Comparative Study of Physicochemical Characteristics and Catalytic Activity of Copper Oxide over Synthetic Silicon Oxide and Silicon Oxide from Rice Husk in Non-Oxidative Dehydrogenation of Ethanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of Catalysts
2.3. Characterization
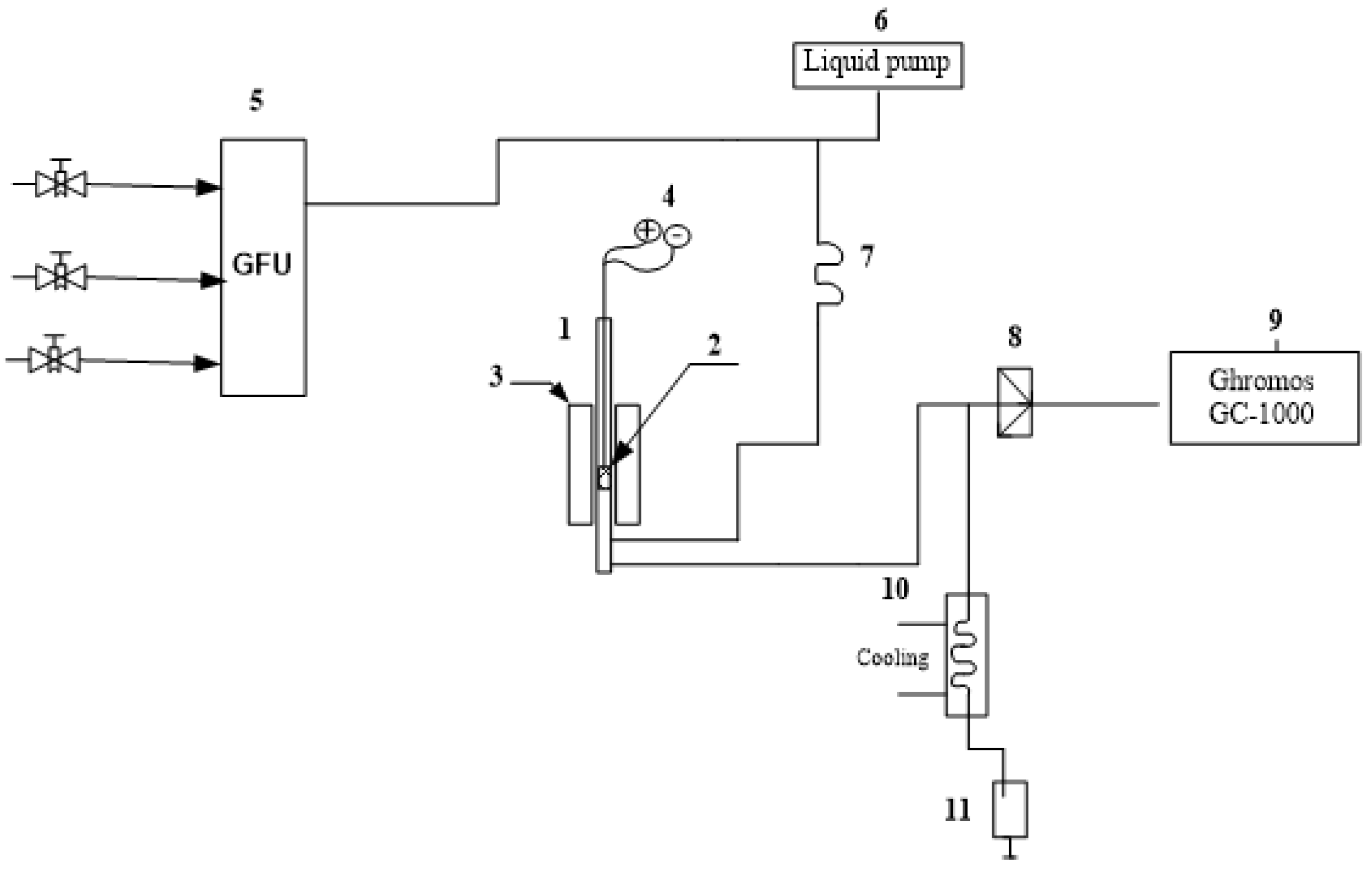
2.4. Testing of Catalysts in Ethanol Conversion
2.5. Analysis of Reaction Products
3. Results
3.1. Characterization
3.2. Catalytic Performance for Non-Oxidation of Ethanol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caro, C.; Thirunavukkarasu, K.; Anilkumar, M.; Shiju, N.R.; Rothenberg, G. Selective Autooxidation of Ethanol over Titania-Supported Molybdenum Oxide Catalysts: Structure and Reactivity. Adv. Synth. Catal. 2012, 354, 1327–1336. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, H.R.; Jaenicke, S.; Chuah, G.K. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. J. Catal. 2020, 389, 19–28. [Google Scholar] [CrossRef]
- Takei, T.; Iguchi, N.; Haruta, M. Synthesis of Acetoaldehyde, Acetic Acid, and Others by the Dehydrogenation and Oxidation of Ethanol. Catal. Surv. Asia 2011, 15, 80–88. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.C.; Yang, X.J.; Han, Y.F. Silver/hydroxyapatite foam as a highly selective catalyst for acetaldehyde production via ethanol oxidation. Catal. Today 2016, 276, 19–27. [Google Scholar] [CrossRef]
- Tang, C.; Zhai, Z.; Li, X.; Sun, L.; Bai, W. Sustainable production of acetaldehyde from lactic acid over the magnesium aluminate spinel. J. Taiwan Inst. Chem. Eng. 2016, 58, 97–106. [Google Scholar] [CrossRef]
- Liu, P.; Hensen, E.J.M. Highly Efficient and Robust Au/MgCuCr2O4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde. J. Am. Chem. Soc. 2013, 135, 14032–14035. [Google Scholar] [CrossRef]
- Santacesaria, E.; Carotenuto, G.; Tesser, R.; Di Serio, M. Ethanol Dehydrogenation to Ethyl Acetate by Using Copper and Copper Chromite Catalysts. Chem. Eng. J. 2012, 179, 209–220. [Google Scholar] [CrossRef]
- Dossumov, K.; Yergazieva, G.Y.; Churina, D.H.; Tayrabekova, S.Z.; Tulebayev, E.M. Effect of the Method of Preparation of a Supported Cerium Oxide Catalyst on Its Activity in the Conversion of Ethanol to Ethylene. Theor. Exp. Chem. 2016, 52, 123–126. [Google Scholar] [CrossRef]
- Dosumov, K.; Ergazieva, G.E.; Churina, D.K.; Tel’baeva, M.M. Cerium-Containing Catalysts for Converting Ethanol into Ethylene. Russ. J. Phys. Chem. A 2014, 88, 1806–1808. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. A Review of the Production of Ethanol from Softwood. Appl. Microbiol. Biotechnol. 2002, 59, 618–628. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of Lignocellulosic Materials for Ethanol Production: A Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Yergaziyeva, G.Y.; Dossumov, K.; Mambetova, M.M.; Strizhak, P.Y.; Kurokawa, H.; Baizhomartov, B. Effect of Ni, La, and Ce Oxides on a Cu/Al2O3 Catalyst with Low Copper Loading for Ethanol Non-oxidative Dehydrogenation. Chem. Eng. Technol. 2021, 44, 1890–1899. [Google Scholar] [CrossRef]
- Pinthong, P.; Praserthdam, P.; Jongsomjit, B. Oxidative Dehydrogenation of Ethanol over Vanadium- and Molybdenum-Modified Mg-Al Mixed Oxide Derived from Hydrotalcite. J. Oleo Sci. 2019, 68, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Sushkevich, V.L.; Ivanova, I.I.; Taarning, E. Mechanistic Study of Ethanol Dehydrogenation over Silica-Supported Silver. ChemCatChem 2013, 5, 2367–2373. [Google Scholar] [CrossRef]
- Amandusson, H.; Ekedahl, L.-G.; Dannetun, H. Isotopic Study of Ethanol Dehydrogenation over a Palladium Membrane. J. Catal. 2000, 195, 376–382. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Cui, Z.D.; Zhu, S.L.; Liu, Y.; Yang, X.J. Silver Nanoparticles Supported on TiO2 Nanotubes as Active Catalysts for Ethanol Oxidation. J. Catal. 2011, 278, 276–287. [Google Scholar] [CrossRef]
- Yu, D.; Dai, W.; Wu, G.; Guan, N.; Li, L. Stabilizing copper species using zeolite for ethanol catalytic dehydrogenation to acetaldehyde. Chin. J. Catal. 2019, 9, 1375–1384. [Google Scholar] [CrossRef]
- Tonner, S.P.; Trimm, D.L.; Wainwright, M.S.; Cant, N.W. Dehydrogenation of Methanol to Methyl Formate over Copper Catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 384–388. [Google Scholar] [CrossRef]
- Guan, Y.; Hensen, E.J.M. Ethanol Dehydrogenation by Gold Catalysts: The Effect of the Gold Particle Size and the Presence of Oxygen. Appl. Catal. Gen. 2009, 361, 49–56. [Google Scholar] [CrossRef]
- Sitthisa, S.; Resasco, D.E. Hydrodeoxygenation of Furfural Over Supported Metal Catalysts: A Comparative Study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784–791. [Google Scholar] [CrossRef]
- Tu, Y.J.; Chen, Y.-W. Effects of Alkaline-Earth Oxide Additives on Silica-Supported Copper Catalysts in Ethanol Dehydrogenation. Ind. Eng. Chem. Res. 1998, 37, 2618–2622. [Google Scholar] [CrossRef]
- Tu, Y.J.; Chen, Y.-W. Effects of Alkali Metal Oxide Additives on Cu/SiO2 Catalyst in the Dehydrogenation of Ethanol. Ind. Eng. Chem. Res. 2001, 40, 5889–5893. [Google Scholar] [CrossRef]
- Wang, C.; Garbarino, G.; Allard, L.F.; Wilson, F.; Busca, G.; Flytzani-Stephanopoulos, M. Low-Temperature Dehydrogenation of Ethanol on Atomically Dispersed Gold Supported on ZnZrOx. ACS Catal. 2016, 6, 210–218. [Google Scholar] [CrossRef]
- Shan, J.; Janvelyan, N.; Li, H.; Liu, J.; Egle, T.M.; Ye, J.; Biener, M.M.; Biener, J.; Friend, C.M.; Flytzani-Stephanopoulos, M. Selective Non-Oxidative Dehydrogenation of Ethanol to Acetaldehyde and Hydrogen on Highly Dilute NiCu Alloys. Appl. Catal. B Environ. 2017, 205, 541–550. [Google Scholar] [CrossRef]
- Tayrabekova, S.; Mäki-Arvela, P.; Peurla, M.; Paturi, P.; Eränen, K.; Ergazieva, G.E.; Aho, A.; Murzin, D.Y.; Dossumov, K. Catalytic Dehydrogenation of Ethanol into Acetaldehyde and Isobutanol Using Mono- and Multicomponent Copper Catalysts. C. R. Chim. 2018, 21, 194–209. [Google Scholar] [CrossRef]
- Witzke, M.E.; Dietrich, J.P.; Ibrahim, M.Y.S.; Al-Bardan, K.; Triezenberg, M.D.; Flaherty, D.W. Spectroscopic evidence for origins of size and support effects on selectivity of Cu nanoparticle dehydrogenation catalysts. Chem. Commun. 2017, 53, 597–600. [Google Scholar] [CrossRef]
- Freitas, I.C.; Damyanova, S.; Oliveira, D.C.; Marques, C.M.P.; Bueno, J.M.C. Effect of Cu Content on the Surface and Catalytic Properties of Cu/ZrO2 Catalyst for Ethanol Dehydrogenation. J. Mol. Catal. Chem. 2014, 381, 26–37. [Google Scholar] [CrossRef]
- Cassinelli, W.H.; Martins, L.; Passos, A.R.; Pulcinelli, S.H.; Rochet, A.; Briois, V.; Santilli, C.V. Correlation between Structural and Catalytic Properties of Copper Supported on Porous Alumina for the Ethanol Dehydrogenation Reaction. ChemCatChem 2015, 7, 1668–1677. [Google Scholar] [CrossRef]
- Cassinelli, W.H.; Martins, L.; Magnani, M.; Pulcinelli, S.H.; Briois, V.; Santilli, C.V. Time-Resolved XAS/MS/Raman Monitoring of Mutual Copper Self-Reduction and Ethanol Dehydrogenation Reactions. RSC Adv. 2016, 6, 20453–20457. [Google Scholar] [CrossRef]
- Rossetti, I.; Biffi, C.; Bianchi, C.L.; Nichele, V.; Signoretto, M.; Menegazzo, F.; Finocchio, E.; Ramis, G.; Di Michele, A. Ni/SiO2 and Ni/ZrO2 Catalysts for the Steam Reforming of Ethanol. Appl. Catal. B Environ. 2012, 117–118, 384–396. [Google Scholar] [CrossRef]
- Ohira, M.; Liu, H.; He, D.; Hirata, Y.; Sano, M.; Suzuki, T.; Miyake, T. Catalytic Performance and Reaction Pathways of Cu/SiO2 and ZnO/SiO2 for Dehydrogenation of Ethanol to Acetaldehyde. J. Jpn. Pet. Inst. 2018, 61, 205–212. [Google Scholar] [CrossRef]
- He, X.; Liu, H. Efficient Synthesis of 1,1-Diethoxyethane via Sequential Ethanol Reactions on Silica-Supported Copper and H-Y Zeolite Catalysts. Catal. Today 2014, 233, 133–139. [Google Scholar] [CrossRef]
- Wang, Q.-N.; Shi, L.; Lu, A.-H. Highly Selective Copper Catalyst Supported on Mesoporous Carbon for the Dehydrogenation of Ethanol to Acetaldehyde. ChemCatChem 2015, 7, 2846–2852. [Google Scholar] [CrossRef]
- Li, M.; Lu, W.; He, L.; Schüth, F.; Lu, A. Tailoring the Surface Structure of Silicon Carbide Support for Copper Catalyzed Ethanol Dehydrogenation. ChemCatChem 2019, 11, 481–487. [Google Scholar] [CrossRef]
- Wang, Q.N.; Shi, L.; Li, W.; Li, W.C.; Si, R.; Schüth, F.; Lu, A.H. Cu supported on thin carbon layer-coated porous SiO2 for efficient ethanol dehydrogenation. Catal. Sci. Technol. 2018, 8, 472–479. [Google Scholar] [CrossRef]
- Shukla, S.S.; Chava, R.; Appari, S.; Bahurudeen, A.; Kuncharam, B.V.R. Sustainable use of rice husk for the cleaner production of value-added products. J. Environ. Chem. Eng. 2022, 10, 106899. [Google Scholar] [CrossRef]
- Materazzi, M.; Foscolo, P.U. The role of waste and renewable gas to decarbonize the energy sector. In Substitute Natural Gas from Waste: Technical Assessment and Industrial Applications of Biochemical and Thermochemical Processes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Ang, T.N.; Ngoh, G.C.; Chua, A.S.M.; Lee, M.G. Elucidation of the effect of ionic liquid pretreatment on rice husk via structural analyses. Biotechnol. Biofuels 2012, 5, 67. [Google Scholar] [CrossRef]
- Materazzi, M. Gasification of Waste Derived Fuels in Fluidized Beds: Fundamental Aspects and Industrial Challenges. In Clean Energy from Wast; Springer: Cham, Switzerland, 2017; pp. 19–63. [Google Scholar] [CrossRef]
- Shah, S.A.; Soomar, M.; Hussain, A. Comparative emission analysis of bituminous coal, Sugarcane Bagasse and Rice Husk. Sind. Univ. Res. J. 2016, 48, 685–688. [Google Scholar]
- Azat, S.; Sartova, Z.; Bekseitova, K.; Askaruly, K. Extraction of high-purity silica from rice husk via hydrochloric acid leachingtreatment. Turk. J. Chem. 2019, 43, 1258–1269. [Google Scholar] [CrossRef]
- Myachina, M.; Gavrilova, N.; Nazarov, V. Formation of Molybdenum Blue Nanoparticles in the Organic Reducing Area. Molecules 2021, 26, 4438. [Google Scholar] [CrossRef]
- Llez, L.T.E.; Rubio, J.; Rubio, F.; Morales, E.; Oteo, J.L. Synthesis of Inorganic-Organic Hybrid Materials from TEOS, TBT and PDMS. J. Mater. Sci. 2003, 38, 1773–1780. [Google Scholar]
- Bakdash, R.S.; Aljundi, I.H.; Basheer, C.; Abdulazeez, I. Rice Husk Derived Aminated Silica for the Efficient Adsorption of Different Gases. Sci. Rep. 2020, 10, 19526. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.-W.; Yang, H.-C.; Roselin, L.S.; Kuo, W.-Y. Ethanol Dehydrogenation over Copper Catalysts on Rice Husk Ash Prepared by Ion Exchange. Appl. Catal. Gen. 2006, 304, 30–39. [Google Scholar] [CrossRef]
- Yalçin, N.; Sevinç, V. Studies on Silica Obtained from Rice Husk. Ceram. Int. 2001, 27, 219–224. [Google Scholar] [CrossRef]
- Guerreiro, E.D.; Gorriz, O.F.; Rivarola, J.B.; Arrúa, L.A. Characterization of Cu/SiO2 Catalysts Prepared by Ion Exchange for Methanol Dehydrogenation. Appl. Catal. Gen. 1997, 165, 259–271. [Google Scholar] [CrossRef]
- Baltes, C.; Vukojevic, S.; Schuth, F. Correlations between Synthesis, Precursor, and Catalyst Structure and Activity of a Large Set of CuO/ZnO/Al2O3 Catalysts for Methanol Synthesis. J. Catal. 2008, 258, 334–344. [Google Scholar] [CrossRef]
- Águila, G.; Gracia, F.; Cortés, J.; Araya, P. Effect of Copper Species and the Presence of Reaction Products on the Activity of Methane Oxidation on Supported CuO Catalysts. Appl. Catal. B Environ. 2008, 77, 325–338. [Google Scholar] [CrossRef]
- Hao, X.-Y.; Zhang, Y.-Q.; Wang, J.-W.; Zhou, W.; Zhang, C.; Liu, S. A Novel Approach to Prepare MCM-41 Supported CuO Catalyst with High Metal Loading and Dispersion. Microporous Mesoporous Mater. 2006, 88, 38–47. [Google Scholar] [CrossRef]
- Ding, J.; Popa, T.; Tang, J.; Gasema, K.A.M.; Fan, M.; Zhong, Q. Highly selective and stable Cu/SiO2 catalysts prepared with a green method for hydrogenation of diethyl oxalate into ethylene glycol. Appl. Catal. B Environ. 2017, 209, 530. [Google Scholar] [CrossRef]
- Dossumov, K.; Ergazieva, G.E.; Ermagambet, B.T.; Telbayeva, M.M.; Mambetova, M.M.; Myltykbayeva, L.K.; Kassenova, Z.M. Role of Ceria in Several Energy-Related Catalytic Transformations. Chem. Pap. 2020, 74, 373–388. [Google Scholar] [CrossRef]
- Dossumov, K.; Ergazieva, G.E.; Ermagambet, B.T.; Myltykbaeva, L.K.; Telbaeva, M.M.; Mironenko, A.V.; Mambetova, M.M.; Kassenova, Z. Morphology and Catalytic Properties of Cobalt-Containing Catalysts Synthesized by Different Means. Russ. J. Phys. Chem. A 2020, 94, 880–882. [Google Scholar] [CrossRef]
- Cavuoto, D.; Zaccheria, A.; Ravasio, N. Some Critical Insights into the Synthesis and Applications of Hydrophobic Solid Catalysts. J. Catal. 2020, 10, 1337. [Google Scholar] [CrossRef]
- Selva Roselin, L.; Chiu, H.-W. Production of hydrogen by oxidative steam reforming of methanol over Cu/SiO2 catalysts. J. Saudi Chem. Soc. 2018, 22, 692. [Google Scholar] [CrossRef]
- Xi, X.; Ma, S.; Chen, J.-F.; Zhang, Y. Promotional Effects of Ce, Mn and Fe Oxides on CuO/SiO2 Catalysts for CO Oxidation. J. Environ. Chem. Eng. 2014, 2, 1011–1017. [Google Scholar] [CrossRef]
- Liu, Z.; Amiridis, M.D.; Chen, Y. Characterization of CuO Supported on Tetragonal ZrO2 Catalysts for N2 O Decomposition to N2. J. Phys. Chem. B 2005, 109, 1251–1255. [Google Scholar] [CrossRef]
- Van Der Grift, C.J.G.; Wielers, A.F.H.; Mulder, A.; Geus, J.W. The reduction behaviour of silica-supported copper catalysts prepared by deposition-precipitation. Thermochim. Acta 1990, 171, 95–113. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Jiao, Y.; Cheng, M.; Wang, L.-L.; Wang, J.-L.; Li, X.-Y.; Chen, Y.-Q. Improved Activity of Ni–Mo/SiO2 Bimetallic Catalyst Synthesized via Sol-Gel Method for Methylcyclohexane Cracking. Pet. Sci. 2021, 18, 1530–1542. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; He, L.; Li, L.; Pan, X.; Wang, A.; Wang, X.; Zhang, T. Upgrading Ethanol to N-Butanol over Highly Dispersed Ni–MgAlO Catalysts. J. Catal. 2016, 344, 184–193. [Google Scholar] [CrossRef]
- Benito, P.; Vaccari, A.; Antonetti, C.; Licursi, D.; Schiarioli, N.; Rodriguez-Castellón, E.; Raspolli Galletti, A.M. Tunable Copper-Hydrotalcite Derived Mixed Oxides for Sustainable Ethanol Condensation to n-Butanol in Liquid Phase. J. Clean. Prod. 2019, 209, 1614–1623. [Google Scholar] [CrossRef]
- Larina, O.V.; Valihura, K.V.; Kyriienko, P.I.; Vlasenko, N.V.; Balakin, D.Y.; Khalakhan, I.; Čendak, T.; Soloviev, S.O.; Orlyk, S.M. Successive Vapour Phase Guerbet Condensation of Ethanol and 1-Butanol over Mg-Al Oxide Catalysts in a Flow Reactor. Appl. Catal. Gen. 2019, 588, 117265. [Google Scholar] [CrossRef]
- Rechi Siqueira, M.; Micali Perrone, O.; Metzker, G.; de Oliveira Lisboa, D.C.; Thoméo, J.C.; Boscolo, M. Highly Selective 1-Butanol Obtained from Ethanol Catalyzed by Mixed Metal Oxides: Reaction Optimization and Catalyst Structure Behavior. Mol. Catal. 2019, 476, 110516. [Google Scholar] [CrossRef]
- Ob-eye, J.; Praserthdam, P.; Jongsomjit, B. Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts. J. Catal. 2019, 9, 66. [Google Scholar] [CrossRef]
- Calles, J.; Carrero, A.; Vizcaíno, A.; Lindo, M. Effect of Ce and Zr Addition to Ni/SiO2 Catalysts for Hydrogen Production through Ethanol Steam Reforming. J. Catal. 2015, 5, 58–76. [Google Scholar] [CrossRef] [Green Version]
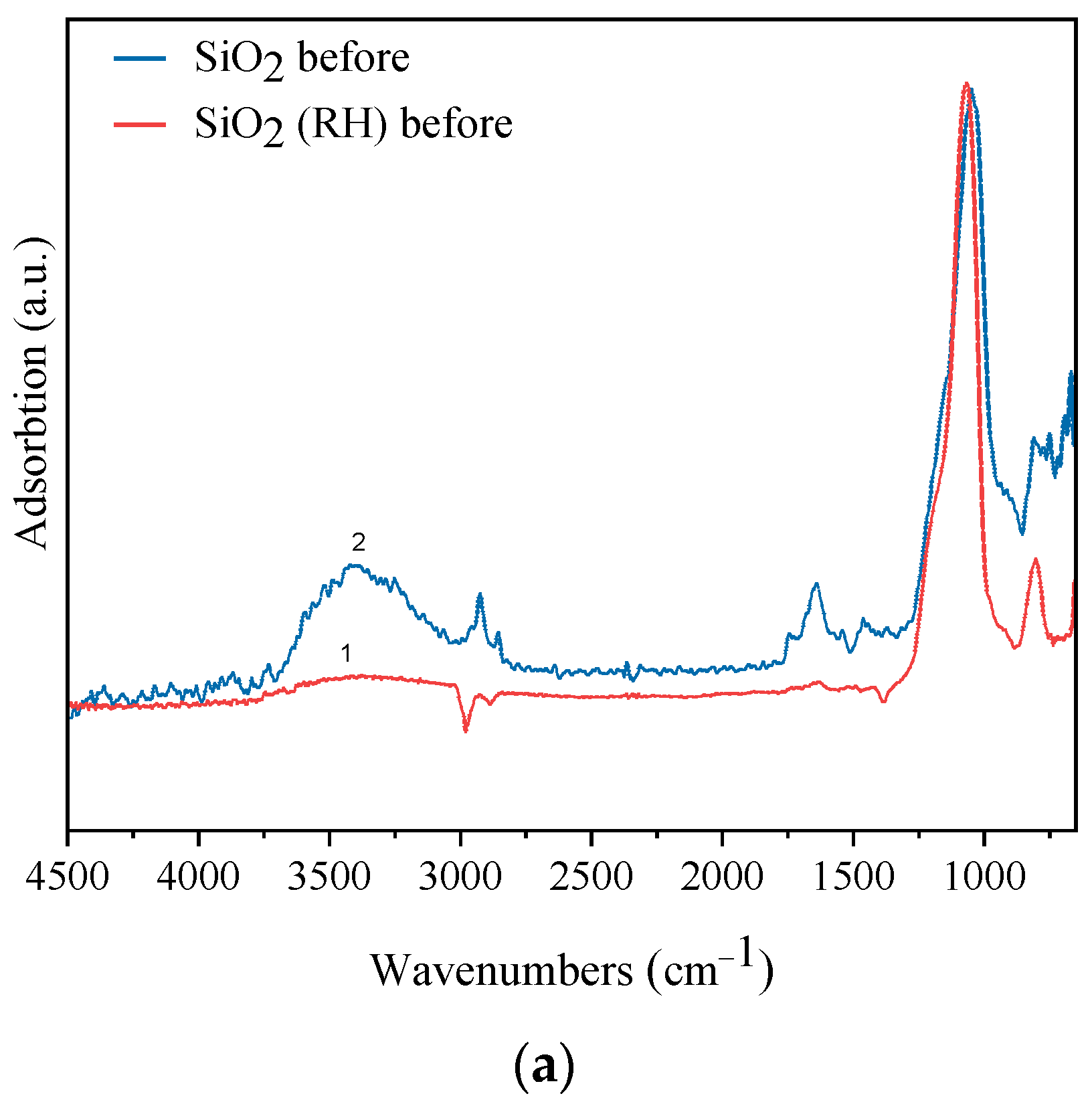

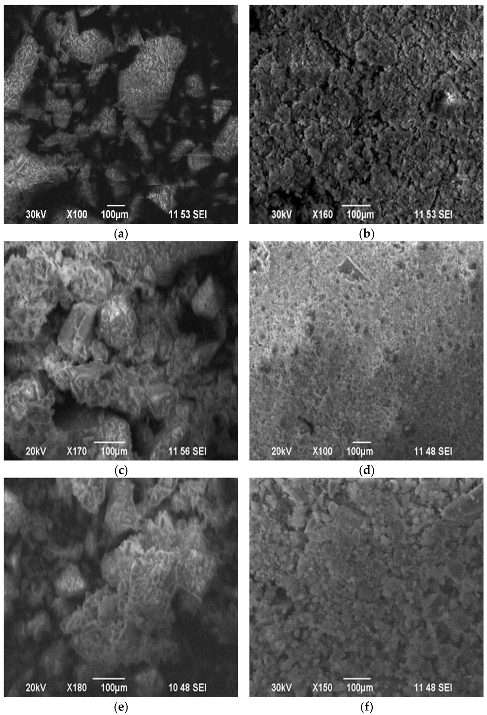

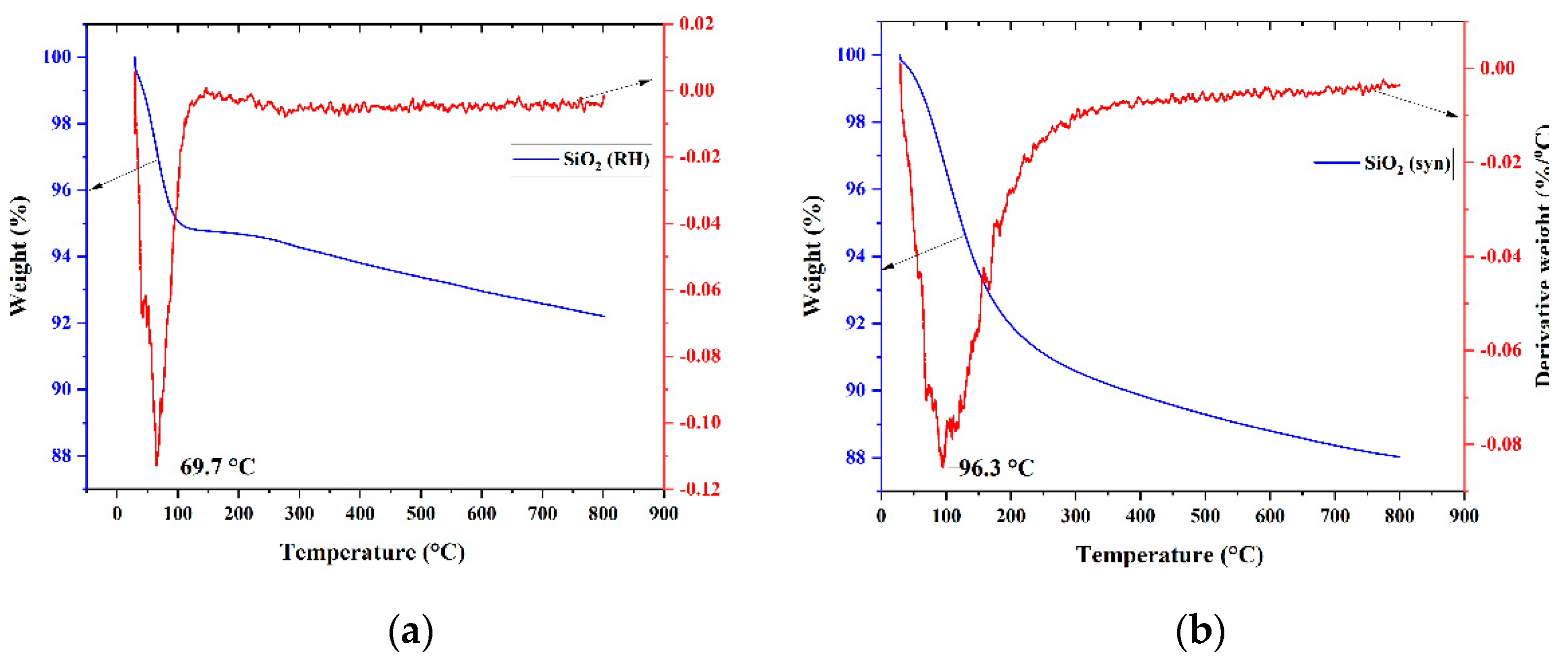

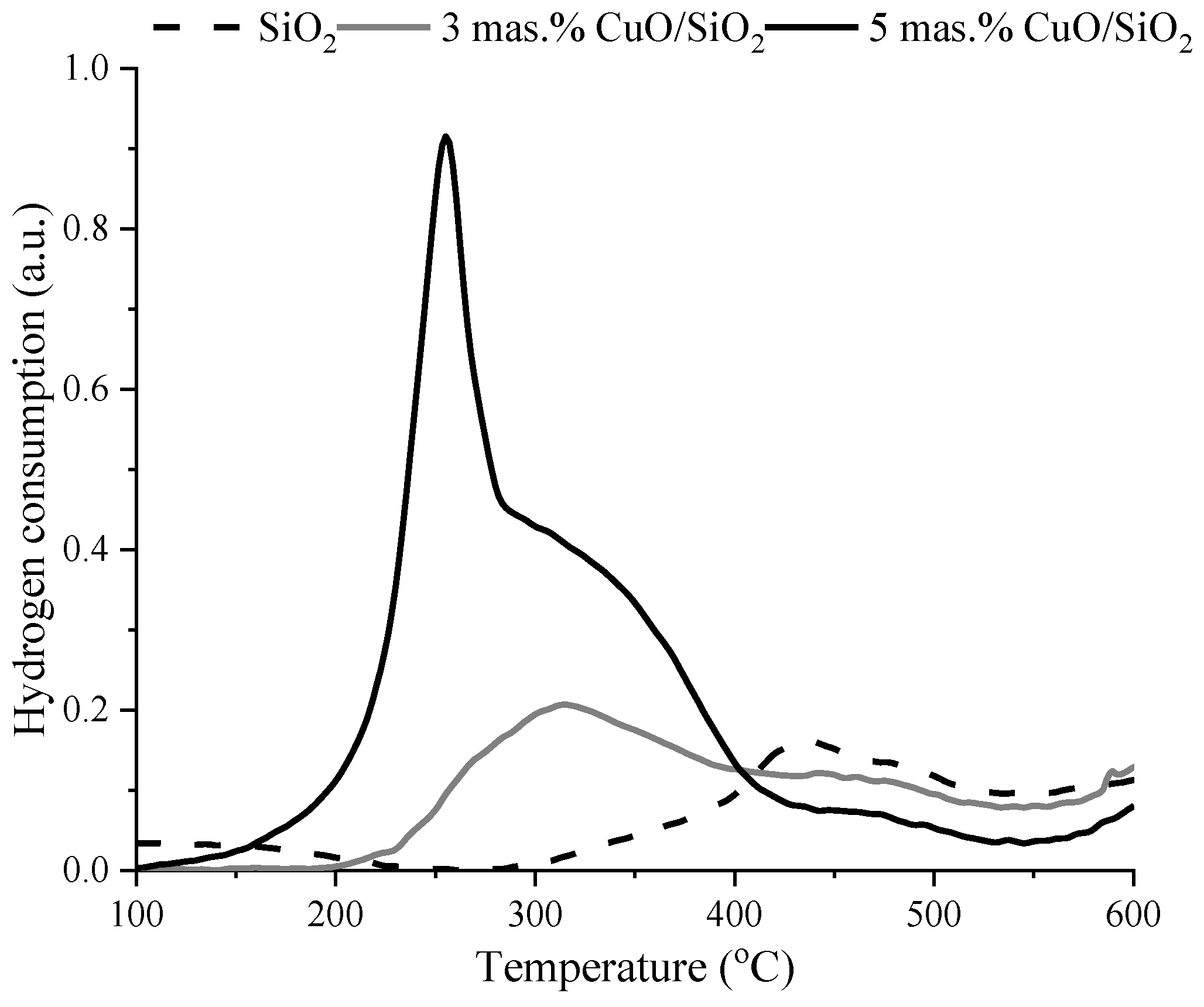
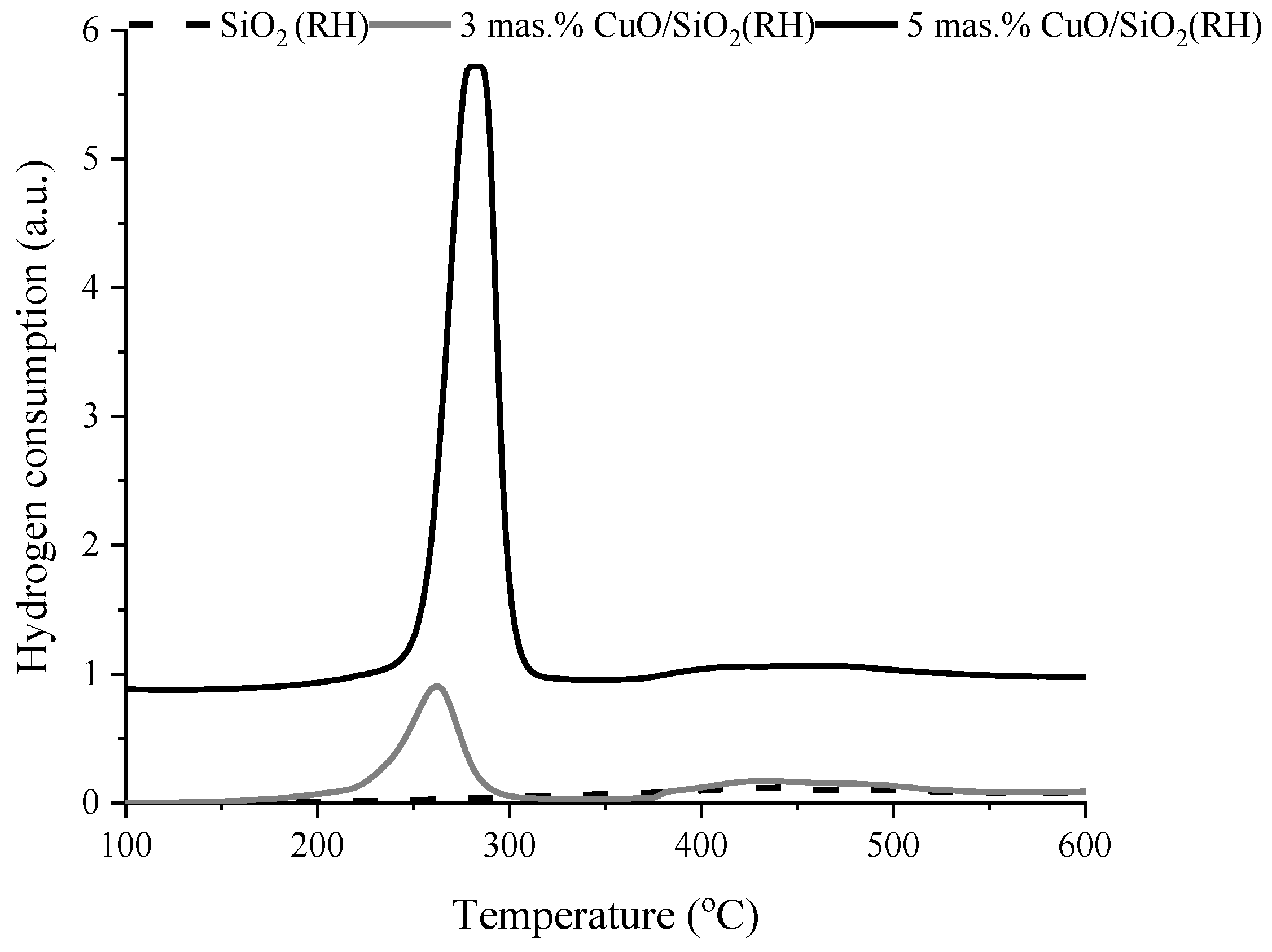
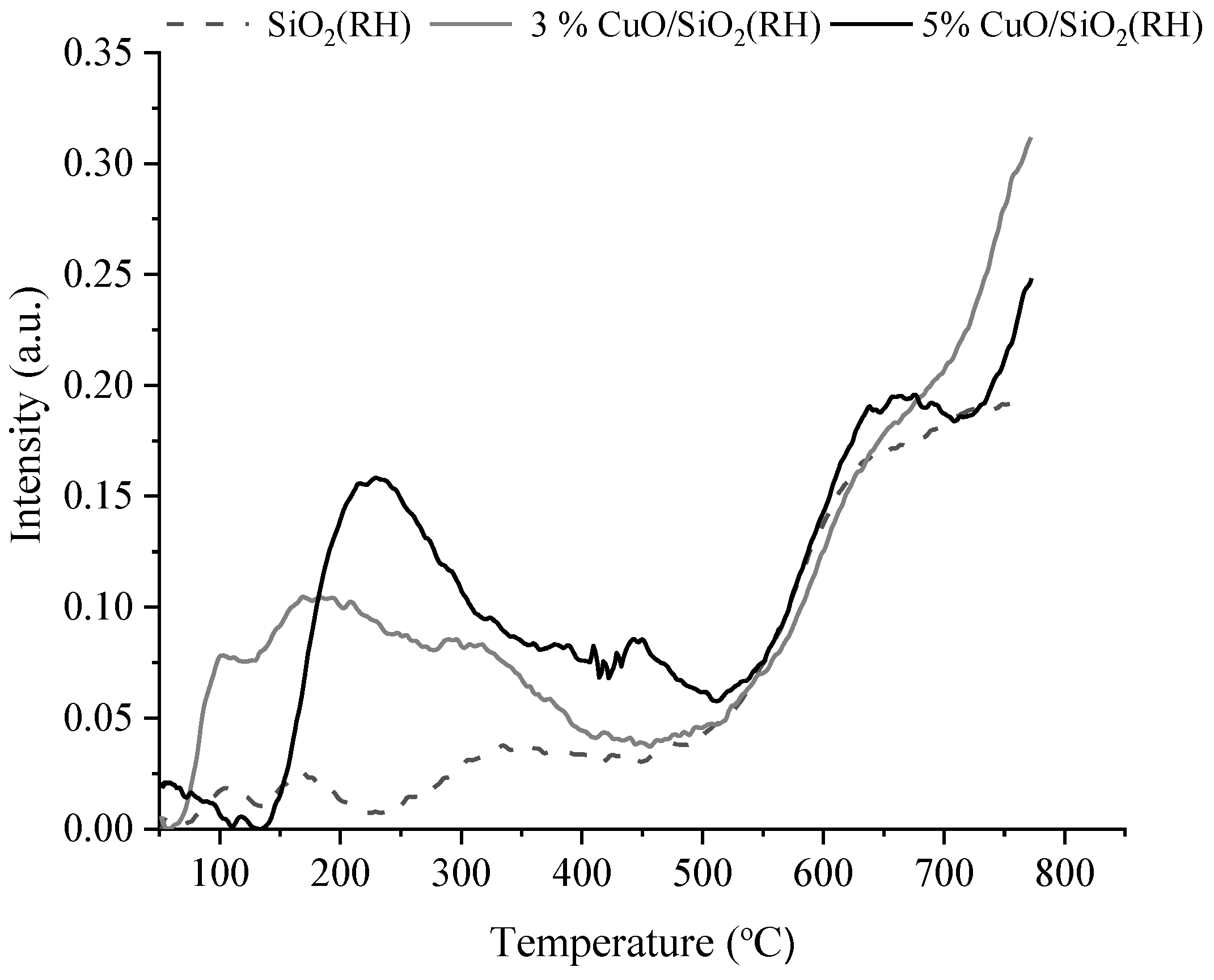
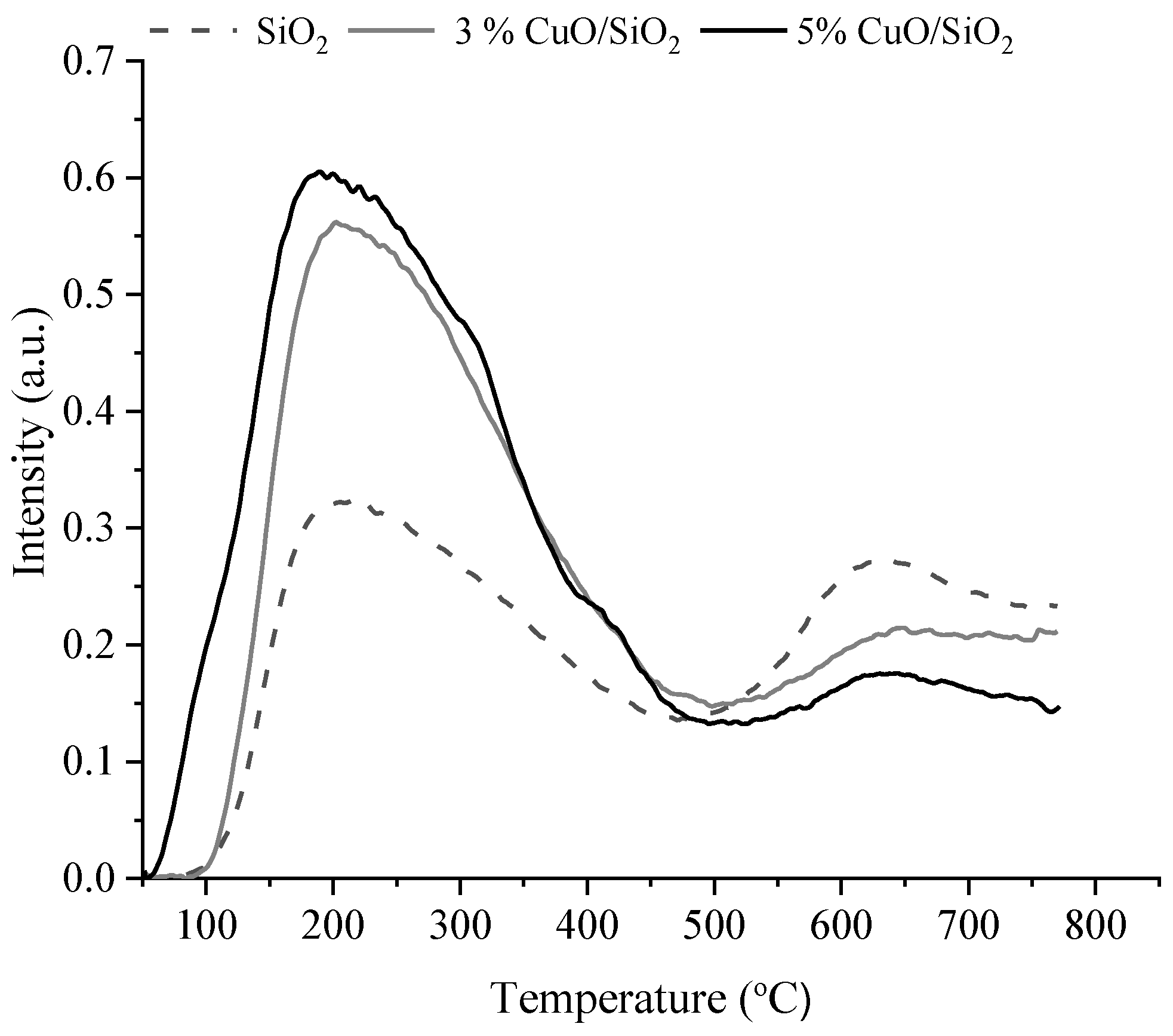
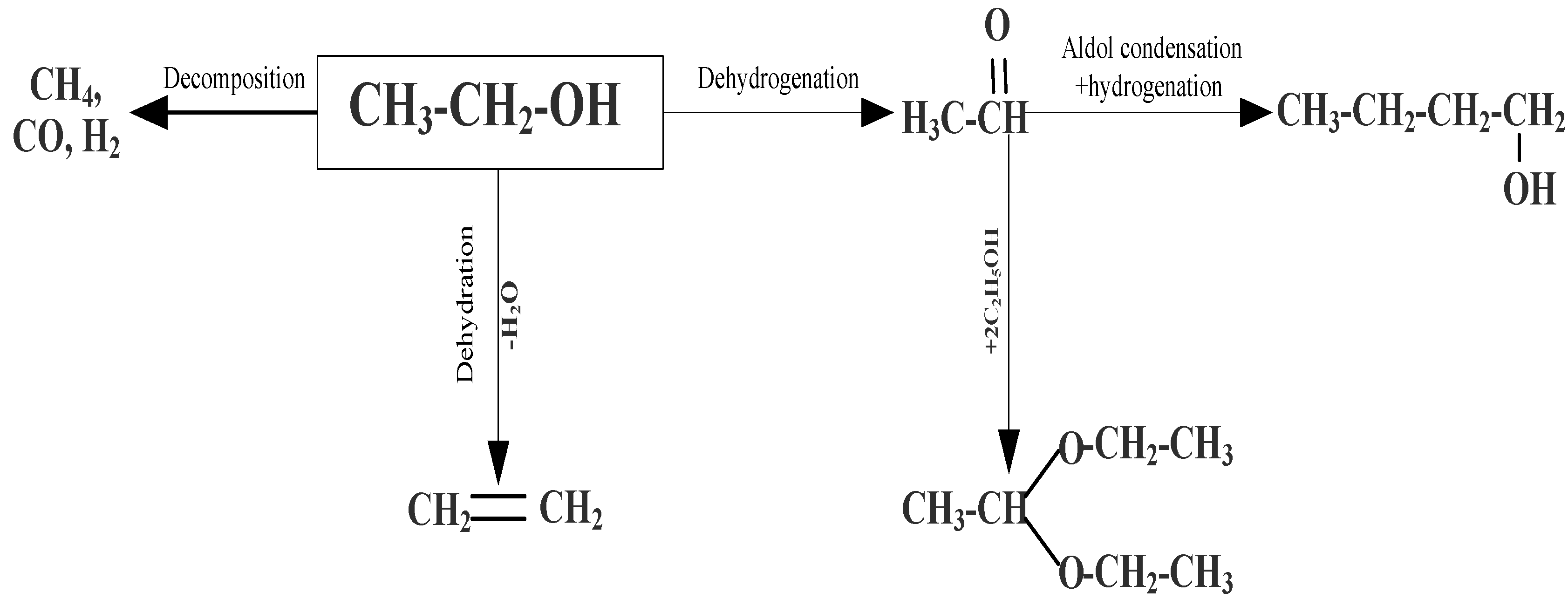
| Sample | Weak Acid Sites, μmol/g | Medium Acid Sites, μmol/g | Strong Acid Sites, μmol/g | Total, μmol/g |
|---|---|---|---|---|
| SiO2 (RH) | 11 | 15 | 52 | 78 |
| 3 wt.% CuO/SiO2 (RH) | 107 | 34 | 12 | 153 |
| 5 wt.% CuO/SiO2 (RH) | 121 | 6 | 34 | 161 |
| SiO2 | 400 | - | 82 | 482 |
| 3 wt.% CuO/SiO2 | 802 | - | 46 | 848 |
| 5 wt.% CuO/SiO2 | 934 | - | 34 | 968 |
| Samples | XEtOH, % | C2H4, C2H6 | AA | CH4 | CO | CO2 | H2 | H2O | Other Products (Dietoxyethane, n-Butanol) |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 30 | 14 | 2 | 25 | 30 | 11 | 17 | 2 | - |
| SiO2 (RH) | 42 | 0.3 | 28 | 13 | 12 | 10 | 30 | 4.3 | 2.0 (DEE), 0.4 (But) |
| 3 wt.% CuO/SiO2 | 63 | 6 | 22 | 13 | 22 | 10 | 25 | 2 | - |
| 3 wt.% CuO/SiO2 (RH) | 70 | 0.4 | 47 | 0.2 | 0.1 | 0.1 | 50 | - | 2.2 (DEE) |
| 5 wt.% CuO/SiO2 | 74 | 1 | 31 | 8 | 10 | 9 | 38 | 3 | - |
| 5 wt.% CuO/SiO2 (RH) | 85 | - | 42 | 0.2 | 0.2 | 0.1 | 51 | - | 5.5(DEE), 1(But) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mambetova, M.; Yergaziyeva, G.; Dossumov, K.; Askaruly, K.; Azat, S.; Bexeitova, K.; Anissova, M.; Baizhomartov, B. Comparative Study of Physicochemical Characteristics and Catalytic Activity of Copper Oxide over Synthetic Silicon Oxide and Silicon Oxide from Rice Husk in Non-Oxidative Dehydrogenation of Ethanol. ChemEngineering 2022, 6, 74. https://doi.org/10.3390/chemengineering6050074
Mambetova M, Yergaziyeva G, Dossumov K, Askaruly K, Azat S, Bexeitova K, Anissova M, Baizhomartov B. Comparative Study of Physicochemical Characteristics and Catalytic Activity of Copper Oxide over Synthetic Silicon Oxide and Silicon Oxide from Rice Husk in Non-Oxidative Dehydrogenation of Ethanol. ChemEngineering. 2022; 6(5):74. https://doi.org/10.3390/chemengineering6050074
Chicago/Turabian StyleMambetova, Manshuk, Gaukhar Yergaziyeva, Kusman Dossumov, Kydyr Askaruly, Seitkhan Azat, Kalampyr Bexeitova, Moldir Anissova, and Bedelzhan Baizhomartov. 2022. "Comparative Study of Physicochemical Characteristics and Catalytic Activity of Copper Oxide over Synthetic Silicon Oxide and Silicon Oxide from Rice Husk in Non-Oxidative Dehydrogenation of Ethanol" ChemEngineering 6, no. 5: 74. https://doi.org/10.3390/chemengineering6050074