Electrocatalysts for the Oxygen Reduction Reaction: From Bimetallic Platinum Alloys to Complex Solid Solutions
Abstract
:1. Introduction
2. Bimetallic Alloys
3. Multicomponent and High-Entropy Alloys
4. Experimental Challenges in the Benchmarking of HEA and MPCA Activity for ORR
4.1. Active sites Distribution and Identification
4.2. Electrochemical Active Surface Area Determination
4.3. Ohmic Drop Correction
4.4. Stability Testing
4.5. Membrane Electrode Assembly vs. Rotating Disc Electrode Studies
5. Concluding Remarks and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gottesfeld, S. Electrocatalysis of Oxygen Reduction in Polymer Electrolyte Fuel Cells: A Brief History and a Critical Examination of Present Theory and Diagnostics. Fuel Cell Catal. Surf. Sci. Approach 2008, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Banham, D.; Ye, S. Current Status and Future Development of Catalyst Materials and Catalyst Layers for Proton Exchange Membrane Fuel Cells: An Industrial Perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Mathias, M.F.; Makharia, R.; Gasteiger, H.A. Conley, J.J.; Fuller, T.J.; Gittleman, C.J.; Kocha, S.S.; Miller, D.P.; Mittelsteadt, C.K.; Xie, T.; Van, S.G.; Yu, P.T. Two fuel cell cars in every garage? Electrochem. Soc. Interface 2005, 14, 24–35. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells. In Fuel Cell Technology; Springer: London, UK, 2006; pp. 27–51. [Google Scholar]
- ALSTO. Successful year and a half of trial operation of the world’s first two hydrogen trains, next project phase begins. Available online: https://www.partners.alstom.com/Assets/View/92a183b6-b12a-4561-b356-76a587d0de4e (accessed on 24 November 2021).
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, B.; Ocon, J.D. Oxygen electrocatalysis in chemical energy conversion and storage technologies. Curr. Appl. Phys. 2012, 13, 309–321. [Google Scholar] [CrossRef]
- Wang, C.; Mei, D.; Wiese, G.; Wang, L.; Deng, M.; Lamaka, S.V.; Zheludkevich, M.L. High rate oxygen reduction reaction during corrosion of ultra-high-purity magnesium. npj Mater. Degrad. 2020, 4, 42. [Google Scholar] [CrossRef]
- Chung, Y.; Ji, J.; Kwon, Y. Performance evaluation of enzymatic biofuel cells using a new cathodic catalyst containing hemin and poly acrylic acid promoting the oxygen reduction reaction. J. Mater. Chem. C 2019, 7, 11597–11605. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. Electrocatalytic Oxygen Reduction Reaction. In PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Zhang, J., Ed.; Springer: London, UK, 2008; pp. 89–134. [Google Scholar]
- Gómez-Marín, A.M.; Rizo, R.; Feliu, J.M. Oxygen reduction reaction at Pt single crystals: A critical overview. Catal. Sci. Technol. 2014, 4, 1685–1698. [Google Scholar] [CrossRef]
- Gómez-Marín, A.M.; Feliu, J. Oxygen Reduction on Platinum Single Crystal Electrodes. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 820–830. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Bretzler, P.; Cleemann, L.N.; Yu, J.; Li, Q. Revealing the genuine stability of the reference Pt/C electrocatalyst toward the ORR. Electrochimica Acta 2021, 391, 138963. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Jaramillo, T.F. Addressing the terawatt challenge: Scalability in the supply of chemical elements for renewable energy. RSC Adv. 2012, 2, 7933–7947. [Google Scholar] [CrossRef] [Green Version]
- Shao, M. Electrocatalysis in Fuel Cells. Catalysts 2015, 5, 2115–2121. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Sun, Y.; Delucchi, M.; Ogden, J. The impact of widespread deployment of fuel cell vehicles on platinum demand and price. Int. J. Hydrogen Energy 2011, 36, 11116–11127. [Google Scholar] [CrossRef]
- James, B.D.; Kalinoski, J.A. Mass Production Cost Estimation for Direct H2 PEM Fuel Cell System for Automotive Applications. DOE Hydrog. Program Rev. 2007. Available online: https://www.energy.gov/sites/prod/files/2019/12/f70/fcto-sa-2018-transportation-fuel-cell-cost-analysis.pdf (accessed on 24 November 2021).
- Appleby, A.J. Electrocatalysis and fuel cells. Catal. Rev. 1971, 4, 221–244. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Paffett, M.T.; Beery, J.G.; Gottesfeld, S. Oxygen reduction at Pt0.65Cr0.35, Pt0.2Cr0.8 and roughened platinum. J. Electrochem. Soc. 1988, 135, 1431–1436. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, S.M.; Nam, S.H.; Seo, M.H.; Choi, S.H.; Kim, W.B. Influence of Sn content on PtSn/C catalysts for electrooxidation of C1-C3 alcohols: Synthesis, characterization, and electrocatalytic activity. Appl. Catal. B Environ. 2008, 82, 89–102. [Google Scholar] [CrossRef]
- Alayoglu, S.; Nilekar, A.U.; Mavrikakis, M.; Eichhorn, B.W. Ru–Pt core–shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen. Nat. Mater. 2008, 7, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guo, S.; Dong, S. PdM (M = Pt, Au) Bimetallic Alloy Nanowires with Enhanced Electrocatalytic Activity for Electro-oxidation of Small Molecules. Adv. Mater. 2012, 24, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.M.P.; Moolhuysen, J. Platinum—Tin catalysts for methanol fuel cells prepared by a novel immersion technique, by electrocodeposition and by alloying. Electrochim. Acta 1976, 21, 861–868. [Google Scholar] [CrossRef]
- Watanabe, M.; Motoo, S. Electrocatalysis by ad-atoms Part I. Enhancement of the oxidation of methanol on platinum and palladium by gold ad-atoms. J. Electroanal. Chem. 1975, 60, 259–266. [Google Scholar] [CrossRef]
- Watanabe, M.; Motoo, S. Electrocatalysis by ad-atoms part II. Enhancement of the oxidation of methanol on platinum by ruthenium ad-atoms. J. Electroanal. Chem. 1975, 60, 267–273. [Google Scholar] [CrossRef]
- Watanabe, M.; Motoo, S. Electrocatalysis by ad-atoms Part III. Enhancement of the oxidation of carbon monoxide on platinum by ruthenium ad-atoms. J. Electroanal. Chem. 1975, 60, 275–283. [Google Scholar] [CrossRef]
- Stamenković, V.; Schmidt, T.J.; Ross, A.P.N.; Marković, N.M. Surface Composition Effects in Electrocatalysis: Kinetics of Oxygen Reduction on Well-Defined Pt3Ni and Pt3Co Alloy Surfaces. J. Phys. Chem. B 2002, 106, 11970–11979. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.Y.; Jun, S.; Yoon, G.; Kwon, S.G.; Shin, D.Y.; Seo, P.; Yoo, J.M.; Shin, H.; Chung, Y.-H.; Kim, H.; et al. Highly Durable and Active PtFe Nanocatalyst for Electrochemical Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485. [Google Scholar] [CrossRef]
- Ohm, V.; Raetz, S.; Sauer, M.; Merkens, M.; Schilder, H.; Lueken, H. Formation of lanthanide-platinum alloys by reaction of platinum with lanthanide iodides Part II. Structural and magnetochemical investigations into the systems with Ln ≜ Nd, Gd, Tb, Dy, Tm2. J. Alloy. Compd. 1996, 238, 95–101. [Google Scholar] [CrossRef]
- Roy, C.; Knudsen, B.P.; Pedersen, C.M.; Palenzuela, A.A.V.; Christensen, L.H.; Damsgaard, C.D.; Stephens, I.E.L.; Chorkendorff, I. Scalable Synthesis of Carbon-Supported Platinum–Lanthanide and—Rare-Earth Alloys for Oxygen Reduction. ACS Catal. 2018, 8, 2071–2080. [Google Scholar] [CrossRef] [Green Version]
- Chu, T.; Xie, M.; Yang, D.; Ming, P.; Li, B.; Zhang, C. Highly active and durable carbon support Pt-rare earth catalyst for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2020, 45, 27291–27298. [Google Scholar] [CrossRef]
- Yoshida, T.; Kojima, K. Toyota MIRAI Fuel Cell Vehicle and Progress Toward a Future Hydrogen Society. Electrochem. Soc. Interface 2015, 24, 45–49. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Chen, A.; Holt-Hindle, P. Platinum-Based Nanostructured Materials: Synthesis, Properties, and Applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef]
- Bing, Y.; Liu, H.; Zhang, L.; Ghosh, D.; Zhang, J. Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem. Soc. Rev. 2010, 39, 2184–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Yang, H. Platinum-Based Oxygen Reduction Electrocatalysts. Accounts Chem. Res. 2013, 46, 1848–1857. [Google Scholar] [CrossRef]
- Timoshenko, J.; Cuenya, B.R. In Situ/Operando Electrocatalyst Characterization by X-ray Absorption Spectroscopy. Chem. Rev. 2020, 121, 882–961. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Bligaard, T.; Rossmeisl, J.; Christensen, C.H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.; Schuhmann, W. Breaking scaling relations in electrocatalysis. J. Solid State Electrochem. 2020, 24, 1–2. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; López, N. Strategies to break linear scaling relationships. Nat. Catal. 2019, 2, 971–976. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Fantauzzi, D.; Zhu, T.; Mueller, J.E.; Filot, I.A.; Hensen, E.J.; Jacob, T. Microkinetic Modeling of the Oxygen Reduction Reaction at the Pt(111)/Gas Interface. Catal. Lett. 2015, 145, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, V.; Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Universality in Oxygen Reduction Electrocatalysis on Metal Surfaces. ACS Catal. 2012, 2, 1654–1660. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Kodama, K.; Hatanaka, T.; Morimoto, Y. First principles based mean field model for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2011, 13, 21070–21083. [Google Scholar] [CrossRef] [PubMed]
- Tripković, V.; Skúlason, E.; Siahrostami, S.; Nørskov, J.K.; Rossmeisl, J. The oxygen reduction reaction mechanism on Pt(111) from density functional theory calculations. Electrochim. Acta 2010, 55, 7975–7981. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Koper, M.T.M.; Bandarenka, A.S. Tailoring the catalytic activity of electrodes with monolayer amounts of foreign metals. Chem. Soc. Rev. 2013, 42, 5210–5230. [Google Scholar] [CrossRef]
- Abild-Pedersen, F.; Greeley, J.; Studt, F.; Rossmeisl, J.; Munter, T.R.; Moses, P.G.; Skúlason, E.; Bligaard, T.; Nørskov, J.K. Scaling Properties of Adsorption Energies for Hydrogen-Containing Molecules on Transition-Metal Surfaces. Phys. Rev. Lett. 2007, 99, 016105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossmeisl, J.; Logadottir, A.; Nørskov, J. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184. [Google Scholar] [CrossRef]
- Stephens, I.E.L.; Bondarenko, A.S.; Perez-Alonso, F.J.; Calle-Vallejo, F.; Bech, L.; Johansson, T.P.; Jepsen, A.K.; Frydendal, R.; Knudsen, B.P.; Rossmeisl, J.; et al. Tuning the Activity of Pt(111) for Oxygen Electroreduction by Subsurface Alloying. J. Am. Chem. Soc. 2011, 133, 5485–5491. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the Activity of Electrocatalysts for Oxygen Reduction by Tuning the Surface Electronic Structure. Angew. Chem. Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef]
- Čolić, V.; Bandarenka, A.S. Pt Alloy Electrocatalysts for the Oxygen Reduction Reaction: From Model Surfaces to Nanostructured Systems. ACS Catal. 2016, 6, 5378–5385. [Google Scholar] [CrossRef]
- Marković, N.M.; Schmidt, T.J.; Stamenković, V.; Ross, P.N. Ross, Oxygen Reduction Reaction on Pt and Pt Bimetallic Surfaces: A Selective Review. Fuel Cells 2001, 1, 105–116. [Google Scholar] [CrossRef]
- Zhang, J.; Mo, Y.; Vukmirovic, M.B.; Klie, R.; Sasaki, K.; Adzic, R.R. Platinum Monolayer Electrocatalysts for O2 Reduction: Pt Monolayer on Pd(111) and on Carbon-Supported Pd Nanoparticles. J. Phys. Chem. B 2004, 108, 10955–10964. [Google Scholar] [CrossRef]
- Zhang, J.; Lima, F.H.B.; Shao, M.H.; Sasaki, K.; Wang, J.X.; Hanson, J.; Adzic, R.R. Platinum Monolayer on Nonnoble Metal−Noble Metal Core−Shell Nanoparticle Electrocatalysts for O2 Reduction. J. Phys. Chem. B 2005, 109, 22701–22704. [Google Scholar] [CrossRef] [PubMed]
- Adzic, R.R.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Shao, M.; Wang, J.X.; Nilekar, A.U.; Mavrikakis, M.; Valerio, J.A.; Uribe, F. Platinum monolayer fuel cell electrocatalysts. Top. Catal. 2007, 46, 249–262. [Google Scholar] [CrossRef]
- Vukmirovic, M.B.; Zhang, J.; Sasaki, K.; Nilekar, A.U.; Uribe, F.; Mavrikakis, M.; Adzic, R.R. Platinum monolayer electrocatalysts for oxygen reduction. Electrochim. Acta 2007, 52, 2257–2263. [Google Scholar] [CrossRef]
- Strasser, P. Dealloyed Core-Shell Fuel Cell Electrocatalysts. Rev. Chem. Eng. 2009, 25. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.V.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Escribano, M.; Malacrida, P.; Hansen, M.H.; Vej-Hansen, U.G.; Velázquez-Palenzuela, A.; Tripkovic, V.; Schiøtz, J.; Rossmeisl, J.; Stephens, I.E.L.; Chorkendorff, I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 2016, 352, 73. [Google Scholar] [CrossRef] [Green Version]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.; Lucas, C.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Stamenković, V.; Schmidt, T.J.; Ross, P.; Marković, N. Surface segregation effects in electrocatalysis: Kinetics of oxygen reduction reaction on polycrystalline Pt3Ni alloy surfaces. J. Electroanal. Chem. 2003, 554-555, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Stamenkovic, V.R.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M. Effect of Surface Composition on Electronic Structure, Stability, and Electrocatalytic Properties of Pt-Transition Metal Alloys: Pt-Skin versus Pt-Skeleton Surfaces. J. Am. Chem. Soc. 2006, 128, 8813–8819. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.P.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]
- Hernandez-Fernandez, P.; Masini, F.; McCarthy, D.N.; Strebel, C.E.; Friebel, D.; Deiana, D.; Malacrida, P.; Nierhoff, A.U.F.; Bodin, A.; Wise, A.M.; et al. Mass-selected nanoparticles of PtxY as model catalysts for oxygen electroreduction. Nat. Chem. 2014, 6, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Mavrikakis, M.; Hammer, B.; Nørskov, J.K. Effect of Strain on the Reactivity of Metal Surfaces. Phys. Rev. Lett. 1998, 81, 2819–2822. [Google Scholar] [CrossRef] [Green Version]
- Lischka, M.; Mosch, C.; Groß, A. Tuning catalytic properties of bimetallic surfaces: Oxygen adsorption on pseudomorphic Pt/Ru overlayers. Electrochimica Acta 2007, 52, 2219–2228. [Google Scholar] [CrossRef]
- Schlapka, A.; Lischka, M.; Groß, A.; Käsberger, U.; Jakob, P. Surface Strain versus Substrate Interaction in Heteroepitaxial Metal Layers: Pt on Ru(0001). Phys. Rev. Lett. 2003, 91, 016101. [Google Scholar] [CrossRef] [Green Version]
- Moffat, T.P.; Fan, F.F.; Bard, A.J. Electrochemical and Scanning Tunneling Microscopic Study of Dealloying of Cu3Au. J. Electrochem. Soc. 1991, 138, 3224–3235. [Google Scholar] [CrossRef]
- Jacobse, L.; Rost, M.J.; Koper, M.T.M. Atomic-Scale Identification of the Electrochemical Roughening of Platinum. ACS Central Sci. 2019, 5, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Stephens, I.E.L.; Bondarenko, A.S.; Grønbjerg, U.; Rossmeisl, J.; Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 2012, 5, 6744–6762. [Google Scholar] [CrossRef] [Green Version]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Role of Strain and Ligand Effects in the Modification of the Electronic and Chemical Properties of Bimetallic Surfaces. Phys. Rev. Lett. 2004, 93, 156801. [Google Scholar] [CrossRef] [Green Version]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Liu, J.; Li, S.; Zuo, Y.; Li, D.; Han, H. Nickel-Ion-Oriented Fabrication of Spiny PtCu Alloy Octahedral Nanoframes with Enhanced Electrocatalytic Performance. ACS Appl. Energy Mater. 2019, 2, 2862–2869. [Google Scholar] [CrossRef]
- Li, W.; Hu, Z.-Y.; Zhang, Z.; Wei, P.; Zhang, J.; Pu, Z.; Zhu, J.; He, D.; Mu, S.; Van Tendeloo, G. Nano-single crystal coalesced PtCu nanospheres as robust bifunctional catalyst for hydrogen evolution and oxygen reduction reactions. J. Catal. 2019, 375, 164–170. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, X.; Wang, X.; Fu, Y.; Zhu, H.; Liu, T. Chemically Ordered Pt–Co–Cu/C as Excellent Electrochemical Catalyst for Oxygen Reduction Reaction. J. Electrochem. Soc. 2020, 167, 024507. [Google Scholar] [CrossRef]
- Deng, Z.; Pang, W.; Gong, M.; Jin, Z.; Wang, X. Revealing the role of mo doping in promoting oxygen reduction reaction performance of Pt3Co nanowires. J. Energy Chem. 2021, 66, 16–23. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Y.; Yang, D.; Zhang, C.; Ming, P.; Li, B.; Yang, S. Efficient synthesis of Pt–Co nanowires as cathode catalysts for proton exchange membrane fuel cells. RSC Adv. 2020, 10, 6287–6296. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, M.; Gao, M.; Jin, J.; van Spronsen, M.; Salmeron, M.B.; Yang, P. High-Performance Pt–Co Nanoframes for Fuel-Cell Electrocatalysis. Nano Lett. 2020, 20, 1974–1979. [Google Scholar] [CrossRef]
- Lu, J.; Yang, L.; Guo, W.; Xiao, S.; Wang, L.; OuYang, Y.; Gao, P. The mechanism of Co oxyhydroxide nano-islands deposited on a Pt surface to promote the oxygen reduction reaction at the cathode of fuel cells. RSC Adv. 2020, 10, 44719–44727. [Google Scholar] [CrossRef]
- Gong, M.; Xiao, D.; Deng, Z.; Zhang, R.; Xia, W.; Zhao, T.; Liu, X.; Shen, T.; Hu, Y.; Lu, Y.; et al. Structure evolution of PtCu nanoframes from disordered to ordered for the oxygen reduction reaction. Appl. Catal. B Environ. 2020, 282, 119617. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Wang, X.; Rosen, A.; Beatrez, W.; Sztaberek, L.; Tan, H.; Zhang, L.; Koenigsmann, C.; Zhao, J. Composition-Dependent Oxygen Reduction Reaction Activity of Pt-Surfaced PtNi Dodecahedral Nanoframes. ACS Appl. Energy Mater. 2020, 3, 768–776. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Huang, L.; Wei, M.; Tsiakaras, P.; Shen, P.K. Highly stable Pt-Co nanodendrite in nanoframe with Pt skin structured catalyst for oxygen reduction electrocatalysis. Appl. Catal. B Environ. 2020, 281, 119460. [Google Scholar] [CrossRef]
- Ma, H.; Zheng, Z.; Zhao, H.; Shen, C.; Chen, H.; Li, H.; Cao, Z.; Kuang, Q.; Lin, H.; Xie, Z. Trimetallic PtNiCo branched nanocages as efficient and durable bifunctional electrocatalysts towards oxygen reduction and methanol oxidation reactions. J. Mater. Chem. A 2021, 9, 23444–23450. [Google Scholar] [CrossRef]
- Sun, K.; Li, J.; Wang, F.; He, W.; Fei, M.; Lu, Z.; Zhang, H.; Liu, J.; Zou, Z. Highly enhanced durability of a graphitic carbon layer decorated PtNi3 alloy electrocatalyst toward the oxygen reduction reaction. Chem. Commun. 2019, 55, 5693–5696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xi, C.; Zhang, R.; Song, L.; Wang, C.; Spendelow, J.S.; Frenkel, A.I.; Yang, J.; Xin, H.L.; Sasaki, K. High-Performance Nitrogen-Doped Intermetallic PtNi Catalyst for the Oxygen Reduction Reaction. ACS Catalysis 2020, 10, 10637–10645. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, L.; Dang, W.; Wang, L.; Xia, M.; Wang, B.; Liu, M.; Gao, F.; Zhang, J.; Zhao, Y. High-Indexed PtNi Alloy Skin Spiraled on Pd Nanowires for Highly Efficient Oxygen Reduction Reaction Catalysis. Small 2019, 15, e1900288. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, Y.; Hong, J.W.; Whang, Y.; Kim, S.; Wi, D.H.; Byon, H.R.; Han, S.W. One-pot production of ceria nanosheet-supported PtNi alloy nanodendrites with high catalytic performance toward methanol oxidation and oxygen reduction. J. Mater. Chem. A 2020, 8, 25842–25849. [Google Scholar] [CrossRef]
- Tang, H.; Su, Y.; Chi, B.; Zhao, J.; Dang, D.; Tian, X.; Liao, S.; Li, G.-R. Nodal PtNi nanowires with Pt skin and controllable Near-Surface composition for enhanced oxygen reduction electrocatalysis in fuel cells. Chem. Eng. J. 2021, 418, 129322. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kwon, T.; Ha, Y.; Jun, M.; Baik, H.; Jeong, H.Y.; Kim, H.; Lee, K.; Joo, S.H. Intermetallic PtCu Nanoframes as Efficient Oxygen Reduction Electrocatalysts. Nano Lett. 2020, 20, 7413–7421. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.; Lou, X.W.; et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Gao, X.; Qin, C.; Yang, D.; Lv, H.; Xiao, Q.; Zhang, C. High performance octahedral PtNi/C catalysts investigated from rotating disk electrode to membrane electrode assembly. Nano Res. 2018, 12, 281–287. [Google Scholar] [CrossRef]
- Kong, F.; Ren, Z.; Banis, M.N.; Du, L.; Zhou, X.; Chen, G.; Zhang, L.; Li, J.; Wang, S.; Li, M.; et al. Active and Stable Pt–Ni Alloy Octahedra Catalyst for Oxygen Reduction via Near-Surface Atomical Engineering. ACS Catal. 2020, 10, 4205–4214. [Google Scholar] [CrossRef]
- Fichtner, J.; Garlyyev, B.; Watzele, S.; El-Sayed, H.A.; Schwämmlein, J.N.; Li, W.-J.; Maillard, F.M.; Dubau, L.; Michalička, J.; Macak, J.M.; et al. Top-Down Synthesis of Nanostructured Platinum–Lanthanide Alloy Oxygen Reduction Reaction Catalysts: PtxPr/C as an Example. ACS Appl. Mater. Interfaces 2019, 11, 5129–5135. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.-Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z.; et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Li, Z.; Zhou, Y.; Ma, X.; Lin, H.; Ying, W.; Peng, X. Fe3Pt intermetallic nanoparticles anchored on N-doped mesoporous carbon for the highly efficient oxygen reduction reaction. Chem. Commun. 2020, 56, 4898–4901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, X.; Kang, Y.; Xia, D.; Gan, L. Rational Development of Structurally Ordered Platinum Ternary Intermetallic Electrocatalysts for Oxygen Reduction Reaction. Catalysts 2019, 9, 569. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Martínez, H.; Tellez-Cruz, M.; Rojas-Chávez, H.; Ramírez-Herrera, C.; Calaminici, P.; Solorza-Feria, O. NiPdPt trimetallic nanoparticles as efficient electrocatalysts towards the oxygen reduction reaction. Int. J. Hydrogen Energy 2018, 44, 12463–12469. [Google Scholar] [CrossRef]
- Dai, S.; Chou, J.-P.; Wang, K.-W.; Hsu, Y.-Y.; Hu, A.; Pan, X.; Chen, T.-Y. Platinum-trimer decorated cobalt-palladium core-shell nanocatalyst with promising performance for oxygen reduction reaction. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Xie, M.; Chen, Z.; Lyu, Z.; Chi, M.; Jin, W.; Xia, Y. Pt-Ir-Pd Trimetallic Nanocages as a Dual Catalyst for Efficient Oxygen Reduction and Evolution Reactions in Acidic Media. Adv. Energy Mater. 2020, 10, 4114. [Google Scholar] [CrossRef]
- Duan, J.-J.; Zheng, X.-X.; Niu, H.-J.; Feng, J.-J.; Zhang, Q.-L.; Huang, H.; Wang, A.-J. Porous dendritic PtRuPd nanospheres with enhanced catalytic activity and durability for ethylene glycol oxidation and oxygen reduction reactions. J. Colloid Interface Sci. 2019, 560, 467–474. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Wang, F.; Yu, J.; Zhu, H. Nickel-introduced structurally ordered PtCuNi/C as high performance electrocatalyst for oxygen reduction reaction. Prog. Nat. Sci. 2020, 30, 905–911. [Google Scholar] [CrossRef]
- Wang, C.; Li, D.; Chi, M.; Pearson, J.; Rankin, R.B.; Greeley, J.; Duan, Z.; Wang, G.; van der Vliet, D.; More, K.L.; et al. Rational Development of Ternary Alloy Electrocatalysts. J. Phys. Chem. Lett. 2012, 3, 1668–1673. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Siahrostami, S.; Björketun, M.E.; Strasser, P.; Greeley, J.; Rossmeisl, J. Tandem cathode for proton exchange membrane fuel cells. Phys. Chem. Chem. Phys. 2013, 15, 9326–9334. [Google Scholar] [CrossRef]
- Busch, M.; Halck, N.B.; Kramm, U.; Siahrostami, S.; Krtil, P.; Rossmeisl, J. Beyond the top of the volcano? A unified approach to electrocatalytic oxygen reduction and oxygen evolution. Nano Energy 2016, 29, 126–135. [Google Scholar] [CrossRef]
- Batchelor, T.; Pedersen, J.K.; Winther, S.H.; Castelli, I.E.; Jacobsen, K.W.; Rossmeisl, J. High-Entropy Alloys as a Discovery Platform for Electrocatalysis. Joule 2019, 3, 834–845. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yeh, J.W.; Chen, S.K.; Shun, T.T. Wear resistance and high-temperature compression strength of Fcc CuCoNiCrAl0.5Fe alloy with boron addition, Metallurgical and Materials Transactions A: Physical. Metall. Mater. Sci. 2004, 35, 1465–1469. [Google Scholar]
- Yeh, J.W. Recent progress in high-entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Batchelor, T.A.; Yan, D.; Skjegstad, L.E.J.; Rossmeisl, J. Surface electrocatalysis on high-entropy alloys. Curr. Opin. Electrochem. 2020, 26, 100651. [Google Scholar] [CrossRef]
- Löffler, T.; Meyer, H.; Savan, A.; Wilde, P.; Garzón Manjón, A.; Chen, Y.T.; Ventosa, E.; Scheu, C.; Ludwig, A.; Schuhmann, W. Discovery of a Multinary Noble Metal–Free Oxygen Reduction Catalyst. Adv. Energy Mater. 2018, 8, 1802269. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Batchelor, T.A.A.; Bagger, A.; Rossmeisl, J. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions. ACS Catal. 2020, 10, 2169–2176. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Katiyar, N.K.; Kumar, R.; Parui, A.; Malviya, K.D.; Pradeep, K.G.; Singh, A.K.; Sharma, S.; Tiwary, C.S.; Biswas, K. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions: Experimental Realization. ACS Catal. 2020, 10, 3658–3663. [Google Scholar] [CrossRef]
- Wang, A.-L.; Wan, H.-C.; Xu, H.; Tong, Y.-X.; Li, G.-R. Quinary PdNiCoCuFe Alloy Nanotube Arrays as Efficient Electrocatalysts for Methanol Oxidation. Electrochim. Acta 2014, 127, 448–453. [Google Scholar] [CrossRef]
- Zhang, G.; Ming, K.; Kang, J.; Huang, Q.; Zhang, Z.; Zheng, X.; Bi, X. High entropy alloy as a highly active and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 279, 19–23. [Google Scholar] [CrossRef]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Nørskov, J.K. Materials for solar fuels and chemicals. Nat. Mater. 2016, 16, 70–81. [Google Scholar] [CrossRef]
- Löffler, T.; Ludwig, A.; Rossmeisl, J.; Schuhmann, W. What Makes High-Entropy Alloys Exceptional Electrocatalysts? Angew. Chem. Int. Ed. 2021, 60, 26894–26903. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Körmann, F. Surface segregation in Cr-Mn-Fe-Co-Ni high entropy alloys. Appl. Surf. Sci. 2020, 533, 147471. [Google Scholar] [CrossRef]
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Ye, Y.; Wang, Q.; Lu, J.; Liu, C.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2015, 19, 349–362. [Google Scholar] [CrossRef]
- Mackay, A.L. On complexity. Crystallogr. Rep. 2001, 46, 524–526. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-Entropy Alloys—A New Era of Exploitation. Mater. Sci. Forum 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.J.; Savan, A.; Ludwig, A. Atomic scale understanding of phase stability and decomposition of a nanocrystalline CrMnFeCoNi Cantor alloy. Appl. Phys. Lett. 2021, 119, 201910. [Google Scholar] [CrossRef]
- Li, Y.; Kostka, A.; Savan, A.; Ludwig, A. Atomic-scale investigation of fast oxidation kinetics of nanocrystalline CrMnFeCoNi thin films. J. Alloy. Compd. 2018, 766, 1080–1085. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Li, T.; Yao, Y.; Huang, Z.; Xie, P.; Liu, Z.; Yang, M.; Gao, J.; Zeng, K.; Brozena, A.H.; Pastel, G.; et al. Denary oxide nanoparticles as highly stable catalysts for methane combustion. Nat. Catal. 2021, 4, 62–70. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Lin, S.-J.; Chin, T.-S.; Gan, J.-Y.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chou, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Met. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Fang, G.; Wen, Y.; Liu, P.; Xie, G.; Liu, X.; Sun, S. Nanoporous high-entropy alloys for highly stable and efficient catalysts. J. Mater. Chem. A 2019, 7, 6499–6506. [Google Scholar] [CrossRef]
- Li, S.; Tang, X.; Jia, H.; Li, H.; Xie, G.; Liu, X.; Lin, X.; Qiu, H.-J. Nanoporous high-entropy alloys with low Pt loadings for high-performance electrochemical oxygen reduction. J. Catal. 2020, 383, 164–171. [Google Scholar] [CrossRef]
- Chen, X.; Si, C.; Gao, Y.; Frenzel, J.; Sun, J.; Eggeler, G.; Zhang, Z. Multi-component nanoporous platinum–ruthenium–copper–osmium–iridium alloy with enhanced electrocatalytic activity towards methanol oxidation and oxygen reduction. J. Power Sources 2015, 273, 324–332. [Google Scholar] [CrossRef]
- Mahmood, A.; Xie, N.; Din, M.A.U.; Saleem, F.; Lin, H.; Wang, X. Shape controlled synthesis of porous tetrametallic PtAgBiCo nanoplates as highly active and methanol-tolerant electrocatalyst for oxygen reduction reaction. Chem. Sci. 2017, 8, 4292–4298. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhan, X.; Bueno, S.L.A.; Shafei, I.H.; Ashberry, H.M.; Chatterjee, K.; Xu, L.; Tang, Y.; Skrabalak, S.E. Synthesis of monodisperse high entropy alloy nanocatalysts from core@shell nanoparticles. Nanoscale Horizons 2021, 6, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Lyu, J.; Zhao, Y.-L.; Li, H.; Lin, X.; Xie, G.; Liu, X.; Kai, J.-J.; Qiu, H.-J. Rugged High-Entropy Alloy Nanowires with in Situ Formed Surface Spinel Oxide As Highly Stable Electrocatalyst in Zn–Air Batteries. ACS Mater. Lett. 2020, 2, 1698–1706. [Google Scholar] [CrossRef]
- Jin, Z.; Lyu, J.; Zhao, Y.-L.; Li, H.; Chen, Z.; Lin, X.; Xie, G.; Liu, X.; Kai, J.-J.; Qiu, H.-J. Top–Down Synthesis of Noble Metal Particles on High-Entropy Oxide Supports for Electrocatalysis. Chem. Mater. 2021, 33, 1771–1780. [Google Scholar] [CrossRef]
- Marković, N.; Ross, P. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 2005, 275–374. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löffler, T.; Waag, F.; Gökce, B.; Ludwig, A.; Barcikowski, S.; Schuhmann, W. Comparing the Activity of Complex Solid Solution Electrocatalysts Using Inflection Points of Voltammetric Activity Curves as Activity Descriptors. ACS Catal. 2021, 11, 1014–1023. [Google Scholar] [CrossRef]
- Perez-Alonso, F.J.; McCarthy, D.N.; Nierhoff, A.; Hernandez-Fernandez, P.; Strebel, C.; Stephens, I.; Nielsen, J.H.; Chorkendorff, I. The Effect of Size on the Oxygen Electroreduction Activity of Mass-Selected Platinum Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 4641–4643. [Google Scholar] [CrossRef] [PubMed]
- Kramm, U.I.; Herrmann-Geppert, I.; Fiechter, S.; Zehl, G.; Zizak, I.; Dorbandt, I.; Schmeißer, D.; Bogdanoff, P. Effect of iron-carbide formation on the number of active sites in Fe–N–C catalysts for the oxygen reduction reaction in acidic media. J. Mater. Chem. A 2013, 2, 2663–2670. [Google Scholar] [CrossRef] [Green Version]
- Batchelor, T.A.A.; Löffler, T.; Xiao, B.; Krysiak, O.A.; Strotkötter, V.; Pedersen, J.K.; Clausen, C.M.; Savan, A.; Li, Y.; Schuhmann, W.; et al. Complex-Solid-Solution Electrocatalyst Discovery by Computational Prediction and High-Throughput Experimentation. Angew. Chem. Int. Ed. 2020, 60, 6932–6937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Wang, S. High-Entropy Alloys for Electrocatalysis: Design, Characterization, and Applications. Small 2021, 2104339. [Google Scholar] [CrossRef] [PubMed]
- Schweinar, K.; Nicholls, R.L.; Rajamathi, C.R.; Zeller, P.; Amati, M.; Gregoratti, L.; Raabe, D.; Greiner, M.; Gault, B.; Kasian, O. Probing catalytic surfaces by correlative scanning photoemission electron microscopy and atom probe tomography. J. Mater. Chem. A 2019, 8, 388–400. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.J.; Savan, A.; Kostka, A.; Stein, H.S.; Ludwig, A. Accelerated atomic-scale exploration of phase evolution in compositionally complex materials. Mater. Horizons 2017, 5, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Marshal, A.; Pradeep, K.; Music, D.; Zaefferer, S.; De, P.; Schneider, J. Combinatorial synthesis of high entropy alloys: Introduction of a novel, single phase, body-centered-cubic FeMnCoCrAl solid solution. J. Alloy. Compd. 2016, 691, 683–689. [Google Scholar] [CrossRef]
- Casalongue, H.S.; Kaya, S.; Viswanathan, V.; Miller, D.J.; Friebel, D.; Hansen, H.A.; Nørskov, J.K.; Nilsson, A.; Ogasawara, H. Direct observation of the oxygenated species during oxygen reduction on a platinum fuel cell cathode. Nat. Commun. 2013, 4, 2817. [Google Scholar] [CrossRef]
- Jacobse, L.; Huang, Y.-F.; Koper, M.T.M.; Rost, M.J. Correlation of surface site formation to nanoisland growth in the electrochemical roughening of Pt(111). Nat. Mater. 2018, 17, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vliet, D.F.; Wang, C.; Li, D.; Paulikas, A.P.; Greeley, J.; Rankin, R.; Strmcnik, D.; Tripkovic, D.; Markovic, N.M.; Stamenkovic, V.R. Unique Electrochemical Adsorption Properties of Pt-Skin Surfaces. Angew. Chem. Int. Ed. 2012, 51, 3139–3142. [Google Scholar] [CrossRef]
- Weaver, M.; Chang, S.-C.; Leung, L.-W.; Jiang, X.; Rubel, M.; Szklarczyk, M.; Zurawski, D.; Wieckowski, A. Evaluation of absolute saturation coverages of carbon monoxide on ordered low-index platinum and rhodium electrodes. J. Electroanal. Chem. 1992, 327, 247–260. [Google Scholar] [CrossRef]
- Cuesta, A.; Couto, A.; Rincón, A.; Pérez, M.C.; López-Cudero, A.; Gutiérrez, C. Potential dependence of the saturation CO coverage of Pt electrodes: The origin of the pre-peak in CO-stripping voltammograms. Part 3: Pt(poly). J. Electroanal. Chem. 2006, 586, 184–195. [Google Scholar] [CrossRef]
- Vidaković, T.; Christov, M.; Sundmacher, K. The use of CO stripping for in situ fuel cell catalyst characterization. Electrochimica Acta 2007, 52, 5606–5613. [Google Scholar] [CrossRef]
- Rudi, S.; Cui, C.; Gan, L.; Strasser, P. Comparative Study of the Electrocatalytically Active Surface Areas (ECSAs) of Pt Alloy Nanoparticles Evaluated by Hupd and CO-stripping voltammetry. Electrocatalysis 2014, 5, 408–418. [Google Scholar] [CrossRef]
- Green, C.L.; Kucernak, A. Determination of the platinum and ruthenium surface areas in platinum-ruthenium alloy electrocatalysts by underpotential deposition of Copper. I. Unsupported catalysts. J. Phys. Chem. B 2002, 106, 1036–1047. [Google Scholar] [CrossRef]
- Nagel, T.; Bogolowski, N.; Baltruschat, H. Towards a determination of the active surface area of polycrystalline and nanoparticle electrodes by Cu upd and CO oxidation. J. Appl. Electrochem. 2006, 36, 1297–1306. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- McCrory, C.; Jung, S.; Ferrer, I.M.; Chatman, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, D.M.; Risch, M. Seven steps to reliable cyclic voltammetry measurements for the determination of double layer capacitance. J. Physics: Energy 2021, 3, 3. [Google Scholar] [CrossRef]
- Čolić, V.; Tymoczko, J.; Maljusch, A.; Ganassin, A.; Schuhmann, W.; Bandarenka, A.S. Experimental Aspects in Benchmarking of the Electrocatalytic Activity. ChemElectroChem 2014, 2, 143–149. [Google Scholar] [CrossRef]
- Lasia, A. Impedance Spectroscopy Applied to the Study of Electrocatalytic Processes. Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, Netherlands, 2018; pp. 241–263. [Google Scholar]
- van der Vliet, D.; Strmcnik, D.S.; Wang, C.; Stamenkovic, V.R.; Markovic, N.M.; Koper, M.T. On the importance of correcting for the uncompensated Ohmic resistance in model experiments of the Oxygen Reduction Reaction. J. Electroanal. Chem. 2010, 647, 29–34. [Google Scholar] [CrossRef]
- Popkirov, G. A technique for series resistance measurement and ohmic drop correction under potentiostatic control. J. Electroanal. Chem. 1993, 359, 97–103. [Google Scholar] [CrossRef]
- DOE Durability Working Group. Rotating Disk-Electrode Aqueous Electrolyte Accelerated Stress Tests for PGM Electrocatalyst/Support Durability Evaluation. 2011. Available online: https://www.energy.gov/sites/default/files/2015/08/f25/fcto_dwg_pgm_electrocatalyst_support_aqueous_ast.pdf (accessed on 24 November 2021).
- Kocha, S.S.; Shinozaki, K.; Zack, J.W.; Myers, D.J.; Kariuki, N.N.; Nowicki, T.; Stamenkovic, V.; Kang, Y.; Li, D.; Papageorgopoulos, D. Best Practices and Testing Protocols for Benchmarking ORR Activities of Fuel Cell Electrocatalysts Using Rotating Disk Electrode. Electrocatalysis 2017, 8, 366–374. [Google Scholar] [CrossRef]
- Du, C.; Tan, Q.; Yin, G.; Zhang, J. 5—Rotating Disk Electrode Method. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Xing, W., Yin, G., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 171–198. [Google Scholar]
- Mayrhofer, K.; Strmcnik, D.; Blizanac, B.; Stamenkovic, V.; Arenz, M.; Markovic, N. Measurement of oxygen reduction activities via the rotating disc electrode method: From Pt model surfaces to carbon-supported high surface area catalysts. Electrochimica Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Martens, S.; Asen, L.; Ercolano, G.; Dionigi, F.; Zalitis, C.; Hawkins, A.; Bonastre, A.M.; Seidl, L.; Knoll, A.C.; Sharman, J.; et al. A comparison of rotating disc electrode, floating electrode technique and membrane electrode assembly measurements for catalyst testing. J. Power Sources 2018, 392, 274–284. [Google Scholar] [CrossRef]
- Topalov, A.A.; Katsounaros, I.; Auinger, M.; Cherevko, S.; Meier, J.C.; Klemm, S.O.; Mayrhofer, K.J.J. Dissolution of Platinum: Limits for the Deployment of Electrochemical Energy Conversion? Angew. Chem. Int. Ed. 2012, 51, 12613–12615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
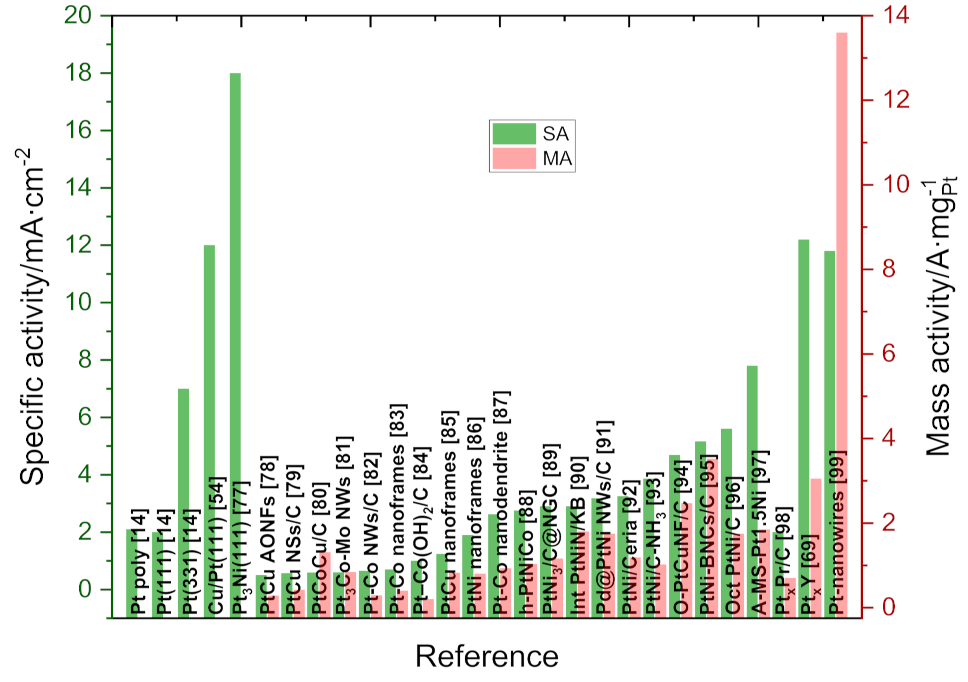

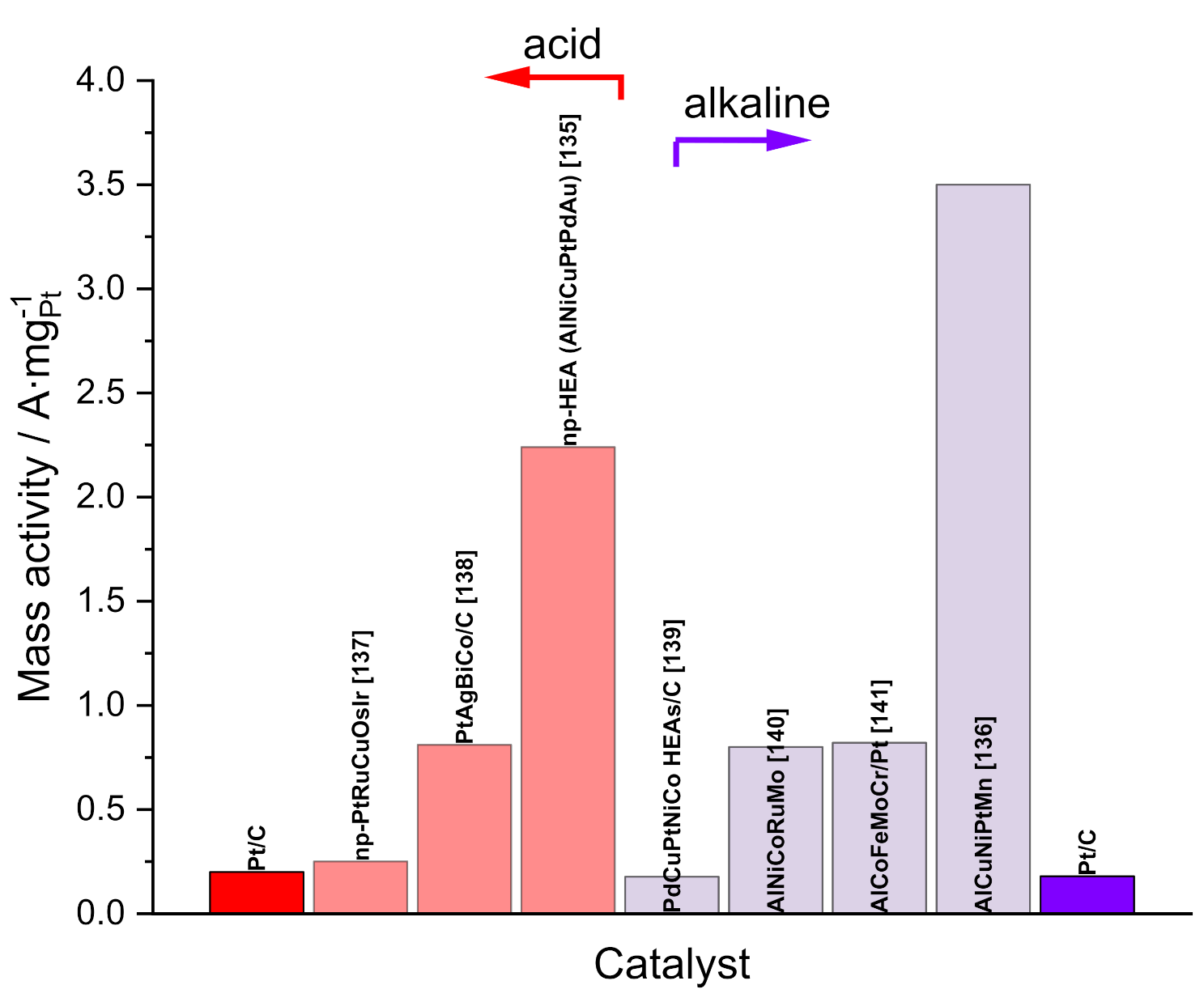



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Hincapié, R.; Čolić, V. Electrocatalysts for the Oxygen Reduction Reaction: From Bimetallic Platinum Alloys to Complex Solid Solutions. ChemEngineering 2022, 6, 19. https://doi.org/10.3390/chemengineering6010019
Martínez-Hincapié R, Čolić V. Electrocatalysts for the Oxygen Reduction Reaction: From Bimetallic Platinum Alloys to Complex Solid Solutions. ChemEngineering. 2022; 6(1):19. https://doi.org/10.3390/chemengineering6010019
Chicago/Turabian StyleMartínez-Hincapié, Ricardo, and Viktor Čolić. 2022. "Electrocatalysts for the Oxygen Reduction Reaction: From Bimetallic Platinum Alloys to Complex Solid Solutions" ChemEngineering 6, no. 1: 19. https://doi.org/10.3390/chemengineering6010019
APA StyleMartínez-Hincapié, R., & Čolić, V. (2022). Electrocatalysts for the Oxygen Reduction Reaction: From Bimetallic Platinum Alloys to Complex Solid Solutions. ChemEngineering, 6(1), 19. https://doi.org/10.3390/chemengineering6010019





