Designing Heat-Set Gels for Crystallizing APIs at Different Temperatures: A Crystal Engineering Approach
Abstract
:1. Introduction
2. Designing Strategy of a Heat-Set Gelator by a Crystal Engineering Approach
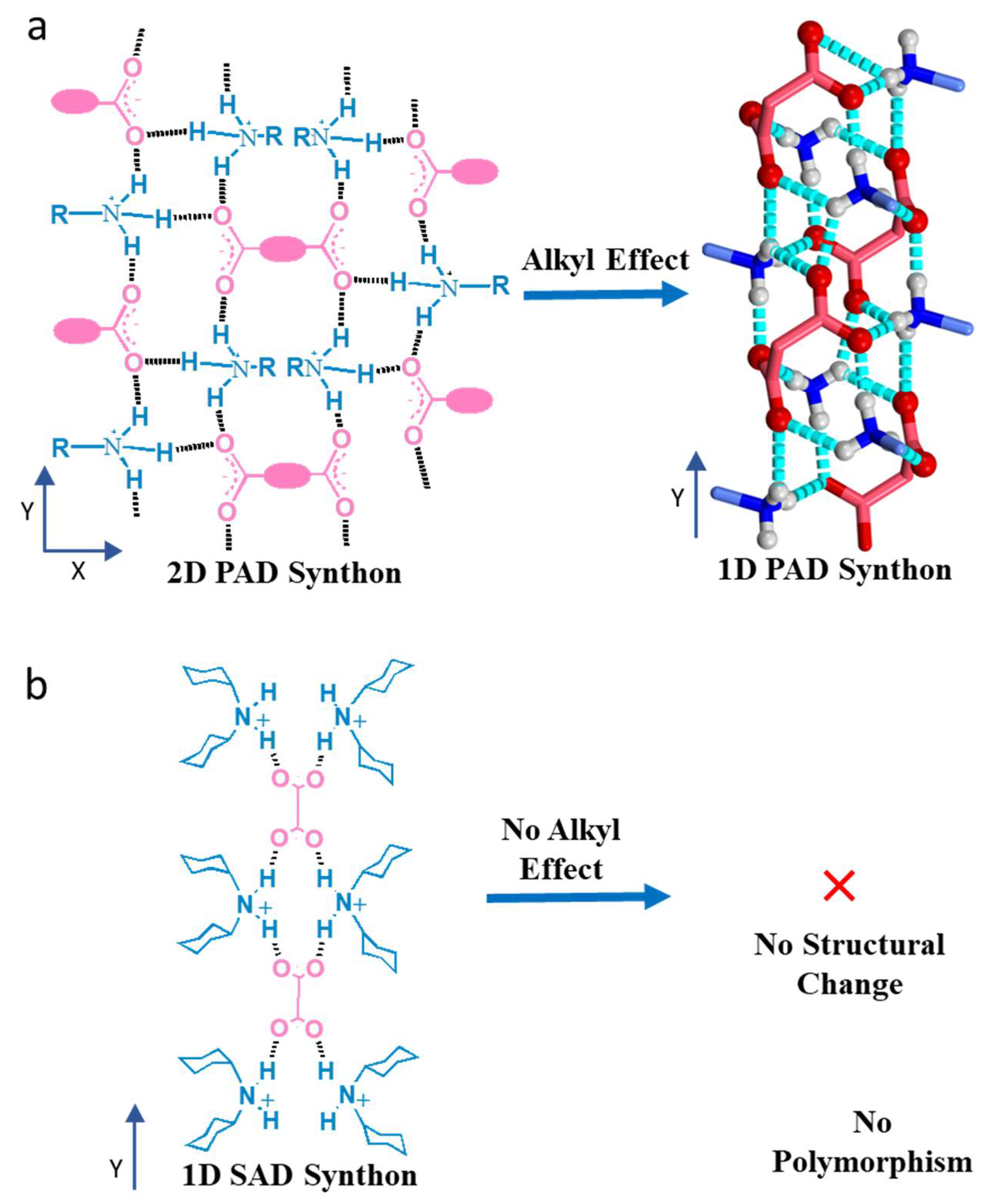
- (a)
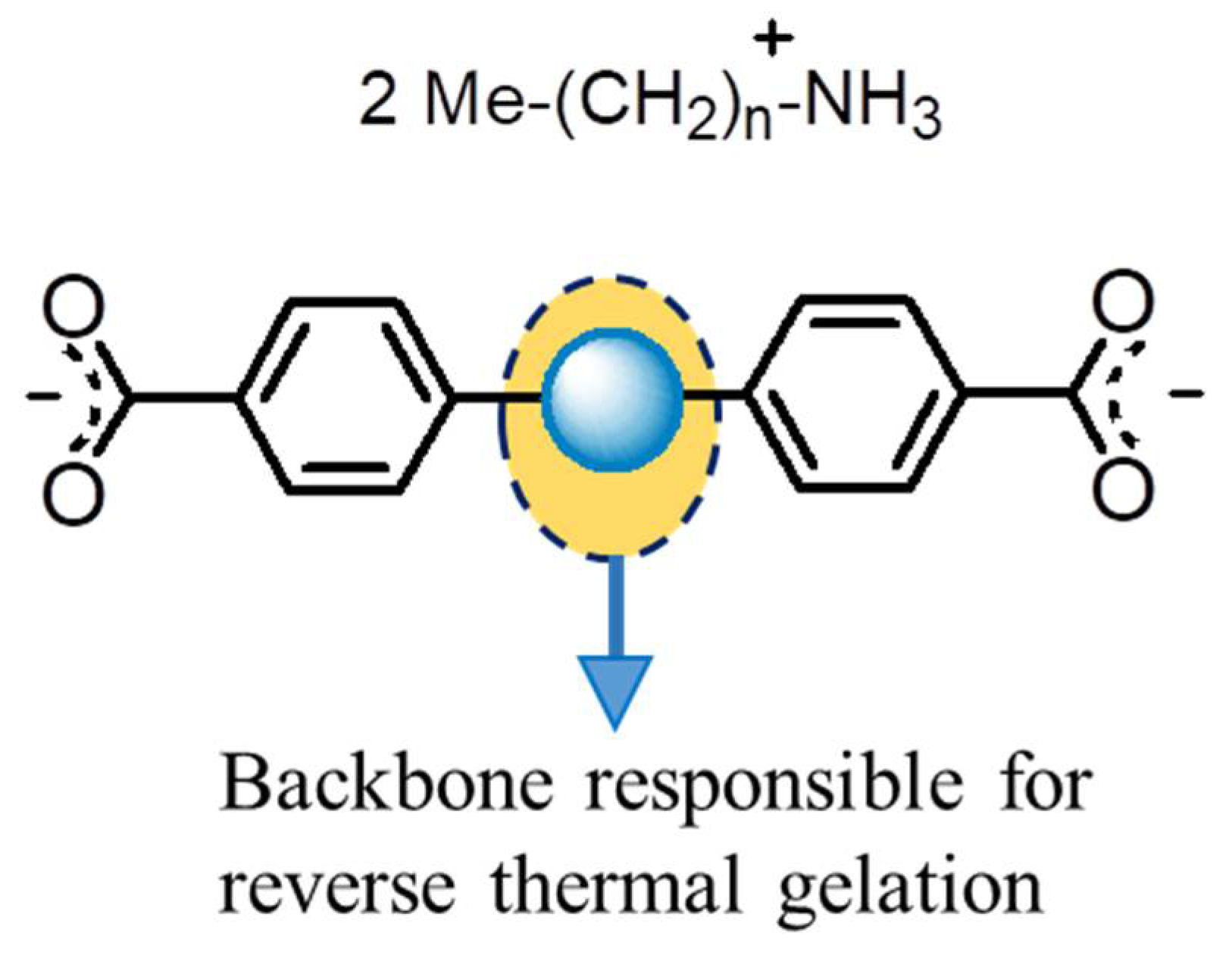
- The dicarboxylic acids with a high melting point (>350 °C) and poor solubility against organic solvent play important roles in designing heat-set gelators. The acids which are insoluble in solvents produce insoluble salts and can not even be dissolved at a high temperature. This feature helps form the Self Assembled Fibrillar Networks (SAFiN) in the solid state to immobilize the gelling solvents.
- (b)
- The primary amines selected here in producing the gelators will not allow the gelators to melt or dissolve below the heat-set gelling temperature, ideally above 350 °C. In addition, the amines should form a weak van der Waals interaction which basically stabilizes the 1D HBN and 2D HBN in different temperature sets.
- (c)
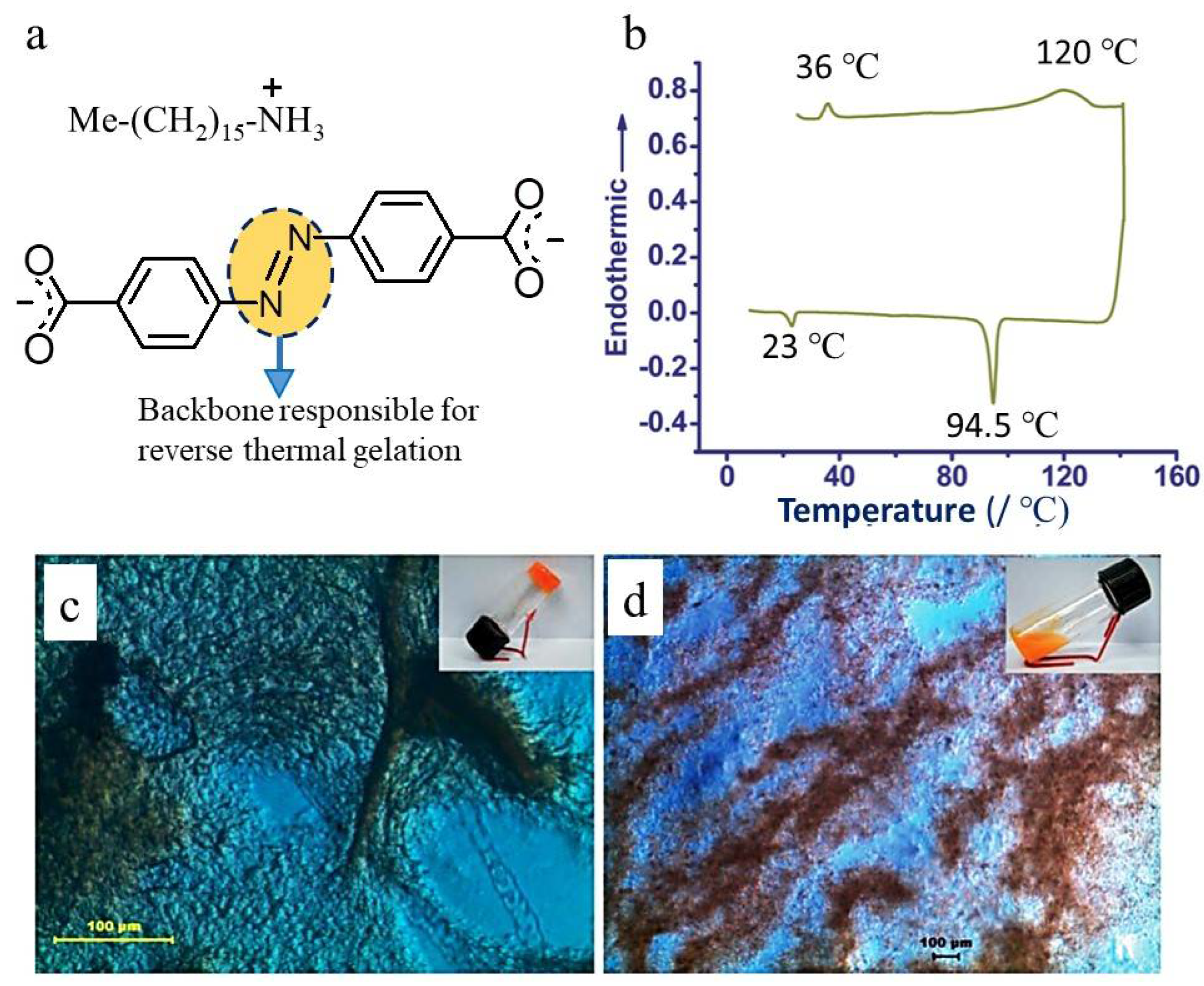
- The gelator structure should exhibit a temperature-dependent crystalline phase transition, where the higher temperature phase should promote the self-assembling of the gelator molecule, and the lower temperature phase should break it [6]. However, the lower-temperature crystalline phase can also form gels in different solvents.
3. Heat-Storing Efficiency
4. Introducing Nucleation Functionality in Gelator Molecule in Growing API Crystals
Precaution of Crystallization in Heat-Set Gel
5. Futuristic Use of Heat-Set Gel
5.1. Crystallizing Bioactive APIs
5.2. Thermal Energy Storage System
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Foster, J.A.; Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Howard, J.A.K.; Steed, J.W. Anion-switchable supramolecular gels for controlling pharmaceutical crystal growth. Nat. Chem. 2010, 2, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Supramolecular Basketry. Nat. Chem. 2017, 9, 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Adarsh, N.N.; Dastidar, P. Coordination polymers: What has been achieved in going from innocent4,4′-bipyridine to bis-pyridyl ligands having a non-innocent backbone? Chem. Soc. Rev. 2012, 41, 3039–3060. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, P. Supramolecular gelling agents: Can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.; Chakraborty, I.; Dastidar, P. Reverse thermal gelation of aromatic solvents by a series of easily accessible organic salt based gelators. Soft Matter 2012, 8, 2595–2598. [Google Scholar] [CrossRef]
- Sahoo, P.; Puranik, V.G.; Patra, A.K.; Sastry, P.U.; Dastidar, P. Ferrocene based organometallic gelators: A supramolecular synthon approach. Soft Matter 2011, 7, 3634. [Google Scholar] [CrossRef]
- Sahoo, P.; Adarsh, N.N.; Chacko, G.E.; Raghavan, S.R.; Puranik, V.G.; Dastidar, P. Combinatorial library of primary alkyl ammonium dicarboxylate gelators: A supramolecular synthon approach. Langmuir 2009, 25, 8742. [Google Scholar] [CrossRef]
- Zhong, D.-C.; Liao, L.-Q.; Wang, K.-J.; Liu, H.-J.; Luo, X.-Z. Heat-set gels formed from easily accessible gelators of a succinamic acid derivative (SAD) and a primary alkyl amine (R-NH2). Soft Matter 2015, 11, 6386–6392. [Google Scholar] [CrossRef]
- Ballabh, A.; Trivedi, D.R.; Dastidar, P. From Nonfunctional Lamellae to Functional Nanotubes. Org. Lett. 2006, 8, 1271–1274. [Google Scholar] [CrossRef]
- Nagy, P.I.; Erhardt, P.W. On the Interaction of Aliphatic Amines and Ammonium Ions with Carboxylic Acids in Solution and in Receptor Pockets. J. Phys. Chem. B 2012, 116, 5425–5436. [Google Scholar] [CrossRef] [PubMed]
- DiStasio, R.A., Jr.; von Lilienfeld, O.A.; Tkatchenko, A. Collective many-body van der Waals interactions in molecular systems. Proc. Natl. Acad. Sci. USA 2012, 109, 14791–14795. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.; Dastidar, P. Secondary Ammonium Dicarboxylate (SAD)—A Supramolecular Synthon in Designing Low Molecular Weight Gelators Derived from Azo-Dicarboxylates. Cryst. Growth Des. 2012, 12, 5917. [Google Scholar] [CrossRef]
- Sahoo, P.; Kumar, D.K.; Trivedi, D.R.; Dastidar, P. An easy access to an organometallic low molecular weight gelator: A crystal engineering approach. Tetrahedron Lett. 2008, 49, 3052. [Google Scholar] [CrossRef]
- Kuroiwa, K.; Shibata, T.; Takada, A.; Nemoto, N.; Kimizuka, N. Heat-Set Gel-like Networks of Lipophilic Co(II) Triazole Complexes in Organic Media and Their Thermochromic Structural Transitions. J. Am. Chem. Soc. 2004, 126, 2016–2021. [Google Scholar] [CrossRef]
- De Hatten, X.; Bell, N.; Yufa, N.; Christmann, G.; Nitschke, J.R. A Dynamic Covalent, Luminescent Metallopolymer that Undergoes Sol-to-Gel Transition on Temperature Rise. J. Am. Chem. Soc. 2011, 133, 3158–3164. [Google Scholar] [CrossRef] [PubMed]
- Pouzot, M.; Nicolai, T.; Durand, D.; Benyahia, L. Structure Factor and Elasticity of a Heat-Set Globular Protein Gel. Macromolecules 2004, 37, 614–620. [Google Scholar] [CrossRef]
- Gavrilov, M.; Gilbert, E.P.; Rowan, A.E.; Lauko, J.; Yakubov, G.E. Structural Insights into the Mechanism of Heat-Set Gel Formation of Polyisocyanopeptide Polymers. Polym. Macromol. Rapid Commun. 2020, 41, 2000304. [Google Scholar] [CrossRef] [PubMed]
- Kaspchak, E.; Oliveira, M.A.S.D.; Simas, F.F.; Franco, C.R.C.; Silveira, J.L.M.; Mafra, M.R.; Igarashi-Mafra, L. Determination of heat-set gelation capacity of a quinoa protein isolate (Chenopodium quinoa) by dynamic oscillatory rheological analysis. Food Chem. 2017, 232, 263–271. [Google Scholar] [CrossRef]
- Martin, A.H.; Nieuwland, M.; de Jong, G.A.H. Characterization of heat-set gels from RuBisCO in comparison to those from other proteins. J. Agric. Food Chem. 2014, 62, 10783–10791. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, X.; Cheng, Y.; Zhao, G.; Zhou, Y. Gelation mechanism of alkali induced heat-set konjac glucomannan gel. Trends Food Sci. Technol. 2021, 116, 244–254. [Google Scholar] [CrossRef]
- Park, D.; Wu, W.; Wang, Y. A functionalizable reverse thermal gel based on a polyurethane/PEG block copolymer. Biomaterials 2011, 32, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Maitia, B.; Bhattacharya, S. First report of charge-transfer induced heat-set hydrogel. Structural insights and remarkable properties. Nanoscale 2016, 8, 11224–11233. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Jalani, K.; George, S.J. Redox-Mediated, Transient Supramolecular Charge-Transfer Gel and Ink. ACS Appl. Mater. Interfaces 2020, 12, 5259–5264. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Chen, X.-J.; Zheng, Y.-S. Heat-set gels and egg-like vesicles using two component gel system based on chiral calix[4]arenes. Chem. Commun. 2007, 5200–5202. [Google Scholar] [CrossRef]
- Sahoo, P. Transformation of 1d to 3d Hydrogen Bonded Network: An Introduction of Competitive Hydrogen Bonding Sites in Supramolecular Synthon; Seth, S.K., Ed.; New Academic Publishers: New Delhi, India, 2016; pp. 144–152. ISBN 9788186772898. [Google Scholar]
- Lee, M.G.; Yoon, W.B. Developing an Effective Method to Determine the Heat Transfer Model in Fish Myofibrillar Protein Paste with Computer Simulation Considering the Phase Transition on Various Dimensions. Int. J. Food Eng. 2016, 12, 889–900. [Google Scholar] [CrossRef]
- Sánchez, F.H.; Mateo, J.M.; Colomer, F.J.R.; Sánchez, M.S.; Ribelles, J.L.G.; Mano, J.F. Influence of Low-Temperature Nucleation on the Crystallization Process of Poly(l-lactide). Biomacromolecules 2005, 6, 3283–3290. [Google Scholar] [CrossRef]
- Blagden, N. Encyclopedia of Supramolecular Chemistry; Atwood, J.L., Steed, J.W., Eds.; Marcel Dekker: New York, NY, USA,, 2004; Volume 1, p. 364. [Google Scholar]
- Velásquez-González, O.; Campos-Escamilla, C.; Flores-Ibarra, A.; Esturau-Escofet, N.; Arreguin-Espinosa, R.; Stojanoff, V.; Cuéllar-Cruz, M.; Moreno, A. Crystal Growth in Gels from the Mechanisms of Crystal Growth to Control of Polymorphism: New Trends on Theoretical and Experimental Aspects. Crystals 2019, 9, 443. [Google Scholar] [CrossRef]
- Dawn, A.; Andrew, K.S.; Yufit, D.S.; Hong, Y.; Reddy, J.P.; Jones, C.D.; Aguilar, J.A.; Steed, J.W. Supramolecular Gel Control of Cisplatin Crystallization: Identification of a New Solvate Form Using a Cisplatin-Mimetic Gelator. Cryst. Growth Des. 2015, 15, 4591–4599. [Google Scholar] [CrossRef]
- Fullam, E.; Prokes, I.; Fütterer, K.; Besra, G.S. Structural and functional analysis of the solute-binding protein UspC from Mycobacterium tuberculosis that is specific for amino sugars. Open Biol. 2016, 6, 160105. [Google Scholar] [CrossRef] [Green Version]
- Amenta, V.; Cook, J.L.; Hunter, C.A.; Low, C.M.R.; Vinter, J.G. Influence of Solvent Polarity on Preferential Solvation of Molecular Recognition Probes in Solvent Mixtures. J. Phys. Chem. B 2012, 116, 14433. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.; Chakraborty, I.; Bandyopadhyaya, A. Designing Supramolecular Pheromone Containers by Crystal Engineering for Replacing Pesticides. Eng. Sci. 2022, 20, 20. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, P. Moisture-Catalyzed Slow Release of Sex Pheromone from Microcrystals in Controlling Phyllophaga Pests. Eng. Sci. 2021, 16, 9–18. [Google Scholar] [CrossRef]
- Sahoo, P.; Sankolli, R.; Lee, H.-Y.; Raghavan, S.R.; Dastidar, P. Gel Sculpture: Moldable, Load-Bearing and Self-Healing Non-Polymeric Supramolecular Gel Derived from a Simple Organic Salt. Chem. Eur. J. 2012, 18, 8057–8063. [Google Scholar] [CrossRef]
- Marwan, A.I.; Williams, S.M.; Bardill, J.R.; Gralla, J.; Abdul-Aziz, N.M.; Park, D. Reverse Thermal Gel for In Utero Coverage of Spina Bifida Defects: An Innovative Bioengineering Alternative to Open Fetal Repair. Macromol. Biosci. 2017, 17, 1600473. [Google Scholar] [CrossRef]
- Sahoo, P.; Ghosh, S. Space and Time Crystal Engineering in Developing Futuristic Chemical Technology. ChemEngineering 2021, 5, 67. [Google Scholar] [CrossRef]
- Cesur, S.; Gokbel, S. Crystallization of mefenamic acid and polymorphs. Cryst. Res. Technol. 2008, 43, 720–728. [Google Scholar] [CrossRef]
- Raza, K.; Kumar, P.; Ratan, S.; Malik, R.; Arora, S. Polymorphism: The phenomenon affecting the performance of drugs. SOJ Pharm. Pharm. Sci. 2014, 1, 10. [Google Scholar]
- Thomas, L.H.; Wales, C.; Wilson, C.C. Selective preparation of elusive and alternative single component polymorphic solid forms through multi-component crystallisation routes. Chem. Commun. 2016, 52, 7372–7375. [Google Scholar]
- Bag, P.P.; Reddy, C.M. Screening and selective preparation of polymorphs by fast evaporation method: A case study of aspirin, anthranilic acid, and niflumic acid. Cryst. Growth Des. 2012, 12, 2740–2743. [Google Scholar] [CrossRef]
- Diao, Y.; Whaley, K.E.; Helgeson, M.E.; Woldeyes, M.A.; Doyle, P.S.; Myerson, A.S.; Hatton, T.A.; Trout, B.L. Gel-Induced Selective Crystallization of Polymorphs. J. Am. Chem. Soc. 2012, 134, 673–684. [Google Scholar] [CrossRef]
- Foster, J.A.; Damodaran, K.K.; Maurin, A.; Day, G.M.; Thompson, H.P.G.; Cameron, G.J.; Bernal, J.C.; Steed, J.W. Pharmaceutical polymorph control in a drug-mimetic supramolecular gel. Chem. Sci. 2017, 8, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, S.R.; Jones, C.D.; Yufit, D.S.; Nicholson, C.E.; Coopera, S.J.; Steed, J.W. Pharmaceutical polymorph control in a drug-mimetic supramolecular gel. Cryst. Eng. Comm. 2018, 20, 1390–1398. [Google Scholar] [CrossRef]
- Amorim, J.A.; Brito, I.C.A.; Vieira, H.M.; Vodianitskaia, P.J.; Gurgel, J.M. Specific heat capacity of water-silica gel adsorbed phase. J. Porous Mater. 2017, 20, 859–863. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, P. Designing Heat-Set Gels for Crystallizing APIs at Different Temperatures: A Crystal Engineering Approach. ChemEngineering 2022, 6, 65. https://doi.org/10.3390/chemengineering6050065
Sahoo P. Designing Heat-Set Gels for Crystallizing APIs at Different Temperatures: A Crystal Engineering Approach. ChemEngineering. 2022; 6(5):65. https://doi.org/10.3390/chemengineering6050065
Chicago/Turabian StyleSahoo, Pathik. 2022. "Designing Heat-Set Gels for Crystallizing APIs at Different Temperatures: A Crystal Engineering Approach" ChemEngineering 6, no. 5: 65. https://doi.org/10.3390/chemengineering6050065
APA StyleSahoo, P. (2022). Designing Heat-Set Gels for Crystallizing APIs at Different Temperatures: A Crystal Engineering Approach. ChemEngineering, 6(5), 65. https://doi.org/10.3390/chemengineering6050065





