A Brief Review of Photocatalytic Reactors Used for Persistent Pesticides Degradation
Abstract
1. Introduction
2. Mechanisms of Pesticides Photochemical Degradation
2.1. General Considerations
2.2. Specific Mechanisms
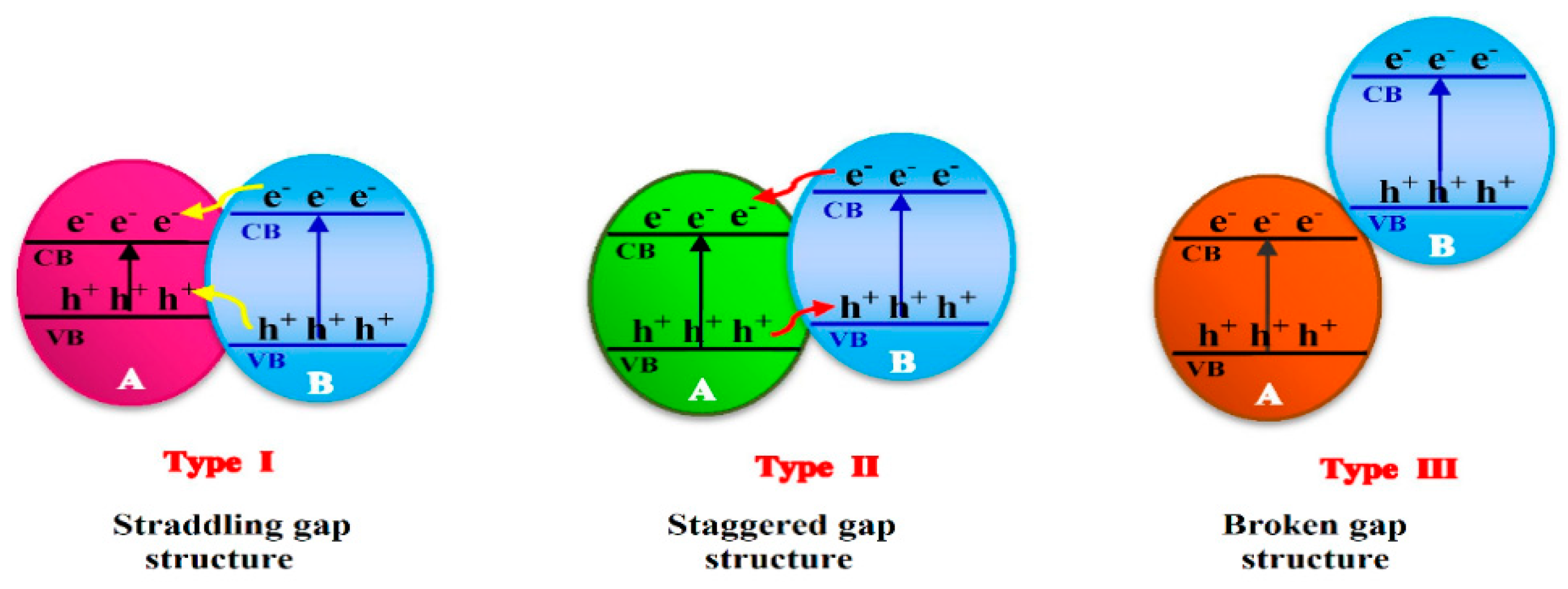
3. Photocatalyst Used for Pesticides Photodegradation
3.1. Pure and Mixed Oxide Semiconductors
3.2. Doped Photocatalysts
3.3. Nanomaterials
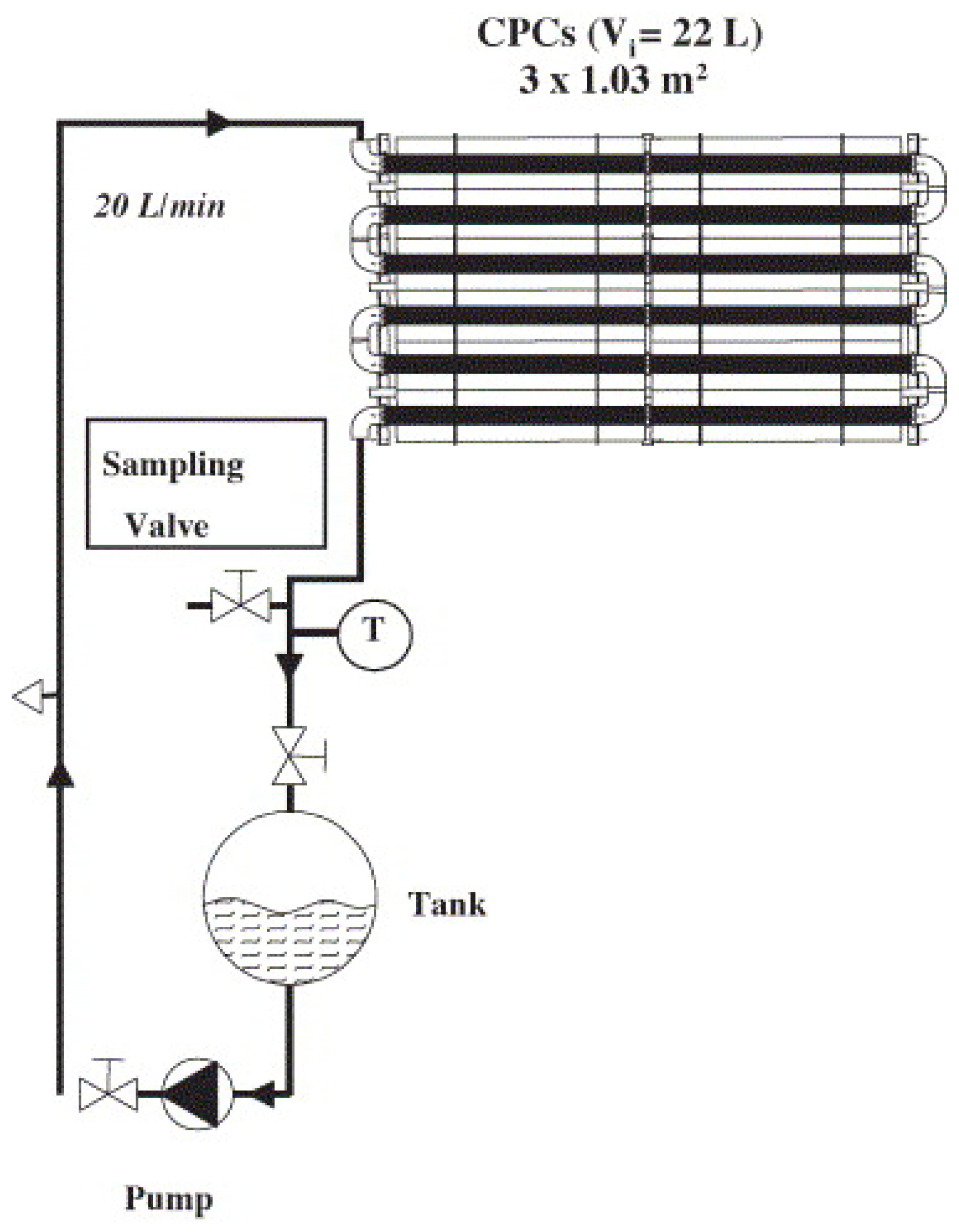
4. Photoreactors Types and Configurations for Pesticides Degradation–Design and Scale-Up Perspectives
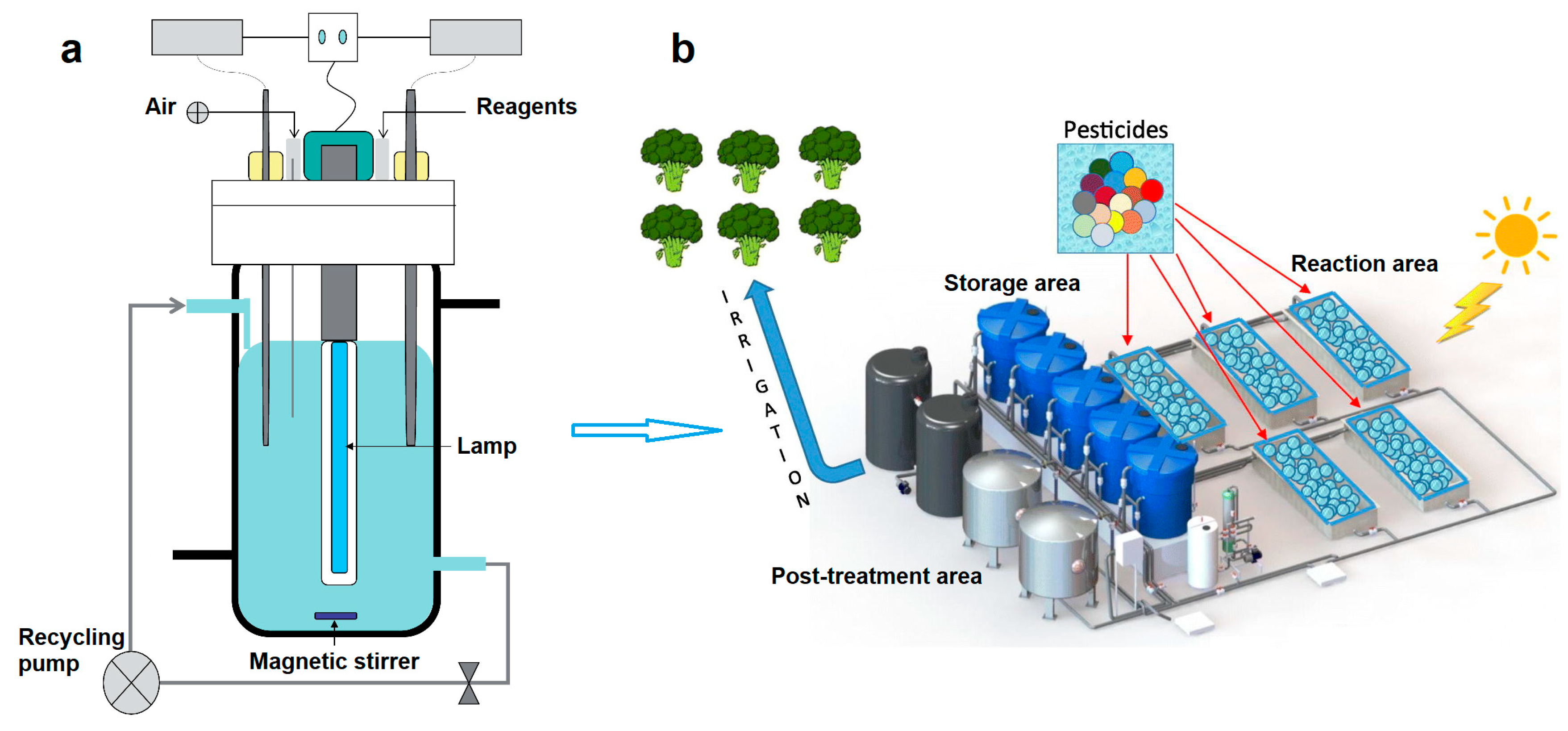
4.1. Slurry Reactors for Pesticides Degradation
4.2. Immobilized Systems for Pesticides Treatment and Agro-Wastewater Reclamation
4.3. Sustainable Approach and Systems Versatility
5. General Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Breggin, L.K.; Falkner, R.; Pendergrass, J.; Porter, R.; Jaspers, N. Addressing the Risks of Nanomaterials under United States and European Union Regulatory Frameworks for Chemicals. In Assessing Nanoparticle Risks to Human Health, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 179–254. [Google Scholar] [CrossRef]
- Tarazona, J.V.; Dohmen, G.P. Ecotoxicology of Rice Pesticides. In Pesticide Risk Assessment in Rice Paddies; Elsevier: Amsterdam, The Netherlands, 2008; pp. 69–90. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Toxics Editorial Farmers’ Exposure to Pesticides: Toxicity Types and Ways of Prevention, (n.d.). Toxics 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Horsak, R.D.; Bedient, P.B.; Hamilton, M.C.; Thomas, F.B. Pesticides. In Environmental Forensics; Elsevier: Amsterdam, The Netherlands, 1964; pp. 143–165. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Margni, M.; Rossier, D.; Crettaz, P.; Jolliet, O. Life cycle impact assessment of pesticides on human health and ecosystems. Agric. Ecosyst. Environ. 2002, 93, 379–392. [Google Scholar] [CrossRef]
- Lemaire, G.; Terouanne, B.; Mauvais, P.; Michel, S.; Rahmani, R. Effect of organochlorine pesticides on human androgen receptor activation in vitro. Toxicol. Appl. Pharmacol. 2004, 196, 235–246. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Colosio, C.; Tiramani, M.; Maroni, M. Neurobehavioral effects of pesticides: State of the art. Neurotoxicology 2003, 24, 577–591. [Google Scholar] [CrossRef]
- Curl, C.L.; Spivak, M.; Phinney, R.; Montrose, L. Synthetic Pesticides and Health in Vulnerable Populations: Agricultural Workers. Curr. Environ. Health Rep. 2020, 7, 13–29. [Google Scholar] [CrossRef]
- Litchfield, M.H. Estimates of Acute Pesticide Poisoning in Agricultural Workers in Less Developed Countries. Toxicol. Rev. 2005, 24, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Araoud, M.; Neffeti, F.; Douki, W.; Hfaiedh, H.B.; Akrout, M.; Hassine, M.; Najjar, M.F.; Kenani, A. Adverse effects of pesticides on biochemical and haematological parameters in Tunisian agricultural workers. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Zikankuba, V.L.; Mwanyika, G.; Ntwenya, J.E.; James, A. Pesticide regulations and their malpractice implications on food and environment safety. Cogent Food Agric. 2019, 5, 1601544. [Google Scholar] [CrossRef]
- Kaur, R.; Mavi, G.K.; Raghav, S.; Khan, I. Pesticides Classification and its Impact on Environment. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1889–1897. [Google Scholar] [CrossRef]
- Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:309:0001:0050:en:PDF (accessed on 15 October 2022).
- Kaltenhäuser, J.; Kneuer, C.; Marx-Stoelting, P.; Niemann, L.; Schubert, J.; Stein, B.; Solecki, R. Relevance and reliability of experimental data in human health risk assessment of pesticides. Regul. Toxicol. Pharmacol. 2017, 88, 227–237. [Google Scholar] [CrossRef]
- Cabrera, L.C.; Pastor, P.M. The 2019 European Union report on pesticide residues in food. EFSA J. 2021, 19, e06491. [Google Scholar] [CrossRef]
- Hall, R.J.; Henry, P.F.P. Assessing effects of pesticides on amphibians and reptiles. Herpetol. J. 1992, 2, 65–71. [Google Scholar]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 2012, 491, 105–108. [Google Scholar] [CrossRef]
- Liess, M.; von der Ohe, P.C. Analyzing effects of pesticides on invertebrate communities in streams. Environ. Toxicol. Chem. 2005, 24, 954–965. [Google Scholar] [CrossRef]
- Carbonell, E.; Valbuena, A.; Xamena, N.; Creus, A.; Marcos, R. Temporary variations in chromosomal aberrations in a group of agricultural workers exposed to pesticides. Mutat. Res./Genet. Toxicol. 1995, 344, 127–134. [Google Scholar] [CrossRef]
- Jung, D.-W.; Jeong, D.-H.; Lee, H.-S. Endocrine disrupting potential of selected azole and organophosphorus pesticide products through suppressing the dimerization of human androgen receptor in genomic pathway. Ecotoxicol. Environ. Saf. 2022, 247, 114246. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.Q.; Zhang, Y.C.; Huang, Q.T.; Jones, A.K.; Han, Z.J.; Zhao, C.Q. Acute toxicity, bioconcentration, elimination, action mode and detoxification metabolism of broflanilide in zebrafish, Danio rerio. J. Hazard. Mater. 2020, 394, 122521. [Google Scholar] [CrossRef] [PubMed]
- Kudsk, P.; Mathiassen, S.K. Pesticide regulation in the European Union and the glyphosate controversy. Weed Sci. 2020, 68, 214–222. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A.B.; Singh, N.; Singh, J. Toxicity, degradation and analysis of the herbicide atrazine. Environ. Chem. Lett. 2017, 16, 211–237. [Google Scholar] [CrossRef]
- Sass, J.B.; Colangelo, A. European Union bans atrazine, while the United States negotiates continued use. Int. J. Occup. Environ. Health 2006, 12, 260–267. [Google Scholar] [CrossRef]
- Agathokleous, E. European Union’s imminent ban on glyphosate: Hormesis should be considered in new chemical screening and selection. J. For. Res. 2022, 33, 1103–1107. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-light photocatalysts and their perspectives for building photocatalytic membrane reactors for various liquid phase chemical conversions. Catalysts 2020, 10, 1334. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a photocatalyst for water splitting—An experimental and theoretical review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef]
- Humayun, M.; Wang, C.; Luo, W. Recent Progress in the Synthesis and Applications of Composite Photocatalysts: A Critical Review. Small Methods 2022, 6, 2101395. [Google Scholar] [CrossRef]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Barzagan, A. Photocatalytic Water and Wastewater Treatment; IWA Publishing: London, UK, 2022. [Google Scholar] [CrossRef]
- Bano, K.; Kaushal, S.; Singh, P.P. A review on photocatalytic degradation of hazardous pesticides using heterojunctions. Polyhedron 2021, 209, 115465. [Google Scholar] [CrossRef]
- Chengli, Z.; Ronghua, M.; Qi, W.; Mingrui, Y.; Rui, C.; Xiaonan, Z. Photocatalytic degradation of organic pollutants in wastewater by heteropolyacids: A review. J. Coord. Chem. 2021, 74, 1751–1764. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, H. Solar driven photocatalysis-an efficient method for removal of pesticides from water and wastewater. Biointerface Res. Appl. Chem. 2021, 11, 9071–9084. [Google Scholar] [CrossRef]
- Sudha, D.; Sivakumar, P. Review on the photocatalytic activity of various composite catalysts. Chem. Eng. Process. Process Intensif. 2015, 97, 112–133. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2-based Z-scheme photocatalysts. Cuihua Xuebao/Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Nano Energy Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Montero-Muñoz, M.; Ramos-Ibarra, J.E.; Rodríguez-Páez, J.E.; Teodoro, M.D.; Marques, G.E.; Sanabria, A.R.; Cajas, P.C.; Páez, C.A.; Heinrichs, B.; Coaquira, J.A.H. Role of defects on the enhancement of the photocatalytic response of ZnO nanostructures. Appl. Surf. Sci. 2018, 448, 646–654. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Lu, J.; Li, Y.; Bao, M. Journal of Colloid and Interface Science Enhanced photocatalytic activity of glyphosate over a combination strategy of GQDs/TNAs heterojunction composites. J. Colloid Interface Sci. 2022, 607, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Soric, A.; Boutin, O. Treatment technologies and degradation pathways of glyphosate: A critical review. Sci. Total Environ. 2020, 742, 140559. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhou, H.; Pan, Z.; Guo, Y.; Yuan, Y.; Yao, G.; Lai, B. Degradation of atrazine by Bi2MoO6 activated peroxymonosulfate under visible light irradiation. J. Hazard. Mater. 2020, 400, 123187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, B.; Chen, H.; Yuan, R. Heterogeneous photocatalytic oxidation for the removal of organophosphorus pollutants from aqueous solutions: A review. Sci. Total Environ. 2023, 856, 159048. [Google Scholar] [CrossRef]
- Miguel, N.; Ormad, M.P.; Mosteo, R.; Ovelleiro, J.L. Photocatalytic degradation of pesticides in natural water: Effect of hydrogen peroxide. Int. J. Photoenergy 2012, 2012, 371714. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Al-Hamdi, A.M.; Rinner, U.; Sillanpää, M. Tin dioxide as a photocatalyst for water treatment: A review. Process Saf. Environ. Prot. 2017, 107, 190–205. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Gebreslassie, T.W.; Pattabi, M.; Pattabi, R.M. Review on the Photocatalytic Degradation of Dyes and Antibacterial Activities of Pure and Doped-ZnO. Int. J. Sci. Res. 2013, 4, 2252–2264. Available online: www.rsisinternational.org%0Awww.ijsr.net (accessed on 28 September 2022).
- Kusiak-Nejman, E.; Morawski, A.W. TiO2/graphene-based nanocomposites for water treatment: A brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl. Catal. B Environ. 2019, 253, 179–186. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Jiang, D.; Otitoju, T.A.; Ouyang, Y.; Shoparwe, N.F.; Wang, S.; Zhang, A.; Li, S. A review on metal ions modified TiO2 for photocatalytic degradation of organic pollutants. Catalysts 2021, 11, 1039. [Google Scholar] [CrossRef]
- Antoniou, M.G.; Zhao, C.; O’Shea, K.E.; Zhang, G.; Dionysiou, D.D.; Zhao, C.; Han, C.; Nadagouda, M.N.; Choi, H.; Fotiou, T.; et al. Photocatalytic degradation of organic contaminants in water: Process optimization and degradation pathways. In Photocatalysis: Applications; The Royal Society of Chemistry: London, UK, 2016. [Google Scholar] [CrossRef]
- El-Saeid, M.H.; Baqais, A.; Alshabanat, M. Study of the Photocatalytic Degradation of Highly Abundant Pesticides in Agricultural Soils. Molecules 2022, 27, 634. [Google Scholar] [CrossRef]
- Djurišić, A.B.; He, Y.; Ng, A.M.C. Visible-light photocatalysts: Prospects and challenges. APL Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; Scirè, S.; Palmisano, L. Effect of the addition of different doping agents on visible light activity of porous TiO2 photocatalysts. Mol. Catal. 2018, 455, 108–120. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Kodom, T.; Rusen, E.; Cəlinescu, I.; Mocanu, A.; Şomoghi, R.; Dinescu, A.; Diacon, A.; Boscornea, C. Silver Nanoparticles Influence on Photocatalytic Activity of Hybrid Materials Based on TiO2 P25. J. Nanomater. 2015, 2015, 210734. [Google Scholar] [CrossRef]
- Diacon, A.; Mocanu, A.; Răducanu, C.E.; Busuioc, C.; Șomoghi, R.; Trică, B.; Dinescu, A.; Rusen, E. New carbon/ZnO/Li2O nanocomposites with enhanced photocatalytic activity. Sci. Rep. 2019, 9, 16840. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef]
- Farhadian, N.; Akbarzadeh, R.; Pirsaheb, M.; Jen, T.C.; Fakhri, Y.; Asadi, A. Chitosan modified N, S-doped TiO2 and N, S-doped ZnO for visible light photocatalytic degradation of tetracycline. Int. J. Biol. Macromol. 2019, 132, 360–373. [Google Scholar] [CrossRef]
- Vaya, D.; Surolia, P.K. Environmental Technology & Innovation Semiconductor based photocatalytic degradation of pesticides: An overview. Environ. Technol. Innov. 2020, 20, 101128. [Google Scholar] [CrossRef]
- Huong, P.T.L.; van Quang, N.; Tran, M.-T.; Trung, D.Q.; Hop, D.T.B.; Tam, T.T.H.; van Dao, D. Excellent visible light photocatalytic degradation and mechanism insight of Co2+-doped ZnO nanoparticles. Appl. Phys. A 2022, 128, 24. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Vasiljevic, Z.Z.; Dojcinovic, M.P.; Vujancevic, J.D.; Jankovic-Castvan, I.; Ognjanovic, M.; Tadic, N.B.; Stojadinovic, S.; Brankovic, G.O.; Nikolic, M.V. Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol-gel method: Methylene blue degradation with Fe2TiO5. R. Soc. Open Sci. 2020, 7, 200708. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.; Fan, J.; Sun, D.; Tang, W.; Yang, X. Biotemplated preparation of CdS nanoparticles/bacterial cellulose hybrid nanofibers for photocatalysis application. J. Hazard. Mater. 2011, 189, 377–383. [Google Scholar] [CrossRef]
- Aritonang, H.F.; Kamea, O.E.; Koleangan, H.; Wuntu, A.D. Biotemplated synthesis of Ag-ZnO nanoparticles/bacterial cellulose nanocomposites for photocatalysis application. Polym. Plast. Technol. Mater. 2020, 59, 1292–1299. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Midoux, N.; Roizard, C.; Andre, J.C. Industrial photochemistry XV: Interests and limits of the buckingham theorem for the design of industrial photoreactors. J. Photochem. Photobiol. A Chem. 1989, 50, 83–102. [Google Scholar] [CrossRef]
- Constantino, D.S.M.; Dias, M.M.; Silva, A.M.T.; Faria, J.L.; Silva, C.G. Intensification strategies for improving the performance of photocatalytic processes: A review. J. Clean. Prod. 2022, 340, 130800. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Hossain, M.F. Water. In Sustainable Design and Build; Elsevier: Amsterdam, The Netherlands, 2019; pp. 301–418. [Google Scholar] [CrossRef]
- Abhang, R.M.; Kumar, D.; Taralkar, S.V. Design of Photocatalytic Reactor for Degradation of Phenol in Wastewater. Int. J. Chem. Eng. Appl. 2011, 2, 337–341. [Google Scholar] [CrossRef]
- Puma, G.L.; Machuca-Martínez, F.; Mueses, M.; Colina-Márquez, J.; Bustillo-Lecompte, C. Scale-Up and Optimization for Slurry Photoreactors. In Advanced Oxidation Processes; IntechOpen: London, UK, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Motegh, M.; Cen, J.; Appel, P.W.; van Ommen, J.R.; Kreutzer, M.T. Diffusion limitations in stagnant photocatalytic reactors. Chem. Eng. J. 2014, 247, 314–319. [Google Scholar] [CrossRef]
- McCullagh, C.; Skillen, N.; Adams, M.; Robertson, P.K.J. Photocatalytic reactors for environmental remediation: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1002–1017. [Google Scholar] [CrossRef]
- Malato, S.; Maldonado, M.I.; Fernández-Ibáñez, P.; Oller, I.; Polo, I.; Sánchez-Moreno, R. Decontamination and disinfection of water by solar photocatalysis: The pilot plants of the Plataforma Solar de Almeria. Mater. Sci. Semicond. Process. 2016, 42, 15–23. [Google Scholar] [CrossRef]
- Herrmann, J.M. Solar photocatalytic detoxification of used waters in ‘plataforma solar de almeria’ (Spain). Appl. Catal. B Environ. 1997, 12, N2–N4. [Google Scholar] [CrossRef]
- Minero, C.; Pelizzetti, E.; Malato, S.; Blanco, J. Large Solar Plant Photocatalytic Water Decontamination: Degradation of Pentachlorophenol. Chemosphere 1993, 26, 2103–2119. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Disdier, J.; Pichat, P.; Malato, S.; Blanco, J. TiO2-based solar photocatalytic detoxification of water containing organic pollutants. Case studies of 2,4-dichlorophenoxyaceticacid (2,4-D) and of benzofuran. Appl. Catal. B Environ. 1998, 17, 15–23. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Richter, C.; Milow, B.; Maldonado, M.I. Pre-industrial experience in solar photocatalytic mineralization of real wastewaters. Application to pesticide container recycling. Water Sci. Technol. 1999, 40, 123–130. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Guillard, C. Photocatalytic degradation of pesticides in agricultural used waters. Comptes Rendus L’academie Des Sci. Ser. IIc Chem. 2000, 3, 417–422. [Google Scholar] [CrossRef]
- Pérez, M.H.; Peñuela, G.; Maldonado, M.I.; Malato, O.; Fernández-Ibáñez, P.; Oller, I.; Gernjak, W.; Malato, S. Degradation of pesticides in water using solar advanced oxidation processes. Appl. Catal. B Environ. 2006, 64, 272–281. [Google Scholar] [CrossRef]
- Luna-Sanguino, G.; Ruíz-Delgado, A.; Duran-Valle, C.J.; Malato, S.; Faraldos, M.; Bahamonde, A. Impact of water matrix and oxidant agent on the solar assisted photodegradation of a complex mix of pesticides over titania-reduced graphene oxide nanocomposites. Catal. Today 2021, 380, 114–124. [Google Scholar] [CrossRef]
- Luna-Sanguino, G.; Ruíz-Delgado, A.; Tolosana-Moranchel, A.; Pascual, L.; Malato, S.; Bahamonde, A.; Faraldos, M. Solar photocatalytic degradation of pesticides over TiO2-rGO nanocomposites at pilot plant scale. Sci. Total Environ. 2020, 737, 140286. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Tang, F.H.M.; la Cecilia, D.; McBratney, A. PEST-CHEMGRIDS, global gridded maps of the top 20 crop-specific pesticide application rates from 2015 to 2025. Sci. Data. 2019, 6, 170. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, C.; Nadagouda, M.N.; Dionysiou, D.D. The fabrication of innovative single crystal N,F-codoped titanium dioxide nanowires with enhanced photocatalytic activity for degradation of atrazine. Appl. Catal. B Environ. 2015, 168–169, 550–558. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Han, C.; Sannino, D.; Dionysiou, D.D. Photocatalytic removal of atrazine using N-doped TiO2 supported on phosphors. Appl. Catal. B Environ. 2015, 164, 462–474. [Google Scholar] [CrossRef]
- Santacruz-chávez, J.A.; Oros-ruiz, S.; Prado, B.; Zanella, R. Journal of Environmental Chemical Engineering Photocatalytic degradation of atrazine using TiO2 superficially modified with metallic nanoparticles. J. Environ. Chem. Eng. 2015, 3, 3055–3061. [Google Scholar] [CrossRef]
- Shamsedini, N.; Dehghani, M.; Nasseri, S.; Baghapour, M.A. Photocatalytic degradation of atrazine herbicide with Illuminated Fe+3-TiO2 Nanoparticles. J. Environ. Heal. Sci. Eng. 2017, 15, 7. [Google Scholar] [CrossRef]
- Wang, W.K.; Chen, J.J.; Gao, M.; Huang, Y.X.; Zhang, X.; Yu, H.Q. Photocatalytic degradation of atrazine by boron-doped TiO2 with a tunable rutile/anatase ratio. Appl. Catal. B Environ. 2016, 195, 69–76. [Google Scholar] [CrossRef]
- Youssef, L.; Younes, G.; Al-Oweini, R. Photocatalytic degradation of atrazine by heteropolyoxotungstates. J. Taibah Univ. Sci. 2019, 13, 274–279. [Google Scholar] [CrossRef]
- Long, T.; Xu, Y.; Lv, X.; Ran, J.; Yang, S.; Xu, L. Fabrication of the annular photocatalytic reactor using large-sized freestanding titania-silica monolithic aerogel as the catalyst for degradation of glyphosate. Mater. Des. 2018, 159, 195–200. [Google Scholar] [CrossRef]
- Russo, M.; Iervolino, G.; Vaiano, V. W-doped zno photocatalyst for the degradation of glyphosate in aqueous solution. Catalysts 2021, 11, 234. [Google Scholar] [CrossRef]
- Suárez-Escobar, A.F.; Guevara-Correa, D.; Méndez-Quintero, M.; Mendoza-Abella, J.F.; Álvarez-Cabrera, J.A. Evaluation of a reactor for the photocatalytic degradation of glyphosate with a catalyst TiO2-Mn. Rev. Colomb. Quim. 2019, 48, 19–25. [Google Scholar] [CrossRef]
- Chen, J.Q.; Hu, Z.J.; Wang, N.X. Photocatalytic mineralization of glyphosate in a small-scale plug flow simulation reactor by UV/TiO2. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2012, 47, 579–588. [Google Scholar] [CrossRef]
- Umar, K.; Aris, A.; Ahmad, H.; Parveen, T.; Jaafar, J.; Majid, Z.A.; Reddy, A.V.B.; Talib, J. Synthesis of visible light active doped TiO2 for the degradation of organic pollutants—Methylene blue and glyphosate. J. Anal. Sci. Technol. 2016, 7, 29. [Google Scholar] [CrossRef]
- Thakur, P.R.; Sharma, S.; Kumar, A.; Sharma, G.; Ghfar, A.A.; Naushad, M.; Stadler, F.J. Fabrication of a Z-scheme Zn3V2O8/g-C3N4 nano-heterojunction with high interfacial charge transfer for superior photocatalytic removal of diazinon pesticide under visible light. Appl. Nanosci. 2022. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, G.; Xu, X.; Yang, X.; Liu, C.; Xu, Y. Preparation of titania nanotubes doped with cerium and their photocatalytic activity for glyphosate. Chem. Eng. J. 2011, 167, 397–402. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, Q.; Yan, W.; Jing, C.; Zhang, Y. Comparative study of glyphosate removal on goethite and magnetite: Adsorption and photo-degradation. Chem. Eng. J. 2018, 352, 581–589. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Huo, R.; Ou, L.Y.; Luo, X.L.; Lv, Y.R.; Xu, Y.H. One-pot synthesis of peony-like Bi2S3/BiVO4(040) with high photocatalytic activity for glyphosate degradation under visible light irradiation. Chin. J. Catal. 2019, 40, 580–589. [Google Scholar] [CrossRef]
- Wu, Z.; He, X.; Xue, Y.; Yang, X.; Li, Y.; Li, Q.; Yu, B. Cyclodextrins grafted MoS2/g-C3N4 as high-performance photocatalysts for the removal of glyphosate and Cr (VI) from simulated agricultural runoff. Chem. Eng. J. 2020, 399, 125747. [Google Scholar] [CrossRef]
- De los Ballari, M.M.; Brandi, R.; Alfano, O.; Cassano, A. Mass transfer limitations in photocatalytic reactors employing titanium dioxide suspensions. I. Concentration profiles in the bulk. Chem. Eng. J. 2008, 136, 50–65. [Google Scholar] [CrossRef]
- De los Ballari, M.M.; Brandi, R.; Alfano, O.; Cassano, A. Mass transfer limitations in photocatalytic reactors employing titanium dioxide suspensions. II. External and internal particle constrains for the reaction. Chem. Eng. J. 2008, 136, 242–255. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Loddo, V.; Palmisano, L.; Parrino, F. Guidelines for the assessment of the rate law of slurry photocatalytic reactions. Catal. Today 2017, 281, 221–230. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Augugliaro, V.; Cardillo, A.G.; Loddo, V.; Palmisano, L.; Parrino, F.; Santarelli, F. A reaction engineering approach to kinetic analysis of photocatalytic reactions in slurry systems. Catal. Today 2016, 259, 87–96. [Google Scholar] [CrossRef]
- Visan, A.; van Ommen, J.R.; Kreutzer, M.T.; Lammertink, R.G.H. Photocatalytic Reactor Design: Guidelines for Kinetic Investigation. Ind. Eng. Chem. Res. 2019, 58, 5349–5357. [Google Scholar] [CrossRef]
- Fan, G.; Ning, R.; Li, X.; Lin, X.; Du, B.; Luo, J.; Zhang, X. Mussel-Inspired Immobilization of Photocatalysts with Synergistic Photocatalytic-Photothermal Performance for Water Remediation. ACS Appl. Mater. Interfaces 2021, 13, 31066–31076. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Alalm, M.G.; Fujii, M.; Ookawara, S.; Ohno, T. Photocatalytic degradation of trimethoprim using S-TiO2 and Ru/WO3/ZrO2 immobilized on reusable fixed plates. J. Water Process Eng. 2020, 33, 101023. [Google Scholar] [CrossRef]
- Alalm, M.G.; Samy, M.; Ookawara, S.; Ohno, T. Immobilization of S-TiO2 on reusable aluminum plates by polysiloxane for photocatalytic degradation of 2,4-dichlorophenol in water. J. Water Process Eng. 2018, 26, 329–335. [Google Scholar] [CrossRef]
- Sivagami, K.; Krishna, R.R.; Swaminathan, T. Photo catalytic degradation of pesticides in immobilized bead photo reactor under solar irradiation. Sol. Energy 2014, 103, 488–493. [Google Scholar] [CrossRef]
- Shorgoli, A.A.; Shokri, M. Photocatalytic degradation of imidacloprid pesticide in aqueous solution by TiO2 nanoparticles immobilized on the glass plate. Chem. Eng. Commun. 2017, 204, 1061–1069. [Google Scholar] [CrossRef]
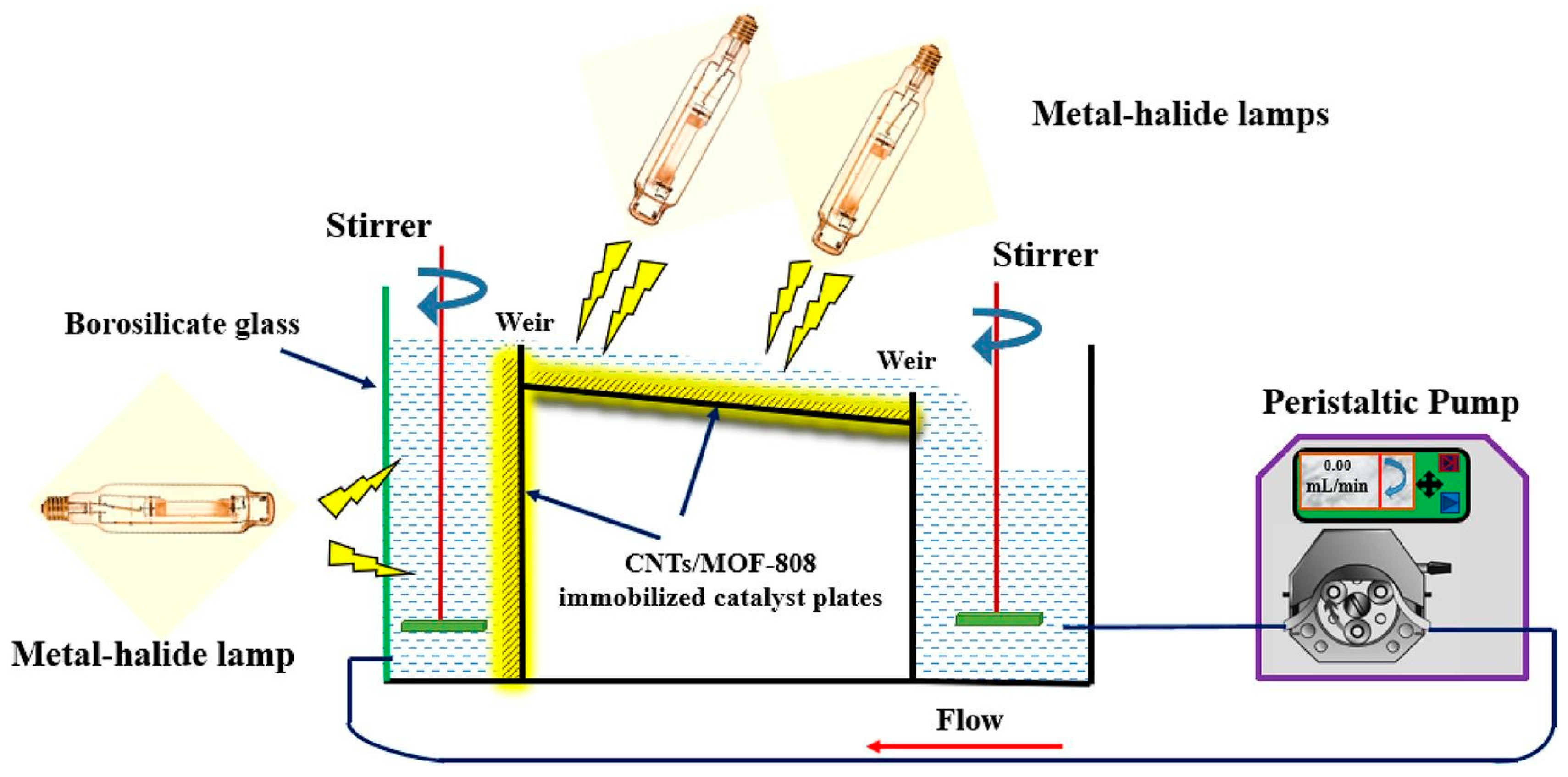
- Samy, M.; Ibrahim, M.G.; Fujii, M.; Diab, K.E.; ElKady, M.; Alalm, M.G. CNTs/MOF-808 painted plates for extended treatment of pharmaceutical and agrochemical wastewaters in a novel photocatalytic reactor. Chem. Eng. J. 2021, 406, 127152. [Google Scholar] [CrossRef]
- Jiménez-Tototzintle, M.; Oller, I.; Hernández-Ramírez, A.; Malato, S.; Maldonado, M.I. Remediation of agro-food industry effluents by biotreatment combined with supported TiO2/H2O2 solar photocatalysis. Chem. Eng. J. 2015, 273, 205–213. [Google Scholar] [CrossRef]
- Chakachaka, V.; Tshangana, C.; Mahlangu, O.; Mamba, B.; Muleja, A. Interdependence of Kinetics and Fluid Dynamics in the Design of Photocatalytic Membrane Reactors. Membranes 2022, 12, 745. [Google Scholar] [CrossRef]
- Sraw, A.; Kaur, T.; Thakur, I.; Verma, A.; Wanchoo, R.K.; Toor, A.P. Photocatalytic degradation of pesticide monocrotophos in water using W-TiO2 in slurry and fixed bed recirculating reactor. J. Mol. Struct. 2022, 1265, 133392. [Google Scholar] [CrossRef]
- Sraw, A.; Wanchoo, R.K.; Toor, A.P. Optimization and kinetic studies for degradation of insecticide monocrotophos using LR grade and P25 TiO2 under UV/Sunlight conditions. Environ. Prog. Sustain. Energy 2014, 33, 1201–1208. [Google Scholar] [CrossRef]
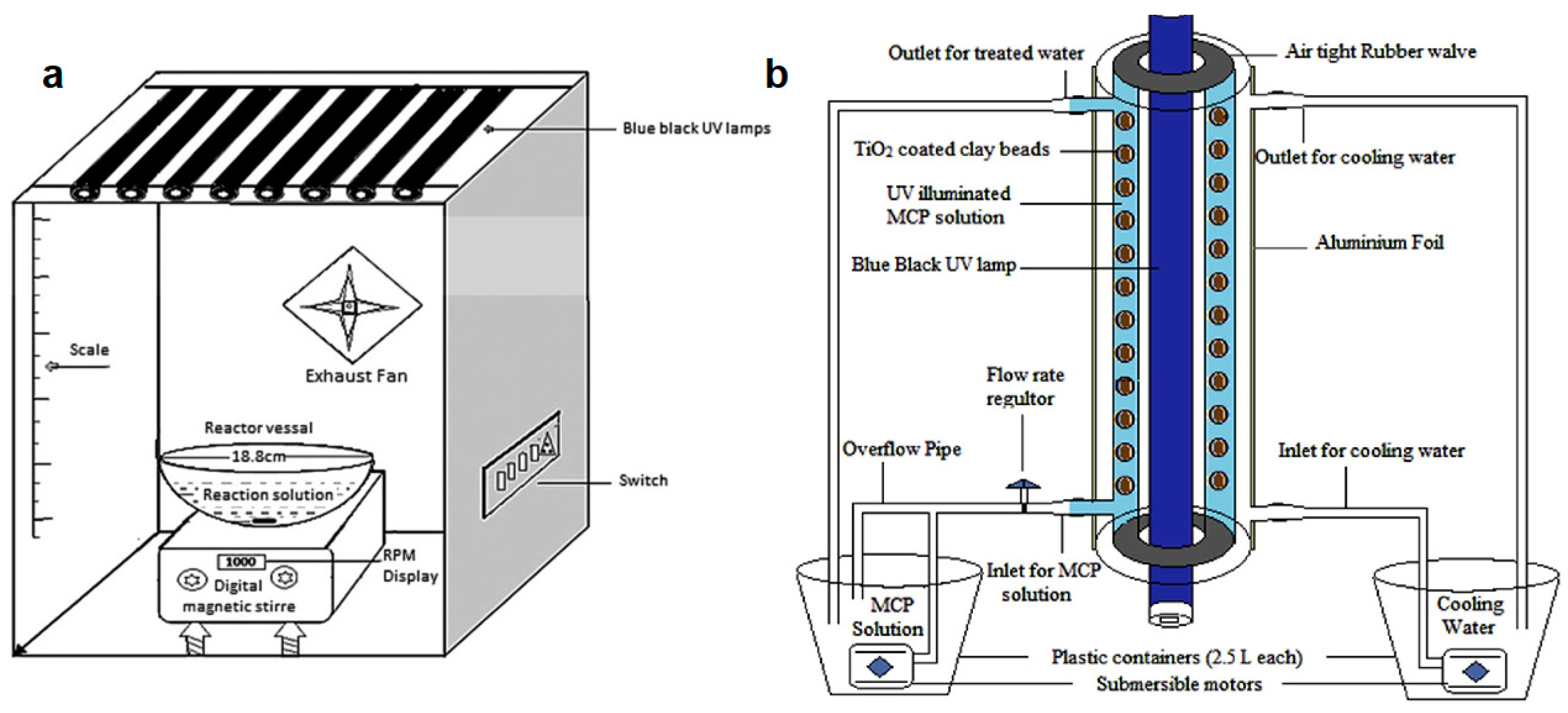
- Sraw, A.; Kaur, T.; Pandey, Y.; Sobti, A.; Wanchoo, R.K.; Toor, A.P. Fixed bed recirculation type photocatalytic reactor with TiO2 immobilized clay beads for the degradation of pesticide polluted water. J. Environ. Chem. Eng. 2018, 6, 7035–7043. [Google Scholar] [CrossRef]
- Vela, N.; Fenoll, J.; Garrido, I.; Navarro, G.; Gambín, M.; Navarro, S. Photocatalytic mitigation of triazinone herbicide residues using titanium dioxide in slurry photoreactor. Catal. Today 2015, 252, 70–77. [Google Scholar] [CrossRef]
- Kushniarou, A.; Garrido, I.; Fenoll, J.; Vela, N.; Flores, P.; Navarro, G.; Hellín, P.; Navarro, S. Solar photocatalytic reclamation of agro-waste water polluted with twelve pesticides for agricultural reuse. Chemosphere 2019, 214, 839–845. [Google Scholar] [CrossRef]
- Vela, N.; Fenoll, J.; Garrido, I.; Pérez-Lucas, G.; Flores, P.; Hellín, P.; Navarro, S. Reclamation of agro-wastewater polluted with pesticide residues using sunlight activated persulfate for agricultural reuse. Sci. Total Environ. 2019, 660, 923–930. [Google Scholar] [CrossRef]
- Stephan, B.; Ludovic, L.; Dominique, W. Modelling of a falling thin film deposited photocatalytic step reactor for water purification: Pesticide treatment. Chem. Eng. J. 2011, 169, 216–225. [Google Scholar] [CrossRef]
- Lou, W.; Kane, A.; Wolbert, D.; Rtimi, S.; Assadi, A.A. Study of a photocatalytic process for removal of antibiotics from wastewater in a falling film photoreactor: Scavenger study and process intensification feasibility. Chem. Eng. Process. Process Intensif. 2017, 122, 213–221. [Google Scholar] [CrossRef]
- Lhomme, L.; Brosillon, S.; Wolbert, D. Photocatalytic degradation of pesticides in pure water and a commercial agricultural solution on TiO2 coated media. Chemosphere 2008, 70, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Zeshan, M.; Bhatti, I.A.; Mohsin, M.; Iqbal, M.; Amjed, N.; Nisar, J.; AlMasoud, N.; Alomar, T.S. Remediation of pesticides using TiO2 based photocatalytic strategies: A review. Chemosphere 2022, 300, 134525. [Google Scholar] [CrossRef] [PubMed]



| Pesticide | Reactor Type | Catalyst | Light Source | Degradation | Ref. |
|---|---|---|---|---|---|
| Atrazine | Suspension, magnetically stirred | N,F-codoped TiO2 NWs | λ 365, 2.5 mW cm−2, 15 W visible light irradiation, 15 W fluorescent lamps UV light irradiation, two 15 W UV light lamps (365 nm wavelength) | Not reported | [93] |
| Suspension | N-TiO2/ZSP | UVA-365 nm radiation was simulated by two 15 W lamps | [94] | ||
| Suspension, air bubbled | Au/TiO2, Cu/TiO2 and Ni/TiO2 | UV–vis UV–PC lamp with primary emission at 254 nm | 60% | [95] | |
| Suspension, mechanically stirred | Fe+3-TiO2 | UV lamp protected by a Quartz tube | 99% | [96] | |
| Suspension | B-doped TiO2 (A/R) | 350 W (15 A) Xenon lamp with a 300 nm cutoff filter | 94% | [97] | |
| Suspension | [α-SiW12O40]4− [α-PW12O40]4− [P8W48O184]40− | Two 8 W UV-Xenon lamps, 254 and 366 nm | 56% 31% 41% | [98] | |
| Glyphosate | Vertical annular photocatalytic reactor, air bubbled | TiO2− SiO2 monolithic aerogel | 16 W UV lamp (254 nm) | >99% | [99] |
| Cylindrical batch reactor, suspension mixed by a peristaltic pump | W-Doped ZnO | Solar simulated lamps, 300–700 nm | 74% | [100] | |
| Continuous packed bed reactor | TiO2 Degussa P25 TiO2-Mn | UV lamp, 70 W, 370–410 nm | 28% 39% | [101] | |
| Plug flow reactor, suspension, magnetic stirrer | TiO2 | 500 W high-pressure mercury lamp with mean wavelength 365 nm | 90% | [102] | |
| Suspension, magnetic stirring | Mn-doped-TiO2 | Visible-light halogen linear lamp (500 W, 9500 Lumens) | 80% | [103] | |
| Cylindrical, suspension, mechanically stirred | Zn3V2O8/40 wt% g-C3N4 | 300 W Xe lamp with a 400 nm cut-off filter Visible light intensity 180 mW cm−2 | 85% | [104] | |
| Suspension, stirred and bubbled with oxygen | Ce–TiO2 nanotubes | 125 W high-pressure mercury lamp | 76% | [105] | |
| Suspension, stirred | Goethite magnetite | Mercury UV lamp (CEL-M500/350, incident light intensity 500–2000 W/m2, equipped with an optical filter for 275 nm) or a xenon Vis lamp (CEL-S500/350, incident light intensity 500–2000 W/m2, wavelength 350–1100 nm) | 41% 71% | [106] | |
| Suspension, ultrasonic stirring | Bi2S3/BiVO4(040) | Visible light irradiation (λ > 400 nm), using a 125-W high-pressure mercury lamp with 180 mL of 2 mol/L NaNO2 solution as the filter liquor | 79% | [107] | |
| Suspension, magnetic stirring | CDs/MoS2/g-C3N4 | Simulated sunlight irradiation with AM 1.5 cut-off filters and the light intensity 1000 mw | 79% | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isopencu, G.O.; Mocanu, A.; Deleanu, I.-M. A Brief Review of Photocatalytic Reactors Used for Persistent Pesticides Degradation. ChemEngineering 2022, 6, 89. https://doi.org/10.3390/chemengineering6060089
Isopencu GO, Mocanu A, Deleanu I-M. A Brief Review of Photocatalytic Reactors Used for Persistent Pesticides Degradation. ChemEngineering. 2022; 6(6):89. https://doi.org/10.3390/chemengineering6060089
Chicago/Turabian StyleIsopencu, Gabriela Olimpia, Alexandra Mocanu, and Iuliana-Mihaela Deleanu. 2022. "A Brief Review of Photocatalytic Reactors Used for Persistent Pesticides Degradation" ChemEngineering 6, no. 6: 89. https://doi.org/10.3390/chemengineering6060089
APA StyleIsopencu, G. O., Mocanu, A., & Deleanu, I.-M. (2022). A Brief Review of Photocatalytic Reactors Used for Persistent Pesticides Degradation. ChemEngineering, 6(6), 89. https://doi.org/10.3390/chemengineering6060089