Effect of Artificial Freeze/Thaw and Thermal Shock Ageing, Combined or Not with Salt Crystallisation on the Colour of Zamora Building Stones (Spain)
Abstract
1. Introduction
2. Materials and Methods
2.1. Zamora Building Stone
2.2. Experimental and Statistical Methods
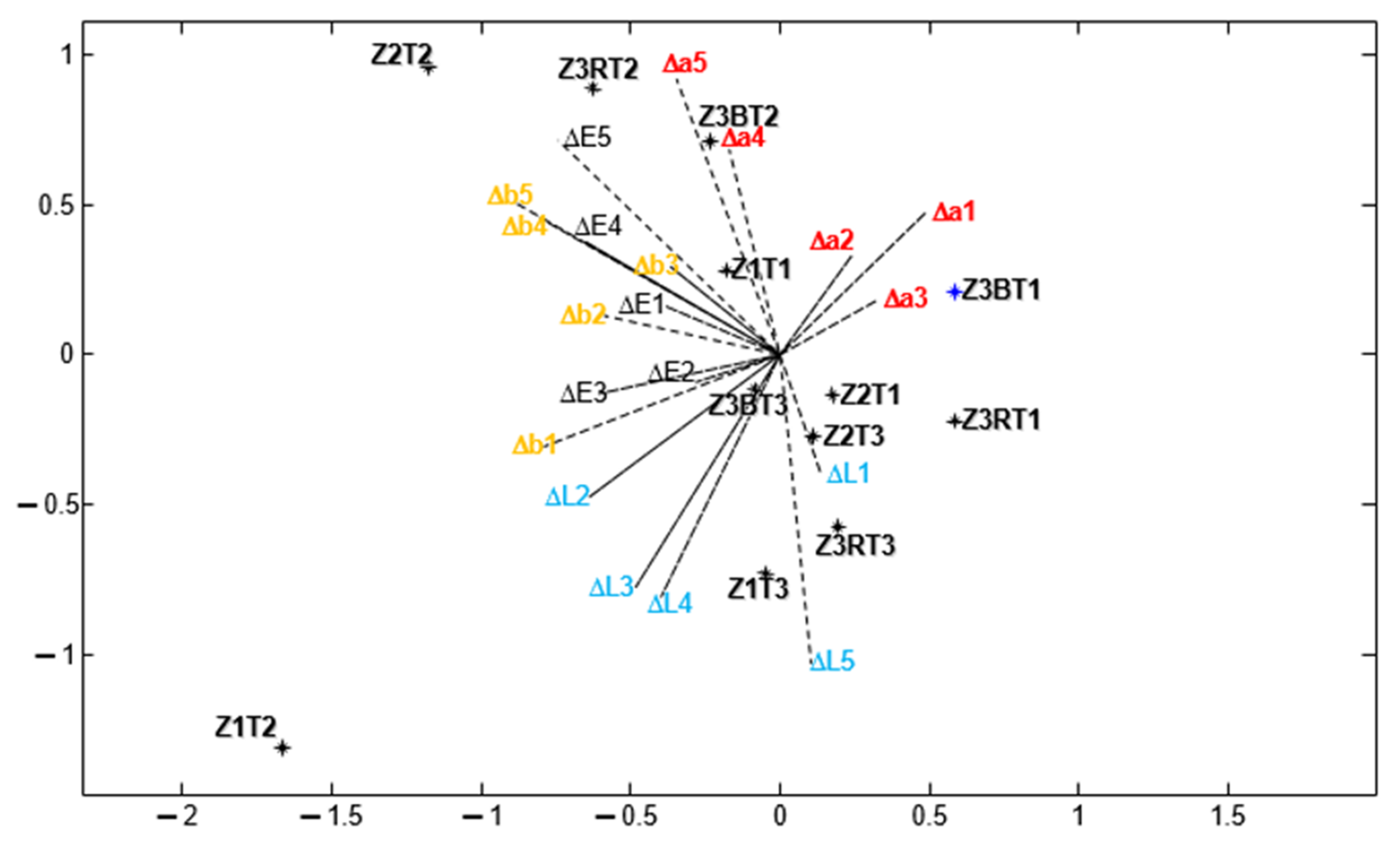
3. Results and Discussion
4. Conclusions
- (a)
- Z1: ∆E* (T2 > T1 ≈ T3), ∆L* (T2 > T3 > T1), ∆a* and ∆b* (T2 > T1 ≈ T3)
- (b)
- Z2: ∆E* (T2 > T1 ≈ T3) and ∆b* (T2 > T1 ≈ T3)
- (c)
- Z3B: ∆L* (T2 ≈ T1 > T3), ∆a* (T2 > T1 > T3) and ∆b* (T2 > T1 ≈ T3)
- (d)
- Z3R: ∆E* (T2 > T1 ≈ T3), ∆L* (there are only differences in T3), ∆a* (T2 ≈ T3 > T1) and ∆b* (T2 > T1 ≈ T3).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Talegón, J.; Iñigo, A.C.; Alonso-Gavilán, G.; Vicente-Tavera, S. Villamayor Stone (Golden Stone) as a Global Heritage Stone Resource from Salamanca (NW of Spain). Geol. Soc. Lond. 2015, 407, 109–120. [Google Scholar] [CrossRef]
- García-Talegon, J.; Vicente, M.A.; Vicente-Tavera, S.; Molina-Ballesteros, E. Assessment of chromatic changes due to artificial ageing and/or conservation treatments of sandstones. Color Res. Appl. 1998, 23, 46–51. [Google Scholar] [CrossRef]
- Occhipinti, R.; Stroscio, A.; Belfiore, C.M.; Barone, G.; Mazzoleni, P. Chemical and colorimetric analysis for the characterization of degradation forms and surface colour modification of building stone materials. Constr. Build. Mater. 2021, 302, 124356. [Google Scholar] [CrossRef]
- Rives, V.; Talegon, J.G. Decay and Conservation of Building Stones on Cultural Heritage Monuments. Mater. Sci. Forum 2006, 514–516, 1689–1694. [Google Scholar] [CrossRef]
- Iñigo, A.C.; Rives, V.; Vicente, M.A. Reproducción en cámara climática de las formas de alteración más frecuentes detectadas en materiales graníticos, en clima de tendencia continental. Mater. Construcc. 2000, 50, 57–60. [Google Scholar] [CrossRef]
- Iñigo, A.C.; Vicente-Tavera, S. Different degrees of stone decay on the inner and outer walls of a Cloister. Build Environ. 2001, 36, 911–917. [Google Scholar] [CrossRef]
- Iñigo, A.C.; Vicente-Tavera, S. Surface-inside (10 cm) thermal gradients in granitic rocks: Effect of environmental conditions. Build. Environ. 2002, 37, 101–108. [Google Scholar] [CrossRef]
- Iñigo, A.; García-Talegón, J.; Vicente-Palacios, V.; Vicente-Tavera, S. Canonical Biplot as a tool to detect microclimates in the inner and outer parts of El Salvador Church in Seville, Spain. Measurement 2019, 136, 745–760. [Google Scholar] [CrossRef]
- García-Talegón, J.; Vicente, M.A.; Molina, E. Decay of granite monuments due to salt crystallization in a non-polluted urban environment. Mater. Construcc. 1999, 49, 17–27. [Google Scholar] [CrossRef]
- Fort, R.; López-de Azcona, M.C.; Mingarro, F. Assessment of protective treatments based on their chromatic evolution: Limestone and granite in the Royal Palace of Madrid, Spain. In 5th International Symposium on the Conservation of Monuments in the Mediterranean Basin; Galán, E., Zezza, F., Eds.; Swets & Zeitlinger B.V.: Lisse, The Netherlands, 2002; pp. 437–441. [Google Scholar]
- Carmona-Quiroga, P.; Martinez-Ramirez, S.; de Rojas, M.S.; Blanco-Varela, M.T. Surface water repellent-mediated change in lime mortar colour and gloss. Constr. Build. Mater. 2010, 24, 2188–2193. [Google Scholar] [CrossRef]
- García, O.; Rz-Maribona, I.; Gardei, A.; Riedl, M.; Vanhellemont, Y.; Santarelli, M.L.; Strupi-Suput, J. Comparative study of the variation of the hydric properties and aspect of natural stone and brick after the application of 4 types of anti-graffiti. Mater. Construcc. 2010, 60, 68–82. [Google Scholar]
- Rivas, T.; Iglesias, J.; Taboada, J.; Vilán, J.A. Sulphide oxidation in ornamental slates: Protective treatment with siloxanes. Mater. Construcc. 2011, 61, 115–130. [Google Scholar] [CrossRef]
- La Russa, M.F.; Barone, G.; Belfiore, C.M.; Mazzoleni, P.; Pezzino, A. Application of protective products to “Noto” calcarenite (south-eastern Sicily): A case study for the conservation of stone materials. Environ. Earth Sci. 2011, 62, 1263–1272. [Google Scholar] [CrossRef]
- Pelin, V.; Sandu, I.; Gurlui, S.; Brânzilă, M.; Vasilache, V.; Borş, E.; Sandu, I.G. Preliminary investigation of various old geomaterials treated with hydrophobic pellicle. Color Res. Appl. 2016, 41, 317–320. [Google Scholar] [CrossRef]
- Iñigo, A.C.; García-Talegón, J.; Vicente-Palacios, V.; Vicente-Tavera, S. Measuring the Effectiveness and Durability of Silicified Sandstones and Conglomerates from Zamora, Spain Subject to Silico-organic Treatments and/or Freezing/Thawing Processes. Rock Mech. Rock Eng. 2021, 54, 2697–2705. [Google Scholar] [CrossRef]
- Grossi, C.M.; Brimblecombe, P.; Esbert, R.M.; Alonso, F.J. Color changes in architectural limestones from pollution and cleaning. Color Res. Appl. 2007, 32, 320–331. [Google Scholar] [CrossRef]
- Alonso, F.J.; Vázquez, P.; Esbert, R.M.; Ordaz, J. Ornamental granite durability: Evaluation of damage caused by salt crystallization test. Mat. Construcc. 2008, 58, 191–201. [Google Scholar]
- Rivas, T.; Prieto, B.; Silva, B. Artificial weathering of granite. Mater. Construcc. 2008, 58, 179–189. [Google Scholar]
- Vazquez, P.; Luque, A.; Alonso, F.J.; Grossi, C.M. Surface changes on crystalline stones due to salt crystallisation. Environ. Earth Sci. 2013, 69, 1237–1248. [Google Scholar] [CrossRef]
- Iñigo, A.C.; García-Talegón, J.; Vicente-Tavera, S. Canonical biplot statistical analysis to detect the magnitude of the effects of phosphates crystallization aging on the color in siliceous conglomerates. Color Res. Appl. 2014, 39, 82–87. [Google Scholar] [CrossRef]
- Aly, N.; Gomez-Heras, M.; Hamed, A.; De Buergo, M.; Soliman, F. The influence of temperature in a capillary imbibition salt weathering simulation test on Mokattam limestone. Mater. Construcc. 2015, 65, e044. [Google Scholar] [CrossRef]
- Navarro, R.; Catarino, L.; Pereira, D.; de Sá Campos Gil, F.P. Effect of UV radiation on chromatic parameters in serpentinites used as dimension stones. Bull. Eng. Geol. Environ. 2019, 78, 5345–5355. [Google Scholar] [CrossRef]
- Martin, J.; Feliu, M.J.; Edreira, M.C.; Villena, A.; Calleja, S.; Pérez, F.; Barros, J.R.; Ortega, P. The original colour of the building facades from “El Pópulo” an old quarter of Cádiz. In 5th International Symposium on the Conservation of Monuments in the Mediterranean Basin; Galan, E., Zezza, F., Eds.; Swets & Zeitlinger B.V.: Lisse, The Netherlands, 2002; pp. 649–653. [Google Scholar]
- Zezza, F. Non-destructive technique for the assessment of the deterioration processes of prehistoric rock art in karstic caves: The paleolithic paintings of Altamira (Spain). In 5th International Symposium on the Conservation of Monuments in the Mediterranean Basin; Galan, E., Zezza, F., Eds.; Swets & Zeitlinger B.V.: Lisse, The Netherlands, 2002; pp. 377–388. [Google Scholar]
- Zezza, F. Inland dispersion of marine spray and its effects on monument stone. In 5th International Symposium on the Conservation of Monuments in the Mediterranean Basin; Galán, E., Zezza, F., Eds.; Swets & Zeitlinger B.V.: Lisse, The Netherlands, 2002; pp. 23–39. [Google Scholar]
- Grossi, C.M.; Brimblecombe, P. Past and future colouring patterns of historic stone buildings. Mater. Construcc. 2008, 58, 143–160. [Google Scholar]
- Aparecida-del Lama, E.; Kazumi-Dehira, L.; Grossi, D.; Kuzmickas, L. The colour of the granite that built the city of São Paulo, Brazil. Color Res. Appl. 2015, 41, 241–245. [Google Scholar] [CrossRef]
- Pelin, V.; Rusu, O.; Sandu, I.; Vasilache, V.; Gurlui, S.; Sandu, A.V.; Cazacu, M.M.; Sandu, I.G. Approaching on Colorimetric Change of Porous Calcareous Rocks Exposed in Urban Environmental Conditions from Iasi–Romania. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012080. [Google Scholar] [CrossRef]
- García-Talegón, J.; Iñigo, A.C.; Vicente-Tavera, S.; Molina-Ballesteros, E. Silicified Granites (Bleeding Stone and Ochre Granite) as Global Heritage Stones Resources from Avila (Central of Spain). Geosci. Can. 2016, 43, 53–62. [Google Scholar] [CrossRef][Green Version]
- García Talegón, J.; Molina, E.; Vicente, M.A. Nature and characteristics of 1:1 phyllosilicates from weathered granite. Central Spain. Clay Miner. 1994, 29, 727–734. [Google Scholar]
- Thiry, M.; Milnes, A.R.; Rayot, V.; Simon-Coinçon, R. Interpretation of palaeoweathering features and successive silicifications in the Tertiary regolith of inland Australia. J. Geol. Soc. Lond. 2006, 163, 723–736. [Google Scholar] [CrossRef]
- Delvigne, J.E. Atlas of micromorphology of mineral alteration and weathering. Mineral. Mag. 1998, 64, 369–370. [Google Scholar]
- Bauluz, B.; Mayayo, M.J.; Yuste, A.; López, J.M.G. Genesis of kaolinite from Albian sedimentary deposits of the Iberian Range (NE Spain): Analysis by XRD, SEM and TEM. Clay Miner. 2008, 43, 459–475. [Google Scholar] [CrossRef]
- Iñigo, A.C.; Supit, J.F.; Prieto, O.; Rives, V. Change in Microporosity of Granitic Building Stones upon Consolidation Treatments. J. Mater. Civ. Eng. 2007, 19, 437–440. [Google Scholar] [CrossRef]
- Robertson, A.R. The CIE 1976 Color-Difference Formulae. Color Res. Appl. 1977, 2, 7–11. [Google Scholar] [CrossRef]
- Sève, R. New formula for the computation of CIE 1976 Hue difference. Color Res. Appl. 1991, 16, 217–218. [Google Scholar] [CrossRef]
- Amaro, I.R.; Vicente-Villardón, J.L.; Galindo-Villardón, M.P. MANOVA Biplot para arreglos de tratamientos con dos factores basado en modelos lineales generales multivariantes. Interciencia 2004, 29, 26–32. [Google Scholar]
- Vicente-Villardón, J.L. MULTBIPLOT: Multivariate Analysis Using Biplots. 2016. Available online: http://biplot.usal.es (accessed on 31 March 2016).
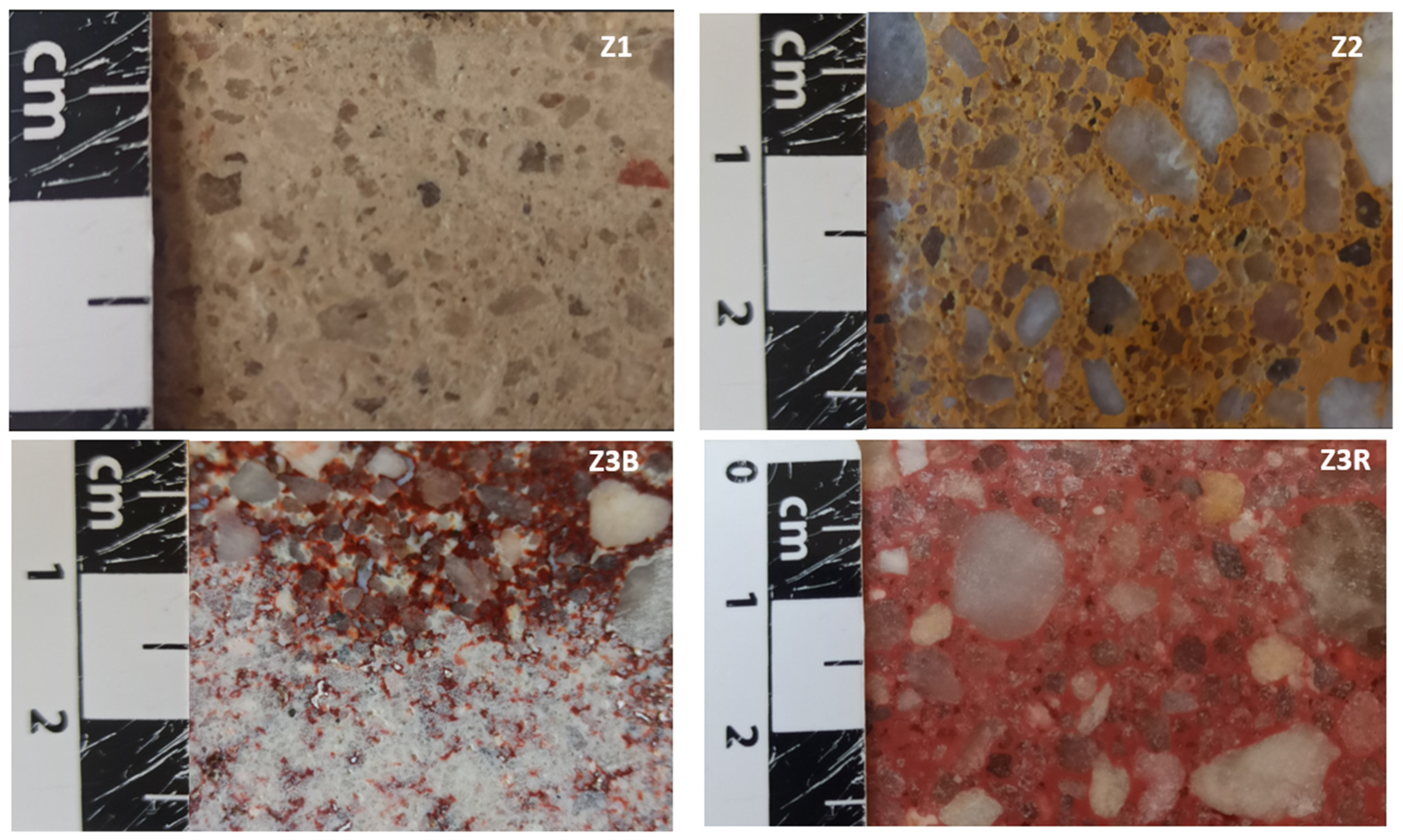


| Samples | FP (%) | TP (%) | AC (%) | RD (g/cm3) | AD (g/cm3) | IC (%) | CAC (g/cm2S½) | P (Kg/m2s) |
|---|---|---|---|---|---|---|---|---|
| Z1 | 11.7 | 14.3 | 82 | 2.57 | 2.28 | 5.4 | 0.001075 | 0.000221 |
| Z2 | 9.6 | 10.1 | 95 | 2.60 | 2.33 | 3.8 | 0.000844 | 0.000195 |
| Z3B | 8.7 | 9.2 | 95 | 2.57 | 2.33 | 4.2 | 0.000927 | 0.000179 |
| Z3R | 9.1 | 9.2 | 99 | 2.56 | 2.33 | 3.7 | 0.000866 | 0.000164 |
| Sample and Treatment | ∆E1* | ∆E2* | ∆E3* | ∆E4* | ∆E5* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | |
| Z1, T1 | 0.505 | 0.133 | 0.657 | 0.175 | 0.918 | 0.172 | 1.199 | 0.194 | 1.011 | 0.198 |
| Z1, T2 | 1.032 | 0.197 | 1.054 | 0.193 | 1.836 | 0.248 | 1.475 | 0.272 | 1.316 | 0.185 |
| Z1, T3 | 0.643 | 0.236 | 0.835 | 0.296 | 0.962 | 0.329 | 0.721 | 0.349 | 0.914 | 0.208 |
| Z2, T1 | 0.410 | 0.065 | 0.595 | 0.101 | 0.628 | 0.107 | 0.743 | 0.148 | 0.691 | 0.123 |
| Z2, T2 | 0.539 | 0.061 | 0.623 | 0.113 | 0.948 | 0.102 | 1.533 | 0.155 | 2.079 | 0.607 |
| Z2, T3 | 0.549 | 0.228 | 0.621 | 0.179 | 0.831 | 0.267 | 0.794 | 0.299 | 0.818 | 0.266 |
| Z3B, T1 | 0.653 | 0.065 | 0.694 | 0.072 | 0.835 | 0.090 | 0.953 | 0.086 | 0.821 | 0.083 |
| Z3B, T2 | 1.189 | 0.128 | 1.181 | 0.167 | 1.228 | 0.242 | 1.317 | 0.178 | 2.423 | 0.279 |
| Z3B, T3 | 0.695 | 0.169 | 0.548 | 0.166 | 0.891 | 0.278 | 0.640 | 0.230 | 0.760 | 0.135 |
| Z3R, T1 | 0.666 | 0.061 | 0.577 | 0.065 | 0.854 | 0.149 | 0.621 | 0.096 | 0.695 | 0.077 |
| Z3R, T2 | 1.377 | 0.130 | 0.621 | 0.081 | 1.251 | 0.149 | 1.092 | 0.101 | 1.687 | 0.066 |
| Z3R, T3 | 0.735 | 0.154 | 0.645 | 0.193 | 0.618 | 0.177 | 0.655 | 0.152 | 0.725 | 0.132 |
| Sample and Treatment | ∆L1* | ∆L2* | ∆L3* | ∆L4* | ∆L5* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | |
| Z1, T1 | −0.295 | 0.098 | −0.409 | 0.118 | −0.703 | 0.124 | −0.892 | 0.123 | −0.703 | 0.094 |
| Z1, T2 | −0.587 | 0.224 | 0.375 | 0.319 | 0.914 | 0.660 | 0.376 | 0.444 | −0.005 | 0.211 |
| Z1, T3 | −0.346 | 0.219 | −0.394 | 0.303 | −0.588 | 0.332 | −0.445 | 0.309 | −0.428 | 0.261 |
| Z2, T1 | −0.324 | 0.061 | −0.397 | 0.102 | −0.528 | 0.101 | −0.506 | 0.124 | −0.482 | 0.108 |
| Z2, T2 | −0.329 | 0.093 | −0.006 | 0.026 | −0.549 | 0.114 | −0.569 | 0.097 | −1.017 | 0.570 |
| Z2, T3 | −0.082 | 0.253 | −0.020 | 0.263 | −0.265 | 0.358 | −0.101 | 0.355 | −0.154 | 0.318 |
| Z3B, T1 | −0.534 | 0.090 | −0.614 | 0.092 | −0.673 | 0.125 | −0.828 | 0.088 | −0.698 | 0.104 |
| Z3B, T2 | −1.028 | 0.181 | −0.932 | 0.183 | −0.751 | 0.310 | −0.972 | 0.170 | −2.177 | 0.280 |
| Z3B, T3 | −0.610 | 0.195 | −0.449 | 0.132 | −0.763 | 0.260 | −0.502 | 0.168 | −0.676 | 0.123 |
| Z3R, T1 | −0.580 | 0.080 | −0.516 | 0.082 | −0.616 | 0.116 | −0.534 | 0.085 | −0.573 | 0.083 |
| Z3R, T2 | −1.310 | 0.128 | −0.332 | 0.166 | −0.983 | 0.286 | −0.573 | 0.220 | −1.347 | 0.191 |
| Z3R, T3 | −0.420 | 0.236 | −0.177 | 0.294 | −0.189 | 0.288 | −0.151 | 0.279 | −0.161 | 0.263 |
| Sample and Treatment | ∆a1* | ∆a2* | ∆a3* | ∆a4* | ∆a5* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | |
| Z1, T1 | 0.000 | 0.019 | 0.096 | 0.024 | 0.251 | 0.027 | 0.326 | 0.030 | 0.166 | 0.026 |
| Z1, T2 | −0.080 | 0.032 | −0.013 | 0.049 | 0.067 | 0.068 | 0.055 | 0.047 | −0.008 | 0.037 |
| Z1, T3 | −0.190 | 0.050 | −0.067 | 0.061 | 0.056 | 0.038 | −0.064 | 0.031 | −0.124 | 0.030 |
| Z2, T1 | 0.017 | 0.025 | 0.079 | 0.029 | 0.080 | 0.029 | 0.095 | 0.032 | 0.033 | 0.039 |
| Z2, T2 | 0.009 | 0.025 | 0.040 | 0.049 | 0.135 | 0.030 | 0.291 | 0.039 | 0.451 | 0.079 |
| Z2, T3 | 0.099 | 0.043 | 0.147 | 0.031 | 0.208 | 0.042 | 0.170 | 0.052 | 0.108 | 0.068 |
| Z3B, T1 | 0.176 | 0.028 | 0.121 | 0.028 | 0.182 | 0.034 | 0.140 | 0.030 | 0.077 | 0.035 |
| Z3B, T2 | 0.350 | 0.110 | 0.473 | 0.108 | 0.499 | 0.135 | 0.515 | 0.119 | 0.660 | 0.097 |
| Z3B, T3 | −0.107 | 0.042 | 0.009 | 0.036 | 0.164 | 0.048 | 0.044 | 0.035 | 0.070 | 0.050 |
| Z3R, T1 | 0.093 | 0.034 | 0.040 | 0.025 | 0.262 | 0.030 | 0.039 | 0.030 | 0.000 | 0.040 |
| Z3R, T2 | −0.240 | 0.106 | −0.320 | 0.075 | −0.305 | 0.154 | −0.232 | 0.125 | −0.108 | 0.165 |
| Z3R, T3 | −0.305 | 0.068 | −0.214 | 0.083 | −0.135 | 0.041 | −0.287 | 0.050 | −0.322 | 0.075 |
| Sample and Treatment | ∆b1* | ∆b2* | ∆b3* | ∆b4* | ∆b5* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | Mean | (S.E.) | |
| Z1, T1 | 0.143 | 0.135 | 0.260 | 0.172 | 0.345 | 0.159 | 0.619 | 0.180 | 0.504 | 0.218 |
| Z1, T2 | 0.759 | 0.148 | 0.684 | 0.243 | 0.607 | 0.402 | 1.092 | 0.290 | 1.222 | 0.218 |
| Z1, T3 | 0.000 | 0.264 | −0.108 | 0.351 | −0.233 | 0.357 | 0.098 | 0.320 | −0.303 | 0.332 |
| Z2, T1 | 0.114 | 0.059 | 0.251 | 0.089 | 0.146 | 0.082 | 0.362 | 0.129 | 0.146 | 0.133 |
| Z2, T2 | 0.367 | 0.081 | 0.613 | 0.111 | 0.733 | 0.083 | 1.385 | 0.139 | 1.535 | 0.469 |
| Z2, T3 | −0.142 | 0.229 | −0.058 | 0.229 | −0.177 | 0.278 | −0.131 | 0.323 | −0.186 | 0.339 |
| Z3B, T1 | −0.170 | 0.034 | 0.105 | 0.040 | 0.234 | 0.051 | 0.397 | 0.045 | 0.204 | 0.069 |
| Z3B, T2 | −0.041 | 0.173 | 0.218 | 0.216 | 0.142 | 0.336 | 0.541 | 0.216 | 0.653 | 0.242 |
| Z3B, T3 | −0.014 | 0.116 | 0.107 | 0.175 | 0.073 | 0.228 | 0.366 | 0.171 | −0.007 | 0.172 |
| Z3R, T1 | −0.106 | 0.050 | 0.015 | 0.038 | 0.288 | 0.149 | 0.205 | 0.071 | 0.006 | 0.092 |
| Z3R, T2 | −0.251 | 0.068 | 0.094 | 0.121 | 0.319 | 0.132 | 0.725 | 0.132 | 0.797 | 0.193 |
| Z3R, T3 | −0.301 | 0.092 | −0.219 | 0.129 | −0.292 | 0.086 | −0.133 | 0.138 | −0.299 | 0.138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Talegón, J.; Iñigo, A.C.; Sepúlveda, R.; Azofra, E. Effect of Artificial Freeze/Thaw and Thermal Shock Ageing, Combined or Not with Salt Crystallisation on the Colour of Zamora Building Stones (Spain). ChemEngineering 2022, 6, 61. https://doi.org/10.3390/chemengineering6040061
García-Talegón J, Iñigo AC, Sepúlveda R, Azofra E. Effect of Artificial Freeze/Thaw and Thermal Shock Ageing, Combined or Not with Salt Crystallisation on the Colour of Zamora Building Stones (Spain). ChemEngineering. 2022; 6(4):61. https://doi.org/10.3390/chemengineering6040061
Chicago/Turabian StyleGarcía-Talegón, Jacinta, Adolfo Carlos Iñigo, Rosa Sepúlveda, and Eduardo Azofra. 2022. "Effect of Artificial Freeze/Thaw and Thermal Shock Ageing, Combined or Not with Salt Crystallisation on the Colour of Zamora Building Stones (Spain)" ChemEngineering 6, no. 4: 61. https://doi.org/10.3390/chemengineering6040061
APA StyleGarcía-Talegón, J., Iñigo, A. C., Sepúlveda, R., & Azofra, E. (2022). Effect of Artificial Freeze/Thaw and Thermal Shock Ageing, Combined or Not with Salt Crystallisation on the Colour of Zamora Building Stones (Spain). ChemEngineering, 6(4), 61. https://doi.org/10.3390/chemengineering6040061






