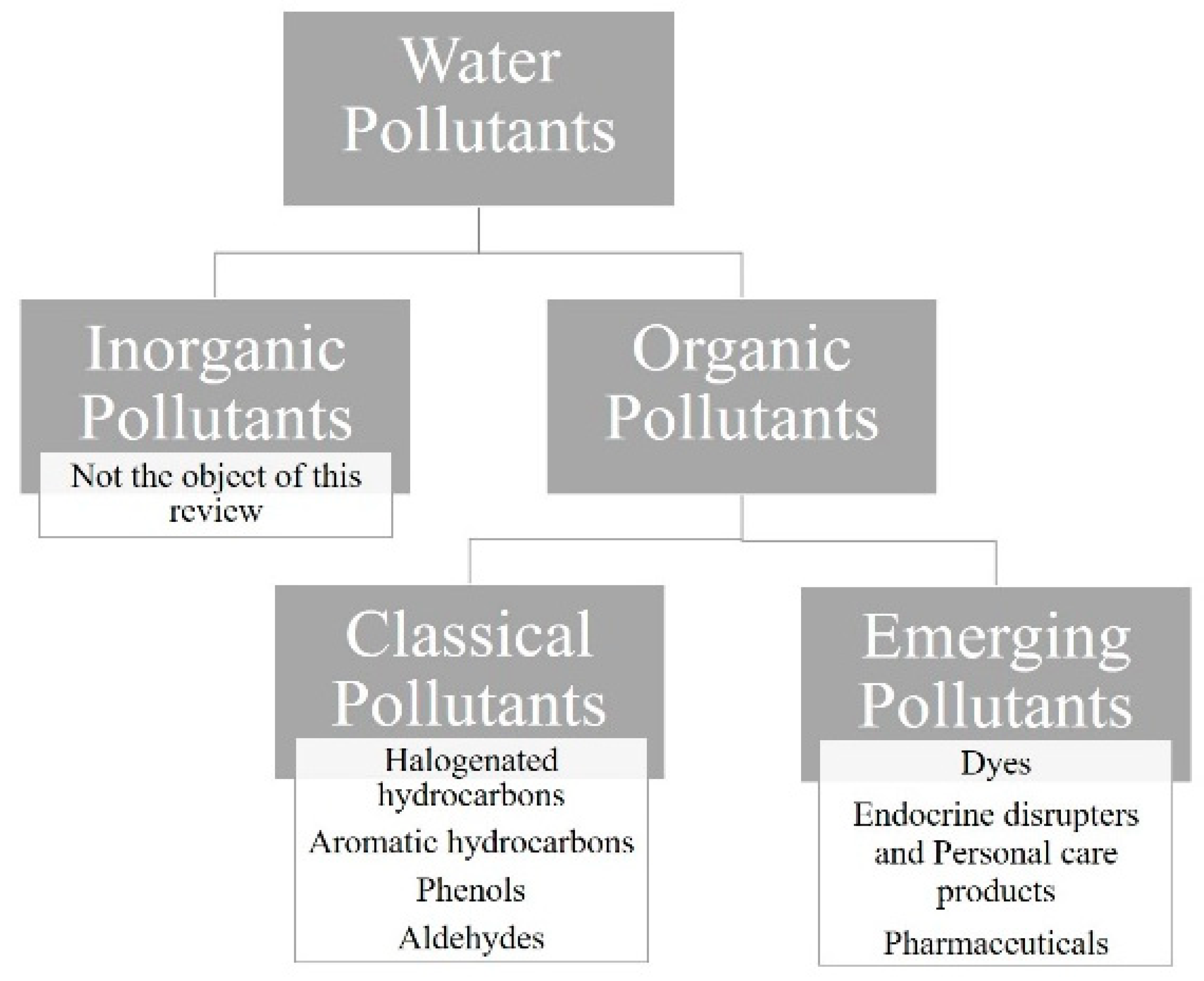
In this review, different categories of organic pollutants were identified, as outlined in Figure 3.
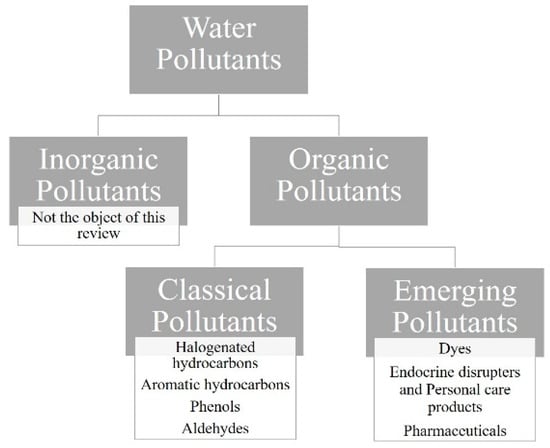
Figure 3.
Main categories of water pollutants.
There are several restrictions about the amount of these compounds that can be discharged by production processes. Moreover, legislation establishes the limit values of each potentially or certainly dangerous compound allowed in waters for human consumption. In Europe, the reference legislation for water is 2000/60/CE, with all its subsequent updates. Through this directive, the European Union organizes the management of inland waters, in particular, surface, underground, transition and coastal areas, to prevent and reduce pollution, to protect the environment and to improve the conditions of aquatic ecosystems. Presently, these restrictions only concern consolidated organic pollutants. Emerging water pollutants are known to be dangerous as well, but no official limits are still present.
The aim of this review is to collect the newest and innovative techniques used to remove classical and emerging organic pollutants from water. Strength and weakness of the different purification techniques are discussed, and their removal performance, tested on various pollutants, is summarized in the form of tables to favor a direct comparison for the reader.
Description of the Main Removal Techniques of Organic Pollutants from Water: Strengths and Weaknesses
The main techniques used for organic pollutant removal from water are adsorption, reductive and oxidative processes, phytoremediation, bioremediation, separation by membranes and liquid–liquid extraction.
Adsorption consists of the physical or chemical interaction between the surface of a solid (adsorbent) and a solute (adsorbate); in this case, the adsorbate is a pollutant in an aqueous solution. This technique allows the treatment of large quantities of water in a simple and compact way, at acceptable costs [2]. Since the removal mechanism takes place on the surfaces of the adsorbent, these materials are characterized by a high specific surface area. Therefore, both surface and pores of the adsorbent play a central role in the removal capacity of the pollutant. For example, activated carbons are generally selected to adsorb organic compounds, thanks to their considerable surface area and affinity towards this category of substances [3,4,5]. One of the advantages of adsorption is the possibility to regenerate and reuse the adsorbent material. For this purpose, a good candidate is a solid whose regeneration is easy and convenient. In general, this step is performed through a chemical or thermal process. There are several possibilities to carry out the first of these two regeneration options: ultrasonication in acetone [6] or in methanol, followed by several washes in deionized water [7]; immersion in ethanol [8], also combined with water [9]; and by using mixtures that include acid/basic solutions [10,11,12,13,14,15]. Thermal evaporation is generally carried out at 100 °C [16,17]. However, this kind of regeneration may damage the adsorbent in case of coated solids, resulting in a lower adsorption efficiency in the following cycles; for this reason, desorption at room temperature is preferred when chemicals with high vapor pressure are involved [17].
Reductive and oxidative processes are valid options to eliminate undesired compounds from water, thanks to their wide applicability, the possibility to completely remove polluting substances without producing other harmful compounds, and to their rapid reaction rates. However, these methods present some disadvantages, such as high costs, necessity to know exactly the pathway that will be followed during the water treatment and the presence of residual traces of reductive and oxidative agents that have to be removed, as in the case of hydrogen peroxide. Among the others, advanced oxidation processes (AOP) are essentially based on radical mechanisms, and O3, H2O2, UV, ultrasound, microwave, gamma-rays, and beam of accelerated electrons can be used as starters; catalysts or electrochemical reactions can also be involved [18]. Photocatalysis, for instance, uses catalysts that can create electron–hole pairs and generate, in this way, free radicals when light is absorbed. For this reason, semiconductor materials are employed. The most common reducing agents are zero valent iron, metallic magnesium in supercritical carbon dioxide, sodium in dry ammonia and sodium biphenyl [18].
Phytoremediation uses vegetable plants to remove pollutants from the environment; this is considered a “green technology” among the available depuration options. Moreover, it is a low-cost and safe method [19]. However, it shows some limits; for instance: long times are required for the depuration process, this technique can be used when pollution levels are low and the contaminated discharge has to be furtherly managed [20]. Therefore, the scientific community is looking for plants characterized by rapid absorption kinetics, with high resistance to the substances to remove and that can easily degrade or transform pollutants into inert or less harmful molecules [21].
Bioremediation is a technique that involves microorganisms to purify contaminated sites, since they can oxidize, immobilize or transform the polluting molecules. This method is appreciated for its low cost and because it is environmentally friendly [22,23]. Among the bioremediation processes, reductive dechlorination, by means of anaerobic bacteria, is the most investigated [23]. However, bioremediation presents some limitations, such as a slow dechlorination rate and long times required for the treatment [24].
Membranes are selective barriers that allow some substances to pass through them (permeate), leaving the other ones on the retentate side. They can be classified by looking at their surface chemistry, structure, morphology and production method; these aspects also determine the kind of molecules that will be able to pass through them. In descending order of pore size, it is possible to identify microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO) membranes [25]. Moreover, pore size distribution of the membranes and surface charge play a decisive role in pollutant rejection as well. Membranes also allow a high level of automation and do not require a relevant usage of chemicals [26]; however, at the same time, a considerable energy consumption can be involved, especially in those processes where pressure is the driving force and fouling phenomena occur. Moreover, attention has to be paid to the couple permeability–selectivity of the membrane [27].
Liquid–liquid extraction (LLE) allows the separation of the components of a mixture, taking advantage of their different solubilities into the extraction solvent. Because of this aspect, the correct choice of the solvent is essential to obtain a successful result. By using LLE, it is possible to obtain high yields and purity of the desired compound, operating with a simple method and at mild conditions [28]. However, the thermodynamics of the extraction process has to be carefully studied and, after processing, the separation of the pollutants from the extraction solvent has to be performed.
2. Removal of Classical Organic Pollutants from Water
The use of a specific technique for water purification cannot be considered as universally valid for all the organic micropollutants, due to the huge variety of compounds and the deep differences that characterize their behavior in water.
The most common depuration solutions consist of steps of air stripping [29,30], adsorption [16,31,32,33], and oxidative [34,35], reductive [36,37] and biological processes [21,38,39]. In some cases, a combination of several methods is required [40,41,42,43], especially when pollutants are particularly difficult to remove till the limits imposed by the law. Since each technique has its limits, researchers are investing lots of energy in this field, both to improve consolidated methods and to find new ones.
The largest amount of classical organic pollutants that can be found in water belongs to the volatile organic compounds (VOCs) category. These substances have vapor pressures of 0.01 kPa or higher at a temperature of 293.15 K, as specified in the Lgs. D. 152/2006. Lots of chemicals of common use belong to this category, such as aliphatic, aromatic and chlorinated hydrocarbons, aldehydes, terpenes, alcohols, esters and ketones. Some examples are summarized in Table 1, according to the Italian Ministry of Health (2015).

Table 1.
Most common VOCs and their usages.
These compounds are strictly monitored in water, since they are extremely dangerous for human health, also when at low concentrations [35,44].
2.1. Halogenated Hydrocarbons
2.1.1. Adsorption
Siggins et al. [31] studied the adsorption capacity of different pyrolyzed waste materials towards trichloroethylene (TCE), for in situ remediation. This compound has been widely used as a degreasing agent since the beginning of the twentieth century [45]; nevertheless, it is known to be a carcinogenic compound, and it belongs to the list of the Environmental Protection Agency priority pollutants. Therefore, its removal from drinking water is crucial. In particular, these authors tested granular activated carbons (GAC), herbal pomace biochar and spruce and oak-derived biochars, obtaining removal efficiencies up to 95%, 93% and larger than 99.5%, respectively.
A halogenated hydrocarbon that is similar in form and usage to TCE is tetrachloroethylene (PCE). This compound has been largely used over time as a degreaser solvent and in dry cleaning; its presence in the environment is a serious problem, since it is suspected of causing cancer and is toxic for the aquatic ecosystem. Gil et al. [32] tried to remove PCE from polluted water through an adsorption process by means of Moroccan stevensite. By adding 0.1 g/L of adsorbent, it was possible to reduce its concentration up to 88.8%. Additionally, Almasi et al. [46] focused on this pollutant in their work, trying to adsorb it by pumice. In particular, both granulated pumice and pumice doped with copper were tested: the results showed a removal efficiency of about 90% in the first case, and 98.4% in the second one.
Adsorption is also the most common method to remove dichloromethane (DCM), trichloromethane (TCM, also known as chloroform) and carbon tetrachloride (CTC). The first solvent is a toxic, potentially carcinogenic compound that is involved in many processes, such as in the removal of paints and greases, and it can also be found in pharmaceutical, chemical, textile, metal-working and petroleum industries’ wastewaters [47]. Chloroform is a cancerogenic molecule used in the production of freon R-22 that is a refrigerant fluid. However, due to its toxicity and the risk of production of phosgene when in contact with light and atmospheric oxygen, this substance has a limited use. CTC was widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent. Nowadays, its toxicity is well known: exposure to high concentrations of this chemical can damage the central nervous system and degenerate the liver and kidneys; a prolonged exposure can even be fatal. Alhooshani [16] tried to remove these three dangerous compounds from water through adsorption by using activated carbons (AC) loaded with cerium oxide nanoparticles (CeO2-NP/AC). Different parameters were investigated to reach the optimum: starting from 10 mg/L of pollutant at 25 °C and by adding 5 g/L of adsorbent, a removal efficiency equal to 82.72%, 99.40% and 89.42% for DCM, TCM and CTC, respectively, was obtained. Carbon tetrachloride was also removed by Wu and Feng [48] using a modified biochar obtained after the immobilization of nanoscale zero-valent iron onto it, and then by attaching the elemental silver to the iron surface (Ag/Fe/MB). Starting from a concentration of pollutants equal to 20 mg/L and at pH 6 and 25 °C, 0.5 g/L of adsorbent was able to remove 93.9% of the compound after 60 min, till the complete adsorption within 90 min. Tongur and Aydin [49] eliminated chloroform from drinking water through adsorption onto activated lignite. To find the optimal operative conditions, they investigated different values of activation and carbonization time during the production of the adsorbent: in 180 min and 120 min, respectively, they removed 99.5% of the initial pollutant. Daniel and Guerra [50] found out that syndiotactic polystyrene (s-PS) and poly (2,6-dimethyl-1,4-phenylene) oxide (PPO), in their nanoporous crystalline phases had a high sorption capacity towards VOCs, even when these substances were present in traces in both air and water. In particular, Daniel et al. [51] obtained s-PS microfibers with a microporous crystalline form, by using eco-friendly solvents [52] that also allowed us to perform a simple and non-polluting regeneration method.
2.1.2. Catalysis
Jung et al. [53] investigated the photodegradation of TCE in water under simulated solar light irradiation using a bismuth oxybromide (BiOBr) photocatalyst. Its performance was improved by the presence of sulfite on its surface: by testing different conditions, they found out that this mechanism could remove 78% of TCE in aqueous solution.
Williams et al. [54] tested the feasibility of the dechlorination of DCM through an electrocatalytic process by using a molecular copper(I) complex with two triazole units (CuT2). The formation of by-products was monitored to evaluate the removal of the pollutant; in particular, a Faradaic efficiency of 70% for methane was measured.
2.1.3. Reductive and Oxidative Processes
Ma et al. [36] removed TCE from water by abiotic reduction using electrospun polyacrylic acid (PAA)/polyvinyl alcohol (PVA) nanofibers, in which Fe/Pd bimetallic nanoparticles were immobilized. This system enabled to remove 99.62% of the pollutant initially present in water in 3.5 h.
Huang et al. [37] used sodium borohydride (NaBH4) as a reductive agent to degrade DCM present in water; to improve the process, zero-valent copper (Cu0) nanoparticles were added as a catalyst. During the first hour, 90% of the pollutant was removed. The complete removal was obtained after 2 h.
Si et al. [55] removed TCM using Fe/Ni nanoparticles. In particular, they resolved the problem of oxidation and sedimentation of these nanoparticles using a green and low-cost polymer, polyethylene glycol (PEG), as a stabilizer. At pH 6 and using 50 mg of PEG-Fe/Ni, the whole pollutant present in water was removed, starting from a concentration of 7 mg/L.
2.1.4. Phytoremediation
Moccia et al. [21] tested Zea Mays in the removal of TCE from polluted water. This vegetable showed a great resistance towards the pollutant; however, a maximum removal efficiency of only 20% was obtained. Additionally, Mouhamad et al. [38] tried to purify water polluted by TCE using transgenic Sesbania grandiflora and Arabidopsis thaliana plants. In particular, these plants were modified to express the cytochrome P450-2E1 that allowed the removal of two times more TCE than the control (about 14 mg/kg fresh weight).
2.1.5. Biodegradation
Wang et al. [56] completely removed PCE present in water by combining nanoscale zero-valent iron (nZVI), properly modified by layered double hydroxide (nZVI-LDH), with a microbial consortium made up of 44.49% Clostridium and other potential PCE degraders. This result was achieved starting from a concentration of pollutant between 0.5 and 2.5 mg/L in 4 days.
Li et al. [39] selected a bacterial community to aerobically degrade TCE by co-metabolism with phenol, using H2O2 as source of oxygen. In particular, Bordetella, Stenotrophomonas sp., Sinorhizobium sp., Variovorax sp. and Sphingobium sp. were tested. Within 12 days and by using 8 mM of hydrogen peroxide, 120 mg/L of TCE was removed, up to 80.6% degradation efficiency. Liu et al. [57] created an anaerobic/aerobic permeable reactive barrier formed of four different layers to eliminate PCE and its intermediates, i.e., TCE, dichloroethylene (DCE) and vinyl chloride (VC). Bioremediation allowed the removal of 99% of PCE, thanks to the initial anaerobic step; the final aerobic one instead mostly removed the toxic by-products: i.e., 98%, 90% and 92% of TCE, DCE and VC, respectively.
2.1.6. Membranes
Abdel-Karim et al. [58] tested six different types of nanofiltration (NF) and reverse osmosis (RO) membranes to control the amount of chloroform present in drinking water. Among these devices, NF-90 removed about 92% of the pollutant, NF-270 only 76%, whereas RO membranes removed between 94% and 98.5% of pollutants.
Ainscough et al. [43] used nanofiltration and reverse osmosis membranes to purify water from both TCE and PCE. Thanks to the first kind of process, they removed 70–93% of the pollutants during laboratory tests, and 100% in case of real groundwater samples. Reverse osmosis allowed the removal instead of 93% of VOCs; however, the elimination of the compounds decreased over time because of the fouling of the membrane.
The main results related to the removal efficiency of halogenated hydrocarbons from water are summarized in Table 2.

Table 2.
Removal of halogenated hydrocarbons from water.
2.2. Aromatic Hydrocarbons
2.2.1. Adsorption
Abbas et al. [59] tested carbon nanotubes (CNTs) loaded with iron oxide nanoparticles to adsorb benzene up to 61% from polluted water. Benzene is a solvent frequently used in industry, since it is involved in the production of several medicines, plastics and dyes. Nowadays, it has been progressively substituted by toluene in many processes and as a fuel additive as well, due to its carcinogenicity and environmental issues.
Tavakoli Dastjerdi et al. [17] removed 55% of toluene by adsorption using manganese oxide nanowires. Although this molecule is not as mutagenic as benzene, it is dangerous for human health since it can damage organs and may be lethal after ingestion. Heydari et al. [60] utilized the metal–organic framework MIL-101(Cr) for the same scope. At optimal conditions (i.e., 25 °C, pH of 4.48, contact time of 64 min, 0.66 g/L of solid and starting from 70 ppm of pollutant), they removed toluene with an efficiency of 97%. Moreover, these authors found out that this adsorbent could be used five times without any regeneration process, maintaining its adsorption capacity. Nefzi et al. [61] tested cellulose diatomite to remove toluene from water. 79% of toluene could be removed by natural diatomite at pH 6, 22 °C and by using 0.1 g of solid when the initial concentration of the pollutant was 3 × 10−3 mol/L. In a second phase of the experimentation and at the same process conditions, the percentage of removal increased to 97.45% by using the modified diatomite.
2.2.2. Photocatalysis
Yuan et al. [62] prepared activated carbon (AC)-supported Fe3+-doped TiO2 nanotubes (Fe-TNTs) to degrade toluene present in water solutions. In particular, by using O3 and UV irradiation, they obtained a removal efficiency of 90.7%. Al-Sabahi et al. [63] studied photocatalytic degradation of benzene and toluene under visible light irradiation by means of supported zinc oxide (ZnO) nanorods. After 3 h processing, a removal efficiency equal to 90% for toluene and 65% for benzene was obtained, but the generation of by-products such as phenol, benzyl alcohol, benzaldehyde and benzoic acid was also detected. Qiu et al. [64] used nano-TiO2 immobilized under 254 nm of UV light irradiation to remove 33% of benzoic acid from polluted water.
2.2.3. Reductive and Oxidative Processes
Jans et al. [65] removed 1-methoxy naphthalene by oxidation with a combination of iron and a tetraamido-macrocyclic ligand (Fe-TAML) and using H2O2 as oxidant reagent. After 15 min, the pollutant concentration in water was reduced by up to 85%.
Among AOPs, the use of a dielectric barrier discharge (DBD) reactor has gained attention in recent years for the treatment of water polluted by organic compounds, thanks to its high efficiency and eco-compatibility [66]. Nawaz et al. [66] used this technology to degrade nitrobenzene, a toxic molecule that is involved in the production of dyes, pharmaceuticals and pesticides. In particular, using a DBD reactor, it was possible to remove all the pollutant in 30 min starting from 10 mg/L, and in 55 min when the initial concentration was 20 mg/L. Additionally, the UV-activated permanganate (UV/PM) process proposed by Ye et al. [67] to remove the benzoic acid from water belongs to the category of AOPs. By using this green oxidant, the authors managed to eliminate 52% of the pollutant (initial concentration of benzoic acid of 1 mg/L) at pH 7.4 and by adding 4 mg/L of PM. This result was then compared with the one related to the process using UV/H2O2; in this case, the removal efficiency was 80%. Guo et al. [68] obtained a removal efficiency of nitrobenzene of up to 96% using low-frequency ultrasound (US) and Zn0-activated persulfate (PS). Farias et al. [69] investigated benzene degradation, catalyzed by Co-MCM-41, in the presence of H2O2. After 5 h of reaction, 82% of the pollutant was removed with an initial amount of benzene equal to 100 mg/L, 2 g of Co-MCM-41 and a H2O2 concentration of 0.1 M. Cesarino et al. [70] used electrocatalytic oxidation to remove benzene from water. In particular, they utilized a multi-walled carbon nanotube-silver (MWCNT-Ag) modified glassy carbon (GC) electrode. Testing an initial concentration of pollutant equal to 10 mg/L and by applying a potential of +2.0 V for 2 h, a removal of 77.9% was obtained.
2.2.4. Liquid–Liquid Extraction (LLE)
Agrahari et al. [71] applied LLE to the removal of benzoic acid from polluted water. In this case, a hydrophobic polypropylene-based hollow fiber membrane contactor (HFMC) was used, together with trioctylamine (TOA) diluted in 1-octanol as the extracting solvent. Starting from a concentration of the compound of interest equal to 200 ppm, they eliminated more than 95% of pollutant.
2.2.5. Combined Methods
Benzene and chlorobenzene were removed by Guo et al. [40] by using both adsorption and free radical oxidation. Iron salt-modified peanut shell biochar (Fe-BC) was used as an activator for persulfate (PS). Within 3 h, a complete depuration of the aqueous solutions was obtained.
The main results related to the removal efficiency of aromatic hydrocarbons from water are summarized in Table 3.

Table 3.
Removal of aromatic hydrocarbons from water.
2.3. Phenols
Phenols are produced by several industrial sectors. They have very harmful effects on ecosystems and human health and are present in 35% of the hazardous sites listed by the Environmental Protection Agency (EPA) in 2008. That is the reason why numerous papers in the literature are focused on the removal of this category of compounds from water.
2.3.1. Adsorption
Al Bsoul et al. [72] used the adsorption process to remove phenol from polluted water. They tested Ziziphus leaves as adsorbent material: the best result, i.e., a removal efficiency equal to 37.5%, was obtained at pH 6, 25 °C and starting from a pollutant concentration of 200 ppm. Liu et al. [7] proposed a new adsorbent for the removal of phenol, prepared by loading cyclodextrin (CD) onto nanofibers of bacterial cellulose. However, they obtained a low removal efficiency of 18%, starting from a 50 mg/L concentration of phenol. Demissie et al. [73] reduced the concentration in water of different phenols by using in situ coated surfactant on Keggin-aluminum nanocluster (SDS-Al30). In particular, this method allowed the destabilization of suspended particles and the extraction of hydrophobic molecules from aqueous solutions; depending on the initial concentration of the pollutant, different removal efficiencies were obtained. The best ones corresponded to the highest amounts of compounds present in water at the beginning of the test, i.e., 85% ÷ 89% for 2,3-dichlorophenol, 2,4-dichlorophenol, 3,4-dichlorophenol, 2,4,6-trichlorophenol, 2,3,4-trichlorophenol and 2,4,5-trichlorophenol, but with 97% and 98% in the case of 2,3,4,6-tetrachlorophenol and pentachlorophenol, respectively.
2.3.2. Photocatalysis
Pentachlorophenol was removed from polluted water with an efficiency of 99% by Yu et al. [74]. They photo-deposited argentum nanoparticles onto anatase TiO2 nanotubes (Ag/TNTs) to photodegrade this molecule under simulated solar light. Jay and Chirwa [75] studied the removal of phenol and the formation and degradation of its aromatic intermediates via photocatalysis. A total of 8 mg/L of TiO2 was added to 1 L of solution with an initial phenol concentration equal to 20 mg/L; experiments were performed under UV irradiation, and a final value of 5 mg/L of pollutant was reached within 100 min (removal efficiency of 75%). Yao et al. [76] combined the photocatalytic capacity of TiO2 with the structure of montmorillonite (MMT) to obtain a TiO2/MMT composite. It was tested under UV irradiation to degrade phenol in wastewaters. Starting from 10 mg/L of pollutant, pH 6 and within 150 min, 63% of the compound was removed.
2.3.3. Reductive and Oxidative Processes
Jans et al. [65] investigated the possibility of reducing the concentration of water pollutants through an oxidative process using iron with a tetraamido-macrocyclic ligand (Fe-TAML) as a catalyst, together with hydrogen peroxide (H2O2). After 15 min of reaction, a phenol removal efficiency equal to 79%, 100%, 98%, 100%, 100% and 100% was obtained in the case of phenol, 4-methyl phenol, 4-chlorophenol, 2,5-dimethyl phenol, 2,4,6-trimethyl phenol and 2,4,6-trichlorophenol, respectively. Horová et al. [77] obtained the complete removal of phenol by wet peroxide oxidation. In particular, they used iron modified zeolites as a catalyst, and a reaction time of 5 h.
Jothinathan et al. [78] studied the purification of petrochemical wastewater combining microbubble and catalytic ozonation (M-O3/Fe/GAC). Several process conditions were investigated. The highest removal efficiency for phenols was equal to 96%. Asgari et al. [79] tested the removal of phenol from polluted water through ozonation, catalyzing it by means of zeolite and pumice modified with copper (CuSO4). An abatement of 51% and 63%, respectively, at pH 8, was measured for the two systems.
The main results related to the removal efficiency of phenols from water are summarized in Table 4.

Table 4.
Removal of phenols from water.
2.3.4. Combined Methods
Several works in the literature describe the possibility of removing phenols from water by coupling different methods and technologies, such as in the case of Fenton, photo-Fenton and electro-Fenton processes [80]. Gernjak et al. [81] described the complete mineralization of phenolic compounds obtained through photo-Fenton treatment, enhanced by UV irradiation. Solutions with an initial concentration of vanillin, protocatechuic acid, syringic acid, p-coumaric acid, gallic acid and L-tyrosine equal to 1 mM were depurated after 1 h, by using an iron concentration of 0.1 mM and H2O2 between 12 and 24 mM. Additionally, Carta and Desogus [82] studied the Fenton process to remove phenol from contaminated water. In this case, the AOP process was coupled with low power microwaves, since they could positively influence reactions taking place in the aqueous solutions. The experiments were carried out testing an initial quantity of phenol between 20 and 120 mg/L, a Fe2+ concentration in the range 0.693–11.112 mg/L and 10 mg/L of hydrogen peroxide at pH 3 and 25 °C. Azizi et al. [83] removed 99.7% of phenol present in a water solution thanks to a Fenton reaction coupled with enzymatic polymerization with immobilized turnip peroxidase. In particular, the second step allowed both to complete the degradation of the pollutant and to remove its oxidation subproducts. The experiments were performed using an initial concentration of phenol equal to 100 mg/L; the Fenton process was carried out at pH 3 and 40 °C with 5 mg/L of iron(II) and 9 mM of hydrogen peroxide; the final treatment took place at the same temperature and at pH 7, also by adding 10.6 mM of H2O2 and 5 U of immobilized peroxidase. Suryaman et al. [84] used biodegradation combined with photocatalysis to eliminate phenol from water. Starting from a pollutant concentration of 50 mg/L, they reached 6.8 mg/L thanks to the biological treatment, and then the mineralization of the compound was continued through photocatalysis by using 0.5 g/L of TiO2. In order to eliminate 98% of phenol, 10.5 h of biological treatment, followed by 30 min of the photocatalytic one, were required. Lu et al. [85] focused on the removal of phenol by using a combination of cavitation water jets and hydrogen peroxide. These authors managed to eliminate 99.85% of the compound of interest, with an initial concentration equal to 100 mg/L at pH 3 and in presence of a H2O2 concentration of 300 mg/L, a confining pressure of 0.5 MPa, and a pumping pressure of 20 MPa.
Adsorption combined with photocatalysis was investigated by Chen et al. [86] They found out that Fe3+ and 8-hydroxyquinoline-7-carboxylic (HQLC) could improve the photocatalytic effect of TiO2. Therefore, they synthesized an Fe-HQLC/TiO2 flower composite to remove phenol from water. A total of 0.05 g of photocatalyst was added to a 10 mg/L phenolic solution; with 300 W of irradiation in the range of 320–750 nm, 99% of the compound of interest was degraded into small organic acids within 20 min; a mineralization rate equal to 81% was reached. Ipek et al. [87] coupled adsorption and membranes to remove phenol present in contaminated water. A total of 3 g/L of Purolite MN 200 and Purolite MN 202 were used as adsorbents, starting from a phenolic concentration of 50 mg/L. Using a flow rate of suspensions of saturated and fresh adsorbents of 6 mL/min, and a flow rate of feed and permeate of 3 mL/min, it was possible to remove 90% of the pollutant by using Purolite MN 200, and 100% in the case of Purolite MN 202.
2.4. Aldehydes
2.4.1. Adsorption
Additionally in the case of aldehydes, adsorption is the favorite method of depurating water. Among these compounds, two main molecules can be identified: formaldehyde and acetaldehyde, since both of them are confirmed carcinogens. Wang et al. [8] efficiently eliminated formaldehyde from water, with up to 99% removal efficiency, using a mesoporous calcium silicate hydrate (CSH). Salehi and Shafie [88] used adsorption to purify water from acetaldehyde by means of strong anionic resins, i.e., AMBERLITE IRA 402-OH, after a pre-treatment with bisulfite. In this way, they obtained an 86% pollutant removal, starting from a concentration of the substance equal to 50 mg/L.
2.4.2. Combined Methods
Talaiekhozani et al. [42] tested UV and ferrate(VI), separately and combined, to remove formaldehyde from water. In particular, they found out that the best conditions were pH 2 and a ferrate(VI) concentration of 1 mg/L. During the first 35 min of the experiment, 87% of the initial pollutant was removed by ferrate(VI) alone, 95% was removed by UV and their combined action removed about 100% of the dangerous compound. Ono et al. [41] combined UV254 and ultrasonic irradiation with H2O2 to completely remove acetaldehyde in aqueous solution within 20 min. This result was obtained starting from a pollutant concentration of 2.70 × 10−5 mol/L, using 430 kHz, a ultrasonic power of 0.08 W mL−1, a UV intensity of 6.3 W and 1 and 10 mmol/L of H2O2.
The main results related to the removal efficiency of aldehydes from water are summarized in Table 5.

Table 5.
Removal of aldehydes from water.
3. Removal of Emerging Organic Pollutants from Water
UNESCO [89] defined emerging pollutants as synthetic or naturally-occurring chemicals or microorganisms that are not commonly monitored or regulated in the environment, and that are dangerous for human health and from an ecological point of view. It is possible to find this kind of substances in pharmaceuticals (anticancer and analgesic compounds, antibiotics, beta blockers), personal care products, pesticides, industrial and household products, metals, surfactants, plasticizers, industrial additives and solvents [89,90].
More than 80 compounds belonging to the category of the emerging pollutants have been detected in the aquatic environment and also in drinking water, with an amount of the order of ng/L [91]. The European Union established 10 ng/L and 10 µg/L as maximum concentrations allowed in surface water and soil in the case of pharmaceuticals and personal care products, respectively [92]. Nevertheless, since the attention towards these molecules in water and their negative health effects are new issues, more specific regulations and limit values are not yet present. On the other hand, concern is rapidly growing, as is the number of studies on emerging pollutants and the possible technologies to depurate contaminated water. In this review, the main proposals reported in the literature to achieve this goal are described; moreover, this macro-category of chemicals has been divided into:
- dyes.
- endocrine disrupters and personal care products (PCP).
- pharmaceuticals (PhACs).
3.1. Dyes
Dyes are mostly detected in wastewaters coming from tanneries and textile industries. These molecules are extremely toxic and not biodegradable; in addition to this, it is not easy to degrade them by using light and oxidation reactions, since they are very stable [93]. For these reasons, several papers investigated the removal of dyes from drinking water by adsorption.
3.1.1. Adsorption
Rego et al. [10] evaluated the adsorption capacity of cerium (Ce)-UiO-66 metal organic framework (MOF) towards inorganic and organic water pollutants. In particular, they focused on two dyes, congo red (CR) and methylene blue (MB), that are an anionic and a cationic compound, respectively. Because of their nature, the adsorption was strongly influenced by the pH of the solution; indeed, a low value of this parameter improved the removal of CR, whereas, in the case of MB, a higher value was preferred. This study showed a maximum removal of CR equal to 99.9% after 60 min, and 90% for MB after 50 min of processing. Gong et al. [93] also tried to depurate water polluted by MB using activated carbons derived from finger citron residue (FAC). Starting from 450 mg/L of pollutant, and by adding 0.4 g/L of solid, they removed 48.9% of dye at pH 7 and within 4 h of contact. Azam et al. [9] focused both on MB and on methyl orange (MO). These authors obtained a removal efficiency equal to 82% for the first dye, starting from 40 mg/L of compound to be adsorbed, and 98.5% for the second one, working with an initial pollutant concentration of 30 mg/L, after 30 min of contact with 2 g/L of magnetic mesoporous activated carbon (MMAC) at pH 3. Zhai et al. [11] studied the possibility to remove MO by adsorption, using chitosan microspheres. They obtained a removal efficiency equal to 98.5%, using 30 mg of the adsorbent at pH 7 and starting from a concentration of 40 mg/L of dye.
Another option to eliminate MO from polluted water is the adsorption of this chemical onto layered double hydroxides (LDHs). Chen et al. [94] selected this material since it is cheap, non-toxic and characterized by structure amenability and anion exchange capability. The adsorbent used in their experiment was a flower-like Ni/Al LDH that decorated porous carbons derived from H3PO4-activated biomass (Ni/Al@PAB). Starting from 80 mg/L of MO, they removed 75% of it by adding 10 mg of solid to 50 mL of aqueous solution. Chaukura et al. [95] obtained a complete removal of MO by means of both biochar (BC) and Fe2O3–BC nano-composites prepared from pulp and paper sludge (PPS), with 5 g/L of solid, and an initial concentration of the chemical equal to 50 mg/L. Rhodamine B (RhB) removal was studied by Peng et al. [12] Adsorption experiments were carried out using Fe3O4 nanoparticles modified with humic acid (HA) (Fe3O4/HA). Within 15 min, a removal efficiency of 98.5% was obtained at pH 2.5 and testing 50 mg of adsorbent in 100 mL of aqueous solution at 50 mg/L of RhB. Kisku et al. [96] removed 75% of disperse orange 25 (DO 25) testing coal fly ash (CFA) as an adsorbent. Markandeya et al. in two works tested the adsorption of DO 25 using chitosan cenosphere nanocomposite [97] and cenosphere nanosyntactic foam [13]; the largest removal efficiency of 97.3% was achieved when cenosphere nanocomposites were tested.
3.1.2. Catalysis
Rhodamine B and methyl orange were removed by CuI/g-C3N4 nanocomposite in the work of Ghanbari and Salavati-Niasari [98] under UV irradiation. Starting from solutions with a dye concentration of 10 ppm, different experimental conditions were tested, until a removal of 98.5% for RhB and 98% for MO was reached.
Abdollahi et al. [99] optimized the quantity of graphene to add to CaCu3Ti4O12 nanocomposite to improve its photocatalytic capacity towards organic pollutants. The best result in the removal of methyl orange was an efficiency of 89%, obtained using 8% of graphene and 0.96 g/L of the photocatalyst, starting from 45 mg/L of dye at pH 5.8 within an irradiation time equal to 288 min.
Nguyen et al. [100] studied palladium-doped titanium dioxide (Pd-TiO2) to catalyze the photodegradation of both MO and MB. In particular, 0.5 wt% of Pd allowed them to obtain the highest removal of the two dyes; mineralization efficiency was equal to 85.9% and 77.1% for MB and MO, respectively, after 180 min of UV irradiation.
3.1.3. Phytoremediation
The biodegradation of methylene blue was investigated by Almaamary et al. [101] These authors removed 87% of MB present in water selecting a Malaysian plant, the Scirpus grossus; within 72 days, the concentration of the pollutant decreased from 200 mg/L to 28 mg/L. Lafta Al-Zurfi et al. [102] tested Lemna minor to remove CR from polluted water. Starting from a concentration of 0.01 µg/L, the aquatic plant allowed them to achieve a removal efficiency up to 51% within 7 days and at room temperature. Sharma et al. [103] used Eichhornia crassipes to eliminate several dyes from water, i.e., rose bengal (RB), methylene blue, crystal violet (CV), auramine O (AO), rhodamine B, xylenol orange (XO), phenol red (PR), cresol red (CrR) and MO. A removal efficiency equal to 87.4%, 90.8%, 87.2%, 79%, 84.8%, 46.2%, 44.4%, 33.3% and 62.8%, respectively, was obtained within 10 days, starting from aqueous solutions prepared by adding 0.25 g of each dye to 2500 mL of water.
3.1.4. Membranes
The removal of dyes using porous membranes was proposed by Zhang et al. [104] They used thin film nanocomposite (TFN) membranes, prepared with graphene oxide quantum dots (GOQDs) dispersed within a tannic acid (TA) film, to treat water polluted by MB and CR. In particular, a reduction of 97.6% and 99.8% was obtained, respectively, and the study was carried out on a feed solution with an initial concentration of 100 mg/L dyes. Modi and Bellare [105] prepared hollow fiber membranes (HFMs) with zinc oxide nanoparticle-dispersed carboxylated graphene oxide nanosheets (ZnO/cGO nanohybrid) to purify water polluted by MB and RhB. Starting from aqueous solutions at 50 mg/L of the first dye and 2 mg/L of the second one, polyether sulfone hollow fiber membranes with 0.50 wt.% ZnO/cGO nanohybrid (ZOGP-50 HFMs) allowed them to obtain a removal efficiency of 98.6% and 98.5%, respectively.
Hu et al. [106] focused on sunset yellow (SY), and selected poly(N-vinyl imidazole) (PVI) gel-filled membrane adsorbers to remove it from water. These authors eliminated more than 99% of the dye starting from a 25-ppm concentration and working at pH 3.
3.1.5. Combined Methods
Mahmoud et al. [107] used adsorption onto γ-Al2O3-SiCl nanosorbent enhanced by microwaves (microwave-enforced sorption, MES) to remove CR. Within 20 s and by adding 5 g/L of solid, it was possible to depurate tap water, sea water and industrial wastewater. Starting from a 30 mg/L concentration of pollutant, a maximum removal efficiency of 99.28%, 96.11%, and 98.41% was obtained, respectively. Additionally, γ-Al2O3 was tested in the same conditions, achieving a removal of 94.86%, 95.01% and 96.14% for the three different types of water. Zhang et al. [108] firstly tested graphitic carbon nitride-titanium dioxide-graphene aerogel (g-C3N4-TiO2-GA) composites to adsorb RhB, and then tried to enhance its removal by irradiating under visible light. In particular, within 1 h, adding 0.2 g/L of solid, a 96.5% removal of the compound of interest was obtained, without the photocatalytic effect. When irradiation was also present, this value reached 98.4%.
The main results related to the removal efficiency of dyes from water are summarized in Table 6.

Table 6.
Removal of dyes from water.
3.2. Endocrine Disrupters (EDs) and Personal Care Products (PCPs)
Endocrine disrupters are all the compounds that may affect the regular hormonal functions of the organism. Different types of substances belong to this category, such as industrial solvents or lubricants and their by-products, detergents, dioxins, plasticizers, pesticides, fungicide, flame retardants and also some pharmaceuticals [109].
Personal care products include cosmetics, steroids, perfumes, shampoos and UV filters [92]. Since these molecules are of common use in everyday life, it is easy to find them in water; as they may be very toxic for both the environment and human health, several studies have focused on their removal from aqueous solutions.
3.2.1. Adsorption
Bisphenol A (BPA) is one of the most diffused and harmful endocrine disrupters. Firstly used as an additive in plastics, it is now well known for the serious damage it causes to human health, and, therefore, it figures among the substances identified as endocrine disruptors at the EU level (2021) [110]. Liu et al. [7] treated a water solution of BPA at 20 mg/L with film-like bacterial cellulose/cyclodextrin oligomer composites, obtaining a very low removal efficiency of 34%. Xu et al. [111] removed 86% of this ED by means of graphene. In particular, 1 mg/L of solid was used to treat a 10 mg/L BPA aqueous solution at pH 6. Gong et al. [6] used a mesoporous magnetic composite material, Fe3O4 with SiO2 coating layers and cetyltrimethylammonium bromide (Fe3O4@SiO2/CTAB-SiO2), to adsorb bisphenol A from water. An efficiency of purification of 93.2% using 500 mg of CTAB, 10 mg adsorbent, a pH 6.5, 15 g/L of NaCl, 35 °C and 30 min of contact was achieved.
Triclosan is an antibacterial compound that was generally used in soaps, toothpastes and other oral hygiene products. Nowadays, its toxicity is quite ascertained; indeed, it appears in the list of molecules that are under evaluation for endocrine disruption, according to EU legislation (2021) [112]. The complete removal of this substance by dioctadecyldimethylammonium-modified bentonite (2C18-BT) was obtained starting from a concentration of 10 mg/L and using 50 mg/L of adsorbent [113].
Adsorption is the most popular method to remove atrazine. This herbicide has been forbidden in many European countries since 1992 because of its toxicity, and the limit imposed by the Council Directive 98/83/EC for its concentration in water is 0.1 µg/L. Moeini et al. [114] tested titanium dioxide encapsulated in salicylaldehyde-NH2-MIL-101 (TS-MIL) to remove atrazine. Starting from an aqueous solution with a pollutant concentration of 30 mg/L, 90% of it was removed using 2 g/L of TS-MIL within 30 min. Moreover, these authors demonstrated that the mechanism responsible for this contaminant removal was adsorption, since the photocatalytic effect under visible light allowed them to obtain a lower removal (78%).
3.2.2. Reductive and Oxidative Processes
Nonylphenol is another compound present in the list of the substances identified as endocrine disruptors at EU level (2021) [110]. Its removal was studied by Limmun et al. [115] by using oxidation with potassium ferrate (K2FeO4), containing hexavalent iron (Fe(VI)), as an oxidant agent. A 98% removal of the pollutant was obtained at pH 4 with an Fe(VI) amount of 5 mg/L and starting from 1 mg/L of ED. Phthalates also belong to the category of EDs; among these, dibutyl phthalate (DBP) is on the list of the endocrine disrupters of the EU (2021) [110]. Akbari-Adergani et al. [116] removed DBP from aqueous solutions at 15 mg/L by using a nanophotocatalytic Fe,Ag-ZnO system, coupled with visible light-emitting diode (LED) irradiation (Fe,Ag-ZnO/VIS-LED). In particular, the reduction of 95% of the pollutants was obtained at pH 3 and using 150 mg/L of photocatalyst.
Parabens are widely used in personal care products such as cosmetics because of their bactericidal properties. However, they have endocrine-disruptive effects, and it is essential to remove them from water. Palharim et al. [117] used advanced oxidative processes to eliminate propylparaben from water. These authors performed a comparison between two methods: in the first one, persulfate (PS) was activated by UVA, whereas the second one was activated by zero-valent iron (ZVI). Starting from 1 mg/L of paraben, the maximum removal efficiency obtained during the experimentation was about 94.8% for the UVA-activated system, with a PS concentration of 10 mmol/L and 13.8 W/m2 of irradiance; the result reached for the other system was 98.5%, with a ZVI concentration equal to 40 mg/L and a PS concentration of 5 mmol/L. Orhon et al. [118] removed all the triclosan contained in polluted water with an initial concentration of 5 mg/L through 20–30 min of ozonation. In particular, a demand of 13.04 mg of ozone/mg of triclosan was estimated.
3.2.3. Membranes
Yüksel et al. [119] removed BPA through different types of membranes, both based on NF and RO. In particular, they put fresh membranes in contact with 15 L of bisphenol A solutions, having an initial concentration of 50 mg/L. In this way, they achieved an almost complete rejection of the endocrine disrupter by using polyamide-based RO membranes (BW30, XLE BWRO and AD SWRO). Rastgar et al. [120] selected forward osmosis thin film composite (FOTFC) membranes to eliminate 97.3% of atrazine present in aqueous solution at 50 mg/L.
Wei et al. [121] tried to purify water polluted by several phthalates by means of hollow-fiber nanofiltration membranes. A removal efficiency of 82.3%, 86.7%, 91.5%, 95.1% and 95.4% for dimethyl phthalate (DMP), diethyl phthalate (DEP), dibutyl phthalate, di-n-octyl phthalate (DnOP) and diethylhexyl phthalate (DEHP) was obtained, respectively.
3.2.4. Combined Methods
López-Ortiz et al. [122] tested a combination of magnetic ion exchange resins (MIEX® DOC and MIEX® GOLD) and nanofiltration membranes (NF-90 and DESAL-HL) to remove methylparaben, ethylparaben, propylparaben and butylparaben. The best results were achieved using DOC resin together with both NF-90 and DESAL-HL membranes. Indeed, in these cases, butylparaben and propylparaben were completely removed; the removal efficiency of methylparaben was 91% using NF-90 membrane and 92% using DESAL-HL one; the removal of ethylparaben reached 96% using NF-90 and 97% using DESAL-HL membrane.
The main results related to the removal efficiency of endocrine disrupters from water are summarized in Table 7.

Table 7.
Removal of EDs and PCPs from water.
3.3. Pharmaceuticals (PhACs)
The presence of pharmaceuticals in water is due to their growing administration to people and animals. Even though their concentrations are of the order of ng/L or µg/L, they may affect the environment and human health. In particular, among them, antibiotics, antiepileptics, analgesics and anti-inflammatories, lipid regulators, betablockers, diuretics, contrast media and antidepressants have been detected in wastewater treatment plants [123].
3.3.1. Adsorption
Rego et al. [10] obtained a removal efficiency of 99.9% for diclofenac sodium using Ce-UiO-66 metal organic framework (MOF) up to a 100-ppm concentration and after 20 min contact between the water solution and the adsorbent. Koutník et al. [124] prepared activated carbons (AC) from the invasive herb Reynoutria japonica and used them to adsorb diclofenac from aqueous solutions at 300 mg/L of pharmaceutical. An amount of 2 g/L of solid determined a removal efficiency of 58%. The elimination of DCF was also studied by Viotti et al. [14], who achieved the removal of 72.4% of the compound of interest using 2 g/L of Moringa oleifera pods. In the work of dos Reis et al. [125], DCF was removed by activated carbons obtained from sewage sludge. The maximum removal efficiency of 96.34% was achieved at pH 7, 25 °C and by adding 0.6 mg/L of adsorbent to a 50 mg/L diclofenac solution.
Ibuprofen was eliminated from aqueous solutions characterized by an initial concentration of 60 μg/L by Ali et al. [15] using a composite iron nano-adsorbent. The optimum (i.e., a removal efficiency of 92%) was reached at pH 7 and 25 °C, after 30 min of contact, and by adding 1.0 g/L of solid to the contaminated water. Ciğeroğlu et al. [126] tested graphene oxide nanopowders (GON) to depurate water from naproxen. The best result was obtained with 0.03 g of adsorbent, starting from 10 mg/L of pharmaceutical compound. At these conditions, the removal efficiency was equal to 65.28%.
Ali et al. [127] removed amoxicillin, an antibiotic, from drinking water using activated carbon prepared from pomegranate peel, coated with zerovalent iron nanoparticles (AC-nZVI). Starting from a pharmaceutical concentration of 10 mg/L, a removal of 97.9% was obtained by adding 1.5 g/L of solid, at pH 5 and within 30 min of contact time.
3.3.2. Catalysis
Changanaqui et al. [128] removed naproxen from aqueous solutions by photoelectrocatalysis (PEC), adopting a ZnO/TiO2/Ag2Se thin-film composite and under visible light. The complete degradation of the pollutant was obtained after 210 min, testing 100 mL of a 5 mg/L naproxen solution with 50 mM Na2SO4.
Martins et al. [129] studied the catalytic activity of nanoparticles of platinum and palladium, biologically synthesized (Bio-Pt ad Bio-Pd) by Desulfovibrio vulgaris, in the removal of ibuprofen and ciprofloxacin. The whole bacterial cell was able to completely degrade only the first pharmaceutical. On the other hand, it removed 70% of the ciprofloxacin.
Jayasree and Remya [130] looked at paracetamol. Through photocatalysis, they aimed at removing the pharmaceutical from polluted water by using TiO2 supported on aluminosilicate, recovered from waste LED panel (ATiO2). After 30 min of UV irradiation, with an initial concentration of the compound of interest equal to 2.74 mg/L, an ATiO2 amount of 2.71 g/L, and at pH 9.5, a removal efficiency of 99% was measured.
3.3.3. Oxidative Processes
Amoxicillin was removed from water by Shi et al. [131] using a three-dimensional electrode system (3DES) in an electrochemical oxidation process. This device was made by granular activated carbon (GAC) packed between the anode and cathode electrodes. Optimal working conditions to achieve 98.8% of pollutant removal within 2 h were: a current density of 5 mA/cm2, 17 mM of NaCl (electrolyte), pH equal to 5.56, and a GAC-quartz sand volume ratio of 9:1.
Tepe et al. [132] focused on the removal of paracetamol from water by an advanced oxidative method. In particular, they used manganese oxide octahedral molecular sieves (OMS-2) and persulfate (PS) and reached a pollutant elimination of 99.5%. Electrooxidation was chosen as the method to degrade paracetamol by Periyasamy and Muthuchamy [133]. Graphite was used as the anode; starting from a pharmaceutical concentration of 20 mg/L, the removal efficiency was larger than 90%, with a current density of 5.1 mA/cm2, 0.1 M of Na2SO4 (as electrolyte), at pH 4 and after 240 min.
He et al. [134] removed ibuprofen from water through catalytic ozonation. An amount of 0.1 g/L of α-MnO2 was used, together with 1 mg/min of ozone, to obtain a removal of 99% of the pharmaceutical present in water, with an initial concentration of 10 mg/L and pH 7.
The main results related to the removal efficiency of PhACs from water are summarized in Table 8.

Table 8.
Removal of PhACs from water.
4. Conclusions
In this review, the newest results found in the scientific literature related to the water purification of classical and emerging pollutants are reported. Adsorption, reductive and oxidative processes, phytoremediation, bioremediation, separation by membranes and liquid–liquid extraction are the most frequently used techniques for this purpose. However, even if ambitious results have been achieved in pollutant removal from water using these techniques (in some cases up to 100%), generally, small volumes of aqueous solution and/or low starting contaminant concentrations were tested. Moreover, these techniques show relevant drawbacks, such as: (i) the production of by-products to eliminate in further processing steps, (ii) batch configuration, (iii) long processing times, (iv) fouling, (v) high cost, (vi) difficulty in operating on a large scale.
Adsorption has emerged as the most promising and versatile water purification technique since it can balance high pollutant removal efficiency and the possibility to treat large quantities of water in a semi-continuous way. Moreover, this approach can also be used with stable pollutant molecules. However, innovation and improvement in adsorbent capacity/selectivity and regeneration techniques of the materials used are required to make this separation method convenient in real cases.
In conclusion, the use of a specific technique for water purification cannot be considered as universally valid for all the organic micropollutants due to the huge variety of compounds and the deep differences that characterize their behavior in water. The adoption of combined methods can be the solution to merge high pollutant removal and acceptable processing time and costs.
Author Contributions
Conceptualization, L.B. and E.R.; methodology, L.B.; investigation, S.S.; resources, E.R.; writing—original draft preparation, S.S.; writing—review and editing, L.B.; visualization, E.R.; supervision, E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Water Use in Europe. Available online: https://www.eea.europa.eu/signals/signals-2018-content-list/infographic/water-use-in-europe/view (accessed on 16 March 2020).
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Alrozi, R. Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 171, 510–516. [Google Scholar] [CrossRef]
- Sweetman, M.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.; Hayball, J. Activated carbon, carbon nanotubes and graphene: Materials and composites for advanced water purification. C J. Carbon Res. 2017, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Qu, F.; Zhu, L.; Yang, K. Adsorption behaviors of volatile organic compounds (VOCs) on porous clay heterostructures (PCH). J. Hazard. Mater. 2009, 170, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, G.; Wang, Q.; Zhu, A.; Liu, P.; Wu, Q. Synthesis of a novel mesoporous Fe3O4@SiO2/CTAB-SiO2 composite material and its application in the efficient removal of bisphenol A from water. Colloid Polym. Sci. 2021, 299, 807–822. [Google Scholar] [CrossRef]
- Liu, F.; Chen, C.; Qian, J. Film-like bacterial cellulose/cyclodextrin oligomer composites with controllable structure for the removal of various persistent organic pollutants from water. J. Hazard. Mater. 2021, 405, 124122. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Fan, B.M.; Wen, B.Y.; Jiang, C. Experimental and theoretical studies on the removal mechanism of formaldehyde from water by mesoporous calcium silicate. Sci. China Technol. Sci. 2020, 63, 2098–2112. [Google Scholar] [CrossRef]
- Azam, K.; Raza, R.; Shezad, N.; Shabir, M.; Yang, W.; Ahmad, N.; Shafiq, I.; Akhter, P.; Razzaq, A.; Hussain, M. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J. Environ. Chem. Eng. 2020, 8, 104220. [Google Scholar] [CrossRef]
- Rego, R.M.; Sriram, G.; Ajeya, K.V.; Jung, H.Y.; Kurkuri, M.D.; Kigga, M. Cerium based UiO-66 MOF as a multipollutant adsorbent for universal water purification. J. Hazard. Mater. 2021, 416, 125941. [Google Scholar] [CrossRef]
- Zhai, L.; Bai, Z.; Zhu, Y.; Wang, B.; Luo, W. Fabrication of chitosan microspheres for efficient adsorption of methyl orange. Chin. J. Chem. Eng. 2018, 26, 657–666. [Google Scholar] [CrossRef]
- Peng, L.; Qin, P.; Lei, M.; Zeng, Q.; Song, H.; Yang, J.; Shao, J.; Liao, B.; Gu, J. Modifying Fe3O4 nanoparticles with humic acid for removal of Rhodamine B in water. J. Hazard. Mater. 2012, 209–210, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Markandeya; Dhiman, N.; Shukla, S.P.; Mohan, D.; Kisku, G.C.; Patnaik, S. Comprehensive remediation study of disperse dyes in wastewater using cenospheres nanosyntactic foam. J. Clean. Prod. 2018, 182, 206–216. [Google Scholar] [CrossRef]
- Viotti, P.V.; Moreira, W.M.; dos Santos, O.A.A.; Bergamasco, R.; Vieira, A.M.S.; Vieira, M.F. Diclofenac removal from water by adsorption on Moringa oleifera pods and activated carbon: Mechanism, kinetic and equilibrium study. J. Clean. Prod. 2019, 219, 809–817. [Google Scholar] [CrossRef]
- Ali, I.; Al-Othman, Z.A.; Alwarthan, A. Synthesis of composite iron nano adsorbent and removal of ibuprofen drug residue from water. J. Mol. Liq. 2016, 219, 858–864. [Google Scholar] [CrossRef]
- Alhooshani, K.R. Adsorption of chlorinated organic compounds from water with cerium oxide-activated carbon composite. Arab. J. Chem. 2019, 12, 2585–2596. [Google Scholar] [CrossRef] [Green Version]
- Dastjerdi, M.H.T.; Habibagahi, G.; Ghahramani, A.; Karimi-Jashni, A.; Zeinali, S. Removal of dissolved toluene in underground water with nanowires of manganese oxide. Adsorpt. Sci. Technol. 2018, 36, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)—A review of recent advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Doty, S.L. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Huang, X.D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Moccia, E.; Intiso, A.; Cicatelli, A.; Proto, A.; Guarino, F.; Iannece, P.; Castiglione, S.; Rossi, F. Use of Zea mays L. in phytoremediation of trichloroethylene. Environ. Sci. Pollut. Res. 2017, 24, 11053–11060. [Google Scholar] [CrossRef]
- Zemb, O.; Lee, M.; Low, A.; Manefield, M. Reactive iron barriers: A niche enabling microbial dehalorespiration of 1,2-dichloroethane. Appl. Microbiol. Biotechnol. 2010, 88, 319–325. [Google Scholar] [CrossRef]
- Wen, L.L.; Zhang, Y.; Chen, J.X.; Zhang, Z.X.; Yi, Y.Y.; Tang, Y.; Rittmann, B.E.; Zhao, H.P. The dechlorination of TCE by a perchlorate reducing consortium. Chem. Eng. J. 2017, 313, 1215–1221. [Google Scholar] [CrossRef]
- Xiu, Z.M.; Jin, Z.H.; Li, T.L.; Mahendra, S.; Lowry, G.V.; Alvarez, P.J.J. Effects of nano-scale zero-valent iron particles on a mixed culture dechlorinating trichloroethylene. Bioresour. Technol. 2010, 101, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M. The use of membrane processes in environmental problems. An introduction. In Membrane Processes in Separation and Purification, 1st ed.; Crespo, J.G., Böddeker, K.W., Eds.; Springer: Enschede, The Netherlands, 1994; pp. 229–262. [Google Scholar]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J.J. Nanotechnology for a safe and sustainable water supply: Enabling integrated water treatment and reuse. Acc. Chem. Res. 2013, 46, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U. The Preparation of Chitosan/PVA/zeolite Electrospun Composite Nanofibrous Membrane for Heavy Metal Removal Application. Master’s Thesis, Engineering Science, University Malaya, Kuala Lumpur, Malaysia, 2016. [Google Scholar]
- Zhang, W.; Tang, S.; Zhang, S.; Chen, Y. Purification of nitrogen-doped graphene quantum dots: Via the liquid-liquid extraction system of tetrahydrofuran-(NH4)2SO4-water and its application to sensitive iron(III) ions determination. Anal. Methods 2017, 9, 5691–5696. [Google Scholar] [CrossRef]
- Cazoir, D.; Fine, L.; Ferronato, C.; Chovelon, J.M. Hydrocarbon removal from bilgewater by a combination of air-stripping and photocatalysis. J. Hazard. Mater. 2012, 235–236, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ghoreyshi, A.A.; Sadeghifar, H.; Entezarion, F. Efficiency assessment of air stripping packed towers for removal of VOCs (volatile organic compounds) from industrial and drinking waters. Energy 2014, 73, 838–843. [Google Scholar] [CrossRef]
- Siggins, A.; Abram, F.; Healy, M.G. Pyrolysed waste materials show potential for remediation of trichloroethylene-contaminated water. J. Hazard. Mater. 2020, 390, 121909. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Elmchaouri, A.; El Mouzdahir, Y.; Korili, S.A. Removal of tetrachloroethylene from aqueous solutions by adsorption on clay minerals. Adsorpt. Sci. Technol. 2015, 33, 355–367. [Google Scholar] [CrossRef]
- Ibrahim, A.O.; Adegoke, K.A.; Adegoke, R.O.; AbdulWahab, Y.A.; Oyelami, V.B.; Adesina, M.O. Adsorptive removal of different pollutants using metal-organic framework adsorbents. J. Mol. Liq. 2021, 333, 115593. [Google Scholar] [CrossRef]
- Aranzabal, A.; Pereda-Ayo, B.; González-Marcos, M.P.; González-Marcos, J.A.; López-Fonseca, R.; González-Velasco, J.R. State of the art in catalytic oxidation of chlorinated volatile organic compounds. Chem. Pap. 2014, 68, 1169–1186. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Suzuki, K. Catalytic oxidation of VOCs over Al- and Fe-pillared montmorillonite. Appl. Clay Sci. 2013, 77–78, 56–60. [Google Scholar] [CrossRef]
- Ma, H.; Huang, Y.; Shen, M.; Guo, R.; Cao, X.; Shi, X. Enhanced dechlorination of trichloroethylene using electrospun polymer nanofibrous mats immobilized with iron/palladium bimetallic nanoparticles. J. Hazard. Mater. 2012, 211–212, 349–356. [Google Scholar] [CrossRef]
- Huang, C.C.; Lo, S.L.; Lien, H.L. Zero-valent copper nanoparticles for effective dechlorination of dichloromethane using sodium borohydride as a reductant. Chem. Eng. J. 2012, 203, 95–100. [Google Scholar] [CrossRef]
- Mouhamad, R.; Ghanem, I.; AlOrfi, M.; Ibrahim, K.; Ali, N.; Al-Daoude, A. Phytoremediation of trichloroethylene and dichlorodiphenyltrichloroethane-polluted water using transgenic sesbania grandiflora and arabidopsis thaliana plants harboring rabbit cytochrome P450 2E1. Int. J. Phytoremediat. 2012, 14, 656–668. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.Y.; Wang, X.L.; Yang, J.; Gu, J.D.; Zhu, R.L.; Wang, P.; Lin, K.F.; Liu, Y. Di Aerobic biodegradation of trichloroethylene and phenol co-contaminants in groundwater by a bacterial community using hydrogen peroxide as the sole oxygen source. Environ. Technol. 2015, 36, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.-S.; Wang, F.; Zhang, X.-L.; Xu, J. Removal mechanism of benzene and chlorobenzene in water by modified biochar activates persulfate. China Environ. Sci. 2020, 40, 5280–5289. [Google Scholar]
- Ono, Y.; Sekiguchi, K.; Sankoda, K.; Nii, S.; Namiki, N. Improved ultrasonic degradation of hydrophilic and hydrophobic aldehydes in water by combined use of atomization and UV irradiation onto the mist surface. Ultrason. Sonochem. 2020, 60, 104766. [Google Scholar] [CrossRef] [PubMed]
- Talaiekhozani, A.; Salari, M.; Talaei, M.R.; Bagheri, M.; Eskandari, Z. Formaldehyde removal from wastewater and air by using UV, ferrate(VI) and UV/ferrate(VI). J. Environ. Manag. 2016, 184, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ainscough, T.J.; Oatley-Radcliffe, D.L.; Barron, A.R. Groundwater remediation of volatile organic compounds using nanofiltration and reverse osmosis membranes—A field study. Membranes 2021, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef]
- De Miranda, B.R.; Greenamyre, J.T. Trichloroethylene, a ubiquitous environmental contaminant in the risk for Parkinson’s disease. Environ. Sci. Process Impacts 2020, 22, 543–554. [Google Scholar] [CrossRef]
- Almasi, A.; Soltanian, M.; Asadi, F.; Nokhasi, P.; Godini, K.; Mohammadi, M.; Azarian, G.; Mohammadi, A. Tetrachloroethylene removal rate from aqueous solutions by pumice doped with copper: An evaluation of the effect of pH. Avicenna J. Environ. Health Eng. 2016, 3. [Google Scholar] [CrossRef]
- Shestakova, M.; Sillanpää, M. Removal of dichloromethane from ground and wastewater: A review. Chemosphere 2013, 93, 1258–1267. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Q. Fabrication of bimetallic Ag/Fe immobilized on modified biochar for removal of carbon tetrachloride. J. Environ. Sci. 2017, 54, 346–357. [Google Scholar] [CrossRef]
- Tongur, S.; Aydin, M.E. Adsorption kinetics of chloroform from aqueous solutions onto activated lignite. Clean Soil Air Water 2013, 41, 32–36. [Google Scholar] [CrossRef]
- Daniel, C.; Guerra, G. Nanoporous crystalline polymer materials for environmental applications. Macromol. Symp. 2016, 369, 19–25. [Google Scholar] [CrossRef]
- Daniel, C.; Antico, P.; Yamaguchi, H.; Kogure, M.; Guerra, G. Microporous-crystalline microfibers by eco-friendly guests: An efficient tool for sorption of volatile organic pollutants. Microporous Mesoporous Mater. 2016, 232, 205–210. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Supercritical assisted electrospray: An improved micronization process. Polymers 2019, 11, 244. [Google Scholar] [CrossRef] [Green Version]
- Jung, B.; Deng, W.; Li, Y.; Batchelor, B.; Abdel-Wahab, A. Simulated solar light-driven photocatalytic degradation of trichloroethylene in water using BiOBr promoted by sulfite addition. Environ. Sci. Eur. 2020, 32, 8. [Google Scholar] [CrossRef]
- Williams, C.K.; McCarver, G.A.; Lashgari, A.; Vogiatzis, K.D.; Jiang, J.J. Electrocatalytic dechlorination of dichloromethane in water using a heterogenized molecular copper complex. Inorg. Chem. 2021, 60, 4915–4923. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Che, M.; Chen, Z.; Qiu, S.; Cui, M.; Huang, R.; Qi, W.; He, Z.; Su, R. Efficient removal of chloroform in groundwater by polyethylene glycol-stabilized Fe/Ni nanoparticles. Environ. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Wang, Q.; Song, X.; Tang, S.; Yu, L. Enhanced removal of tetrachloroethylene from aqueous solutions by biodegradation coupled with nZVI modified by layered double hydroxide. Chemosphere 2020, 243, 125260. [Google Scholar] [CrossRef]
- Liu, S.J.; Yang, Q.M.; Yang, Y.K.; Ding, H.; Qi, Y. In situ remediation of tetrachloroethylene and its intermediates in groundwater using an anaerobic/aerobic permeable reactive barrier. Environ. Sci. Pollut. Res. 2017, 24, 26615–26622. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Karim, A.; Gad-Allah, T.A.; Badawy, M.I.; Khalil, A.S.G.; Ulbricht, M. Removal of humic acid and chloroform from drinking water by using commercial nanofiltration and reverse osmosis membranes. Desalin. Water Treat. 2017, 59, 48–54. [Google Scholar] [CrossRef]
- Abbas, A.; Abussaud, B.A.; Al-Baghli, N.A.H.I.; Khraisheh, M.; Atieh, M.A. Benzene removal by iron oxide nanoparticles decorated carbon nanotubes. J. Nanomater. 2016, 2016, 5654129. [Google Scholar] [CrossRef] [Green Version]
- Heydari, M.; Sabbaghi, S.; Zeinali, S. Adsorptive removal of toluene from aqueous solution using metal-organic framework MIL-101(Cr): Removal optimization by response surface methodology. Int. J. Environ. Sci. Technol. 2019, 16, 6217–6226. [Google Scholar] [CrossRef]
- Nefzi, H.; Salaberria, A.M.; Abderrabba, M.; Ayadi, S.; Labidi, J. Cellulose modified diatomite for toluene removal from aqueous solution. Desalin. Water Treat. 2019, 150, 228–236. [Google Scholar] [CrossRef]
- Yuan, R.; Zhou, B.; Ma, L. Removal of toluene from water by photocatalytic oxidation with activated carbon supported Fe3+-doped TiO2 nanotubes. Water Sci. Technol. 2014, 70, 642–648. [Google Scholar] [CrossRef]
- Al-Sabahi, J.; Bora, T.; Al-Abri, M.; Dutta, J. Efficient visible light photocatalysis of benzene, toluene, ethylbenzene and xylene (BTEX) in aqueous solutions using supported zinc oxide nanorods. PLoS ONE 2017, 12, e0189276. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Yang, Q.; Liu, W. Photocatalytic degradation of phytotoxic substances in waste nutrient solution by various immobilized levels of Nano-TiO2. Water Air Soil Pollut. 2013, 224, 1461. [Google Scholar] [CrossRef]
- Jans, U.; Prasse, C.; Von Gunten, U. Enhanced treatment of municipal wastewater effluents by Fe-Taml/H2O2: Efficiency of micropollutant abatement. Environ. Sci. Technol. 2021, 55, 3313–3321. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.I.; Yi, C.; Zhao, H.; Asilevi, P.J.; Yin, L.; Yi, R.; Javed, Q.; Wang, H. Experimental study of nitrobenzene degradation in water by strong ionization dielectric barrier discharge. Environ. Technol. 2021, 42, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Li, C.; Liu, X.; Wang, L.; Chen, R. UV-activated permanganate process for micro-organic pollutant degradation: Efficiency, mechanism and influencing factors. Water Sci. Technol. 2021, 83, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, L.; Sun, N.; Lan, Y. Degradation of nitrobenzene by sodium persulfate activated with zero-valent zinc in the presence of low frequency ultrasound. J. Taiwan Inst. Chem. Eng. 2017, 78, 137–143. [Google Scholar] [CrossRef]
- Farias, M.F.; Domingos, Y.S.; Fernandes, G.J.T.; Castro, F.L.; Fernandes, V.J.; Costa, M.J.F.; Araujo, A.S. Effect of acidity in the removal-degradation of benzene in water catalyzed by Co-MCM-41 in medium containing hydrogen peroxide. Microporous Mesoporous Mater. 2018, 258, 33–40. [Google Scholar] [CrossRef]
- Cesarino, I.; Cesarino, V.; Moraes, F.C.; Ferreira, T.C.R.; Lanza, M.R.V.; Mascaro, L.H.; Machado, S.A.S. Electrochemical degradation of benzene in natural water using silver nanoparticle-decorated carbon nanotubes. Mater. Chem. Phys. 2013, 141, 304–309. [Google Scholar] [CrossRef]
- Agrahari, G.K.; Verma, N.; Bhattacharya, P.K. Removal of benzoic acid from water by reactive extraction using hollow fiber membrane contactor: Experiment and modeling. Clean Soil Air Water 2014, 42, 901–908. [Google Scholar] [CrossRef]
- Al Bsoul, A.; Hailat, M.; Abdelhay, A.; Tawalbeh, M.; Al-Othman, A.; Al-kharabsheh, I.N.; Al-Taani, A.A. Efficient removal of phenol compounds from water environment using Ziziphus leaves adsorbent. Sci. Total Environ. 2021, 761, 143229. [Google Scholar] [CrossRef]
- Demissie, H.; An, G.; Jiao, R.; Ma, G.; Liu, L.; Sun, H.; Wang, D. Removal of phenolic contaminants from water by in situ coated surfactant on Keggin-aluminum nanocluster and biodegradation. Chemosphere 2021, 269, 128692. [Google Scholar] [CrossRef]
- Yu, L.; Yang, X.; Ye, Y.; Peng, X.; Wang, D. Silver nanoparticles decorated anatase TiO2 nanotubes for removal of pentachlorophenol from water. J. Colloid Interface Sci. 2015, 453, 100–106. [Google Scholar] [CrossRef]
- Jay, L.; Chirwa, E.E.M.N. Pathway analysis of phenol degradation by UV/TiO2 photocatalysis utilising the C-13 isotopic labelling technique. Chem. Eng. Trans. 2018, 70, 181–186. [Google Scholar] [CrossRef]
- Yao, Z.; Li, H.; Zhou, X.; Dong, Y.; Hang, Y.; Wang, Y. Preparation and photocatalysis degradation performances for phenol of TiO2/montmorillonite composites. Fuhe Cailiao Xuebao 2015, 32, 1581–1589. [Google Scholar] [CrossRef]
- Horová, D.; Nováková, J.; Pelíšková, L.; Kohout, J.; Šafář, J.; Hrachovcová, K.; Tokarová, V. Synthesis of MFI structured iron silicates and their catalytic performance in phenol removal by wet peroxide oxidation. React. Kinet. Mech. Catal. 2020, 130, 1077–1092. [Google Scholar] [CrossRef]
- Jothinathan, L.; Cai, Q.Q.; Ong, S.L.; Hu, J.Y. Organics removal in high strength petrochemical wastewater with combined microbubble-catalytic ozonation process. Chemosphere 2021, 263, 127980. [Google Scholar] [CrossRef]
- Asgari, G.; Rahmani, A.; Askari, F.B.; Godini, K. Catalytic ozonation of phenol using copper coated pumice and zeolite as catalysts. J. Res. Health Sci. 2012, 12, 93–97. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Gernjak, W.; Krutzler, T.; Glaser, A.; Malato, S.; Caceres, J.; Bauer, R.; Fernández-Alba, A.R. Photo-fenton treatment of water containing natural phenolic pollutants. Chemosphere 2003, 50, 71–78. [Google Scholar] [CrossRef]
- Carta, R.; Desogus, F. The enhancing effect of low power microwaves on phenol oxidation by the Fenton process. J. Environ. Chem. Eng. 2013, 1, 1292–1300. [Google Scholar] [CrossRef]
- Azizi, A.; Abouseoud, M.; Amrane, A. Phenol removal by a sequential combined fenton-enzymatic process. Nat. Environ. Pollut. Technol. 2017, 16, 321–330. [Google Scholar]
- Suryaman, D.; Hasegawa, K.; Kagaya, S. Combined biological and photocatalytic treatment for the mineralization of phenol in water. Chemosphere 2006, 65, 2502–2506. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Xia, B.; Zuo, W. Phenol Oxidation by Combined Cavitation Water Jet and Hydrogen Peroxide. Chin. J. Chem. Eng. 2012, 20, 760–767. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Wang, L.; Cheng, X.; Shang, Q. Rapid removal of phenol/antibiotics in water by Fe-(8-hydroxyquinoline-7-carboxylic)/TiO2 flower composite: Adsorption combined with photocatalysis. Chem. Eng. J. 2020, 402. [Google Scholar] [CrossRef]
- Ipek, I.Y.; Kabay, N.; Yüksel, M.; Yapici, D.; Yüksel, Ü. Application of adsorption-ultrafiltration hybrid method for removal of phenol from water by hypercrosslinked polymer adsorbents. Desalination 2012, 306, 24–28. [Google Scholar] [CrossRef]
- Salehi, E.; Shafie, M. Adsorptive removal of acetaldehyde from water using strong anionic resins pretreated with bisulfite: An efficient method for spent process water recycling in petrochemical industry. J. Water Process Eng. 2020, 33, 101025. [Google Scholar] [CrossRef]
- Emerging Pollutants in Water and Wastewater. Available online: https://en.unesco.org/emergingpollutantsinwaterandwastewater (accessed on 8 June 2021).
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.G.T.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Gong, R.; Ye, J.; Dai, W.; Yan, X.; Hu, J.; Hu, X.; Li, S.; Huang, H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with finger-citron-residue-based activated carbon. Ind. Eng. Chem. Res. 2013, 52, 14297–14303. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Han, X.; Wu, Z.; Lai, C.; Wang, J.; Deng, Q.; Zeng, Z.; Deng, S. Simultaneous and efficient removal of Cr(VI) and methyl orange on LDHs decorated porous carbons. Chem. Eng. J. 2018, 352, 306–315. [Google Scholar] [CrossRef]
- Chaukura, N.; Murimba, E.C.; Gwenzi, W. Synthesis, characterisation and methyl orange adsorption capacity of ferric oxide-biochar nano-composites derived from pulp and paper sludge. Appl. Water Sci. 2017, 7, 2175–2186. [Google Scholar] [CrossRef] [Green Version]
- Kisku, G.C.; Markandeya; Shukla, S.P.; Singh, D.S.; Murthy, R.C. Characterization and adsorptive capacity of coal fly ash from aqueous solutions of disperse blue and disperse orange dyes. Environ. Earth Sci. 2015, 74, 1125–1135. [Google Scholar] [CrossRef]
- Markandeya; Dhiman, N.; Shukla, S.P.; Kisku, G.C. Statistical optimization of process parameters for removal of dyes from wastewater on chitosan cenospheres nanocomposite using response surface methodology. J. Clean. Prod. 2017, 149, 597–606. [Google Scholar] [CrossRef]
- Ghanbari, M.; Salavati-Niasari, M. Copper iodide decorated graphitic carbon nitride sheets with enhanced visible-light response for photocatalytic organic pollutant removal and antibacterial activities. Ecotoxicol. Environ. Saf. 2021, 208, 111712. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, Y.; Sabbaghi, S.; Abouzari-Lotf, E.; Jahangirian, H.; Sairi, N.A. A new achievement in green degradation of aqueous organic pollutants under visible-light irradiation. Water Sci. Technol. 2018, 77, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Almaamary, E.A.S.; Abdullah, S.R.S.; Hasan, H.A.; Rahim, R.A.A.; Idris, M. Rawatan metilena biru dalam air sisa menggunakan Scirpus grossus. Malays. J. Anal. Sci. 2017, 21, 182–187. [Google Scholar] [CrossRef]
- Al-Zurfi, S.K.L.; Albdairi, N.A.; Almadany, S.A. Phytoremediation of Congo red and brilliant green using Lemna minor. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 1–10. [Google Scholar]
- Sharma, R.; Saini, H.; Paul, D.R.; Chaudhary, S.; Nehra, S.P. Removal of organic dyes from wastewater using Eichhornia crassipes: A potential phytoremediation option. Environ. Sci. Pollut. Res. 2021, 28, 7116–7122. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, K.; Zhang, W.; Bai, Y.; Sun, Y.; Gu, J. Graphene oxide quantum dots incorporated into a thin film nanocomposite membrane with high flux and antifouling properties for low-pressure nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 11082–11094. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Efficient removal of dyes from water by high flux and superior antifouling polyethersulfone hollow fiber membranes modified with ZnO/cGO nanohybrid. J. Water Process Eng. 2019, 29, 100783. [Google Scholar] [CrossRef]
- Hu, F.; Wang, Z.; Zhu, B.; Zhu, L.; Xu, Y. Poly (N-vinyl imidazole) gel-filled membrane adsorbers for highly efficient removal of dyes from water. J. Chromatogr. A 2018, 1563, 198–206. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdou, A.E.H.; Shehata, A.K.; Header, H.M.A.; Hamed, E.A. Sustainable super fast adsorptive removal of Congo red dye from water by a novel technique based on microwave-enforced sorption process. J. Ind. Eng. Chem. 2018, 57, 28–36. [Google Scholar] [CrossRef]
- Zhang, J.J.; Fang, S.S.; Mei, J.Y.; Zheng, G.P.; Zheng, X.C.; Guan, X.X. High-efficiency removal of rhodamine B dye in water using g-C3N4 and TiO2 co-hybridized 3D graphene aerogel composites. Sep. Purif. Technol. 2018, 194, 96–103. [Google Scholar] [CrossRef]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Substances Identified as Endocrine Disruptors at EU Level. Available online: https://edlists.org/the-ed-lists/list-i-substances-identified-as-endocrine-disruptors-by-the-eu (accessed on 10 June 2021).
- Xu, J.; Wang, L.; Zhu, Y. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef]
- Substances under Evaluation for Endocrine Disruption under an EU Legislation. Available online: https://edlists.org/the-ed-lists/list-ii-substances-under-eu-investigation-endocrine-disruption (accessed on 10 June 2021).
- Phuekphong, A.F.; Imwiset, K.J.; Ogawa, M. Organically modified bentonite as an efficient and reusable adsorbent for triclosan removal from water. Langmuir 2020, 36, 9025–9034. [Google Scholar] [CrossRef] [PubMed]
- Moeini, Z.; Azhdarpoor, A.; Yousefinejad, S.; Hashemi, H. Removal of atrazine from water using titanium dioxide encapsulated in salicylaldehyde–NH2–MIL-101 (Cr): Adsorption or oxidation mechanism. J. Clean. Prod. 2019, 224, 238–245. [Google Scholar] [CrossRef]
- Limmun, W.; Ito, A.; Ishikawa, N.; Momotori, J.; Kawamura, Y.; Majima, Y.; Sasamoto, M.; Umita, T. Removal of nonylphenol and nonylphenol monoethoxylate from water and anaerobically digested sewage sludge by Ferrate(VI). Chemosphere 2019, 236, 124399. [Google Scholar] [CrossRef]
- Akbari-Adergani, B.; Saghi, M.H.; Eslami, A.; Mohseni-Bandpei, A.; Rabbani, M. Removal of dibutyl phthalate from aqueous environments using a nanophotocatalytic Fe, Ag-ZnO/VIS-LED system: Modeling and optimization. Environ. Technol. 2018, 39, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Palharim, P.H.; Graça, C.A.L.; Teixeira, A.C.S.C. Comparison between UVA- and zero-valent iron-activated persulfate processes for degrading propylparaben. Environ. Sci. Pollut. Res. 2020, 27, 22214–22224. [Google Scholar] [CrossRef]
- Orhon, K.B.; Orhon, A.K.; Dilek, F.B.; Yetis, U. Triclosan removal from surface water by ozonation—Kinetics and by-products formation. J. Environ. Manag. 2017, 204, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef]
- Rastgar, M.; Shakeri, A.; Karkooti, A.; Asad, A.; Razavi, R.; Sadrzadeh, M. Removal of trace organic contaminants by melamine-tuned highly cross-linked polyamide TFC membranes. Chemosphere 2020, 238, 124691. [Google Scholar] [CrossRef]
- Wei, X.; Shi, Y.; Fei, Y.; Chen, J.; Lv, B.; Chen, Y.; Zheng, H.; Shen, J.; Zhu, L. Removal of trace phthalate esters from water by thin-film composite nanofiltration hollow fiber membranes. Chem. Eng. J. 2016, 292, 382–388. [Google Scholar] [CrossRef]
- López-Ortiz, C.M.; Sentana-Gadea, I.; Varó-Galvañ, P.; Maestre-Pérez, S.E.; Prats-Rico, D. The use of combined treatments for reducing parabens in surface waters: Ion-exchange resin and nanofiltration. Sci. Total Environ. 2018, 639, 228–236. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Koutník, I.; Vráblová, M.; Bednárek, J. Reynoutria japonica, an invasive herb as a source of activated carbon for the removal of xenobiotics from water. Bioresour. Technol. 2020, 309, 123315. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Mahbub, M.K.B.; Wilhelm, M.; Lima, E.C.; Sampaio, C.H.; Saucier, C.; Dias, S.L.P. Activated carbon from sewage sludge for removal of sodium diclofenac and nimesulide from aqueous solutions. Korean J. Chem. Eng. 2016, 33, 3149–3161. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; Özdemir, O.K.; Şahin, S.; Haşimoğlu, A. Naproxen adsorption onto graphene oxide nanopowders: Equilibrium, kinetic, and thermodynamic studies. Water Air Soil Pollut. 2020, 231. [Google Scholar] [CrossRef]
- Ali, I.; Afshinb, S.; Poureshgh, Y.; Azari, A.; Rashtbari, Y.; Feizizadeh, A.; Hamzezadeh, A.; Fazlzadeh, M. Green preparation of activated carbon from pomegranate peel coated with zero-valent iron nanoparticles (nZVI) and isotherm and kinetic studies of amoxicillin removal in water. Environ. Sci. Pollut. Res. 2020, 27, 36732–36743. [Google Scholar] [CrossRef]
- Changanaqui, K.; Alarcón, H.; Brillas, E.; Sirés, I. Blue LED light-driven photoelectrocatalytic removal of naproxen from water: Kinetics and primary by-products. J. Electroanal. Chem. 2020, 867, 114192. [Google Scholar] [CrossRef]
- Martins, M.; Mourato, C.; Sanches, S.; Noronha, J.P.; Crespo, M.T.B.; Pereira, I.A.C. Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds. Water Res. 2017, 108, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Jayasree, P.; Remya, N. Photocatalytic degradation of paracetamol using aluminosilicate supported TiO2. Water Sci. Technol. 2020, 82, 2114–2124. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Ni, J.; Xu, Y.; Song, N.; Gao, M. Highly efficient removal of amoxicillin from water by three-dimensional electrode system within granular activated carbon as particle electrode. J. Water Process Eng. 2020, 38, 101656. [Google Scholar] [CrossRef]
- Tepe, O.; Tunç, Z.; Yıldız, B.; Şahin, M. Efficient removal of paracetamol by manganese oxide octahedral molecular sieves (OMS-2) and persulfate. Water Air Soil Pollut. 2020, 231, 238. [Google Scholar] [CrossRef]
- Periyasamy, S.; Muthuchamy, M. Electrochemical oxidation of paracetamol in water by graphite anode: Effect of pH, electrolyte concentration and current density. J. Environ. Chem. Eng. 2018, 6, 7358–7367. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.; Chen, Z.; Shen, B.; Wei, J.; Zeng, P.; Wen, X. Catalytic ozonation for metoprolol and ibuprofen removal over different MnO2 nanocrystals: Efficiency, transformation and mechanism. Sci. Total Environ. 2021, 785, 147328. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).