Review of the Application of Hydrotalcite as CO2 Sinks for Climate Change Mitigation
Abstract
:1. Introduction
2. Calcined or Uncalcined Hydrotalcite to Capture CO2?
2.1. Thermal Behaviour of Hydrotalcite by TGA/DTA
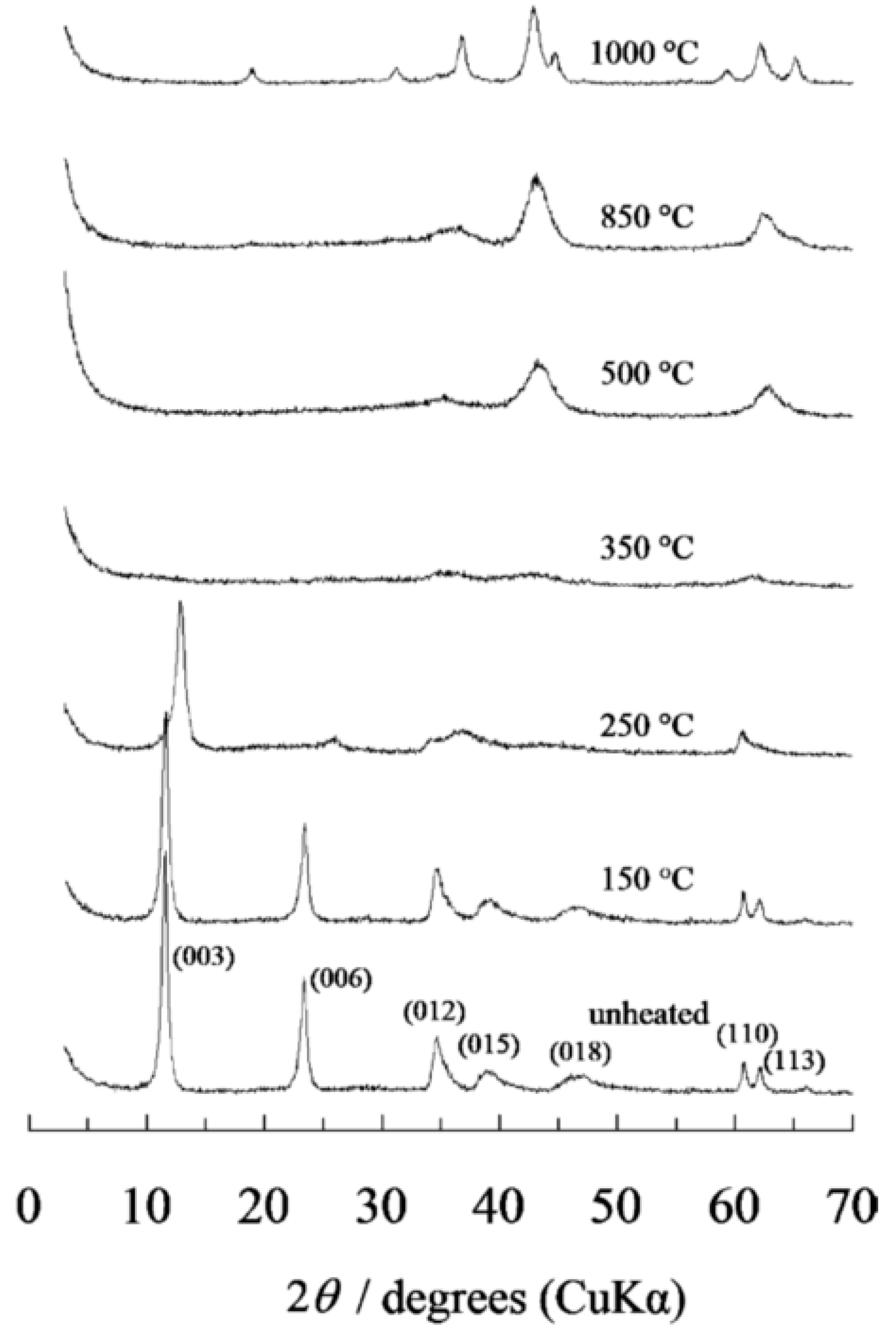
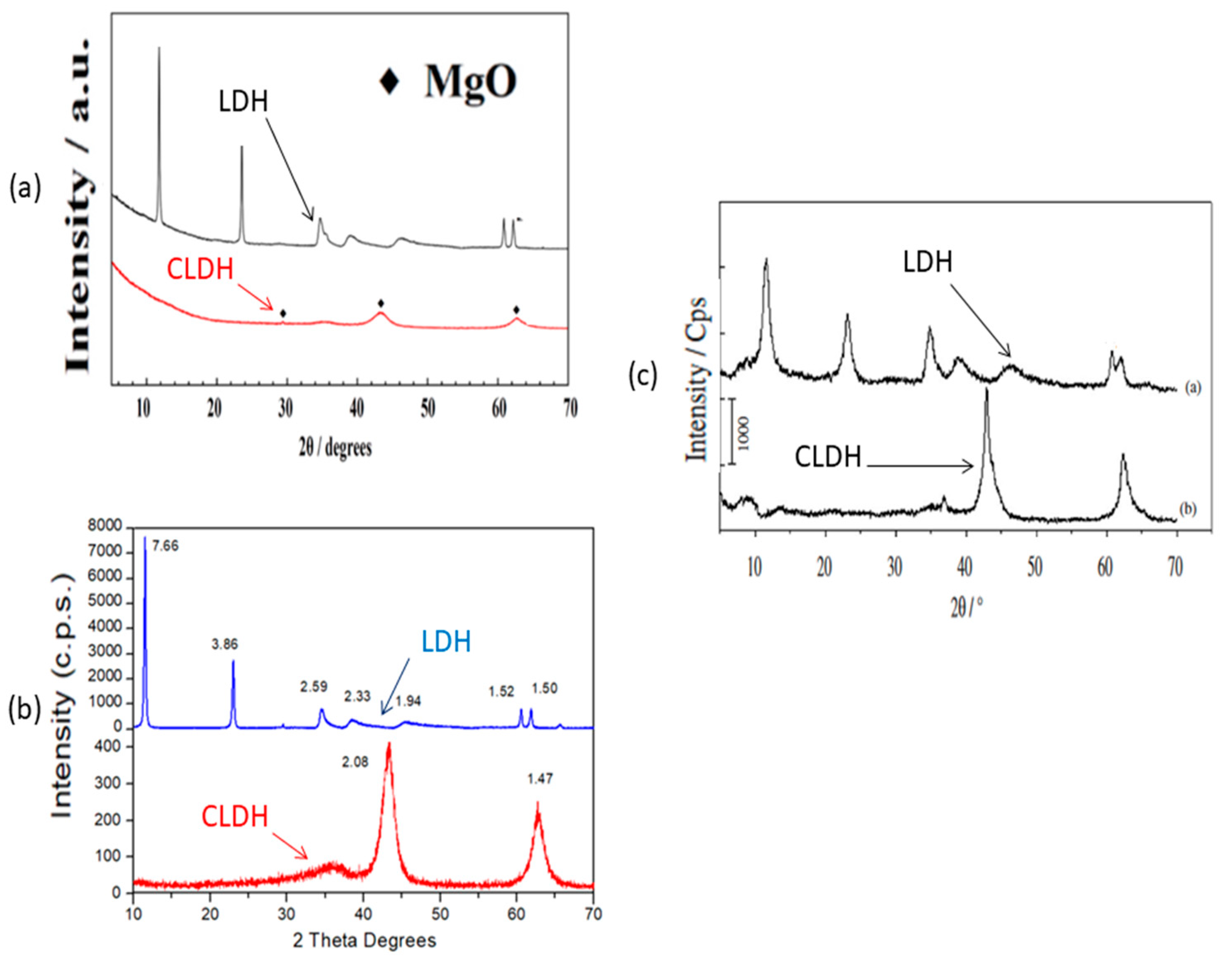
2.2. Thermal Behaviour of Hydrotalcite by XRD
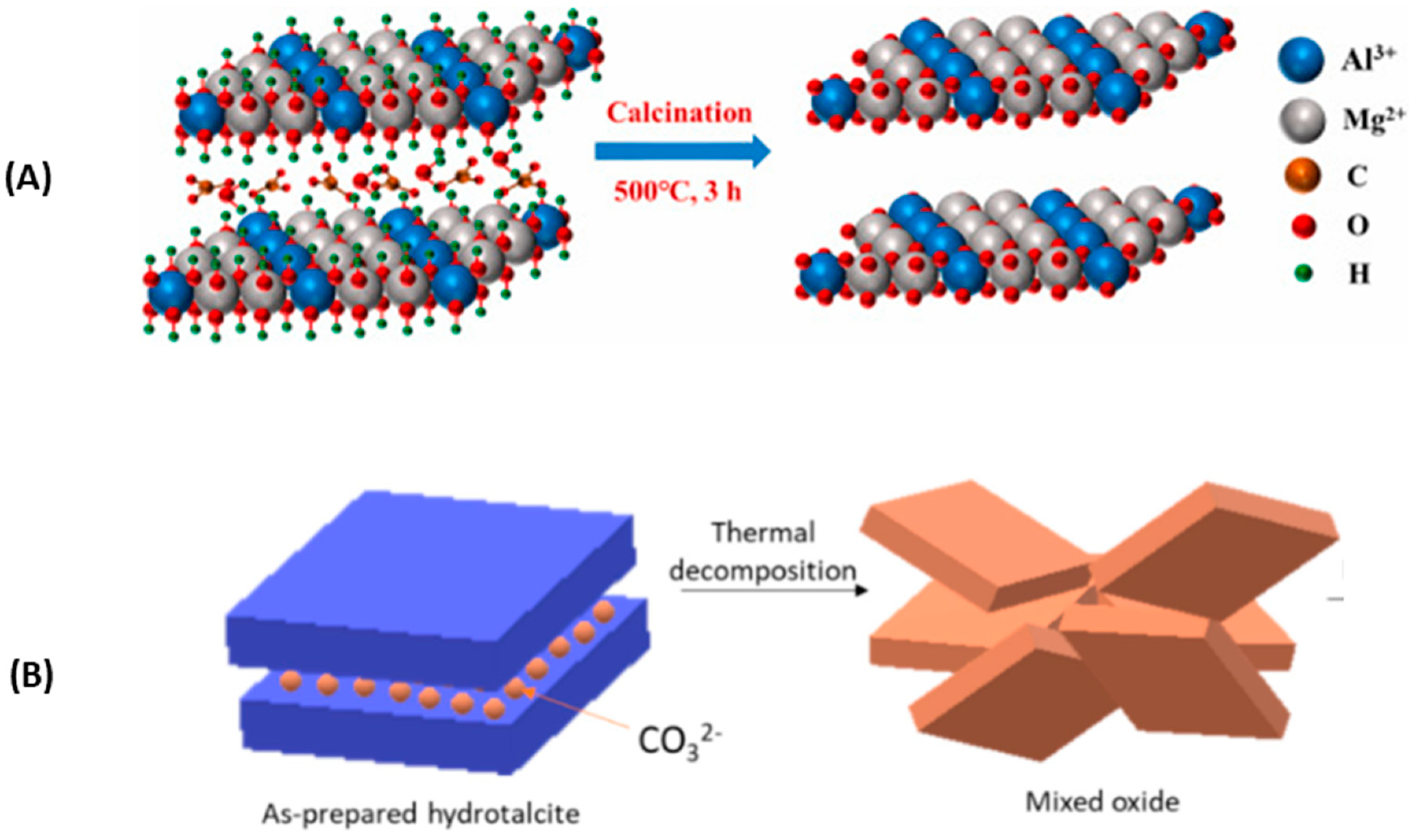
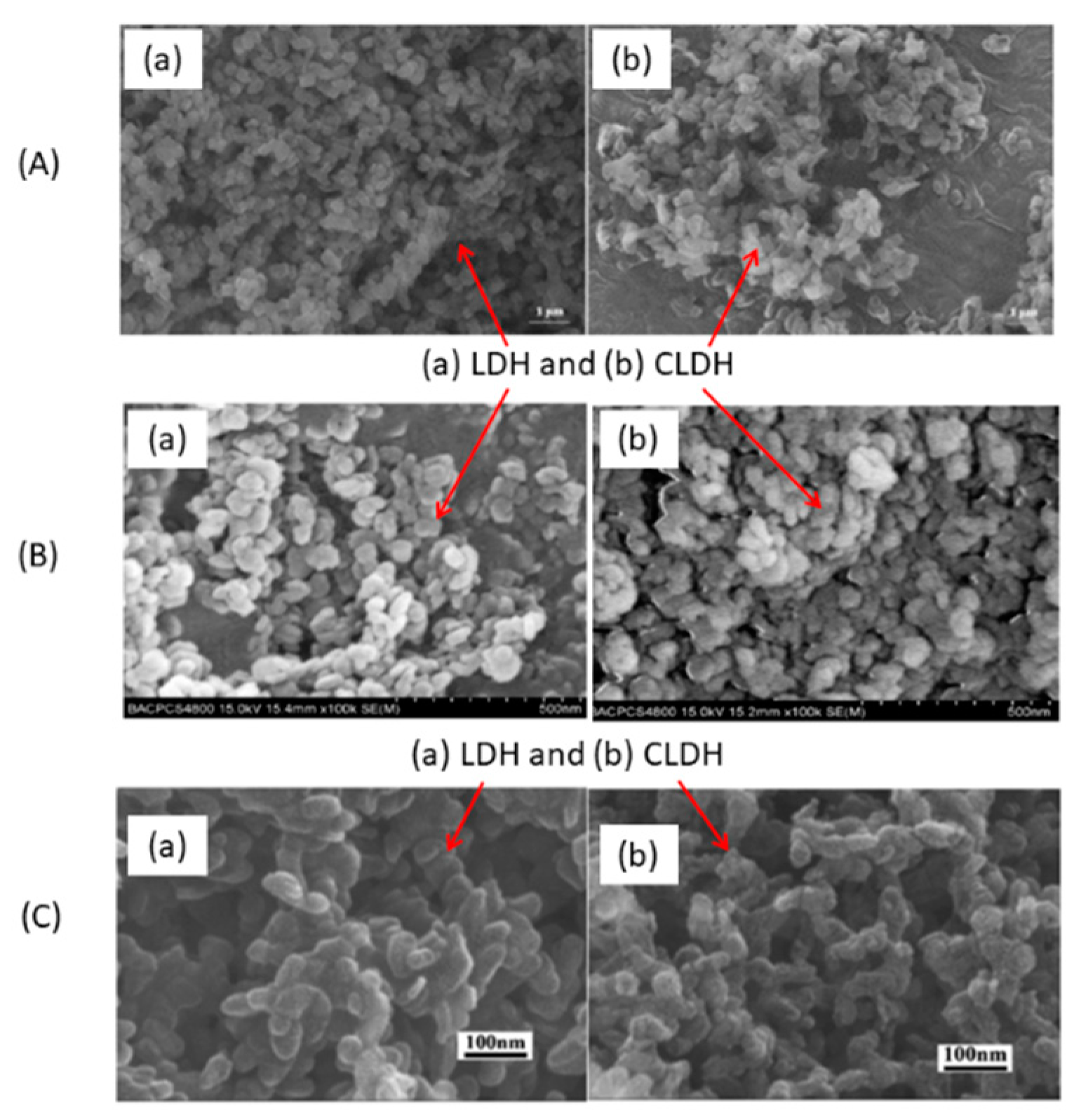
2.3. Thermal Behaviour of Hydrotalcite by SEM/TEM
3. Influence of the pH Used in the Synthesis in CO2 Capture
4. Influence of the Molar Ratio (Mg/Al) of Its Main Elements in CO2 Capture
5. Ways to Increase the Specific Area of Hydrotalcites
6. Pressure, Temperature, and Capacity in CO2 Absorption and Use of CO2 Captured
7. Combined Use of Hydrotalcites and Cement-Based Materials
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USEP Agency. Global Greenhouse Gas Emissions Data. 2022. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 24 March 2022).
- Van Selow, E.R.; Cobden, P.D.; Verbraeken, P.A.; Hufton, J.R.; Brink, R.W.V.D. Carbon Capture by Sorption-Enhanced Water−Gas Shift Reaction Process using Hydrotalcite-Based Material. Ind. Eng. Chem. Res. 2009, 48, 4184–4193. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.R.; Fernández, J.M. Potential CO2 capture in one-coat limestone mortar modified with Mg3Al–CO3 calcined hydrotalcites using ultrafast testing technique. Chem. Eng. J. 2021, 415, 129077. [Google Scholar] [CrossRef]
- Martins, V.F.D.; Miguel, C.V.; Gonçalves, J.C.; Rodrigues, A.E.; Madeira, L.M. Modeling of a cyclic sorption–desorption unit for continuous high temperature CO2 capture from flue gas. Chem. Eng. J. 2022, 434, 134704. [Google Scholar] [CrossRef]
- IEA. Global Energy and CO2 Status Report 2017. Glob. Energy CO2 Status Rep. 2017. Available online: https://www.iea.org/publications/freepublications/publication/GECO2017.pdf (accessed on 1 April 2022).
- Yadav, S.; Mondal, S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2021, 308, 122057. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.; Madeira, L.M. Effect of interlayer anion on the CO2 capture capacity of hydrotalcite-based sorbents. Sep. Purif. Technol. 2019, 219, 290–302. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Mondal, S.S. A complete review based on various aspects of pulverized coal combustion. Int. J. Energy Res. 2019, 43, 3134–3165. [Google Scholar] [CrossRef]
- Christensen, T.H.; Bisinella, V. Climate change impacts of introducing carbon capture and utilisation (CCU) in waste incineration. Waste Manag. 2021, 126, 754–770. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Kalinowska-Wichrowska, K.; Fernández, J.M.; Jiménez, J.R. Accelerated carbonation of fresh cement-based products containing recycled masonry aggregates for CO2 sequestration. J. CO2 Util. 2021, 46, 101461. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Fernández-Rodríguez, J.M.; Jiménez, J.R. Use of carbonated water to improve the mechanical properties and reduce the carbon footprint of cement-based materials with recycled aggregates. J. CO2 Util. 2022, 57, 101886. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2013, 7, 130–189. [Google Scholar] [CrossRef]
- Jung, W.; Lee, J. Economic evaluation for four different solid sorbent processes with heat integration for energy-efficient CO2 capture based on PEI-silica sorbent. Energy 2021, 238, 121864. [Google Scholar] [CrossRef]
- Jung, W.; Lee, M.; Hwang, G.S.; Kim, E.; Lee, K.S. Thermodynamic modeling and energy analysis of a polyamine-based water-lean solvent for CO2 capture. Chem. Eng. J. 2020, 399, 125714. [Google Scholar] [CrossRef]
- Jung, W.; Park, J.; Won, W.; Lee, K.S. Simulated moving bed adsorption process based on a polyethylenimine-silica sorbent for CO2 capture with sensible heat recovery. Energy 2018, 150, 950–964. [Google Scholar] [CrossRef]
- Jung, W.; Park, S.; Lee, K.S.; Jeon, J.-D.; Lee, H.K.; Kim, J.-H.; Lee, J.S. Rapid thermal swing adsorption process in multi-beds scale with sensible heat recovery for continuous energy-efficient CO2 capture. Chem. Eng. J. 2019, 392, 123656. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.-K. Process-integrated design of a sub-ambient membrane process for CO2 removal from natural gas power plants. Appl. Energy 2019, 260, 114255. [Google Scholar] [CrossRef]
- Bhatta, L.K.G.; Subramanyam, S.; Chengala, M.D.; Olivera, S.; Venkatesh, K. Progress in hydrotalcite like compounds and metal-based oxides for CO2 capture: A review. J. Clean. Prod. 2015, 103, 171–196. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Quesada Carballo, L.; Perez Perez, M.; Cantador Fernández, D.; Caballero Amores, A.; Fernández Rodríguez, J.M. Optimum Particle Size of Treated Calcites for CO2 Capture in a Power Plant. Materials 2019, 12, 1284. [Google Scholar] [CrossRef] [Green Version]
- Halabi, M.; de Croon, M.; van der Schaaf, J.; Cobden, P.; Schouten, J. High capacity potassium-promoted hydrotalcite for CO2 capture in H2 production. Int. J. Hydrog. Energy 2012, 37, 4516–4525. [Google Scholar] [CrossRef]
- Kou, X.; Guo, H.; Ayele, E.G.; Li, S.; Zhao, Y.; Wang, S.; Ma, X. Adsorption of CO2 on MgAl-CO3 LDHs-Derived Sorbents with 3D Nanoflower-like Structure. Energy Fuels 2018, 32, 5313–5320. [Google Scholar] [CrossRef]
- Williams, G.R.; O’Hare, D. Towards understanding, control and application of layered double hydroxide chemistry. J. Mater. Chem. 2006, 16, 3065–3074. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Bennici, S.; Vega, A.; Ordóñez, S.; Auroux, A. Adsorption of CO2 on Hydrotalcite-Derived Mixed Oxides: Sorption Mechanisms and Consequences for Adsorption Irreversibility. Ind. Eng. Chem. Res. 2010, 49, 3663–3671. [Google Scholar] [CrossRef]
- Torres-Rodríguez, D.A.; Lima, E.; Valente, J.S.; Pfeiffer, H. CO2 Capture at Low Temperatures (30–80 °C) and in the Presence of Water Vapor over a Thermally Activated Mg–Al Layered Double Hydroxide. J. Phys. Chem. A 2011, 115, 12243–12250. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.M.; Campos, J.; Souza, M.M.V.M. CO2 capture by Mg–Al and Zn–Al hydrotalcite-like compounds. Adsorption 2015, 22, 151–158. [Google Scholar] [CrossRef]
- Hutson, N.D.; Attwood, B.C. High temperature adsorption of CO2 on various hydrotalcite-like compounds. Adsorption 2008, 14, 781–789. [Google Scholar] [CrossRef]
- Oliveira, E.L.; Grande, C.A.; Rodrigues, A.E. CO2 sorption on hydrotalcite and alkali-modified (K and Cs) hydrotalcites at high temperatures. Sep. Purif. Technol. 2008, 62, 137–147. [Google Scholar] [CrossRef]
- Allmann, R. The crystal structure of pyroaurite. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1968, 24, 972–977. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Crystal structures of some double hydroxide minerals. Miner. Mag. 1973, 39, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Miyata, S. The syntheses of hydrotalcite-like compounds and their structure and physico-chemical properties—I: The systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.; Fernández, J. Mitigation of CO2 emissions by hydrotalcites of Mg3Al-CO3 at 0 °C and high pressure. Appl. Clay Sci. 2020, 202, 105950. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Morales, D.S.; Jiménez, J.R.; Fernández-Rodriguez, J.M. CO2 adsorption by organohydrotalcites at low temperatures and high pressure. Chem. Eng. J. 2021, 431, 134324. [Google Scholar] [CrossRef]
- Faria, A.C.; Trujillano, R.; Rives, V.; Miguel, C.; Rodrigues, A.; Madeira, L.M. Alkali metal (Na, Cs and K) promoted hydrotalcites for high temperature CO2 capture from flue gas in cyclic adsorption processes. Chem. Eng. J. 2021, 427, 131502. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Costantino, U.; Nocchetti, M.; Sisani, M.; Vivani, R. Recent progress in the synthesis and application of organically modified hydrotalcites. Zeitschrift für Kristallographie 2009, 224, 273–281. [Google Scholar] [CrossRef]
- Ding, Y.; Alpay, E. Equilibria and kinetics of CO2 adsorption on hydrotalcite adsorbent. Chem. Eng. Sci. 2000, 55, 3461–3474. [Google Scholar] [CrossRef]
- Schulze, K.; Makowski, W.; Chyzy, R. Nickel doped hydrotalcites as catalyst precursors for the partial oxidation of light paraffins. Appl. Clay Sci. 2001, 18, 59–69. [Google Scholar] [CrossRef]
- Reichle, W. Catalytic reactions by thermally activated, synthetic, anionic clay minerals. J. Catal. 1985, 94, 547–557. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Rives, V.; Ulibarri, M.A. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- Ishihara, Y.; Okabe, S. Effects of cholestyramine and synthetic hydrotalcite on acute gastric or intestinal lesion formation in rats and dogs. Am. J. Dig. Dis. 1981, 26, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Barlattani, M.; Mantera, G.; Fasani, R.; Carosi, M. Efficacy of antacid treatment in peptic ulcerative patients: Therapeutic value of synthetic hydrotalcite (Talcid). Clin. Trials J. 1982, 19, 359–367. [Google Scholar]
- Ookubo, A.; Ooi, K.; Hayashi, H. Hydrotalcites as Potential Adsorbents of Intestinal Phosphate. J. Pharm. Sci. 1992, 81, 1139–1140. [Google Scholar] [CrossRef] [PubMed]
- Rives, V.; Carriazo, D.; Martín, C. Heterogeneous Catalysis by Polyoxometalate-Intercalated Layered Double Hydroxides. In Pillared Clays and Related Catalysts; Springer: New York, NY, USA, 2010. [Google Scholar]
- Mori, K.; Nakamura, Y.; Kikuchi, I. Modification of poly(vinyl chloride). XLI. Effect of hydrotalcite on the stabilization of poly(vinyl chloride) by 6-anilino-1,3,5-triazine-2,4-dithiol and zinc stearate. J. Polym. Sci. Part C Polym. Lett. 1981, 19, 623–628. [Google Scholar] [CrossRef]
- Van der Ven, L.; van Gemert, M.; Batenburg, L.; Keern, J.; Gielgens, L.; Koster, T.; Fischer, H. On the action of hydrotalcite-like clay materials as stabilizers in polyvinylchloride. Appl. Clay Sci. 2000, 17, 25–34. [Google Scholar] [CrossRef]
- Hermosín, M.; Pavlovic, I.; Ulibarri, M.; Cornejo, J. Hydrotalcite as sorbent for trinitrophenol: Sorption capacity and mechanism. Water Res. 1996, 30, 171–177. [Google Scholar] [CrossRef]
- Cheng, W.; Wan, T.; Wang, X.; Wu, W.; Hu, B. Plasma-grafted polyamine/hydrotalcite as high efficient adsorbents for retention of uranium (VI) from aqueous solutions. Chem. Eng. J. 2018, 342, 103–111. [Google Scholar] [CrossRef]
- Ogata, F.; Ueta, E.; Kawasaki, N. Characteristics of a novel adsorbent Fe–Mg-type hydrotalcite and its adsorption capability of As(III) and Cr(VI) from aqueous solution. J. Ind. Eng. Chem. 2018, 59, 56–63. [Google Scholar] [CrossRef]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Abdelouas, A. Formation of Hydrotalcite-like Compounds During R7T7 Nuclear Waste Glass and Basaltic Glass Alteration. Clays Clay Miner. 1994, 42, 526–533. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Faucon, P.; Le Bescop, P.; Adenot, F.; Bonville, P.; Jacquinot, J.; Pineau, F.; Felix, B. Leaching of cement: Study of the surface layer. Cem. Concr. Res. 1996, 26, 1707–1715. [Google Scholar] [CrossRef]
- Paul, M.; Glasser, F. Impact of prolonged warm (85 °C) moist cure on Portland cement paste. Cem. Concr. Res. 2000, 30, 1869–1877. [Google Scholar] [CrossRef]
- Scheidegger, A.M.; Wieland, E.; Scheinost, A.; Dähn, R.; Tits, J.; Spieler, P. Ni phases formed in cement and cement systems under highly alkaline conditions: An XAFS study. J. Synchrotron Radiat. 2001, 8, 916–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suescum-Morales, D.; Fernández, D.C.; Fernández, J.M.; Jiménez, J.R. The combined effect of CO2 and calcined hydrotalcite on one-coat limestone mortar properties. Constr. Build. Mater. 2021, 280, 122532. [Google Scholar] [CrossRef]
- He, P.; Shi, C.; Tu, Z.; Poon, C.S.; Zhang, J. Effect of further water curing on compressive strength and microstructure of CO2-cured concrete. Cem. Concr. Compos. 2016, 72, 80–88. [Google Scholar] [CrossRef]
- Erans, M.; Jeremias, M.; Zheng, L.; Yao, J.G.; Blamey, J.; Manovic, V.; Fennell, P.S.; Anthony, E.J. Pilot testing of enhanced sorbents for calcium looping with cement production. Appl. Energy 2018, 225, 392–401. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Shoro, D.; Murakami, K.; Honda, M.; Kawabata, T.; Takaki, K. Preparation of egg-shell type Ni-loaded catalyst by adopting “Memory Effect” of Mg–Al hydrotalcite and its application for CH4 reforming. Catal. Commun. 2004, 5, 209–213. [Google Scholar] [CrossRef]
- Gao, Z.; Sasaki, K.; Qiu, X. Structural Memory Effect of Mg–Al and Zn–Al layered Double Hydroxides in the Presence of Different Natural Humic Acids: Process and Mechanism. Langmuir 2018, 34, 5386–5395. [Google Scholar] [CrossRef]
- Yong, Z.; Rodrigues, E. Hydrotalcite like compounds as adsorbents for carbon dioxide. Energy Convers. Manag. 2002, 43, 1865–1876. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.; Madeira, L.M. Doping of hydrotalcite-based sorbents with different interlayer anions for CO2 capture. Sep. Purif. Technol. 2019, 235, 116140. [Google Scholar] [CrossRef]
- Qu, Z.; Yu, Q.; Brouwers, H. Relationship between the particle size and dosage of LDHs and concrete resistance against chloride ingress. Cem. Concr. Res. 2018, 105, 81–90. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Cerezo, J.; Mol, J.M.C.; Polder, R. Aminobenzoate modified MgAAl hydrotalcites as a novel smart additive of reinforced concrete for anticorrosion applications. Constr. Build. Mater. 2013, 47, 1436–1443. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Polder, R. Synthesis and characterization of modified hydrotalcites and their ion exchange characteristics in chloride-rich simulated concrete pore solution. Cem. Concr. Compos. 2014, 47, 87–93. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Polder, R. Modified hydrotalcites as a new emerging class of smart additive of reinforced concrete for anticorrosion applications: A literature review. Mater. Corros. 2013, 64, 1066–1074. [Google Scholar] [CrossRef]
- Lozano-Lunar, A.; Álvarez, J.I.; Navarro-Blasco, Í.; Jiménez, J.R.; Fernández-Rodriguez, J.M. Optimisation of mortar with Mg-Al-Hydrotalcite as sustainable management strategy lead waste. Appl. Clay Sci. 2021, 212, 106218. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Fireproof performance of the intumescent fire retardant coatings with layered double hydroxides additives. Constr. Build. Mater. 2020, 256, 119445. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, P.; Yan, C. Role of layered double hydroxides in setting, hydration degree, microstructure and compressive strength of cement paste. Appl. Clay Sci. 2018, 158, 123–131. [Google Scholar] [CrossRef]
- Lauermannová, A.-M.; Paterová, I.; Patera, J.; Skrbek, K.; Jankovský, O.; Bartůněk, V. Hydrotalcites in Construction Materials. Appl. Sci. 2020, 10, 7989. [Google Scholar] [CrossRef]
- Gomes, C.; Mir, Z.; Sampaio, R.; Bastos, A.; Tedim, J.; Maia, F.; Rocha, C.; Ferreira, M. Use of ZnAl-Layered Double Hydroxide (LDH) to Extend the Service Life of Reinforced Concrete. Materials 2020, 13, 1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, Z.M.; Bastos, A.; Höche, D.; Zheludkevich, M.L. Recent Advances on the Application of Layered Double Hydroxides in Concrete—A Review. Materials 2020, 13, 1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Guo, J.; Tian, J.; Xu, Y.; Hu, M.; Wang, M.; Fan, J. Preparation of Ca/Al-Layered Double Hydroxide and the influence of their structure on early strength of cement. Constr. Build. Mater. 2018, 184, 203–214. [Google Scholar] [CrossRef]
- Long, W.-J.; Xie, J.; Zhang, X.; Fang, Y.; Khayat, K.H. Hydration and microstructure of calcined hydrotalcite activated high-volume fly ash cementitious composite. Cem. Concr. Compos. 2021, 123, 104213. [Google Scholar] [CrossRef]
- Long, W.; Xie, J.; Zhang, X.; Kou, S.; Xing, F.; He, C. Accelerating effect of calcined hydrotalcite-Na2SO4 binary system on hydration of high volume fly ash cement. Constr. Build. Mater. 2022, 328, 127068. [Google Scholar] [CrossRef]
- Ma, J.; Duan, P.; Ren, D.; Zhou, W. Effects of layered double hydroxides incorporation on carbonation resistance of cementitious materials. J. Mater. Res. Technol. 2019, 8, 292–298. [Google Scholar] [CrossRef]
- Vágvölgyi, V.; Palmer, S.J.; Kristóf, J.; Frost, R.L.; Horváth, E. Mechanism for hydrotalcite decomposition: A controlled rate thermal analysis study. J. Colloid Interface Sci. 2007, 318, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Yahyaoui, R.; Jimenez, P.E.S.; Maqueda, L.A.P.; Nahdi, K.; Luque, J.M.C. Synthesis, characterization and combined kinetic analysis of thermal decomposition of hydrotalcite (Mg6Al2(OH)16CO3·4H2O). Thermochim. Acta 2018, 667, 177–184. [Google Scholar] [CrossRef]
- Miyata, S. Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Kannan, V.R.S.; Velu, S. Synthesis and physicochemical properties of cobalt aluminium hydrotalcites. J. Mater. Sci. 1995, 30, 1462–1468. [Google Scholar] [CrossRef]
- Cocheci, L.; Barvinschi, P.; Pode, R.; Popovici, E.; Seftel, E.M. Structural Characterization of Some Mg/Zn-Al Type Hy-drotalcites Prepared for Chromate Sorption from Wastewater. Chem. Bull. 2010, 55, 40–45. [Google Scholar]
- Palmer, S.J.; Spratt, H.J.; Frost, R.L. Thermal decompostition of hydrotalcites with variable cationic ratios. J. Therm. Anal. Calorim. 2009, 95, 123–129. [Google Scholar] [CrossRef]
- Garcia-Gallastegui, A.; Iruretagoyena, D.; Gouvea, V.; Mokhtar, M.; Asiri, A.M.; Basahel, S.N.; Al-Thabaiti, S.A.; Alyoubi, A.O.; Chadwick, D.; Shaffer, M.S.P. Graphene Oxide as Support for Layered Double Hydroxides: Enhancing the CO2 Adsorption Capacity. Chem. Mater. 2012, 24, 4531–4539. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Lu, G.Q.; Diniz da Costa, J.C. Layered Double Hydroxides for CO2 Capture: Structure Evolution and Regeneration. Ind. Eng. Chem. Res. 2006, 45, 7504–7509. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Z.; Tay, H.H.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. High temperature adsorption of CO2 on Mg–Al hydrotalcite: Effect of the charge compensating anions and the synthesis pH. Catal. Today 2011, 164, 198–203. [Google Scholar] [CrossRef]
- Hibino, T. Decarbonation Behavior of Mg-Al-CO3 Hydrotalcite-like Compounds during Heat Treatment. Clays Clay Miner. 1995, 43, 427–432. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, Y.; Zhang, X.; Yuan, P.; He, H. Synthesis and characterization of layered double hydroxides with a high aspect ratio. J. Solid State Chem. 2006, 179, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Garcés-Polo, S.; Villarroel-Rocha, J.; Sapag, K.; Korili, S.; Gil, A. Adsorption of CO2 on mixed oxides derived from hydrotalcites at several temperatures and high pressures. Chem. Eng. J. 2018, 332, 24–32. [Google Scholar] [CrossRef]
- Martunus; Othman, M.R.; Fernando, W.J.N. Elevated temperature carbon dioxide capture via reinforced metal hydrotalcite. Microporous Mesoporous Mater. 2011, 138, 110–117. [Google Scholar] [CrossRef]
- Aschenbrenner, O.; McGuire, P.; Alsamaq, S.; Wang, J.; Supasitmongkol, S.; Al-Duri, B.; Styring, P.; Wood, J. Adsorption of carbon dioxide on hydrotalcite-like compounds of different compositions. Chem. Eng. Res. Des. 2011, 89, 1711–1721. [Google Scholar] [CrossRef] [Green Version]
- Forano, C.T.-G.C.; Hibino, T.; Leroux, F. Layered double hydroxides. In Hand-Book of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Newnes, Australia, 2006; ISBN 978-0-08-044183-2. [Google Scholar]
- Cai, P.; Zheng, H.; Wang, C.; Ma, H.; Hu, J.; Pu, Y.; Liang, P. Competitive adsorption characteristics of fluoride and phosphate on calcined Mg–Al–CO3 layered double hydroxides. J. Hazard. Mater. 2012, 213–214, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Xu, T.; Li, Y.; Chang, Z.; Sun, X.; Lei, X. Effect of synthesis method on selective adsorption of thiosulfate by calcined MgAl-layered double hydroxides. Chem. Eng. J. 2013, 232, 510–518. [Google Scholar] [CrossRef]
- Hobbs, C.; Jaskaniec, S.; McCarthy, E.K.; Downing, C.; Opelt, K.; Güth, K.; Shmeliov, A.; Mourad, M.C.D.; Mandel, K.; Nicolosi, V. Structural transformation of layered double hydroxides: An in situ TEM analysis. npj 2D Mater. Appl. 2018, 2, 4. [Google Scholar] [CrossRef]
- Luo, S.; Guo, Y.; Yang, Y.; Zhou, X.; Peng, L.; Wu, X.; Zeng, Q. Synthesis of calcined La-doped layered double hydroxides and application on simultaneously removal of arsenate and fluoride. J. Solid State Chem. 2019, 275, 197–205. [Google Scholar] [CrossRef]
- Elhalil, A.; Qourzal, S.; Mahjoubi, F.; Elmoubarki, R.; Farnane, M.; Tounsadi, H.; Sadiq, M.; Abdennouri, M.; Barka, N. Defluoridation of groundwater by calcined Mg/Al layered double hydroxide. Emerg. Contam. 2016, 2, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Harizi, I.; Chebli, D.; Bouguettoucha, A.; Rohani, S.; Amrane, A. A New Mg–Al–Cu–Fe-LDH Composite to Enhance the Adsorption of Acid Red 66 Dye: Characterization, Kinetics and Isotherm Analysis. Arab. J. Sci. Eng. 2018, 44, 5245–5261. [Google Scholar] [CrossRef]
- Chebli, D.; Bouguettoucha, A.; Reffas, A.; Tiar, C.; Boutahala, M.; Gulyas, H.; Amrane, A. Removal of the anionic dye Biebrich scarlet from water by adsorption to calcined and non-calcined Mg–Al layered double hydroxides. Desalin. Water Treat. 2016, 57, 22061–22073. [Google Scholar] [CrossRef]
- Li, R.; Zhan, W.; Song, Y.; Lan, J.; Guo, L.; Zhang, T.C.; Du, D. Template-free synthesis of an eco-friendly flower-like Mg/Al/Fe-CLDH for efficient arsenate removal from aqueous solutions. Sep. Purif. Technol. 2021, 282, 120011. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Yu, Q.; Fan, J.; Brouwers, H. Effect of MgO, Mg-Al-NO3 LDH and calcined LDH-CO3 on chloride resistance of alkali activated fly ash and slag blends. Constr. Build. Mater. 2020, 250, 118865. [Google Scholar] [CrossRef]
- Singh, R.; Reddy, M.R.; Wilson, S.; Joshi, K.; da Costa, J.C.D.; Webley, P. High temperature materials for CO2 capture. Energy Procedia 2009, 1, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Ebner, A.D.; Reynolds, S.P.; Ritter, J.A. Nonequilibrium Kinetic Model That Describes the Reversible Adsorption and Desorption Behavior of CO2 in a K-Promoted Hydrotalcite-like Compound. Ind. Eng. Chem. Res. 2007, 46, 1737–1744. [Google Scholar] [CrossRef]
- Wang, Q.; Tay, H.H.; Guo, Z.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. Morphology and composition controllable synthesis of Mg–Al–CO3 hydrotalcites by tuning the synthesis pH and the CO2 capture capacity. Appl. Clay Sci. 2012, 55, 18–26. [Google Scholar] [CrossRef]
- Yong, Z.; Mata, V.; Rodrigues, E. Adsorption of Carbon Dioxide onto Hydrotalcite-like Compounds (HTlcs) at High Temperatures. Ind. Eng. Chem. Res. 2001, 40, 204–209. [Google Scholar] [CrossRef]
- Yang, W.; Kim, Y.; Liu, P.K.T.; Sahimi, M.; Tsotsis, T.T. A study by in situ techniques of the thermal evolution of the structure of a Mg–Al–CO3 layered double hydroxide. Chem. Eng. Sci. 2002, 57, 2945–2953. [Google Scholar] [CrossRef]
- Peng, J.; Iruretagoyena, D.; Chadwick, D. Hydrotalcite/SBA15 composites for pre-combustion CO2 capture: CO2 adsorption characteristics. J. CO2 Util. 2018, 24, 73–80. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, S.G.; Lee, K.B. High-Temperature CO2 Sorption on Hydrotalcite Having a High Mg/Al Molar Ratio. ACS Appl. Mater. Interfaces 2016, 8, 5763–5767. [Google Scholar] [CrossRef]
- Macedo, M.S.; Soria, M.; Madeira, L.M. High temperature CO2 sorption using mixed oxides with different Mg/Al molar ratios and synthesis pH. Chem. Eng. J. 2021, 420, 129731. [Google Scholar] [CrossRef]
- Silva, J.; Trujillano, R.; Rives, V.; Soria, M.; Madeira, L.M. High temperature CO2 sorption over modified hydrotalcites. Chem. Eng. J. 2017, 325, 25–34. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Wu, J.; Yi, X.; Zheng, A.; Umar, A.; O’Hare, D.; Wang, Q. Comprehensive investigation of CO2 adsorption on Mg–Al–CO3 LDH-derived mixed metal oxides. J. Mater. Chem. A 2013, 1, 12782–12790. [Google Scholar] [CrossRef]
- Walspurger, S.; de Munck, S.; Cobden, P.; Haije, W.; Brink, R.V.D.; Safonova, O. Correlation between structural rearrangement of hydrotalcite-type materials and CO2 sorption processes under pre-combustion decarbonisation conditions. Energy Procedia 2011, 4, 1162–1167. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Tay, H.H.; Zhong, Z.; Luo, J.; Borgna, A. Synthesis of high-temperature CO2 adsorbents from organo-layered double hydroxides with markedly improved CO2 capture capacity. Energy Environ. Sci. 2012, 5, 7526–7530. [Google Scholar] [CrossRef]
- Li, S.; Shi, Y.; Yang, Y.; Zheng, Y.; Cai, N. High-Performance CO2 Adsorbent from Interlayer Potassium-Promoted Stearate-Pillared Hydrotalcite Precursors. Energy Fuels 2013, 27, 5352–5358. [Google Scholar] [CrossRef]
- Hanif, A.; Dasgupta, S.; Divekar, S.; Arya, A.; Garg, M.O.; Nanoti, A. A study on high temperature CO2 capture by improved hydrotalcite sorbents. Chem. Eng. J. 2014, 236, 91–99. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Large-scale synthesis of highly dispersed layered double hydroxide powders containing delaminated single layer nanosheets. Chem. Commun. 2013, 49, 6301–6303. [Google Scholar] [CrossRef]
- Othman, M.; Rasid, N.; Fernando, W. Mg–Al hydrotalcite coating on zeolites for improved carbon dioxide adsorption. Chem. Eng. Sci. 2006, 61, 1555–1560. [Google Scholar] [CrossRef]
- Wu, K.; Ye, Q.; Wang, L.; Meng, F.; Dai, H. Mesoporous alumina-supported layered double hydroxides for efficient CO2 capture. J. CO2 Util. 2022, 60, 101982. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, C.; Suo, H.; Wang, Q.; Shi, Y.; O’Hare, D.; Cai, N. Synthesis of elevated temperature CO2 adsorbents from aqueous miscible organic-layered double hydroxides. Energy 2018, 167, 960–969. [Google Scholar] [CrossRef]
- Meis, N.N.A.H.; Bitter, J.H.; de Jong, K.P. On the Influence and Role of Alkali Metals on Supported and Unsupported Activated Hydrotalcites for CO2 Sorption. Ind. Eng. Chem. Res. 2010, 49, 8086–8093. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Shinall, B.D.; Iretskii, A.V.; White, M.G.; Golden, T.; Atilhan, M.; Ford, P.C.; Stucky, G.D. Markedly Improved CO2 Capture Efficiency and Stability of Gallium Substituted Hydrotalcites at Elevated Temperatures. Chem. Mater. 2009, 21, 3473–3475. [Google Scholar] [CrossRef]
- Lwin, Y.; Abdullah, F. High temperature adsorption of carbon dioxide on Cu–Al hydrotalcite-derived mixed oxides: Kinetics and equilibria by thermogravimetry. J. Therm. Anal. 2009, 97, 885–889. [Google Scholar] [CrossRef]
- Raki, L.; Beaudoin, J.; Mitchell, L. Layered double hydroxide-like materials: Nanocomposites for use in concrete. Cem. Concr. Res. 2004, 34, 1717–1724. [Google Scholar] [CrossRef] [Green Version]

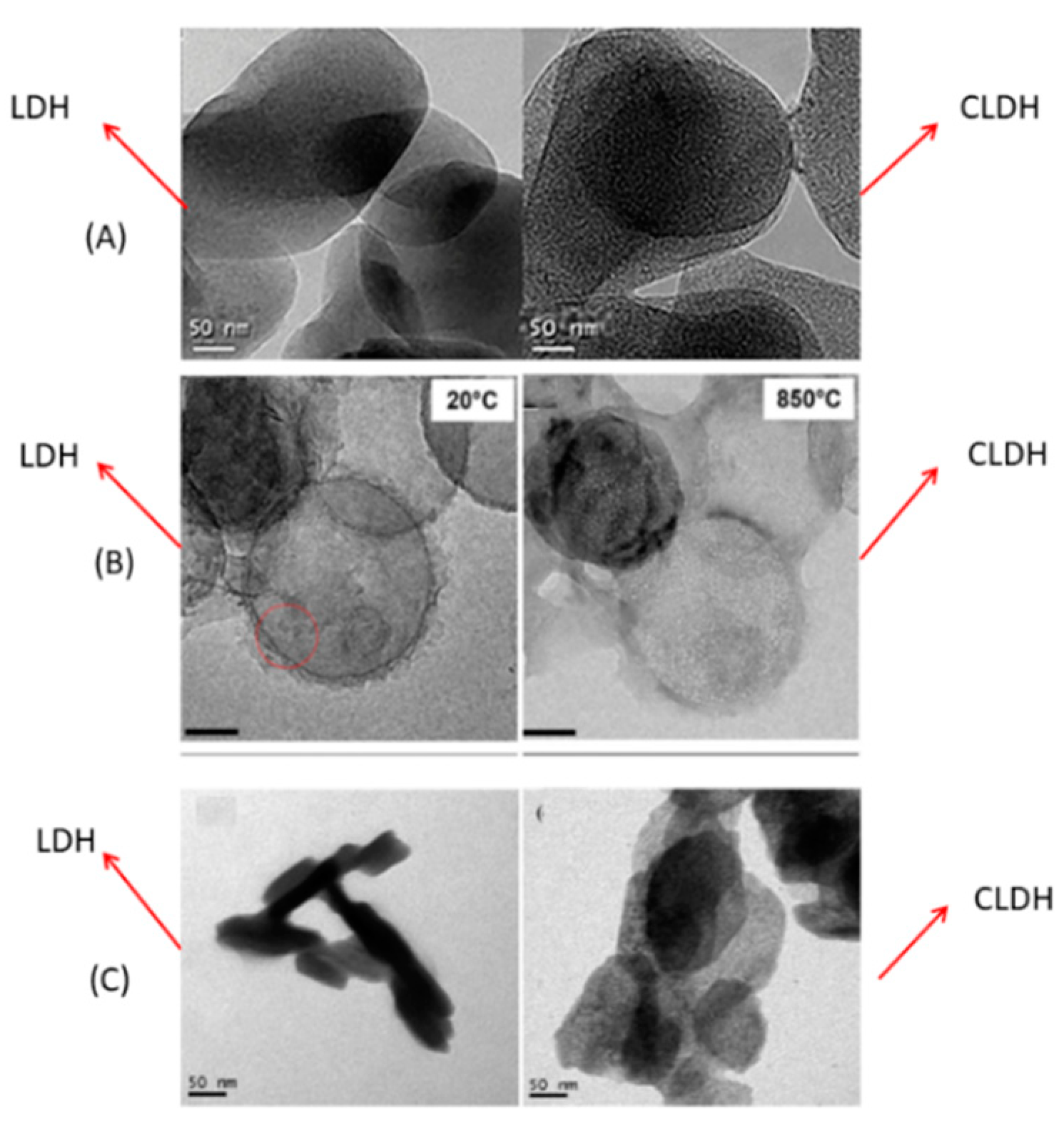


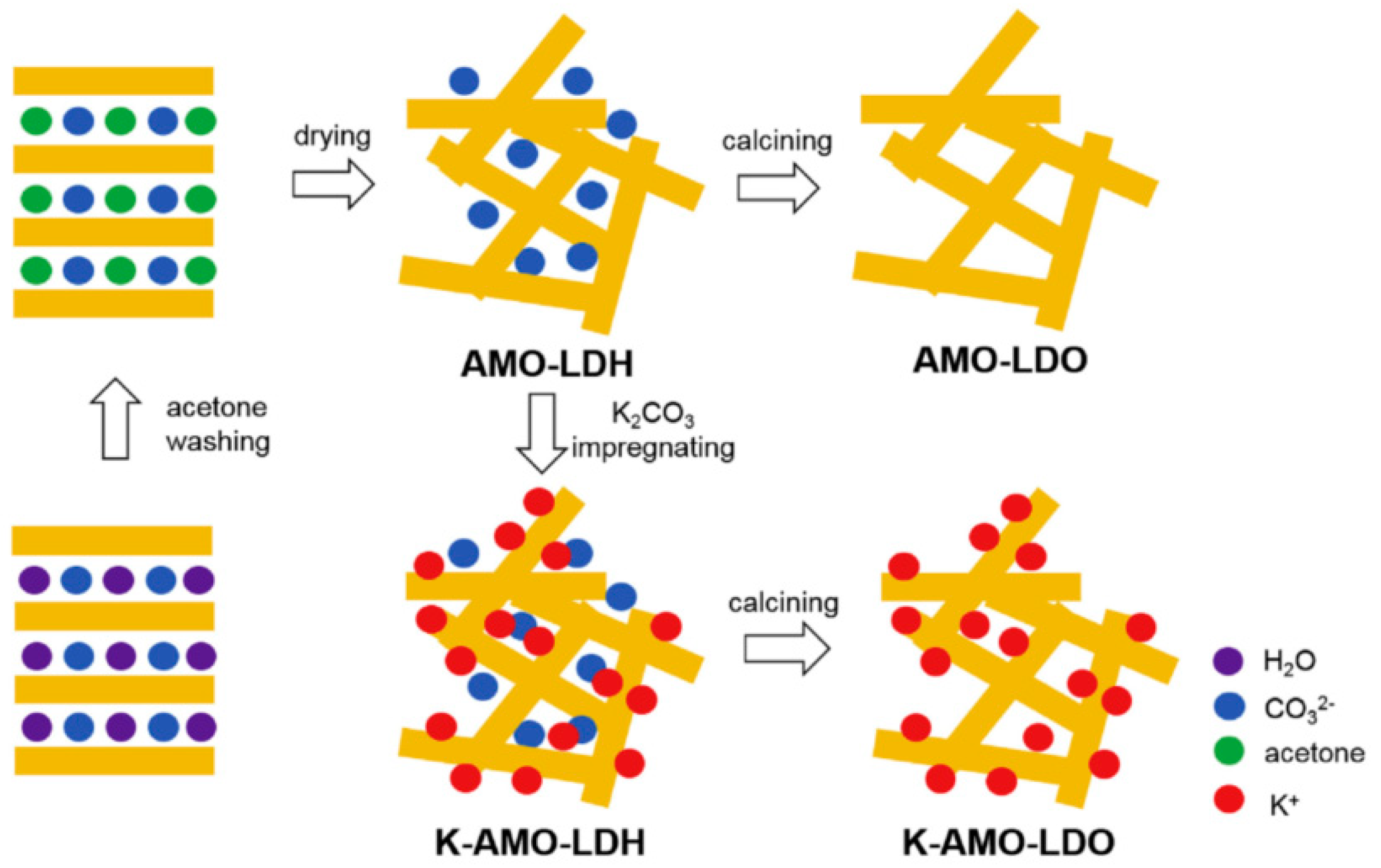

| Refs. | Type | LDH to CLDH? | Mg/Al Molar Ratio | Pressure (Atm.) | Temperature Isotherm (°C) | Capacity Adsorption (mg/g) |
|---|---|---|---|---|---|---|
| [29] | Alkali-modified (K and CS) | 300 °C for 3 h | - | 2 | 400 | 25.52 |
| [38] | K promoted * Mg3AlCO3 | LDH | - | 16.50 | 400 | 28.60 |
| [122] | * Mg3AlCO3 with treatment AMOST | 450 °C for 3 h | 3 | 1 | 400 | 30.58 |
| [22] | K promoted * Mg3AlCO3 | 400 °C for 4 h | - | 3.5 | 400 | 41.80 |
| [64] | K promoted * Mg3AlCO3 | 400 °C for 3 h | - | 10 | 400 | 25.68 |
| [2] | K promoted commercial hydrotalcite | 400 °C for 6 h | - | 30 | 400 | 21.18 |
| [117] | * Mg3AlCO3 | 450 °C for 10 h | 2 | 13 | 350 | 44.95 |
| [114] | K promoted * Mg3AlCO3 | 450 °C for 3 h | 2.9 | 20 | 350 | 44.94 |
| [28] | * Mg3AlCO3 | 400 °C for 4h | 3 | 1 | 300 | 26.4 |
| [7] | * Mg3AlCO3 | 400 °C for 2h | 2 | 1 | 300 | 46.21 |
| [91] | * Mg3AlCO3 | 500 °C for 4 h | 3 | 43.42 | 300 | 144.32 |
| [92] | Hydrotalcite of K-Na | 650 °C for 6 h (100 g) | 3 (K/Na ratio) | 1.34 | 300 | 34.03 |
| [112] | * Mg3AlCO3 | 400 °C for 2h | 2 | 1 | 300 | 41.53 |
| [116] | * K-Mg-Al | 400 °C for 6 h | 3 | - | 300 | 54.57 |
| [86] | * Mg-Al with graphene oxide | 400 °C for 4 h | - | - | 300 | 12.84 |
| [35] | Alkali metal (Na, Cs and K) with * Mg3AlCO3 | 300 °C for -h | 2 | 0.15 | 300 | 21.12 |
| [123] | K-loaded CNF supported hydrotalcite | 500 °C for 4 h | - | 1.1 | 250 | 62.27 |
| [110] | * (Mg/Al = 20) | No information | 20 | 1 | 240 | 407.97 |
| [88] | * Mg3AlCO3 | - | 3 | 1 | 200 | 23.32 |
| [106] | * Mg3AlCO3 | 400 °C for 1 h | 3.1 | 1 | 200 | 23.76 |
| [23] | * Mg3AlCO3 | 400 °C for 4 h | 1 | 200 | 39.60 | |
| [106] | * Mg3AlCO3 | 400 °C for 6 h | 3 | 1 | 200 | 36.52 |
| [124] | K promoted Gallium substituted hydrotalcite | 400 °C | - | - | 200 | 39.80 |
| [121] | Mesoporous alumina with Mg-Al LDH | No | 4 | 1 | 200 | 68.64 |
| [26] | * Mg3AlCO3 | 550 °C for 1 h | 3 | 1 | 80 | 93.72 |
| [25] | * Mg3AlCO3 | 450 °C for 10 h | 3 | 1 | 50 | 45.76 |
| [27] | * Mg3AlCO3 | 400 °C for 1 h | 3 | 1 | 50 | 40.04 |
| [120] | * Mg-Al with coated zeolites | 400 °C for 15 h | 3 | 1 | 30 | 197.73 |
| [93] | * Ni-Mg-Al | 650 °C for 7 h (1 g) | - | 1 | 20 | 70.62 |
| [125] | * Cu-Al | 600 °C for 75 min | 3 (Cu/Al ratio) | 1 | 20 | 20.54 |
| [33] | * Mg3AlCO3 | 500 °C for 2 h | 3 | 34.28 | 0 | 142.02 |
| [34] | Organohydrotalcites TDD 1 | 500 °C for 2 h | 3 | 35 | 0 | 176.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suescum-Morales, D.; Jiménez, J.R.; Fernández-Rodríguez, J.M. Review of the Application of Hydrotalcite as CO2 Sinks for Climate Change Mitigation. ChemEngineering 2022, 6, 50. https://doi.org/10.3390/chemengineering6040050
Suescum-Morales D, Jiménez JR, Fernández-Rodríguez JM. Review of the Application of Hydrotalcite as CO2 Sinks for Climate Change Mitigation. ChemEngineering. 2022; 6(4):50. https://doi.org/10.3390/chemengineering6040050
Chicago/Turabian StyleSuescum-Morales, David, José Ramón Jiménez, and José María Fernández-Rodríguez. 2022. "Review of the Application of Hydrotalcite as CO2 Sinks for Climate Change Mitigation" ChemEngineering 6, no. 4: 50. https://doi.org/10.3390/chemengineering6040050
APA StyleSuescum-Morales, D., Jiménez, J. R., & Fernández-Rodríguez, J. M. (2022). Review of the Application of Hydrotalcite as CO2 Sinks for Climate Change Mitigation. ChemEngineering, 6(4), 50. https://doi.org/10.3390/chemengineering6040050







