Effects of Constant Electric Field on Biodegradation of Phenol by Free and Immobilized Cells of Bradyrhizobium japonicum 273
Abstract
:1. Introduction
- How does the electric field affect the microbial growth of this strain?
- How is the optimum anode potential for bioelectrochemical oxidation of phenol established?
- How does the application of an electric field affect enzyme oxidation of phenol and catechol?
- How is enzyme activity affected by the electric field for free cultures?
- What is the impact of the electric field on the microbial cells immobilized on activated carbon to degrade phenol combined with bioelectrochemical stimulation?
2. Materials and Methods
2.1. Free Cell Cultivation
2.2. Immobilization Technique
2.3. Experimental Conditions
2.4. Effect of Physical Adsorption
2.5. Analyses
2.5.1. Phenol Concentration
2.5.2. Biomass
2.5.3. Phenol Hydroxylase Activity
2.5.4. Catechol 1,2-Dioxygenase Activity
2.5.5. Catechol 2,3-Dioxygenase Activity
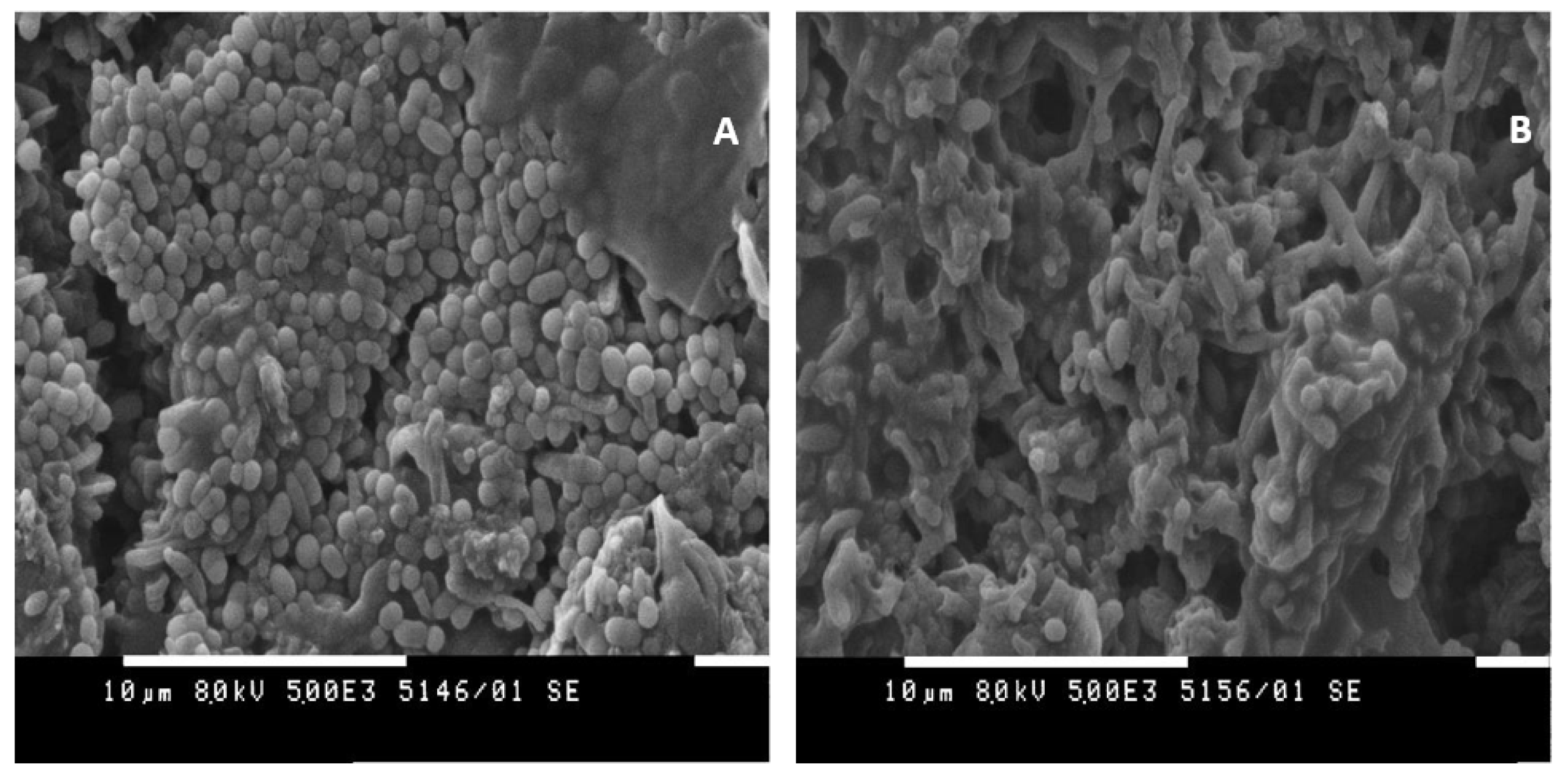
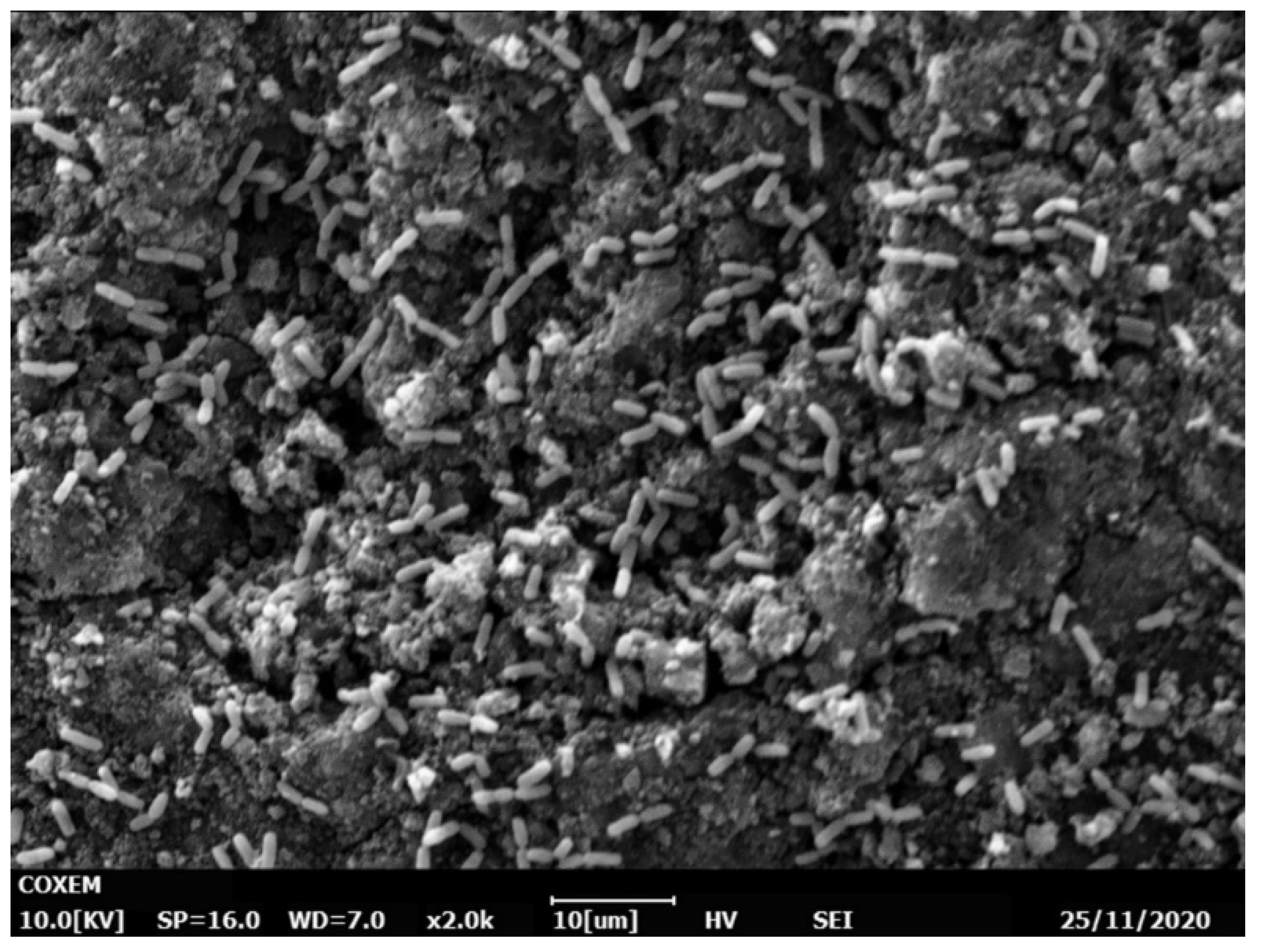
2.5.6. Scanning Electron Microscopy (SEM)
3. Results
3.1. Physical Adsorption of Phenol by Granulated Activated Carbon
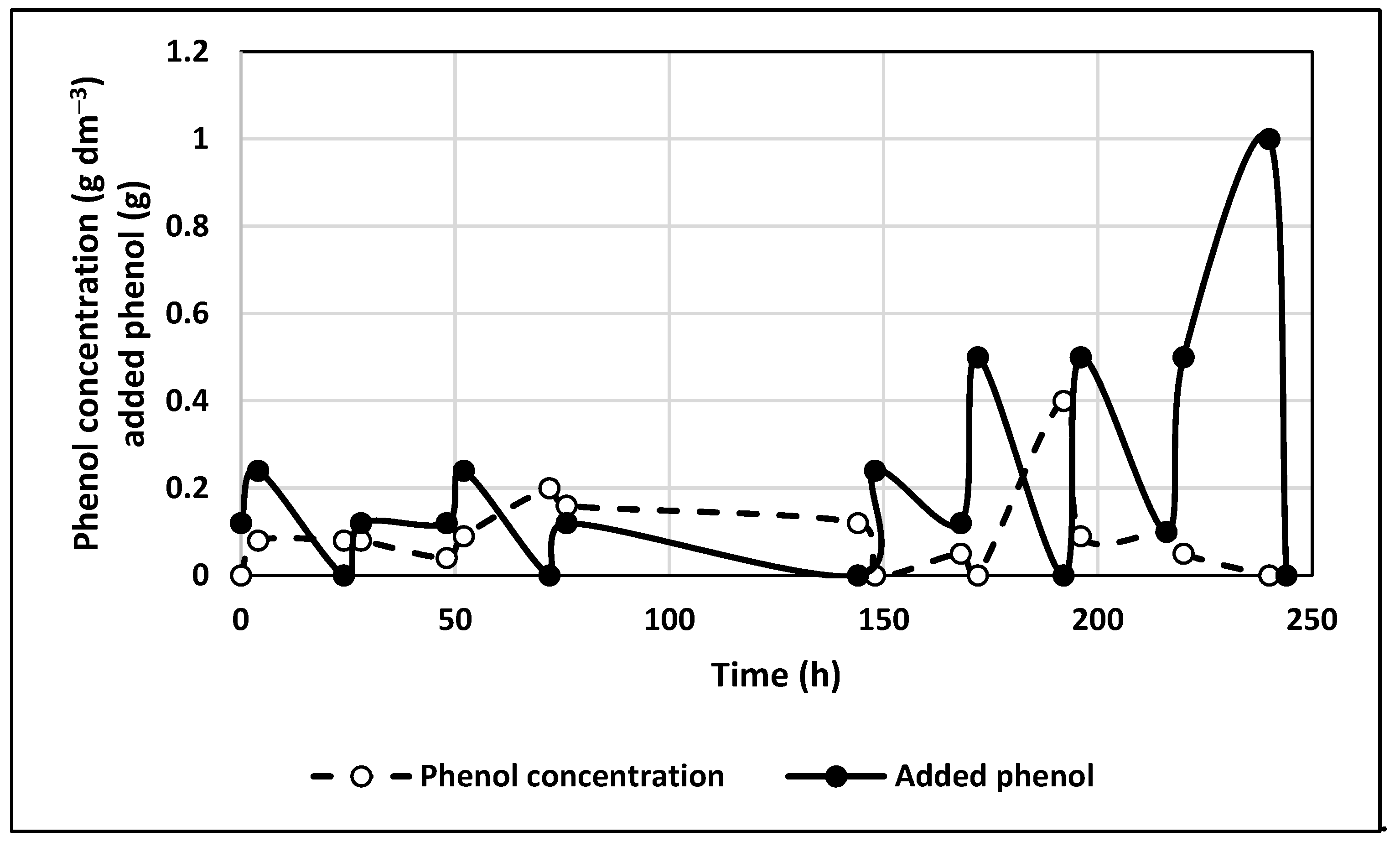
3.2. Experiments with Free Cells without a Constant Electric Field
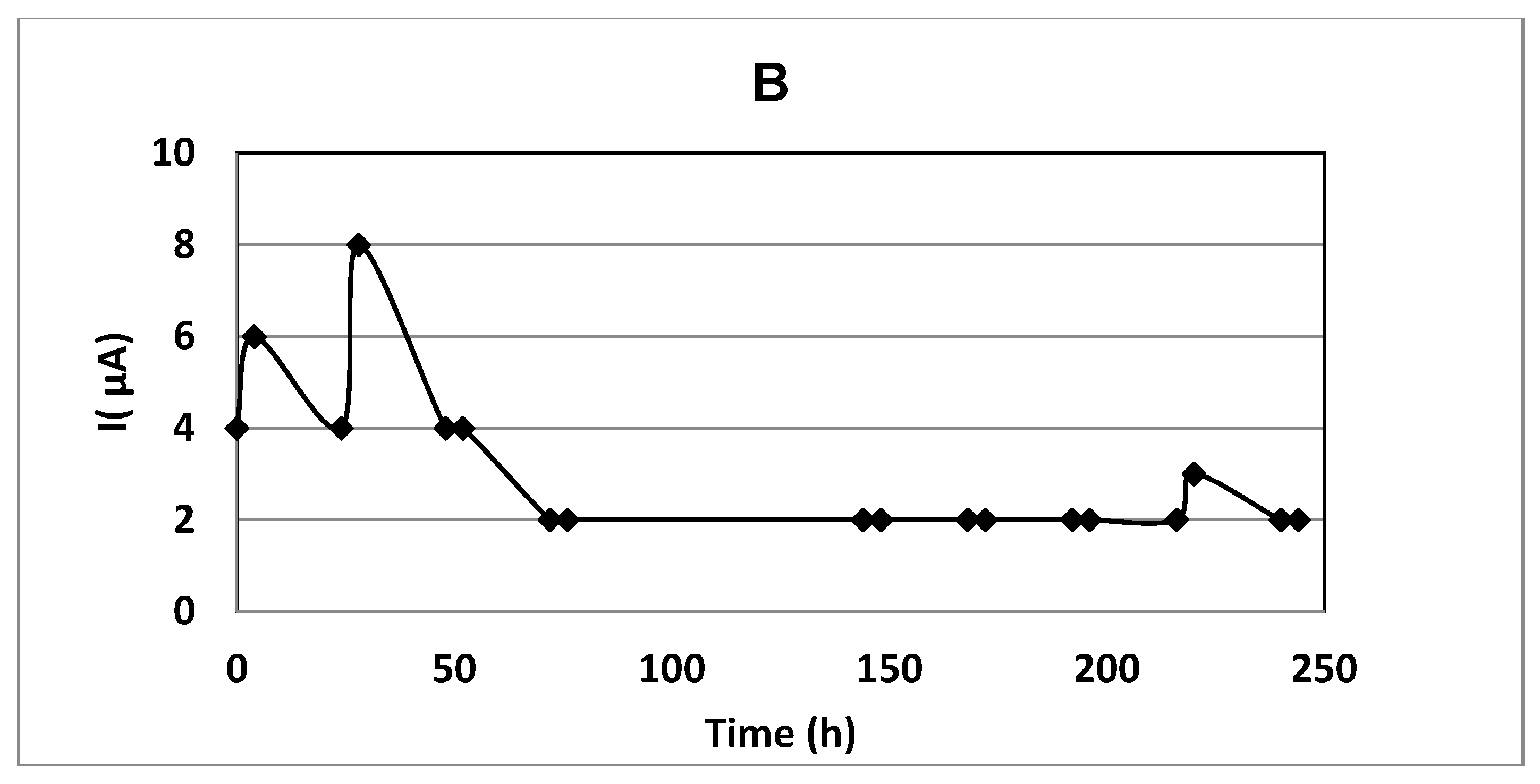
3.3. Experiments with Free Cells with a Constant Electric Field
3.4. Effect of Electric Field on Enzyme Activity of Free Cells
3.5. Experiments with Immobilized Cells without a Constant Electric Field
3.6. Experiments in Immobilized Culture with the Application of a Constant Electric Field
4. Discussion
5. Conclusions
- The bacteria Bradyrhizobium japonicum 273 were capable to degrade phenol at moderate concentrations either in a free culture or as immobilized ones on granulated activated carbon particles. The amount of degraded phenol was greater in an immobilized cell preparation than in a free culture. This fact could be explained by the additional effects of physical adsorption and, therefore, the facilitated biodegradation.
- The constant electric field applied during cells cultivation led to increased phenol degradation in both free and immobilized cells cultures. The best results were observed for an anode potential of 1.0 V/S.H.E. The effect was relatively better pronounced in the case of free cultures: the biodegraded amount of phenol was more than 4 times greater when an electric field was applied at an anode potential of 1.0 V/S.H.E, whereas for immobilized cells, the increase in this amount was 67%. The explanation is that the biodegradation rate by immobilized cells without an electric field application was already more than 4 times higher, compared with the case of the free ones. The combination of immobilization and application of a constant electric field provided synergic results: the degraded amount of phenol was 7.3 times higher than for free culture when no electric field was applied.
- It was observed that the enzyme activities for the first two steps of the phenol catabolic pathway of phenol biodegradation—phenol oxidation (phenol hydroxylase) and benzene ring cleavage (catechol-1,2-dioxygenase and catechol-2,3-dioxygenase)—in free cells were very sensitive to the anode potential. The enzyme activities varied in time and with the anode potential. It was even observed that at an anode potential of 0.8 V/S.H.E., the meta-pathway of cleavage of the benzene ring catalyzed by catechol-2,3-dioxygenase became competitive with the ortho-pathway, catalyzed by catechol-1,2-dioxygenase.
- The increased enzyme activities, together with the calculated biodegraded amounts of phenol under the influence of electric current, compared with the analytical data, showed that the positive effect on the biodegradation of phenol in a constant electric field was due to electrical stimulation of enzyme activity rather than electrochemical anode oxidation. The obtained synergic results for phenol biodegradation may induce researchers to conduct further studies on continuous processes with constant electric field application on immobilized cell cultures.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, M. The Merck Index: An Encyclopedia of Chemical, Drugs, and Biologicals, 15th ed.; O’Neil, M.J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; ISBN 9781849736701. [Google Scholar] [CrossRef]
- Phenol. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000; ISBN 9783527306732. [Google Scholar] [CrossRef]
- World Health Organization/International Labour Organization: International Chemical Safety Cards. 2017. Available online: http://www.inchem.org/documents/icsc/icsc/eics0070.htm (accessed on 18 August 2021).
- Caetano, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Phenol removal from aqueous solution by adsorption and ion exchange mechanisms onto polymeric resins. J. Colloid Interface Sci. 2009, 338, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.L.; Moure, A.; Dominguez, H.; Parajу, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Victor-Ortega, M.D.; Ochando-Pulido, J.M.; Martinez-Ferez, A. Modeling of fixed bed column studies for iron removal from industrial effluents through ion exchange process. Chem. Eng. Trans. 2016, 47, 253–258. [Google Scholar] [CrossRef]
- Allsop, P.J.; Chisti, Y.; Moo-Young, M.; Sullivan, G.R. Dynamics of phenol degradation by Pseudomonas putida. Biotechnol. Bioeng. 1993, 41, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Neumegen, R.A.; Fernandez-Alba, A.R.; Chisti, Y. Toxicities of triclosan, phenol, and copper sulfate in activated sludge. Environ. Toxicol. 2005, 20, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Sridevi, V.; Lakshmi, M.V.V.C.; Manasa, M.; Sravani, M. Metabolic pathways for the biodegradation of phenol. Int. J. Eng. Sci. Adv. Technol. 2012, 2, 695–705. [Google Scholar]
- Hinteregger, C.; Leitner, R.; Loidl, M.; Ferschl, A.; Streichsbier, F. Degradation of phenol and phenolic compounds by Pseudomonas putida EKII. Appl. Microbiol. Biotechnol. 1992, 37, 252–259. [Google Scholar] [CrossRef]
- Beschkov, V.; Alexieva, Z.; Parvanova-Mancheva, T.; Vasileva, E.; Gerginova, M.; Peneva, N.; Stoyanova, K. Phenol biodegradation by the strain Pseudomonas putida affected by constant electric field. Int. J. Environ. Sci. Technol. 2020, 17, 1929–1936. [Google Scholar] [CrossRef]
- Lakshmi, M.V.V.C.; Sridevi, V. A review on biodegradation of phenol from industrial effluents. J. Ind. Pollut. Control 2009, 25, 13–27. [Google Scholar]
- Li, Q.; Kang, C.; Zhang, C. Waste water produced from an oilfield and continuous treatment with an oil-degrading bacterium. Process Biochem. 2005, 40, 873–877. [Google Scholar] [CrossRef]
- Dey, S.K.; Mukherjee, A. Catechol oxidase and phenoxazinone synthase: Biomimetic functional models and mechanistic studies. Coord. Chem. Rev. 2016, 310, 80–115. [Google Scholar] [CrossRef]
- Hopper, W.; Mahadevan, A. Degradation of catechin by Bradyrhizobium japonicum. Biodegradation 1997, 8, 159–165. [Google Scholar] [CrossRef]
- Vasileva, E.; Parvanova-Mancheva, T.; Beschkov, V. The Bradyrhizobium japonicum 273 strain’ s ability to degrade phenol. Part 1. J. Int. Sci. Publ. Mater. Methods Technol. 2020, 14, 165–176. Available online: https://www.scientific-publications.net/en/article/1002058/ (accessed on 8 October 2021).
- Jobin, L.; Namour, P. Bioremediation in water environment: Controlled electro-stimulation of organic matter self-purification in aquatic environments. Adv. Microbiol. 2017, 7, 813–852. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Tong, S.; Chen, N.; Liu, Y.; Feng, C.; Hu, Q. Effect of electro-stimulation on activity of heterotrophic denitrifying bacteria and denitrification performance. Bioresour. Technol. 2015, 196, 123–128. [Google Scholar] [CrossRef]
- Ailijiang, N.; Chang, J.; Wu, Q.; Li, P.; Liang, P.; Zhang, X.X.; Huang, X. Phenol degradation by suspended biomass in aerobic/anaerobic electrochemical reactor. Water Air Soil Pollut. 2016, 227, 233. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, X.; Yan, Y.; Li, T.; An, J.; Liao, C.M.; Li, N.; Wang, X. Electrode potential regulates phenol degradation pathways in oxygen-diffused microbial electrochemical system. Chem. Eng. J. 2020, 381, 122663. [Google Scholar] [CrossRef]
- Nakazawa, T.; Nakazawa, A. [64] Pyrocatechase (pseudomonas). Methods Enzymol. 1970, 17, 518–522. [Google Scholar]
- Baggi, G.; Barbieri, P.; Galli, E.; Tollari, S. Isolation of a Pseudomonas stutzeri strain that degrades o-xylene. Appl. Environ. Microbiol. 1987, 53, 2129–2132. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC204069/ (accessed on 8 October 2021).
- Lazarkevich, I.; Sotirova, T.; Avramova, S.; Stoitsova, S.; Paunova-Krasteva, T.; Galabova, D. Antibacterial activity of methyltiosulfonate and its complexes with rhamnolipid and trehalose lipid against Pseudomonas aeroginosa NBMCC 1390. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 282–290. [Google Scholar]
- Sotirova, A.; Avramova, T.; Stoitsova, S.; Lazarkevich, I.; Lubenets, V.; Karpenko, E.; Galabova, D. The importance of rhamnolipid-biosurfactant-induced changes in bacterial membrane lipids of Bacillus subtilis for the antimicrobial activity of thiosulfonates, Curr. Microbiol. 2012, 65, 534–541. [Google Scholar] [CrossRef]
- Field, S.J.; Thornton, N.P.; Anderson, L.J.; Gates, A.J.; Reilly, A.; Jepson, B.J.N.; Richardson, D.J.; George, S.J.; Cheesman, M.R.; Butt, J.N. Reductive activation of nitrate reductases. Dalton Trans. 2005, 21, 3580–3586. [Google Scholar] [CrossRef] [PubMed]




| Free Culture/Anode Potential, V/S.H.E. | Immobilized Cells | |||||
|---|---|---|---|---|---|---|
| No Electric Field | 0.8 | 0.9 | 1.0 | No Electric Field | 1.0 V/S.H.E. | |
| Total amount phenol degraded, g | 0.9 ± 0.04 | 1.0 ± 0.2 | 2.2 ± 0.4 | 4.2 ± 0.4 | 3.9 + 0.4 | 6.6 + 0.6 |
| Specific microbial growth rate, h−1 | 0.09 ± 0.005 | 0.07 ± 0.01 | 0.05 ± 0.005 | 0.08 ± 0.005 | Not detected | Not detected |
| Anode Potential, V/S.H.E | Time, h | Enzyme Activity, U/mg Protein | ||
|---|---|---|---|---|
| Phenol Hydroxylase | Catechol-1,2-dioxygenase | Catechol-2,3-dioxygenase | ||
| 72 | 0.025 ± 0.004 | 0.033 ± 0.019 | 0 | |
| No field | 144 | 0.058 ± 0.003 | 0.358 ± 0.013 | 0.019 ± 0.008 |
| 240 | 0.110 ± 0.003 | 0.93 ± 0.013 | 0.094 ± 0.003 | |
| 76 | 0.118 ± 0.009 | 0.124 ± 0.010 | 0.113 ± 0.002 | |
| 0.8 | 144 | 0.184 ± 0.006 | 0.571 ± 0.014 | 0.187 ± 0.005 |
| 240 | 0.102 ± 0.005 | 0.514 ± 0.013 | 0.431 ± 0.002 | |
| 0.9 | 144 | 0.1 ± 0.003 | 0.127 ± 0.002 | 0.006 ± 0.006 |
| 240 | 0.285 ± 0.006 | 0.371 ± 0.005 | 0.976 ± 0.005 | |
| 76 | 0.044 ± 0.006 | 0.057 ± 0.012 | 0.015 ± 0.006 | |
| 1.0 | 144 | 0.116 ± 0.003 | 0.372 ± 0.009 | 0.026 ± 0.005 |
| 240 | 0.270 ± 0.009 | 1.67 ± 0.007 | 0.071 ± 0.003 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, E.; Parvanova-Mancheva, T.; Beschkov, V.; Alexieva, Z.; Gerginova, M.; Peneva, N. Effects of Constant Electric Field on Biodegradation of Phenol by Free and Immobilized Cells of Bradyrhizobium japonicum 273. ChemEngineering 2021, 5, 75. https://doi.org/10.3390/chemengineering5040075
Vasileva E, Parvanova-Mancheva T, Beschkov V, Alexieva Z, Gerginova M, Peneva N. Effects of Constant Electric Field on Biodegradation of Phenol by Free and Immobilized Cells of Bradyrhizobium japonicum 273. ChemEngineering. 2021; 5(4):75. https://doi.org/10.3390/chemengineering5040075
Chicago/Turabian StyleVasileva, Evgenia, Tsvetomila Parvanova-Mancheva, Venko Beschkov, Zlatka Alexieva, Maria Gerginova, and Nadejda Peneva. 2021. "Effects of Constant Electric Field on Biodegradation of Phenol by Free and Immobilized Cells of Bradyrhizobium japonicum 273" ChemEngineering 5, no. 4: 75. https://doi.org/10.3390/chemengineering5040075
APA StyleVasileva, E., Parvanova-Mancheva, T., Beschkov, V., Alexieva, Z., Gerginova, M., & Peneva, N. (2021). Effects of Constant Electric Field on Biodegradation of Phenol by Free and Immobilized Cells of Bradyrhizobium japonicum 273. ChemEngineering, 5(4), 75. https://doi.org/10.3390/chemengineering5040075







