Hydrothermal Synthesis of Biphasic Calcium Phosphate from Cuttlebone Assisted by the Biosurfactant L-rhamnose Monohydrate for Biomedical Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
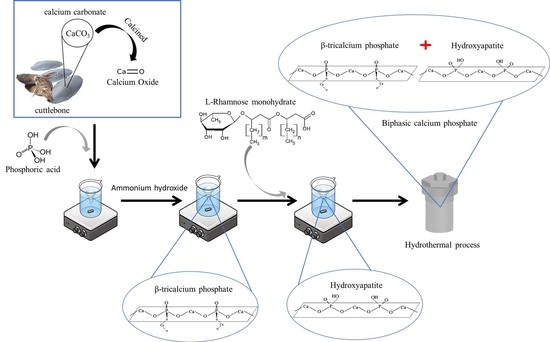
2.2.1. Synthesis of Biphasic Calcium Phosphate from Cuttlebone by a Surfactant-Assisted Hydrothermal Process
2.2.2. Characterization
Fourier Transform Infrared Spectroscopy (FTIR)
X-ray Diffraction
Scanning Electron Microscopy
Dynamic Light Scattering (DLS)
BET Analysis
Zeta Potential
2.2.3. Cytotoxicity Test
3. Results and Discussion
- σ = population standard deviation.
- μ = assumed mean.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Population on 1 January by Broad Age Group and Sex [Internet]. Available online: http://appsso.eurostat.ec.europa.eu/nui/show.do (accessed on 2 December 2021).
- Wani, S.U.D.; Gautam, S.P.; Qadrie, Z.L.; Gangadharappa, H. Silk fibroin as a natural polymeric based bio-material for tissue engineering and drug delivery systems—A review. Int. J. Biol. Macromol. 2020, 163, 2145–2161. [Google Scholar] [CrossRef] [PubMed]
- Pawelzik, P.; Carus, M.; Hotchkiss, J.; Narayan, R.; Selke, S.; Wellisch, M.; Weiss, M.; Wicke, B.; Patel, M. Critical aspects in the life cycle assessment (LCA) of bio-based materials—Reviewing methodologies and deriving recommendations. Resour. Conserv. Recycl. 2013, 73, 211–228. [Google Scholar] [CrossRef]
- Ladu, L.; Blind, K. Overview of policies, standards and certifications supporting the European bio-based economy. Curr. Opin. Green Sustain. Chem. 2017, 8, 30–35. [Google Scholar] [CrossRef]
- Ladu, L.; Morone, P. Holistic approach in the evaluation of the sustainability of bio-based products: An Integrated Assessment Tool. Sustain. Prod. Consum. 2021, 28, 911–924e6. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Shekhirev, M.A.; Tretyakov, Y.D.; Kuznetsov, A.V.; Belyakov, A.V. Densification additives for hydroxyapatite ceramics. J. Eur. Ceram. Soc. 2009, 29, 1925–1932. [Google Scholar] [CrossRef]
- Khalid, H.; Chaudhry, A.A. 4-Basics of hydroxyapatite—Structure, synthesis, properties, and clinical applications. In Handbook of Ionic Substituted Hydroxyapatites; Khan, A.S., Chaudhry, A.A., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 85–115. [Google Scholar]
- Huang, L.-H.; Sun, X.-Y.; Ouyang, J.-M. Shape-dependent toxicity and mineralization of hydroxyapatite nanoparticles in A7R5 aortic smooth muscle cells. Sci. Rep. 2019, 9, 18979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamil, M.; Elouahli, A.; Khallok, H.; El Ouatli, B.; Hatim, Z. Characterization of β-tricalcium phosphate-clay mineral composite obtained by sintering powder of apatitic calcium phosphate and montmorillonite. Surf. Interfaces 2019, 17, 100380. [Google Scholar] [CrossRef]
- Liu, B.; Lun, D.-X. Current Application of β-tricalcium Phosphate Composites in Orthopaedics. Orthop. Surg. 2012, 4, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit, A.E.-D.; Ali, A.; Sun, Z. Synthesis of nano-hydroxyapatite (nHA) from waste mussel shells using a rapid microwave method. Mater. Chem. Phys. 2015, 149–150, 607–616. [Google Scholar] [CrossRef]
- Shavandi, A.; Wilton, V.; Bekhit, A.E.-D.A. Synthesis of macro and micro porous hydroxyapatite (HA) structure from waste kina (Evechinus chloroticus) shells. J. Taiwan Inst. Chem. Eng. 2016, 65, 437–443. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Rajendran, A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O.; Pattanayak, D.K. Synthesis of organic derived hydroxyapatite scaffold from pig bone waste for tissue engineering applications. Adv. Powder Technol. 2018, 29, 1–8. [Google Scholar] [CrossRef]
- Ferro, A.C.; Guedes, M. Mechanochemical synthesis of hydroxyapatite using cuttlefish bone and chicken eggshell as calcium precursors. Mater. Sci. Eng. C 2019, 97, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Checa, A.G.; Cartwright, J.; Sánchez-Almazo, I.; Andrade, J.; Ruiz-Raya, F. The cuttlefish Sepia officinalis (Sepiidae, Cephalopoda) constructs cuttlebone from a liquid-crystal precursor. Sci. Rep. 2015, 5, 11513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kangkan, S.; Arpornmaeklong, P.; Ummartyotin, S. Synthesis of hydroxyapatite from cuttlebone under various pH conditions: An approach for medical materials. J. Metals Mater. Miner. 2020, 30, 136–141. [Google Scholar]
- Kangkan, S.; Pongprayoon, T.; Ummartyotin, S. Morphologically controlled synthesis of (β-tricalcium phosphate) β-TCP particles from cuttlebone by sodium dodecyl sulfate (SDS) anionic surfactant: In vitro behavior of mouse osteoblast cells. J. Mater. Res. Technol. 2020, 9, 3121–3127. [Google Scholar] [CrossRef]
- Cameotra, S.S.; Makkar, R.S.; Kaur, J.; Mehta, S.K. Synthesis of Biosurfactants and Their Advantages to Microorganisms and Mankind. Adv. Exp. Med. Biol. 2010, 672, 261–280. [Google Scholar] [CrossRef]
- Vijayakuma, S.; Varatharajan, S. Biosurfactants-Types, Sources and Applications. Res. J. Microbiol. 2015, 10, 181–192. [Google Scholar]
- Tan, Y.N.; Li, Q. Microbial production of rhamnolipids using sugars as carbon sources. Microb. Cell Fact. 2018, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater. Sci. Eng. C 2017, 71, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Khiri, M.Z.A.; Matori, K.A.; Zaid, M.H.M.; Abdullah, C.A.C.; Zainuddin, N.; Alibe, I.M.; Rahman, N.A.A.; Wahab, S.A.A.; Azman, A.Z.K.; Effendy, N. Crystallization behavior of low-cost biphasic hydroxyapatite/β-tricalcium phosphate ceramic at high sintering temperatures derived from high potential calcium waste sources. Results Phys. 2019, 12, 638–644. [Google Scholar] [CrossRef]
- Wan, J.; Zeng, G.; Huang, D.; Hu, L.; Xu, P.; Huang, C.; Deng, R.; Xue, W.; Lai, C.; Zhou, C.; et al. Rhamnolipid stabilized nano-chlorapatite: Synthesis and enhancement effect on Pb-and Cd-immobilization in polluted sediment. J. Hazard. Mater. 2018, 343, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Sarkar, R. Synthesis and characterization of sintered beta-tricalcium phosphate: A comparative study on the effect of preparation route. Mater. Sci. Eng. C 2016, 67, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Suresh, S.; Dakshanamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Pinjari, D.V.; Pandit, A.B. Room temperature synthesis of crystalline CeO2 nanopowder: Advantage of sonochemical method over conventional method. Ultrason. Sonochem. 2011, 18, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, J.A.; Weinand, W.R.; Neto, A.M.; Palácios, R.S.; Sales, A.J.M.; Prezas, P.R.; Costa, A.A.; Graça, M.P.F. Low-Cost Hydroxyapatite Powders from Tilapia Fish. JOM 2020, 72, 1435–1442. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.; Ibrahim, M.L.; Hin, T.-Y.Y. Synthesis and application of waste egg shell derived CaO supported W-Mo mixed oxide catalysts for FAME production from waste cooking oil: Effect of stoichiometry. Energy Convers. Manag. 2017, 151, 216–226. [Google Scholar] [CrossRef]
- Xia, J.; Yuan, Y.; Wu, H.; Huang, Y.; Weitz, D.A. Decoupling the effects of nanopore size and surface roughness on the attachment, spreading and differentiation of bone marrow-derived stem cells. Biomaterials 2020, 248, 120014. [Google Scholar] [CrossRef]
- Guzelsu, N.; Wienstien, C.; Kotha, S. A new streaming potential chamber for zeta potential measurements of particles. Rev. Sci. Instrum. 2010, 81, 015106. [Google Scholar] [CrossRef]
- Rahimi, K.; Lotfabad, T.B.; Jabeen, F.; Ganji, S.M. Cytotoxic effects of mono- and di-rhamnolipids from Pseudomonas aeruginosa MR01 on MCF-7 human breast cancer cells. Colloids Surf. B Biointerfaces 2019, 181, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, Y.; Alfred, A.T.; Xin, X.; Yang, S. Chemical structures and biological activities of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa M14808. J. Chem. Pharm. Res. 2013, 5, 177–182. [Google Scholar]






| CMC | Crystallite Size (nm) |
|---|---|
| 0 | 16.8 |
| 10 | 18.4 |
| 20 | 18.5 |
| 100 | 18.4 |
| 500 | 18.4 |
| 1000 | 18.4 |
| CMC | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Radius (nm) |
|---|---|---|---|
| 0 | 4.15 | 0.006 | 5.71 |
| 10 | 6.26 | 0.009 | 5.72 |
| 20 | 6.83 | 0.009 | 5.55 |
| 100 | 6.04 | 0.009 | 5.76 |
| 500 | 6.31 | 0.009 | 5.86 |
| 1000 | 8.55 | 0.013 | 6.11 |
| CMC | Zeta Potential (mV) |
|---|---|
| 0 | −1.35 |
| 10 | −1.07 |
| 20 | −0.85 |
| 100 | −1.68 |
| 500 | −1.22 |
| 1000 | −1.51 |
| Sample | OD 570 nm | % Viability |
|---|---|---|
| Blank | 0.621 | 100 |
| Negative control | 0.625 | 100 |
| Positive control | 0 | 0 |
| 0 CMC | 0.570 | 91 |
| 10 CMC | 0.459 | 74 |
| 20 CMC | 0.466 | 75 |
| 100 CMC | 0.469 | 75 |
| 500 CMC | 0.460 | 74 |
| 1000 CMC | 0.439 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tattanon, T.; Arpornmaeklong, P.; Ummartyotin, S.; Pongprayoon, T. Hydrothermal Synthesis of Biphasic Calcium Phosphate from Cuttlebone Assisted by the Biosurfactant L-rhamnose Monohydrate for Biomedical Materials. ChemEngineering 2021, 5, 88. https://doi.org/10.3390/chemengineering5040088
Tattanon T, Arpornmaeklong P, Ummartyotin S, Pongprayoon T. Hydrothermal Synthesis of Biphasic Calcium Phosphate from Cuttlebone Assisted by the Biosurfactant L-rhamnose Monohydrate for Biomedical Materials. ChemEngineering. 2021; 5(4):88. https://doi.org/10.3390/chemengineering5040088
Chicago/Turabian StyleTattanon, Thamonwan, Premjit Arpornmaeklong, Sarute Ummartyotin, and Thirawudh Pongprayoon. 2021. "Hydrothermal Synthesis of Biphasic Calcium Phosphate from Cuttlebone Assisted by the Biosurfactant L-rhamnose Monohydrate for Biomedical Materials" ChemEngineering 5, no. 4: 88. https://doi.org/10.3390/chemengineering5040088