Study of the Rheological Properties of PVC Composites Plasticized with Butoxyethyl Adipates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Methods
2.2.1. Synthesis of Ethoxylated Butanol
2.2.2. The Synthesis of Esters of Adipic Acid and Ethoxylated Butanol
2.3. Characterization
2.3.1. Analysis of the Physico-Chemical Characteristics of Adipates
2.3.2. Characterization of Adipates
2.3.3. High Performance Liquid Chromatography
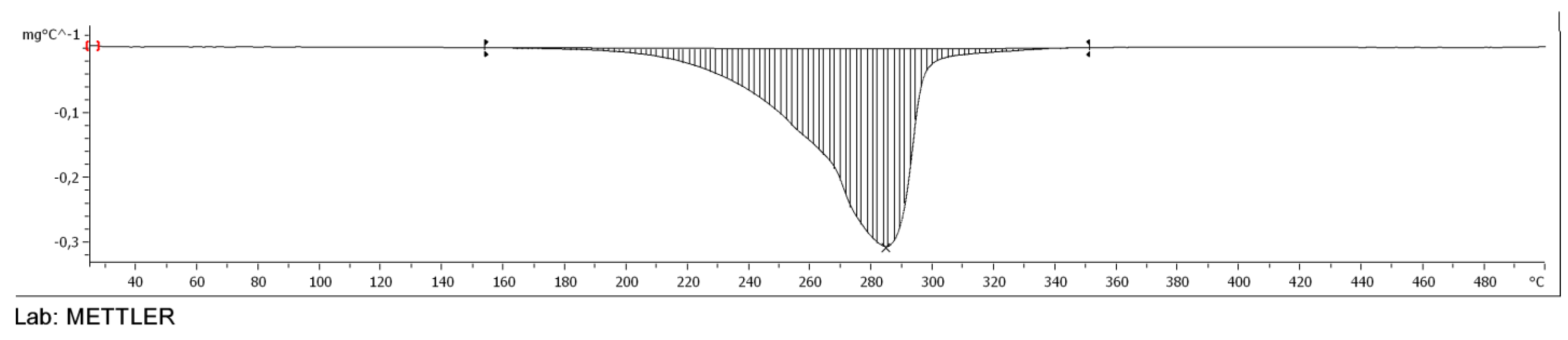
2.3.4. TGA of Esters
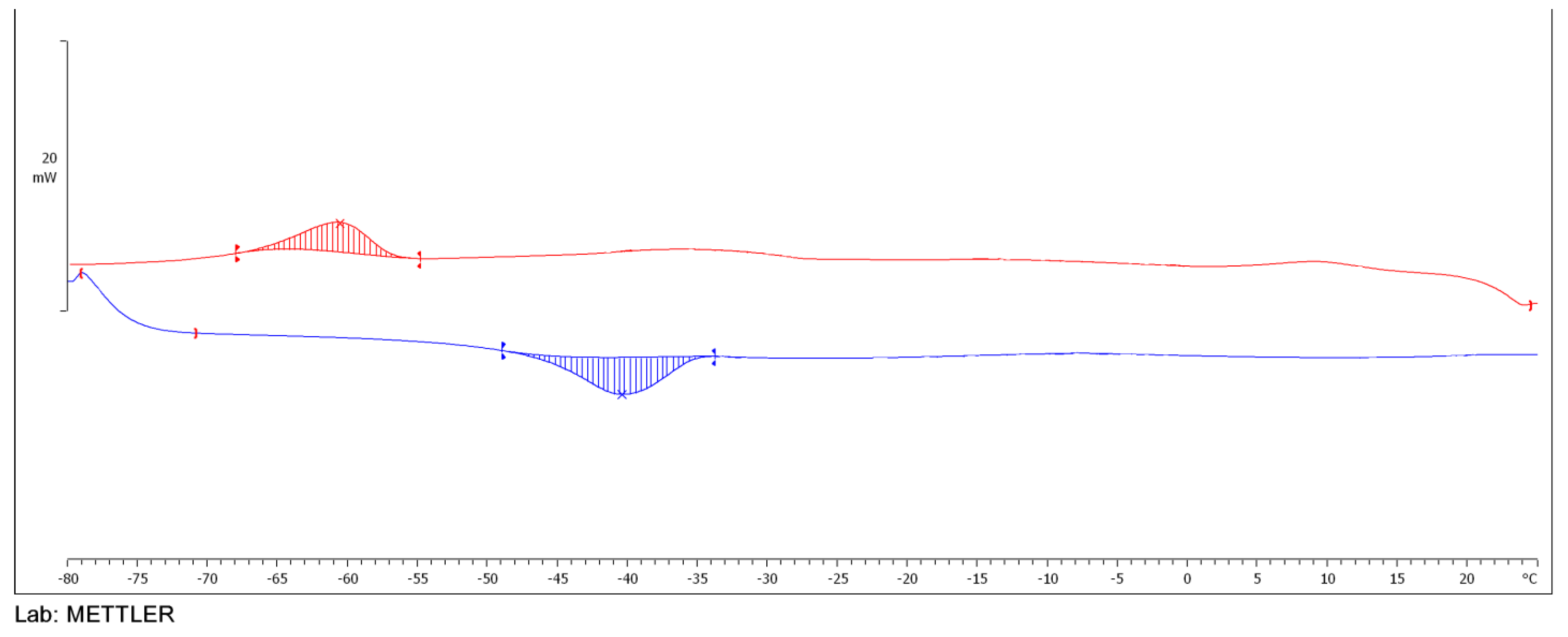
2.3.5. DSC Analysis of Esters
2.3.6. Determination of the Melt Flow Rate of Polymer Composition
2.4. Preparation of Film Samples
3. Results and Discussion
3.1. Synthesis of Butoxyethanol
3.2. The Synthesis of Esters of Adipic Acid and Butoxyethanol
3.3. High Performance Liquid Chromatography
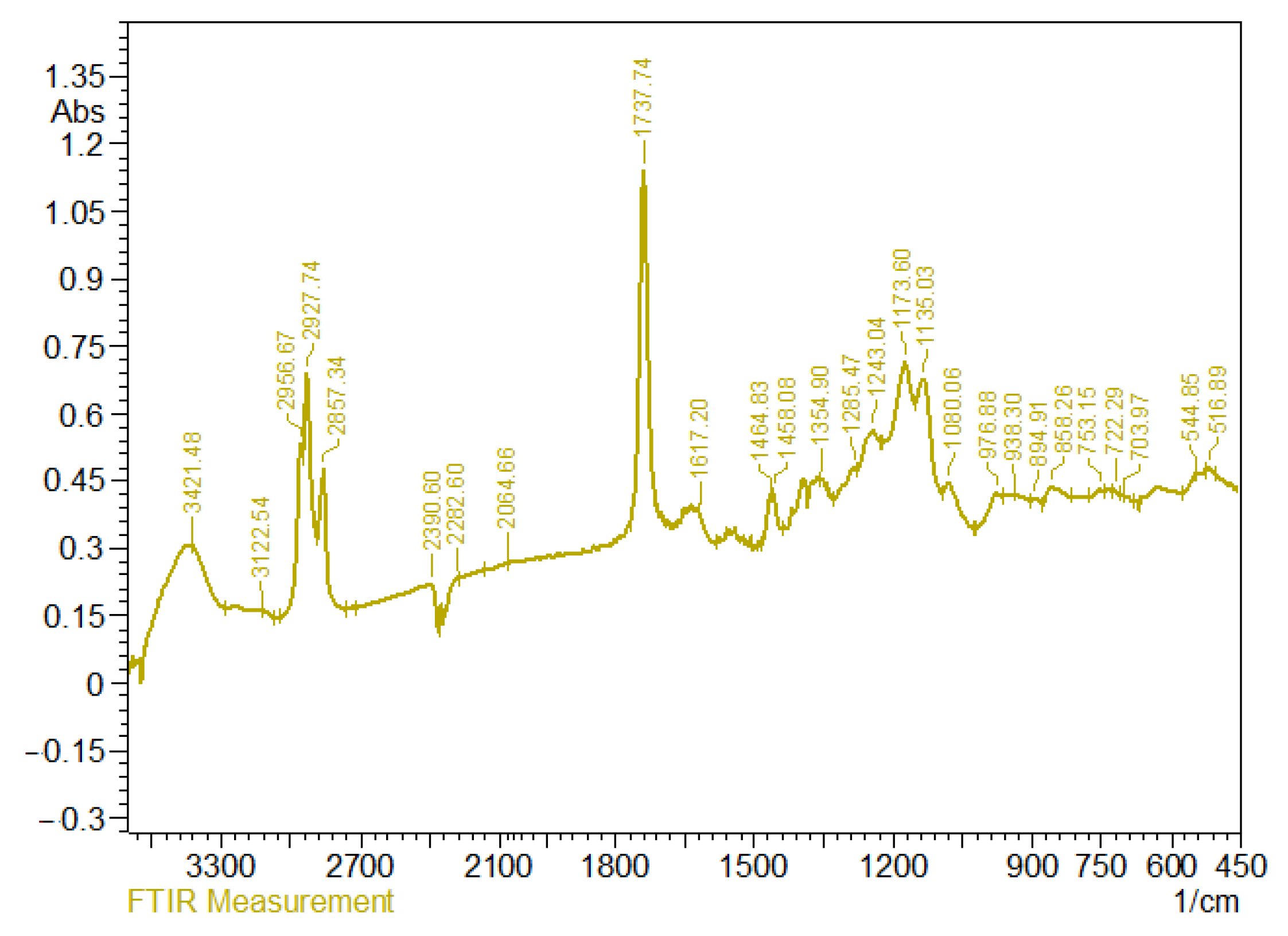
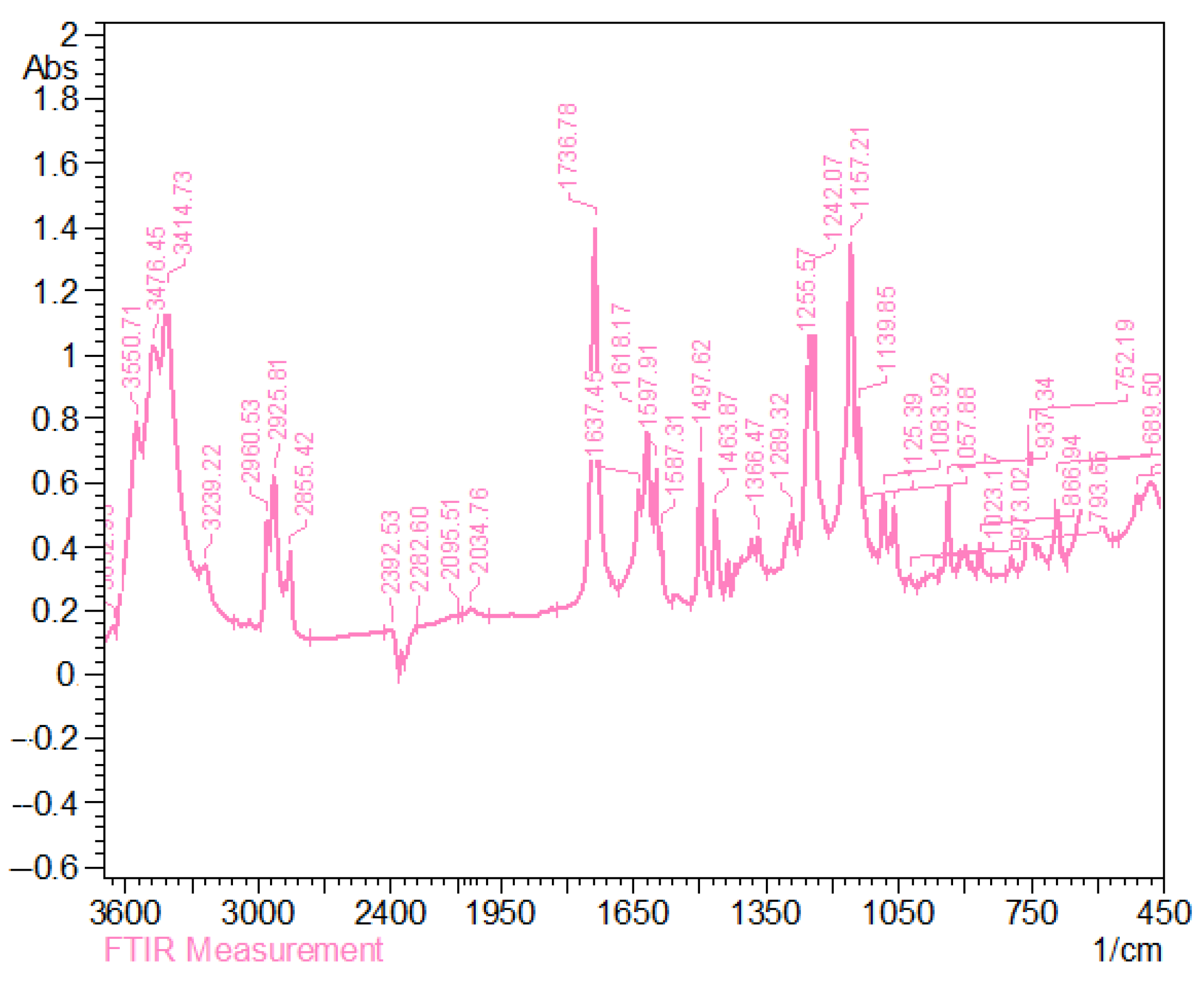
3.4. IR Spectra
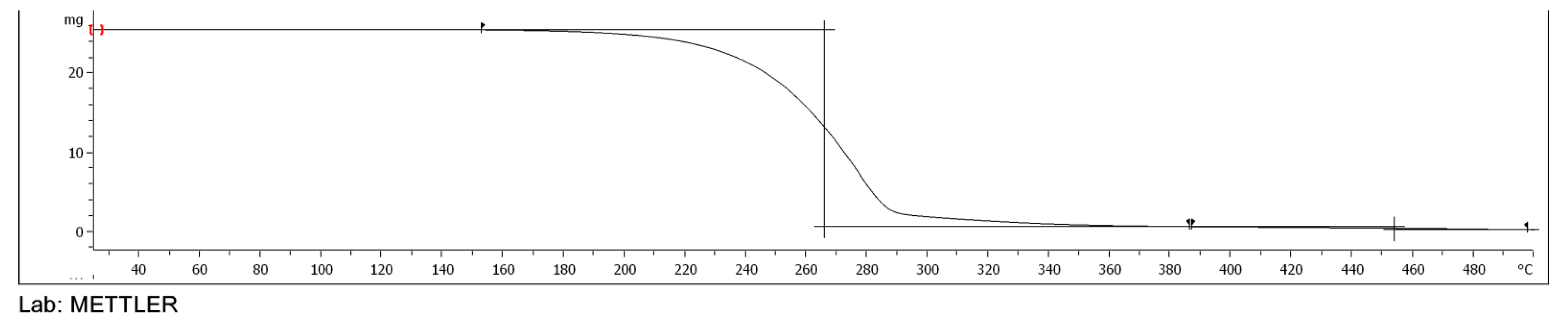
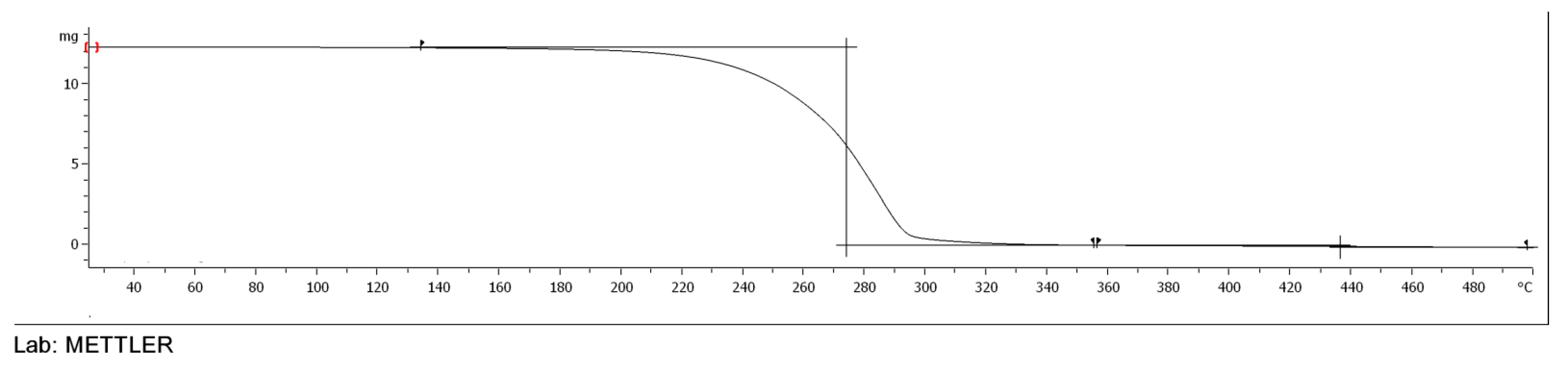
3.5. TGA of Esters of Adipic Acid
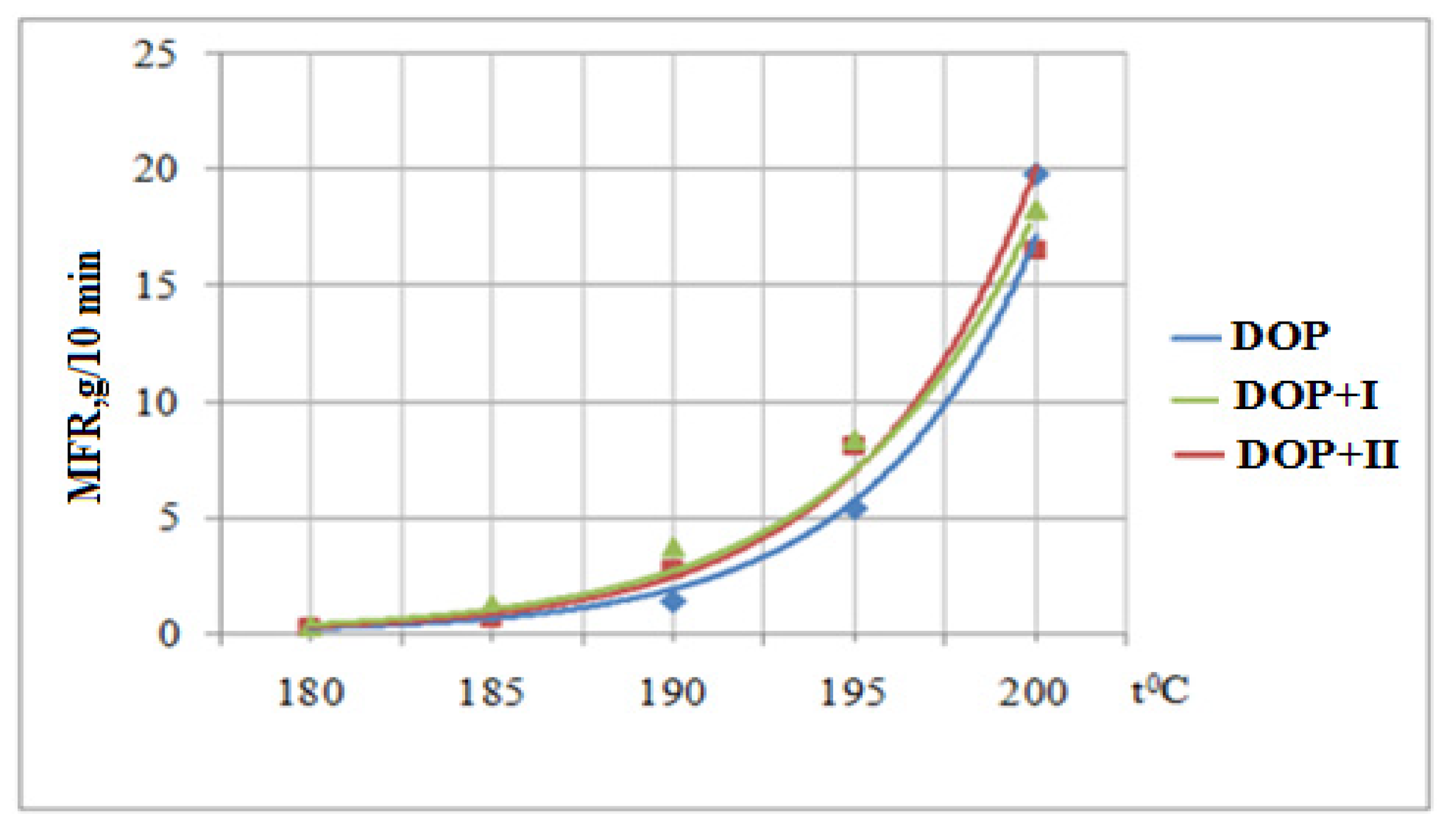
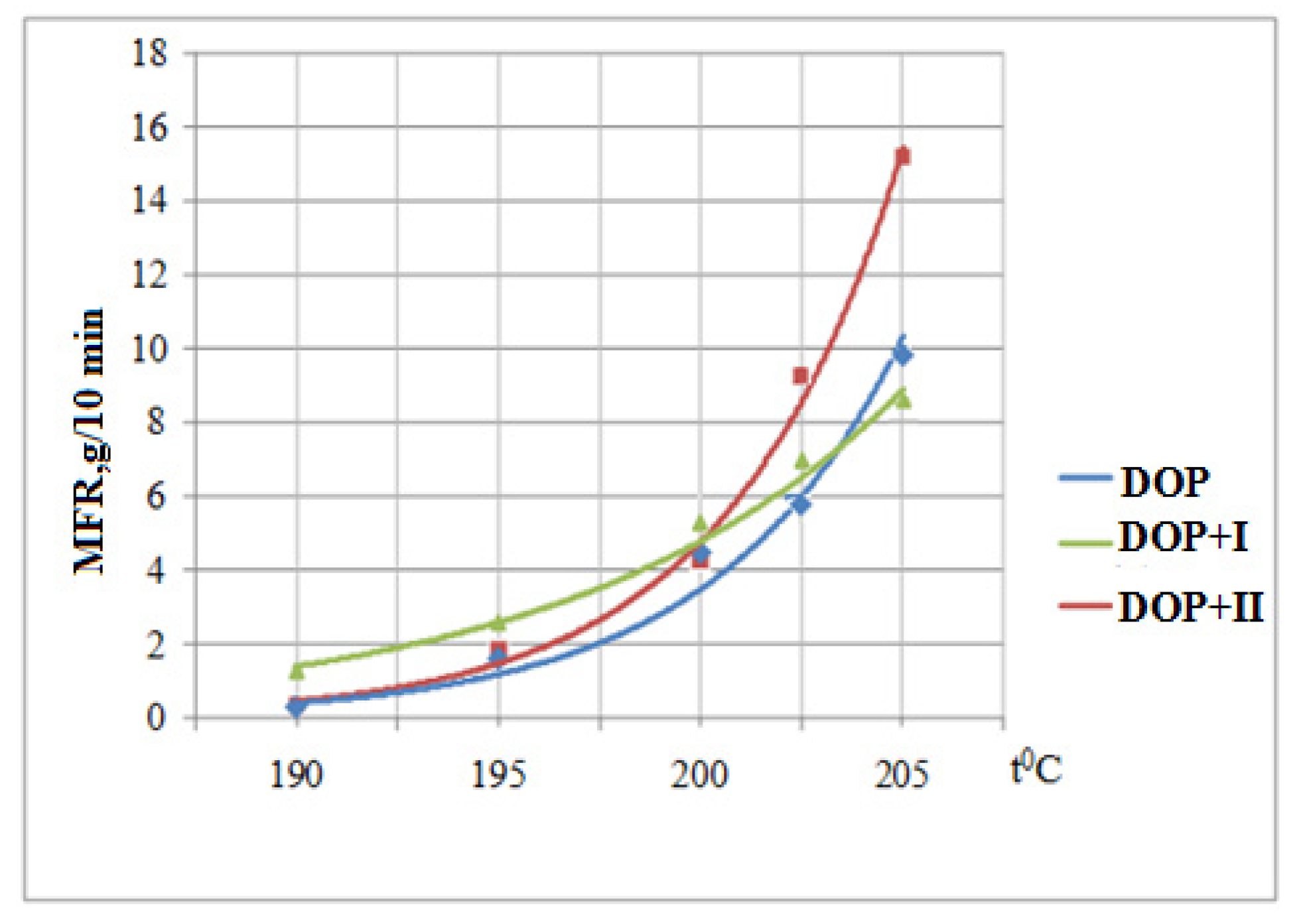
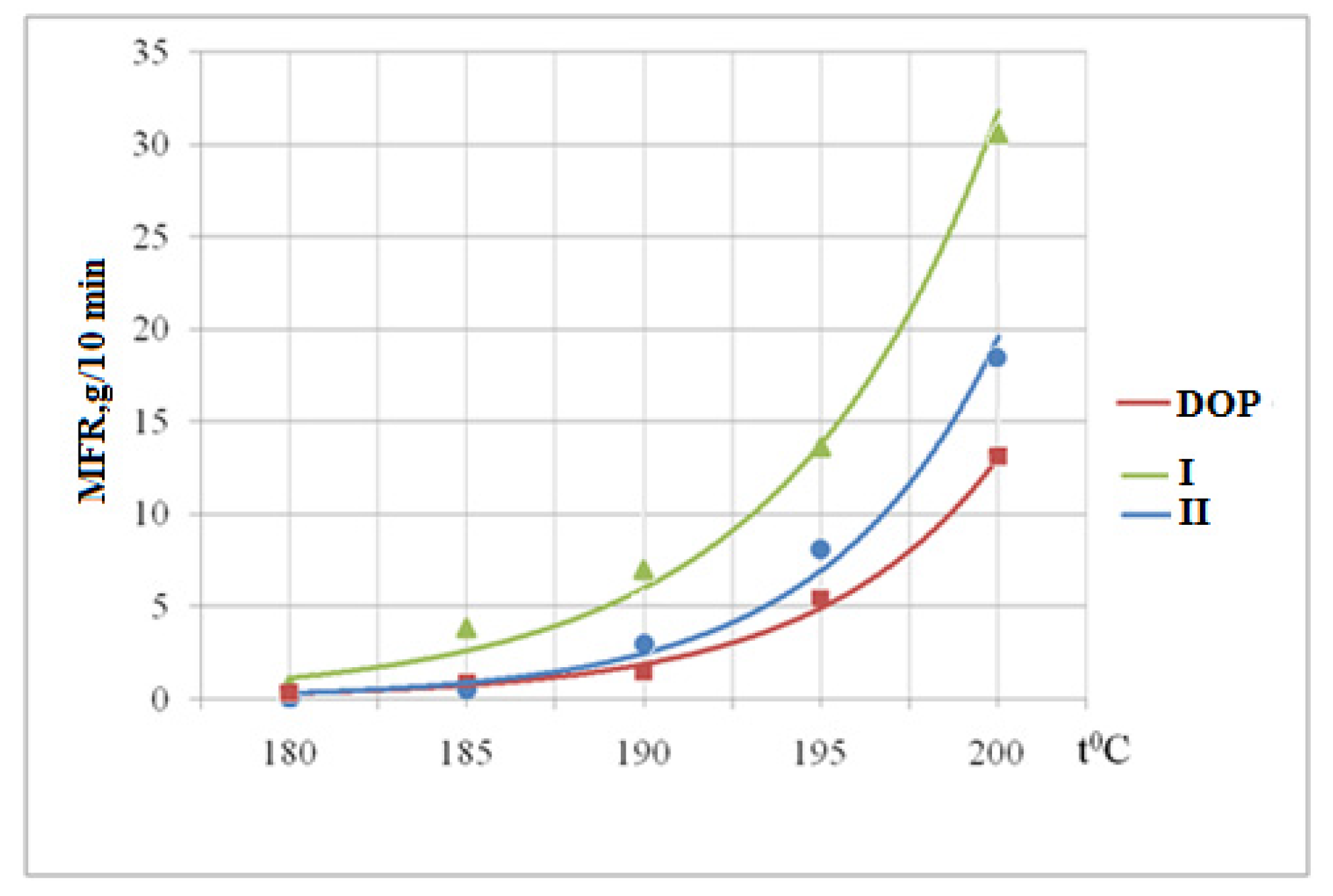
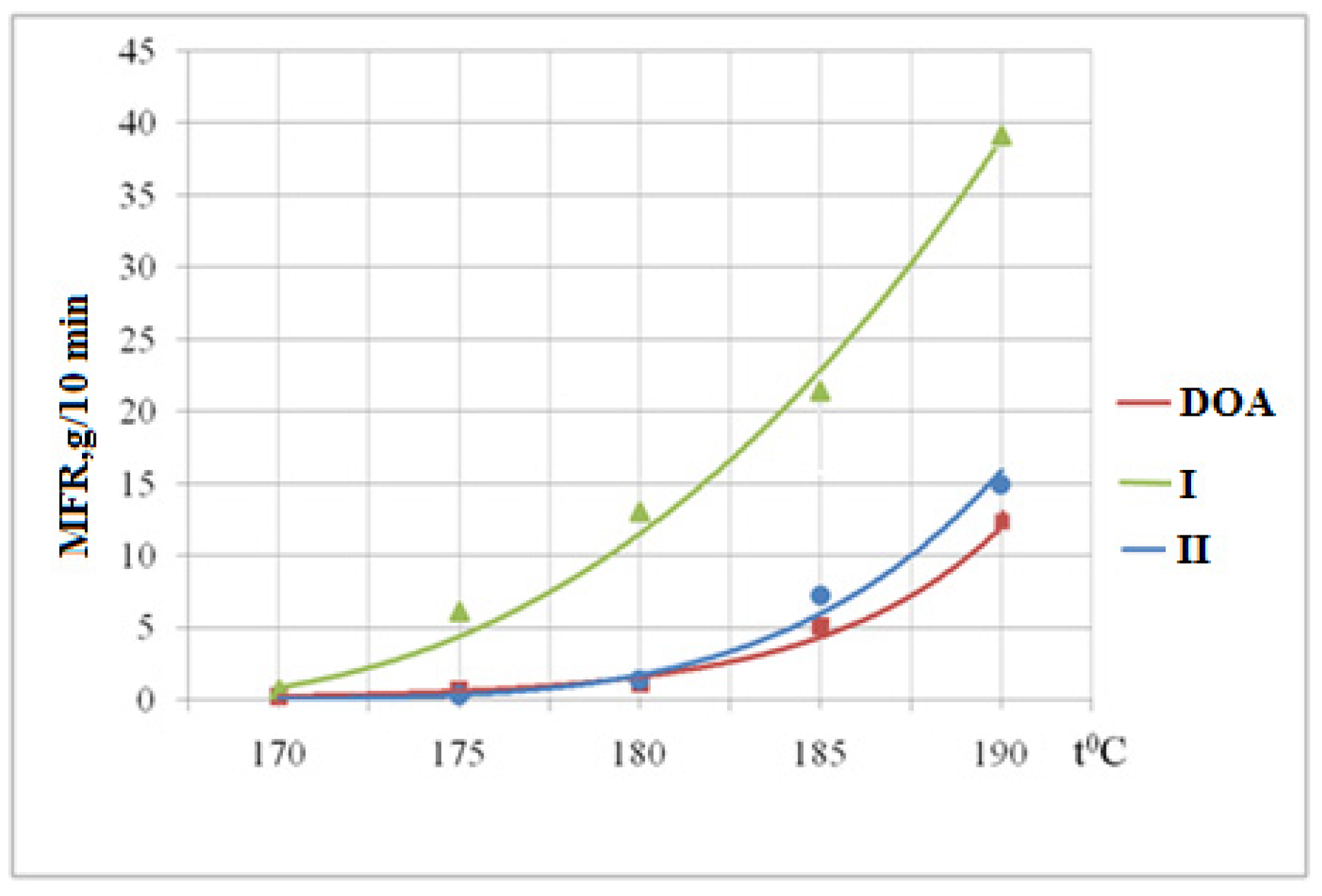
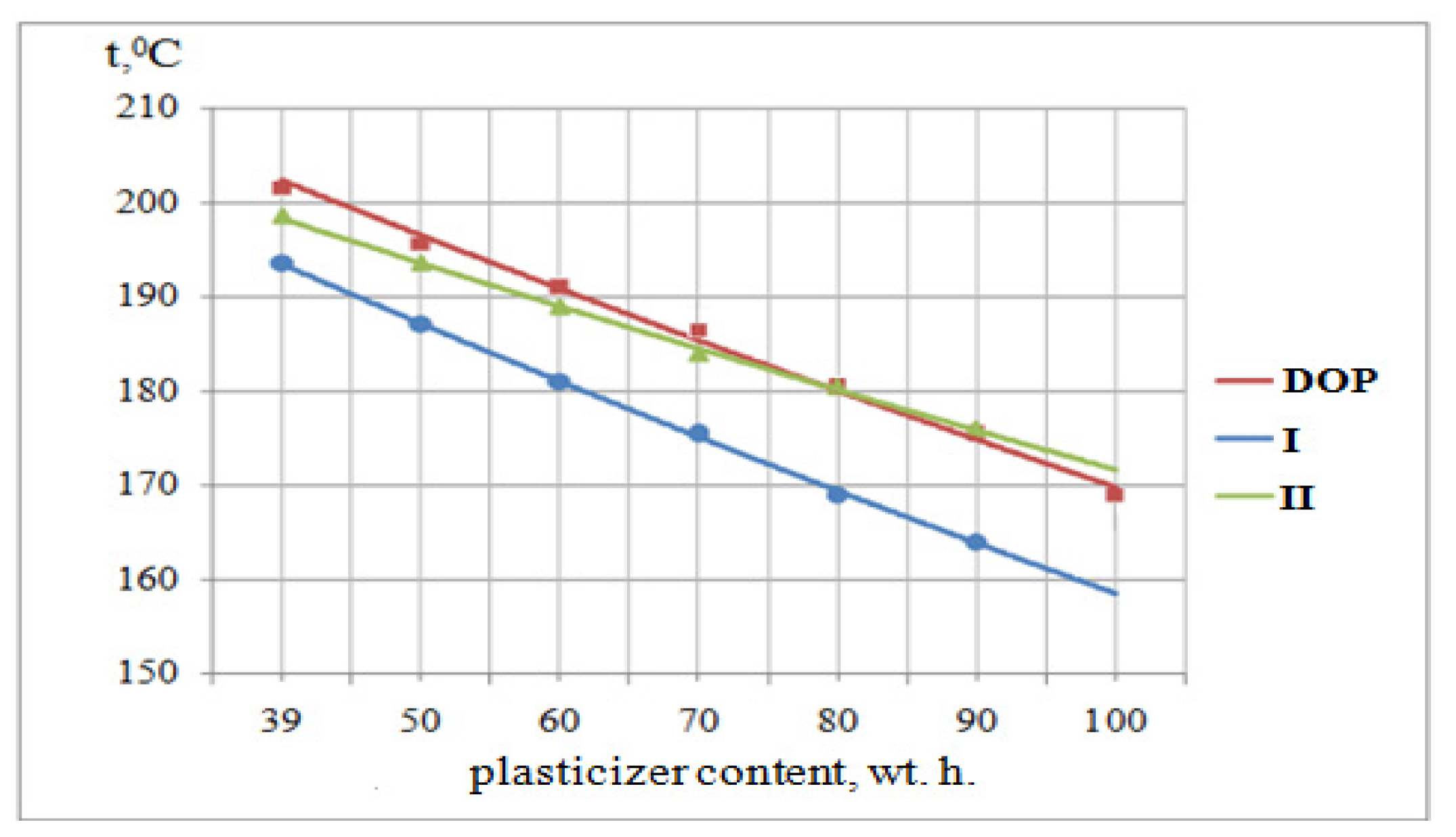
3.6. Determination of the Melt Flow Rate
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statista. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 30 August 2020).
- Fadina, Y.I. Analysis of the Russian polymer market and further ways of its development. Bus. Educ. Knowl. Econ. 2017, 1, 99–101. [Google Scholar]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Pospıšil, J.; Horák, Z.; Kruliš, Z.; Nešpůrek, S.; Kuroda, S. Degradation and aging of polymer blends. Thermomechanical and thermal degradation. Polym. Degrad. Stab. 1999, 65, 405–414. [Google Scholar] [CrossRef]
- Ulyanov, V.M.; Rybkin, E.P.; Gudkovich, A.D.; Pishin, G.A. Polyvinyl Chloride; Chemistry: Moscow, Russia, 2000; p. 288. [Google Scholar]
- Chodak, I. Degradable Polymers; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Erceg, M.; Kovacic, T.; Klaric, I. Thermal degradation of poly(3-hydroxybutyrate) plasticized with acetyl tributyl citrate. Polym. Degrad. Stab. 2005, 90, 313–318. [Google Scholar] [CrossRef]
- Pakharenko, V.A.; Pakharenko, V.V.; Yakovleva, R.A. Plastics in construction; Scientific Fundamentals and Technologies: St. Petersburg, Russia, 2010; p. 349. [Google Scholar]
- Schiller, M. Additives to PVC. In PVC Additives. Performance, Chemistry, Developments, and Sustainability; Translated from English by Tikhonov, N.N.; Profession: Moscow, Russia, 2017; p. 400. [Google Scholar]
- Grossman, R.F. Handbook of Vinyl Formulating, 2nd ed.; Grossman, F., Ed.; Translated from English by Guzeev, V.V.; Scientific Foundations and Technologies: St. Petersburg, Russia, 2009; p. 608. [Google Scholar]
- Wypych, G. Handbook of Plasticizers; ChemTec Publishin: Scarborough, ON, Canada, 2004; p. 687. [Google Scholar]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer composite materials: A comprehensive review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Stipek, J.; Daoust, H. Additives for Plastics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; p. 243. [Google Scholar]
- Lakeev, S.N.; Maydanova, I.O.; Mullakhmetov, R.F. Ester plasticizers for polyvinyl chloride. Russ. J. Appl. Chem. 2016, 89, 1–15. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef] [Green Version]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667. [Google Scholar] [CrossRef]
- Fromme, H.; Kücher, T.; Otto, T.; Pilz, K.; Müller, J.; Wenzel, A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002, 36, 1429. [Google Scholar] [CrossRef]
- Fernandez, M.P.; Ikonomou, M.G.; Buchanan, I. An assessment of estrogenic organic contaminants in Canadian wastewaters. Sci. Total Environ. 2007, 373, 250. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Cui, K.; Xie, Z.; Wu, L.; Luo, D.; Chen, L.; Lin, Y.; Liu, M.; Sun, G. Distribution of phthalate esters in urban soils of subtropical city. J. Hazard. Mater. 2009, 164, 1171. [Google Scholar] [CrossRef]
- Felder, J.D.; Adams, W.J.; Saege, V.W. Assessment of the safety of dioctyl adipate in freshwater environments. Environ. Toxicol. Chem. 1986, 5, 777–784. [Google Scholar] [CrossRef]
- Gu, J.D. Biodegradability of plastics: The issues, recent advances, and future perspectives. Environ. Sci. Pollut. Res. 2021, 28, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Rakhimov, M.A.; Rakhimova, G.M.; Imanov, E.M. Problems of utilization of polymer waste. Fundam. Res. 2014, 8, 331–332. [Google Scholar]
- Gogol, E.V.; Mingazetdinov, I.K.; Gumerova, G.I. Analysis of existing methods of utilization and processing of polymer waste. Bull. Kazan Technol. Univ. 2013, 10, 163–167. [Google Scholar]
- Gu, G.D. Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. Int. Biodeterior. Biodegrad. 2003, 53, 69–91. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Janeschitz-Kriegl, H. Polymer Melt Rheology and Flow Birefringence; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; p. 524. [Google Scholar]
- Aydin, I. Evaluation of Rheological Properties during Extrusion Compounding of Soft PVC Powder Blends. Int. J. Emerg. Technol. Adv. Eng. 2016, 6, 210–214. [Google Scholar]
- Han, C.D. Rheology in Polymer Processing; Vinogradov, G.V., Fridman, M.L., Eds.; Chemistry: Moscow, Russia, 1979; p. 368. [Google Scholar]
- Yershov, S.V. Investigation of rheological properties of electrical insulating polymers. Bull. PNRPU 2012, 2, 88–98. [Google Scholar]
- Yankov, V.I. Non-isothermal flow of polymer liquids in screw seals with longitudinal circulation. Chem. Oil Gas Eng. 2006, 3, 12–15. [Google Scholar]
- Mazitova, A.K.; Vikhareva, I.N.; Aminova, G.K.; Savicheva, J.N. Application of Zinc Oxide to Obtain and Modify Properties of Adipate Plasticizer of Polyvinyl Chloride. Polymers 2020, 12, 1728. [Google Scholar] [CrossRef]
- Vikhareva, I.N.; Aminova, G.K.; Moguchev, A.I.; Mazitova, A.K. The effect of a zinc-containing additive on the properties of PVC compounds. Adv. Polym. Technol. 2021, 2021, 5593184. [Google Scholar] [CrossRef]
- Mazitova, A.K.; Vikhareva, I.N.; Aminova, G.K. Designing of green plasticizers and assessment of the effectiveness of their use. Polymers 2021, 13, 1761. [Google Scholar] [CrossRef]
- Interstate Standard 8728-88. Plasticizers Specifications; IPK Publishing House of Standards: Moscow, Russia, 2003; p. 11. [Google Scholar]
- Interstate Standard 18329-2014. Liquid Resins and Plasticizers, Methods for Determination of Density; FSA Standartinform: Moscow, Russia, 2015; p. 8. [Google Scholar]
- Interstate Standard 11645-73. Plastics Method for Determining the Melt Flow Index of Thermoplastics (with Changes N 1, 2, 3) M.; Publishing House of Standards: Moscow, Russia, 1994. [Google Scholar]



| Ester | Indicators | |||
|---|---|---|---|---|
| Molecular Weight | Acid Number, mg KOH/g | Ester Number, mg KOH/g | d204 | |
| Butyl butoxyethyl adipate (BBEA) (I) | 302 | 0.1 | 369 | 1.0452 |
| Decyl butoxyethyl adipate (DBEA) (II) | 386 | 0.1 | 288 | 1.1003 |
| Plasticizer Composition | Granule Color | ||||
|---|---|---|---|---|---|
| 180 °C | 185 °C | 190 °C | 195 °C | 200 °C | |
| DOP | Yellow | Red | Red | Red | Red |
| DOP + I | White | White | Yellow | Yellow | Yellow-Red |
| DOP + II | White | White | White | Yellow | Red |
| Plasticizer Composition | Granule Color | ||||
|---|---|---|---|---|---|
| 190 °C | 195 °C | 200 °C | 202.5 °C | 205 °C | |
| DOP | Yellow | Red | Red | Red | Red |
| DOP + I | Yellow | Yellow | Yellow | Yellow | Yellow |
| DOP + II | Yellow | Yellow | Yellow-Red | Red | Red |
| Plasticizer Composition | Granule Color | ||||
|---|---|---|---|---|---|
| 180 °C | 185 °C | 190 °C | 195 °C | 200 °C | |
| DOP | Yellow | Red | Red | Red | Red |
| DOP + I | Yellow | Yellow | Yellow | Yellow | Yellow-Red |
| DOP + II | Yellow | Yellow | Yellow | Yellow-Red | Yellow-Red |
| Plasticizer Composition | Granule Color | ||||
|---|---|---|---|---|---|
| 170 °C | 175 °C | 180 °C | 185 °C | 190 °C | |
| DOA | White | White | White | White | White |
| I | White | White | White | White | Yellow |
| II | White | White | White | Yellow | Yellow |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikhareva, I.N.; Aminova, G.K.; Mazitova, A.K. Study of the Rheological Properties of PVC Composites Plasticized with Butoxyethyl Adipates. ChemEngineering 2021, 5, 85. https://doi.org/10.3390/chemengineering5040085
Vikhareva IN, Aminova GK, Mazitova AK. Study of the Rheological Properties of PVC Composites Plasticized with Butoxyethyl Adipates. ChemEngineering. 2021; 5(4):85. https://doi.org/10.3390/chemengineering5040085
Chicago/Turabian StyleVikhareva, Irina N., Guliya K. Aminova, and Aliya K. Mazitova. 2021. "Study of the Rheological Properties of PVC Composites Plasticized with Butoxyethyl Adipates" ChemEngineering 5, no. 4: 85. https://doi.org/10.3390/chemengineering5040085
APA StyleVikhareva, I. N., Aminova, G. K., & Mazitova, A. K. (2021). Study of the Rheological Properties of PVC Composites Plasticized with Butoxyethyl Adipates. ChemEngineering, 5(4), 85. https://doi.org/10.3390/chemengineering5040085






