Heterogeneous Photodegradation Reaction of Phenol Promoted by TiO2: A Kinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
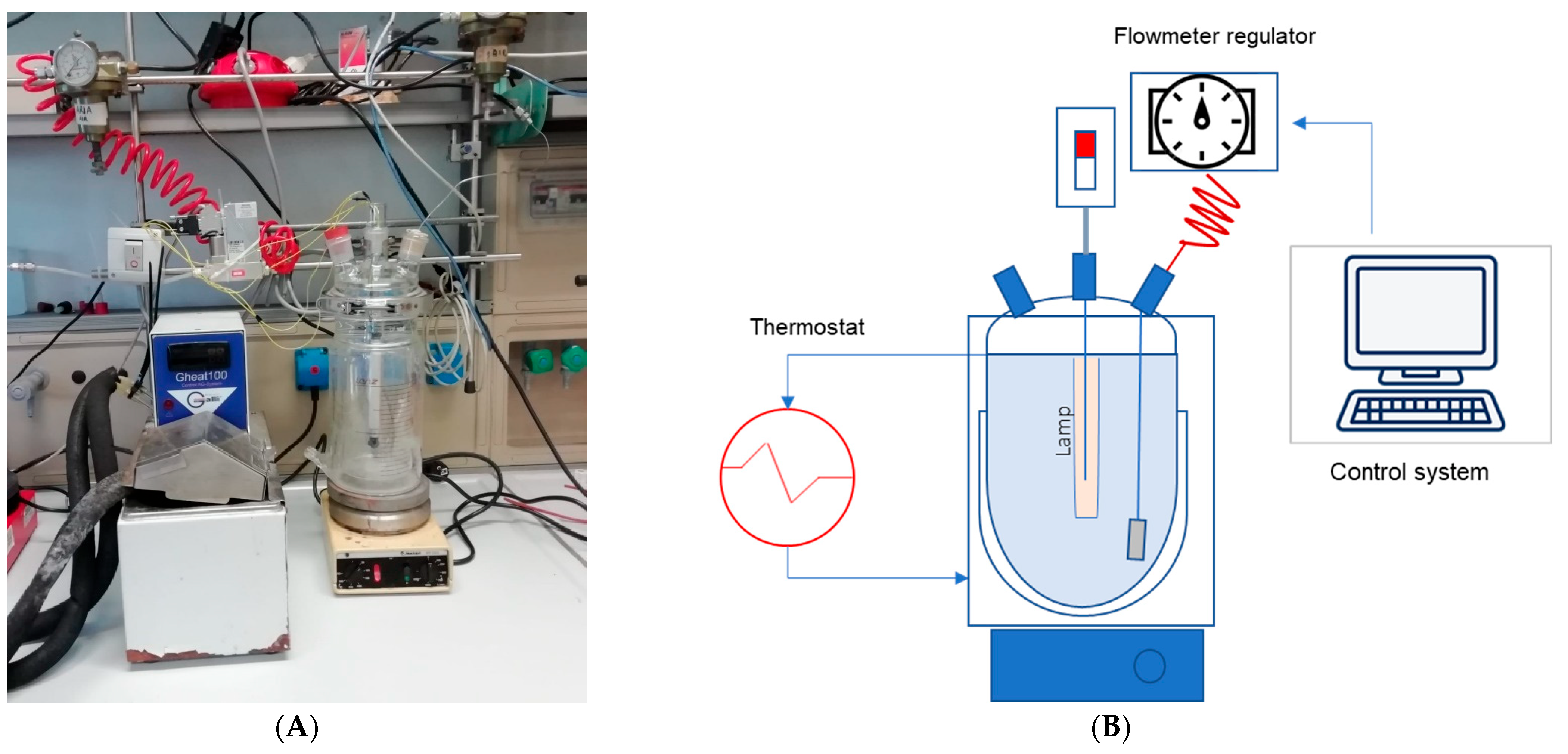
2.2.1. Experimental Procedures
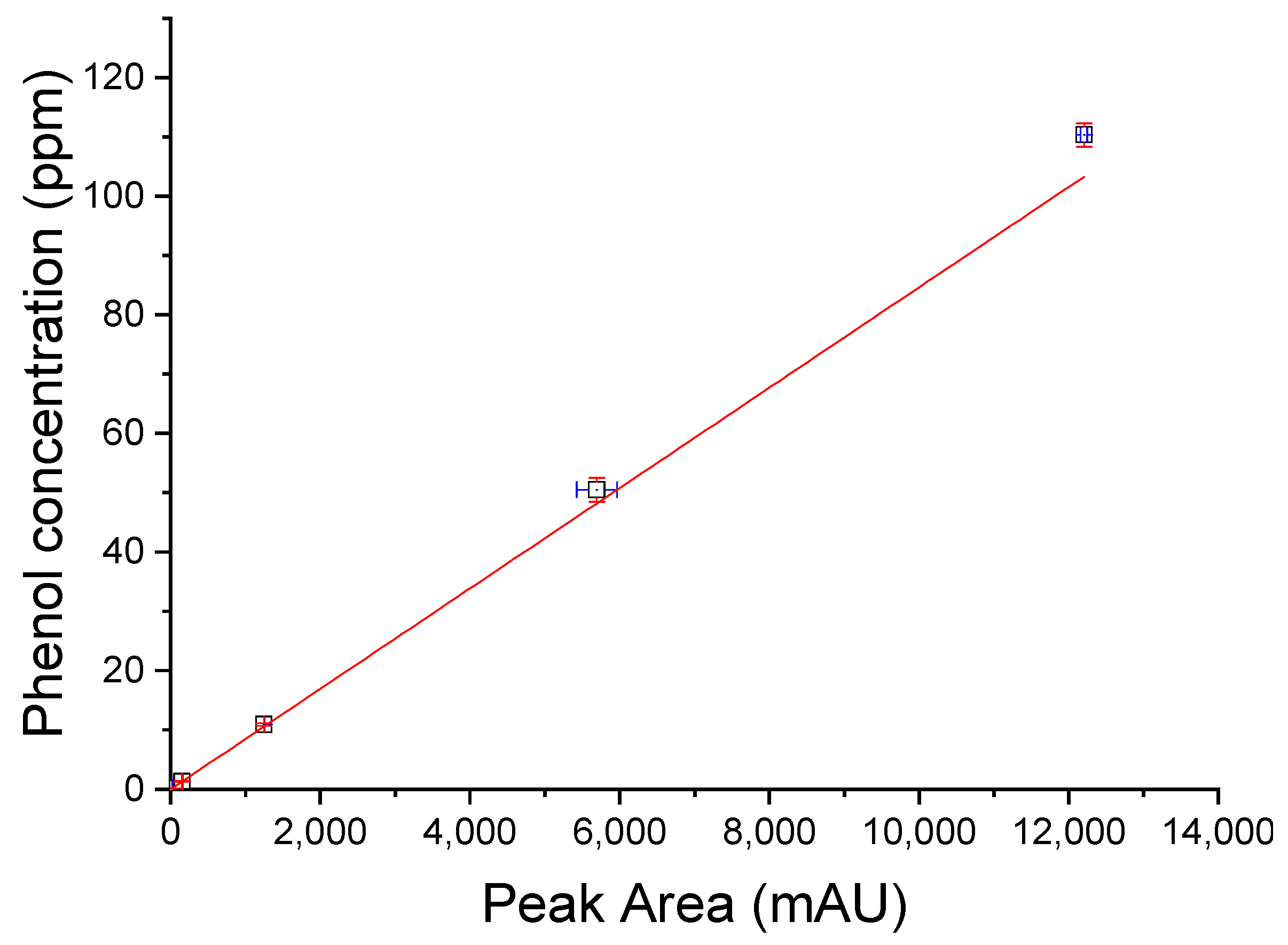
2.2.2. Analytical Method
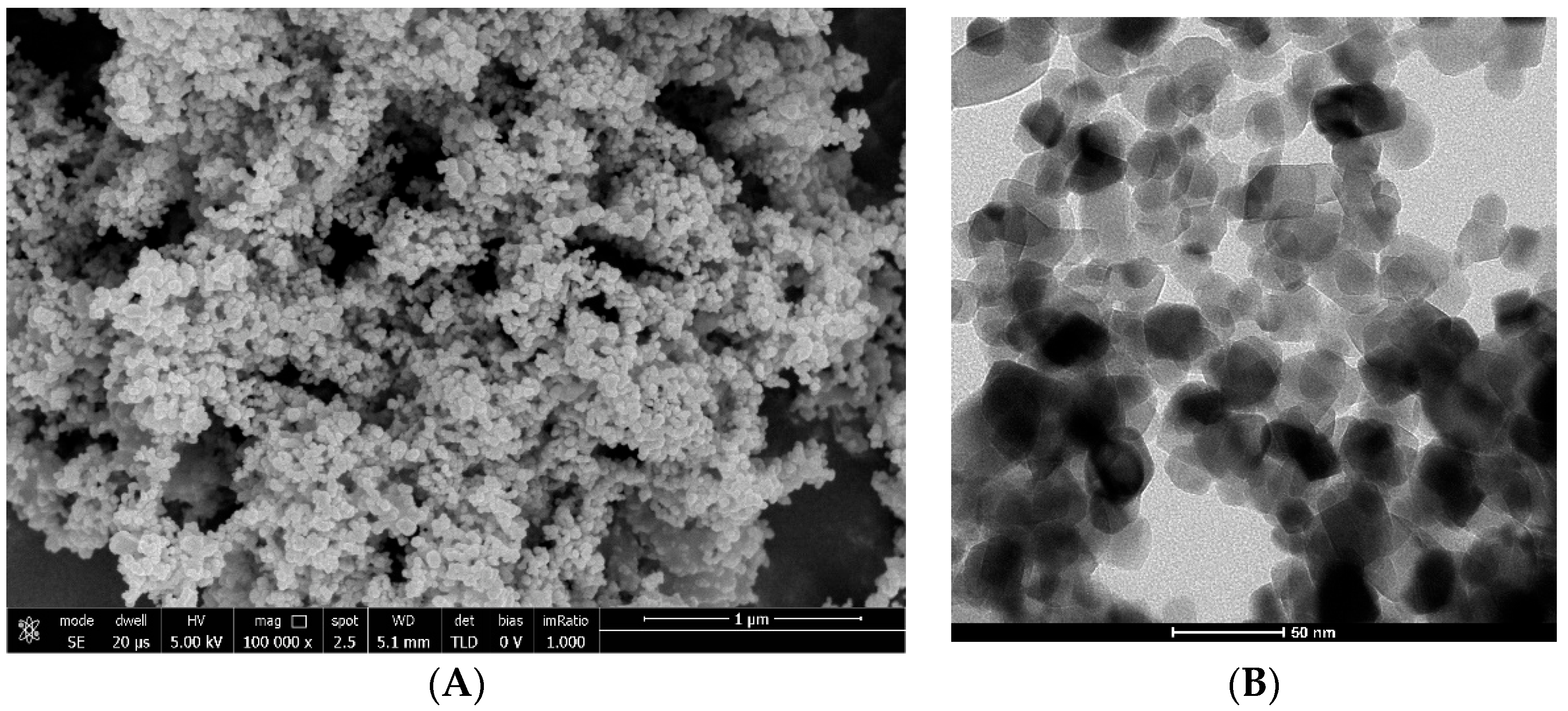
2.2.3. Characterization of the Photocatalyst
3. Results and Discussion
3.1. Aeroxide P-25 Characterization
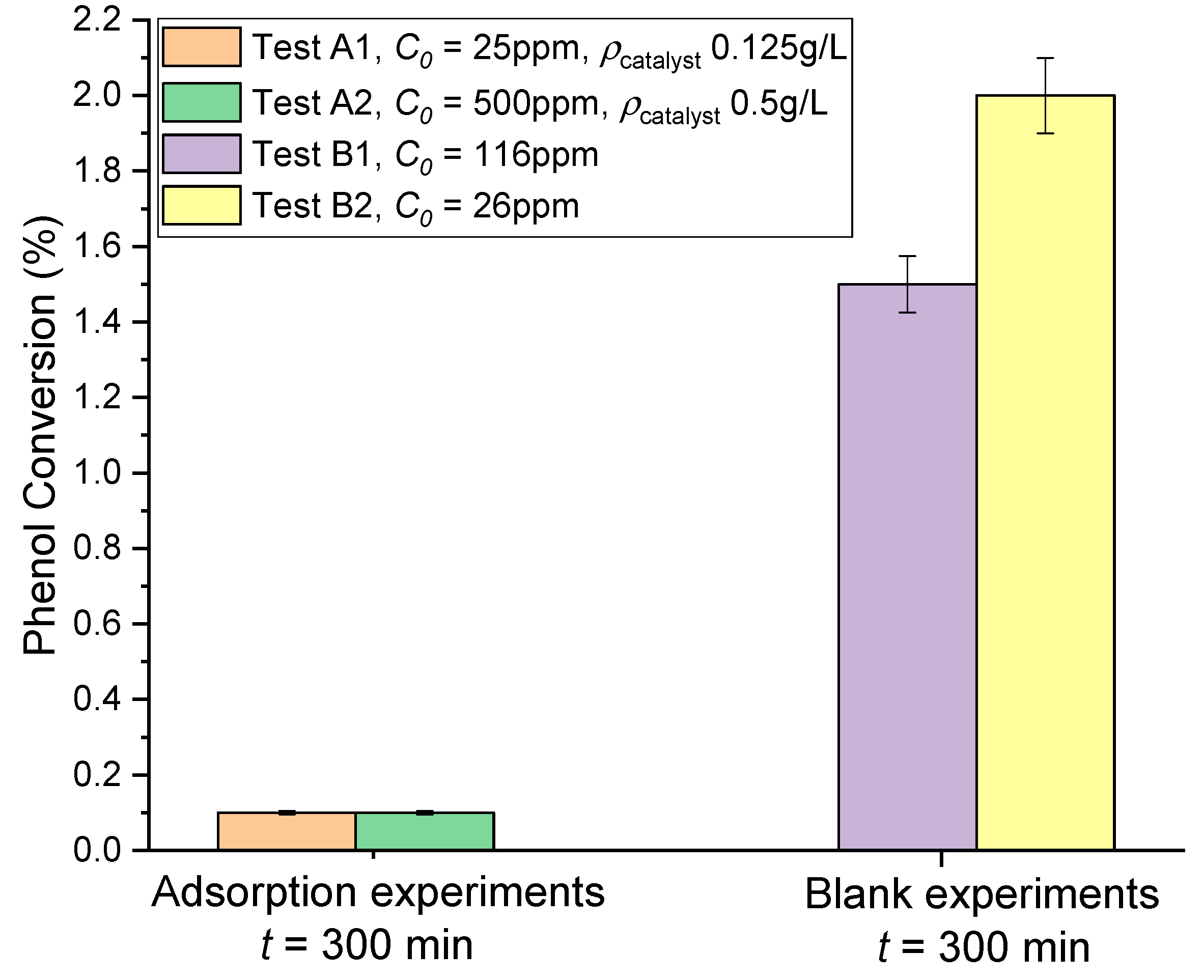
3.2. Adsorption and Blank Tests
3.3. Photodegradation Tests
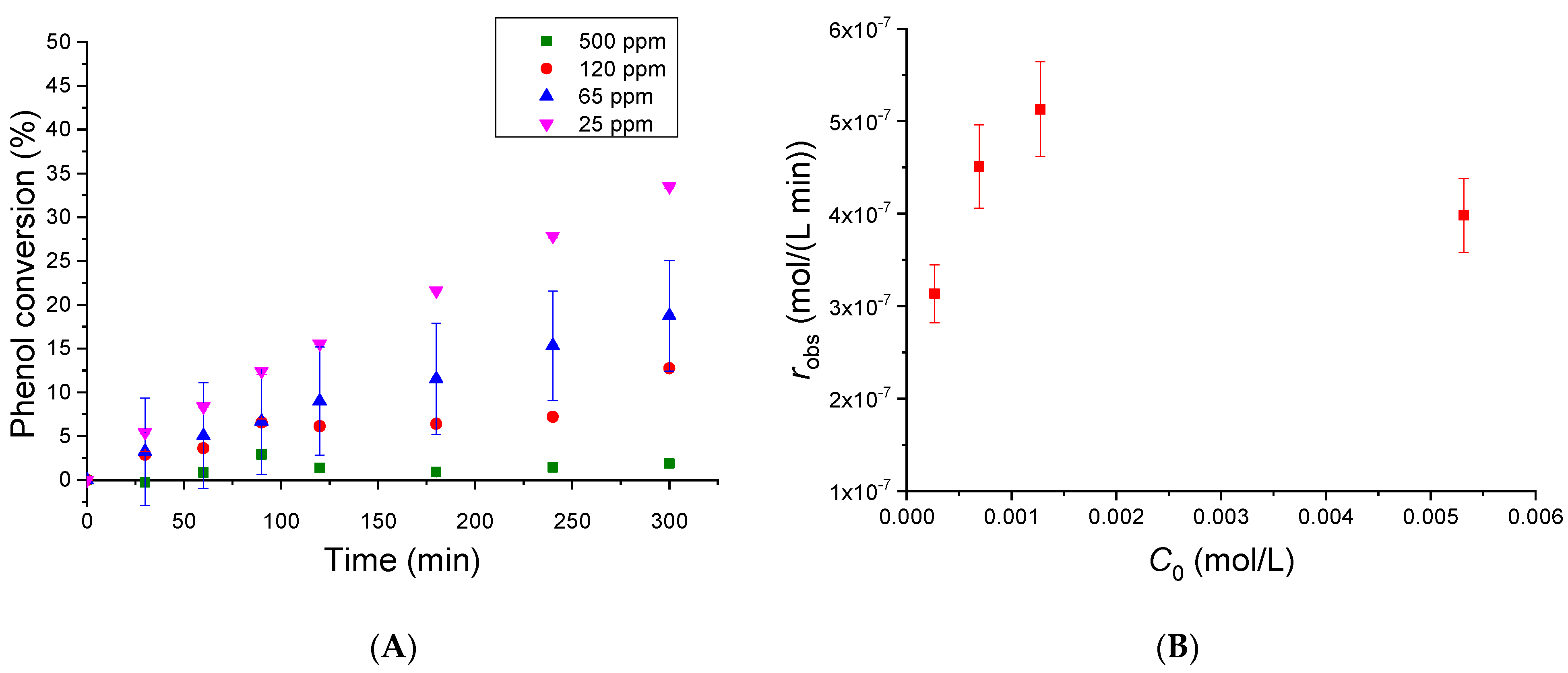
3.3.1. Initial Phenol Concentration Effect
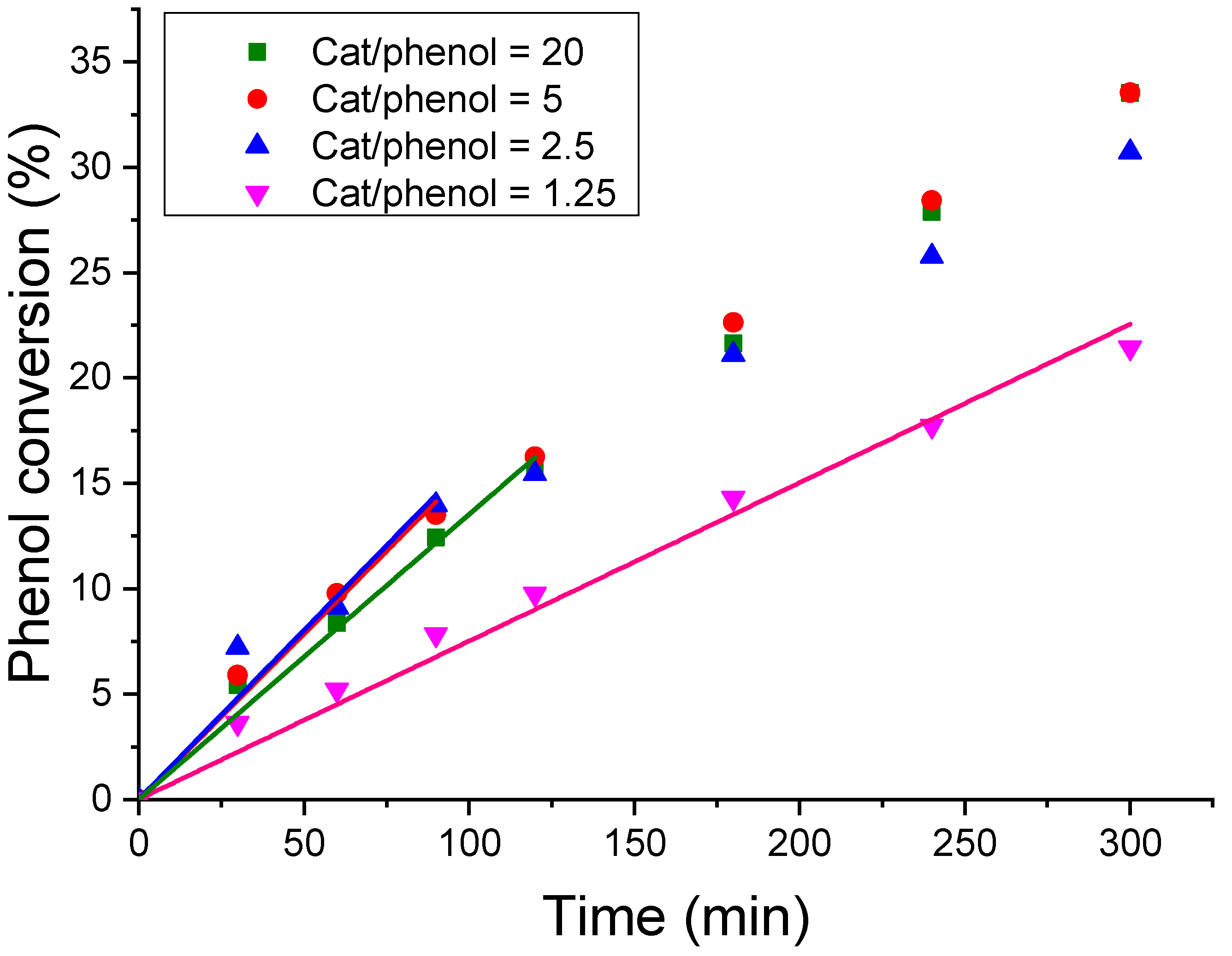
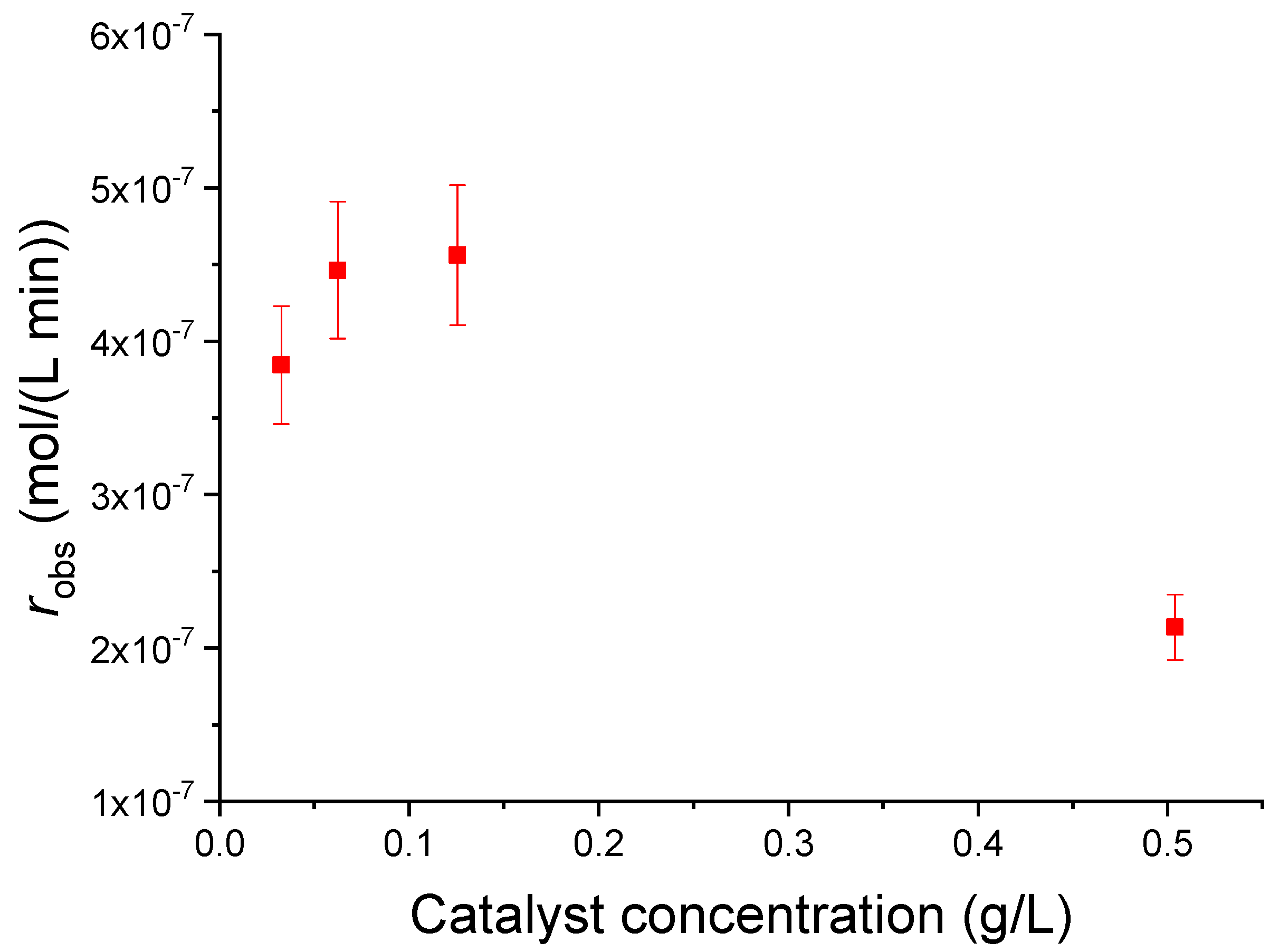
3.3.2. Effect of the Catalyst/Phenol Concentration Ratio
3.3.3. Evaluation of the Kinetic Parameters
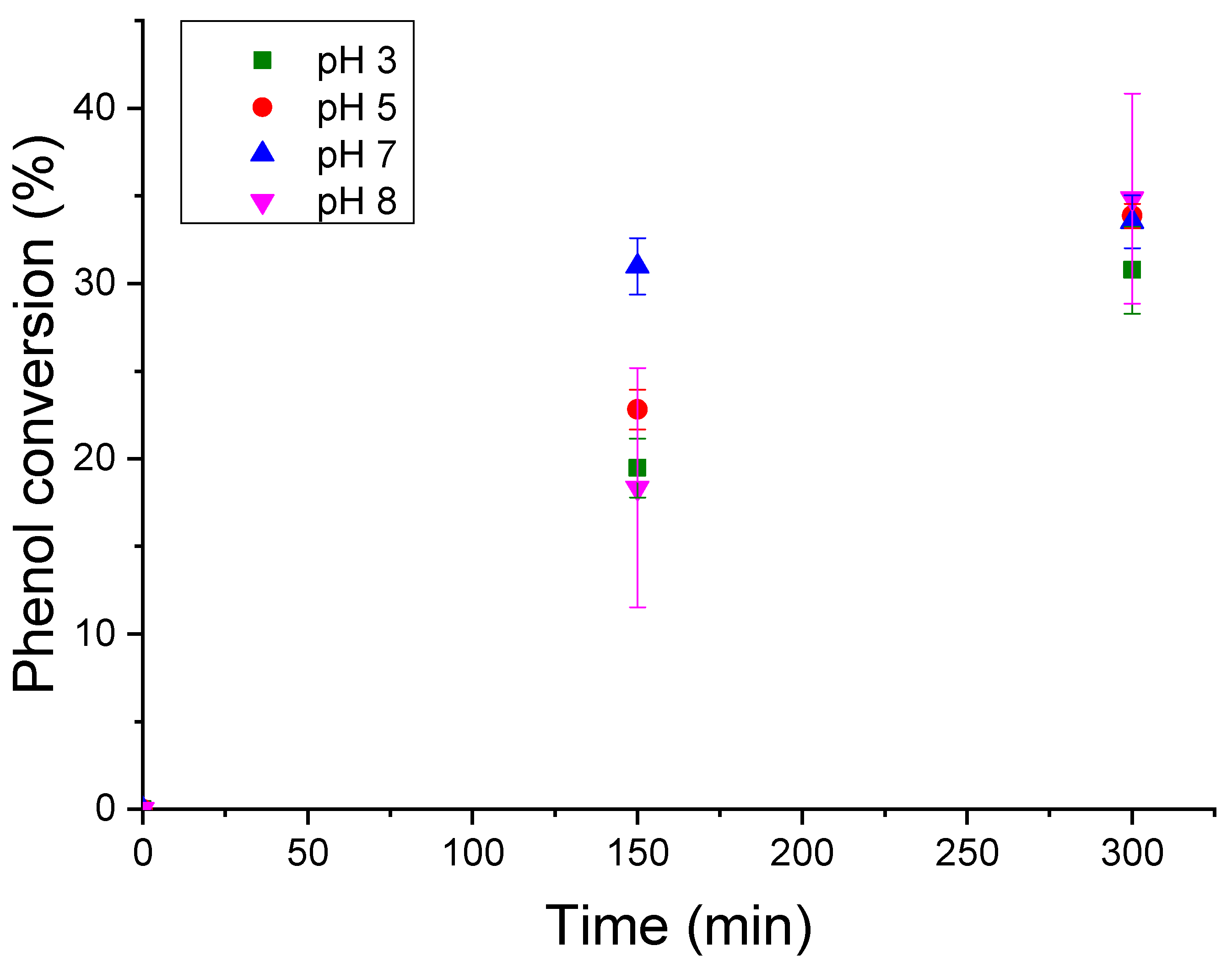
3.3.4. Effect of pH
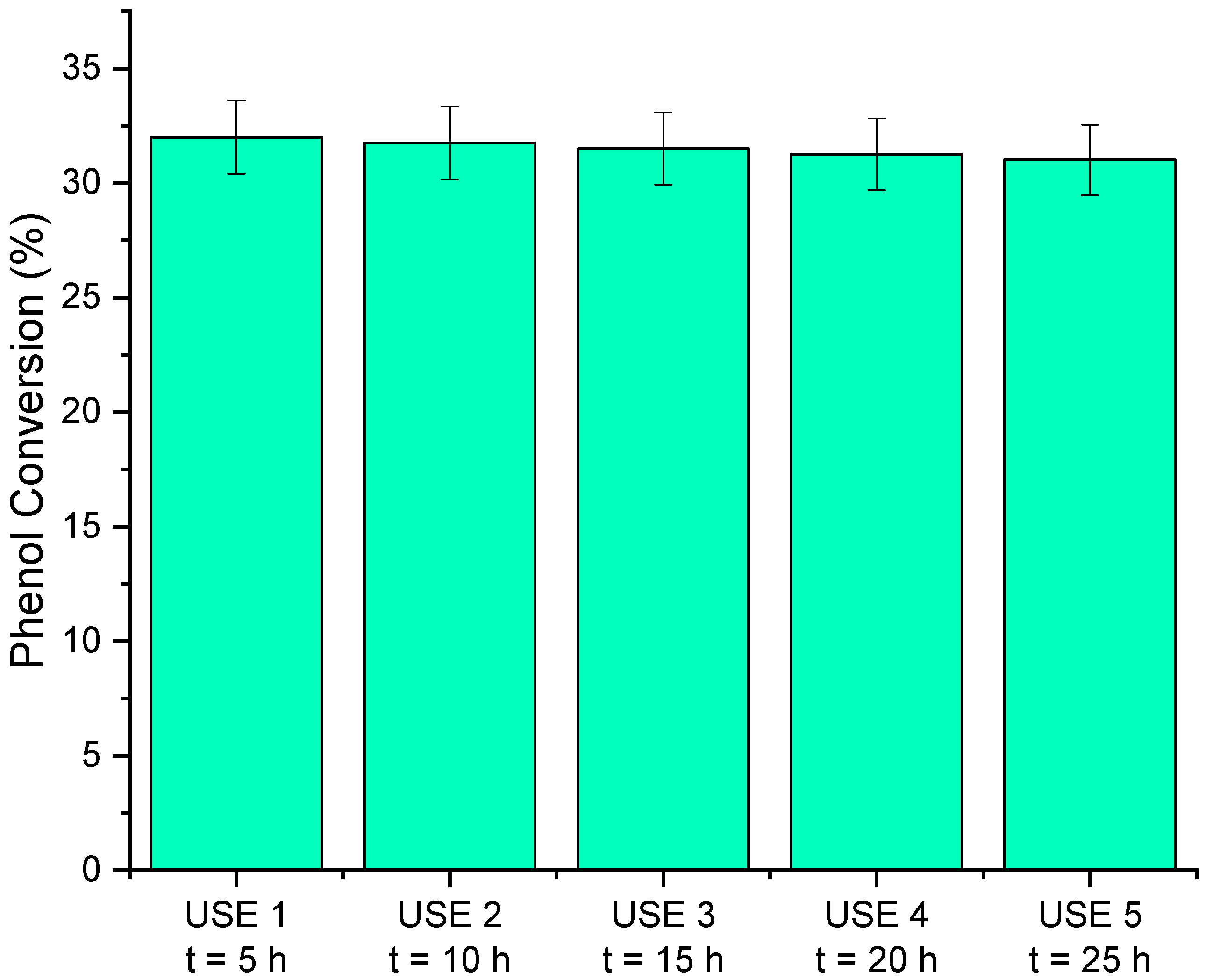
3.3.5. Reusability Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Filipowicz, N.; Momotko, M.; Boczkaj, G.; Pawlikowski, T.; Wanarska, M.; Cieśliński, H. Isolation and Characterization of Phenol-Degrading Psychrotolerant Yeasts. Water Air Soil Pollut. 2017, 228, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swarts, H.J.; Verhagen, F.J.; Field, J.A.; Wijnberg, J.B. Trichlorinated phenols from hypholoma elongatum. Phytochemistry 1998, 49, 203–206. [Google Scholar] [CrossRef]
- Michałowicz, J.; Duda, W. Phenols—Sources and Toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- US EPA. Ambient Water Quality Criteria DOC: Phenol US EPA-440/5-80-066 (PB 81-117772); US Environmental Protection Agency: Washington, DC, USA, 1980; pp. 1100–1156. [Google Scholar]
- Tsuruta, Y.; Watanabe, S.; Inoue, H. Fluorometric determination of phenol and p-cresol in urine by precolumn high-performance liquid chromatography using 4-(N-phthalimidinyl)benzenesulfonyl chloride. Analyt. Biochem. 1996, 243, 86–91. [Google Scholar] [CrossRef]
- Bruce, R.M.; Santodonato, J.; Neal, M.W. Summary Review of the Health Effects Associated With Phenol. Toxicol. Ind. Health 1987, 3, 535–568. [Google Scholar] [CrossRef]
- Bernats, M.; Juhna, T. Factors Governing Degradation of Phenol in Pharmaceutical Wastewater by White-rot Fungi: A Batch Study. Open Biotechnol. J. 2015, 9, 93–99. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Babich, H.; Davis, D. Phenol: A review of environmental and health risks. Regul. Toxicol. Pharmacol. 1981, 1, 90–109. [Google Scholar] [CrossRef]
- Faggiano, A.; De Carluccio, M.; Fiorentino, A.; Ricciardi, M.; Cucciniello, R.; Proto, A.; Rizzo, L. Photo-Fenton like process as polishing step of biologically co-treated olive mill wastewater for phenols removal. Sep. Purif. Technol. 2023, 305, 122525. [Google Scholar] [CrossRef]
- Galgale, A.D.; Shah, N.B.; Shah, N.G. Treatment of Wastewater Containing High Concentration of Phenol & Total Dissolved Solids in Moving Bed Biofilm Reactor. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 10924–10930. [Google Scholar]
- Gogate, P.R.; Aniruddha, B.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Fedorak, P.M.; Hrudey, S.E. The effects of phenol and some alkyl phenolics on batch anaerobic methanogenesis. Water Res. 1984, 18, 361–367. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Russo, V.; Hmoudah, M.; Broccoli, F.; Iesce, M.R.; Di Serio, M. Applications of Metal Organic Frameworks in Wastewater Treatment: A Review on Adsorption and Photodegradation. Front. Chem. Eng. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W.; Chapin, D.H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and UV-radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Ameta, S.C. Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Academic Press: Cambridge, MA, USA, 2018; pp. 1–12. [Google Scholar]
- Guillard, C.; Disdier, J.; Herrmann, J.-M.; Lehaut, C.; Chopin, T.; Malato, S.; Blanco, J. Comparison of various titania samples of industrial origin in the solar photocatalytic detoxification of water containing 4-chlorophenol. Catal. Today 1999, 54, 217–228. [Google Scholar] [CrossRef]
- Li, S.; Shan, S.; Chen, S.; Li, H.; Li, Z.; Liang, Y.; Fei, J.; Xie, L.; Li, J. Photocatalytic degradation of hazardous organic pollutants in water by Fe-MOFs and their composites: A review. J. Environ. Chem. Eng. 2021, 9, 105967. [Google Scholar] [CrossRef]
- Araña, J.; Nieto, J.L.M.; Meli án, J.A.H.; Rodriguez, J.M.D.; Diaz, O.G.; Perna, J.P.; Bergasa, C.A.; Mendez, J. Photocatalytic degradation of formaldehyde containing wastewater from veterinarian laboratories. Chemosphere 2004, 55, 893–904. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Lv, K.; Chen, X. S-Scheme photocatalyst TaON/Bi2WO6 nanofibers with oxygen vacancies for efficient abatement of antibiotics and Cr(VI): Intermediate eco-toxicity analysis and mechanistic insights. Chin. J. Catal. 2022, 43, 2652–2664. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Zhang, Y.; Cai, J.; He, M.; Li, M.; Chen, Z.; Zhang, L. Growth of BiOBr/ZIF-67 Nanocomposites on Carbon Fiber Cloth as Filter-Membrane-Shaped Photocatalyst for Degrading Pollutants in Flowing Wastewater. Adv. Fiber Mater. 2022, 4, 1620–1631. [Google Scholar] [CrossRef]
- Curcó, D.; Giménez, J.; Addardak, A.; Cervera-March, S.; Esplugas, S. Effects of radiation absorption and catalyst concentration on the photocatalytic degradation of pollutants. Catal. Today 2002, 76, 177–188. [Google Scholar] [CrossRef]
- Qamar, M.; Muneer, M.; Bahnemann, D. Heterogeneous photocatalysed degradation of two selected pesticide derivatives, triclopyr and daminozid in aqueous suspensions of titanium dioxide. J. Environ. Manag. 2006, 80, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Visible light-induced photocatalytic degradation of Acid Orange 7 in aqueous TiO2 suspensions. Appl. Catal. B Environ. 2004, 47, 189–201. [Google Scholar] [CrossRef]
- Abu Tariq, M.; Faisal, M.; Muneer, M.; Bahnemann, D. Photochemical reactions of a few selected pesticide derivatives and other priority organic pollutants in aqueous suspensions of titanium dioxide. J. Mol. Catal. A Chem. 2007, 265, 231–236. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Kamble, S.P.; Sawant, S.B.; Pangarkar, V.G. Photocatalytic and photochemical degradation of nitrobenzene using artificial ultraviolet light. Chem. Eng. J. 2004, 102, 283–290. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Kogo, K.; Yoneyama, H.; Tamura, H. Photocatalytic oxidation of cyanide on platinized titanium dioxide. J. Phys. Chem. 1980, 84, 1705–1710. [Google Scholar] [CrossRef]
- Dinga, H.; Suna, H.; Shan, Y. Preparation and characterization of mesoporous SBA-15 supported dye-sensitized TiO2 photocatalyst. J. Photochem. Photobiol. A 2005, 169, 101–107. [Google Scholar] [CrossRef]
- Maira, A.; Yeung, K.; Soria, J.; Coronado, J.; Belver, C.; Lee, C.; Augugliaro, V. Gas-phase photo-oxidation of toluene using nanometer-size TiO2 catalysts. Appl. Catal. B Environ. 2001, 29, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Krýsa, J.; Keppert, M.; Jirkovský, J.; Štengl, V.; Šubrt, J. The effect of thermal treatment on the properties of TiO2 photocatalyst. Mater. Chem. Phys. 2004, 86, 333–339. [Google Scholar] [CrossRef]
- Chun, H.; Yizhong, W.; Hongxiao, T. Destruction of phenol aqueous solution by photocatalysis or direct photolysis. Chemosphere 2000, 41, 1205–1209. [Google Scholar] [CrossRef]
- Haque, M.; Muneer, M. Photodegradation of norfloxacin in aqueous suspensions of titanium dioxide. J. Hazard. Mater. 2007, 145, 51–57. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Sun, J.; Sun, R.; Sun, S.; Qiao, L. Photocatalytic degradation and kinetics of Orange G using nano-sized Sn (IV)/TiO2/AC photocatalyst. J. Mol. Catal. A 2006, 260, 241–246. [Google Scholar] [CrossRef]
- Alnaizy, R.; Akgerman, A. Advanced oxidation of phenolic compounds. Adv. Environ. Res. 2000, 4, 233–244. [Google Scholar] [CrossRef]
- Evgenidou, E.; Fytianos, K.; Poulios, I. Semiconductor-sensitized photodegradation of dichlorvos in water using TiO2 and ZnO as catalysts. Appl. Catal. B Environ. 2005, 59, 81–89. [Google Scholar] [CrossRef]
- Pitre, S.P.; Yoon, T.P.; Scaiano, J.C. Titanium dioxide visible light photocatalysis: Surface association enables photocatalysis with visible light irradiation. Chem. Commun. 2017, 53, 4335–4338. [Google Scholar] [CrossRef] [Green Version]
- Pirkanniemi, K.; Sillanpää, M. Heterogeneous water phase catalysis as an environmental application: A review. Chemosphere 2002, 48, 1047–1060. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Zhou, D.; Luo, H.; Zhang, F.; Wu, J.; Yang, J.; Wang, H. Efficient Photocatalytic Degradation of the Persistent PET Fiber-Based Microplastics over Pt Nanoparticles Decorated N-Doped TiO2 Nanoflowers. Adv. Fiber Mater. 2022, 4, 1094–1107. [Google Scholar] [CrossRef]
- Gerischer, H.; Heller, A. Photocatalytic Oxidation of Organic Molecules at TiO2 Particles by Sunlight in Aerated Water. J. Electrochem. Soc. 1992, 139, 113–118. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, R.; Li, G. Degradation of phenol by nanomaterial TiO2 in wastewater. Chem. Eng. J. 2006, 119, 55–59. [Google Scholar] [CrossRef]
- AEROXIDE TiO2 P 25. Available online: http://glenncorp.com/wp-content/uploads/2013/12/AEROXIDE-TiO2-P-25.pdf (accessed on 14 February 2023).
- Carneiro, J.O.; Vasconcelos, G.; Azevedo, S.; Jesus, C.; Palha, C.; Gomes, N.; Teixeira, V. The evaluation of the thermal behaviour of a mortar based brick masonry wall coated with TiO2 nanoparticles: An experimental assessment towards energy efficient buildings. Energy Build. 2014, 81, 1–8. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Barakat, M.A.; Al-Hutailah, R.I.; Qayyum, E.; Rashid, J.; Kuhn, J.N. Pt nanoparticles/TiO2 for photocatalytic degradation of phenols in wastewater. Environ. Technol. 2013, 35, 137–144. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Laoufi, N.A.; Tassalit, D.; Benthahar, F. The degradation of phenol in water solution by TiO2 photocatalysis in a helical reactor. Glob. NEST J. 2008, 10, 404–418. [Google Scholar]
- Barka, N.; Bakas, I.; Qourzal, S.; Ait-Ichou, A.A.A.Y. Degradation of Phenol in Water by Titanium Dioxide Photocatalysis. Orient. J. Chem. 2013, 29, 1055–1060. [Google Scholar] [CrossRef] [Green Version]
- Peiró, A.M.; Ayllón, J.A.; Peral, J.; Doménech, X. TiO2-photocatalyzed degradation of phenol and ortho-substituted phenolic compounds. Appl. Catal. B Environ. 2001, 30, 359–373. [Google Scholar] [CrossRef]



| Property | Value |
|---|---|
| CAS-No. | 13463-67-7 |
| Specific surface [m2/g] | 35–65 |
| pH (in 4% dispersion) | 3.5–4.5 |
| Density [g/cm3] | 4.1 |
| Average particle size [nm] | 21 |
| Tamped density [g/L] | 130 |
| Anatase: rutile weight ratio | 80:20 |
| TiO2 content [wt.-%] | ≥99.5 |
| Al2O3 content [wt.-%] | ≤0.3 |
| SiO2 content [wt.-%] | ≤0.2 |
| Fe2O3 content [wt.-%] | ≤0.01 |
| HCl content [wt.-%] | ≤0.3 |
| Test | C0 (ppm) | ρcatalyst (g/L) | T (°C) | pH |
|---|---|---|---|---|
| A1 | 25 | 0.125 | 40 | 7 |
| A2 | 500 | 0.5 | 40 | 7 |
| B1 | 116 | 0 | 40 | 7 |
| B2 | 26 | 0 | 40 | 7 |
| 1 | 500 | 0.50 | 40 | 7 |
| 2 | 120 | 0.50 | 40 | 7 |
| 3 | 65 | 0.50 | 40 | 7 |
| 4 | 25 | 0.50 | 40 | 7 |
| 5 | 25 | 0.125 | 40 | 7 |
| 6 | 25 | 0.065 | 40 | 7 |
| 7 | 25 | 0.031 | 40 | 7 |
| 8 * | 25 | 0.125 | 30 | 7 |
| 9 | 25 | 0.125 | 50 | 7 |
| 10 | 25 | 0.125 | 40 | 3 |
| 11 | 25 | 0.125 | 40 | 5 |
| 12 | 25 | 0.125 | 40 | 7 |
| 13 | 25 | 0.125 | 70 | 8 |
| T (°C) | C0 (mol L−1) | robs (mol L−1 min−1) | k (min−1) |
|---|---|---|---|
| 30 | 2.84·10−4 | 3.14·10−7 | 1.10·10−3 |
| 40 | 2.79·10−4 | 3.74·10−7 | 1.34·10−3 |
| 50 | 2.80·10−4 | 4.39·10−7 | 1.57·10−3 |
| ln(k0) | Ea/R (kJ mol−1) | R2 |
|---|---|---|
| −1.1 ± 0.2 | 1.7 ± 0.6 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broccoli, F.; Paparo, R.; Iesce, M.R.; Di Serio, M.; Russo, V. Heterogeneous Photodegradation Reaction of Phenol Promoted by TiO2: A Kinetic Study. ChemEngineering 2023, 7, 27. https://doi.org/10.3390/chemengineering7020027
Broccoli F, Paparo R, Iesce MR, Di Serio M, Russo V. Heterogeneous Photodegradation Reaction of Phenol Promoted by TiO2: A Kinetic Study. ChemEngineering. 2023; 7(2):27. https://doi.org/10.3390/chemengineering7020027
Chicago/Turabian StyleBroccoli, Francesco, Rosanna Paparo, Maria Rosaria Iesce, Martino Di Serio, and Vincenzo Russo. 2023. "Heterogeneous Photodegradation Reaction of Phenol Promoted by TiO2: A Kinetic Study" ChemEngineering 7, no. 2: 27. https://doi.org/10.3390/chemengineering7020027
APA StyleBroccoli, F., Paparo, R., Iesce, M. R., Di Serio, M., & Russo, V. (2023). Heterogeneous Photodegradation Reaction of Phenol Promoted by TiO2: A Kinetic Study. ChemEngineering, 7(2), 27. https://doi.org/10.3390/chemengineering7020027







