Recovery of Polyphenolic Antioxidants from Coffee Silverskin Using Acid-Catalyzed Ethanol Organosolv Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Coffee Silverskin
2.3. Hydroethanolic Solvent Testing and Organosolv Treatments
2.4. Treatment Severity Determination
2.5. Response Surface Treatment Optimization
2.6. Total Polyphenol Measurement and Antioxidant Activity Tests
2.7. Determination of the Analytical Polyphenolic Composition
2.8. Statistical Analysis
3. Results and Discussion
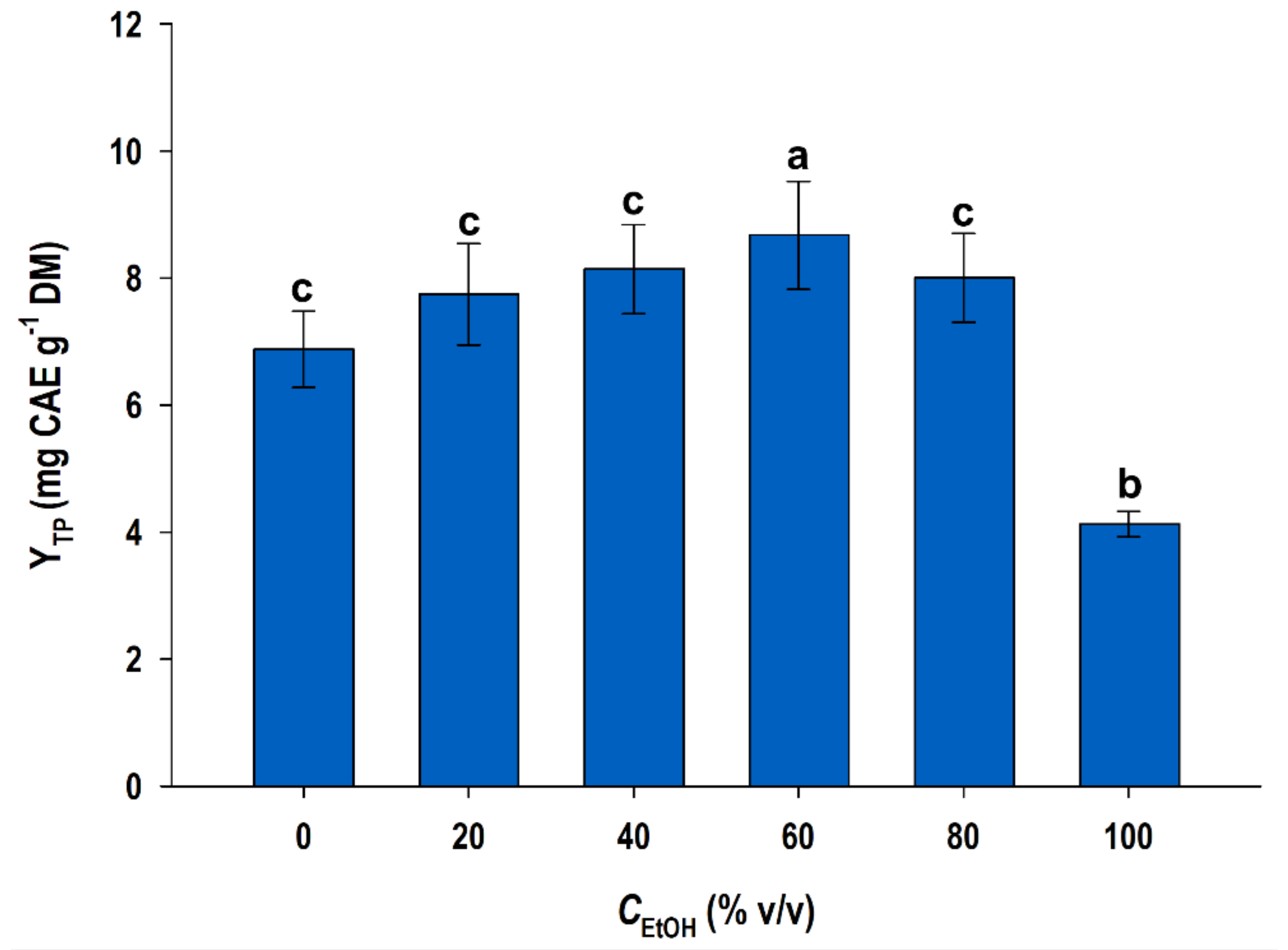
3.1. Effect of Ethanol Concentration
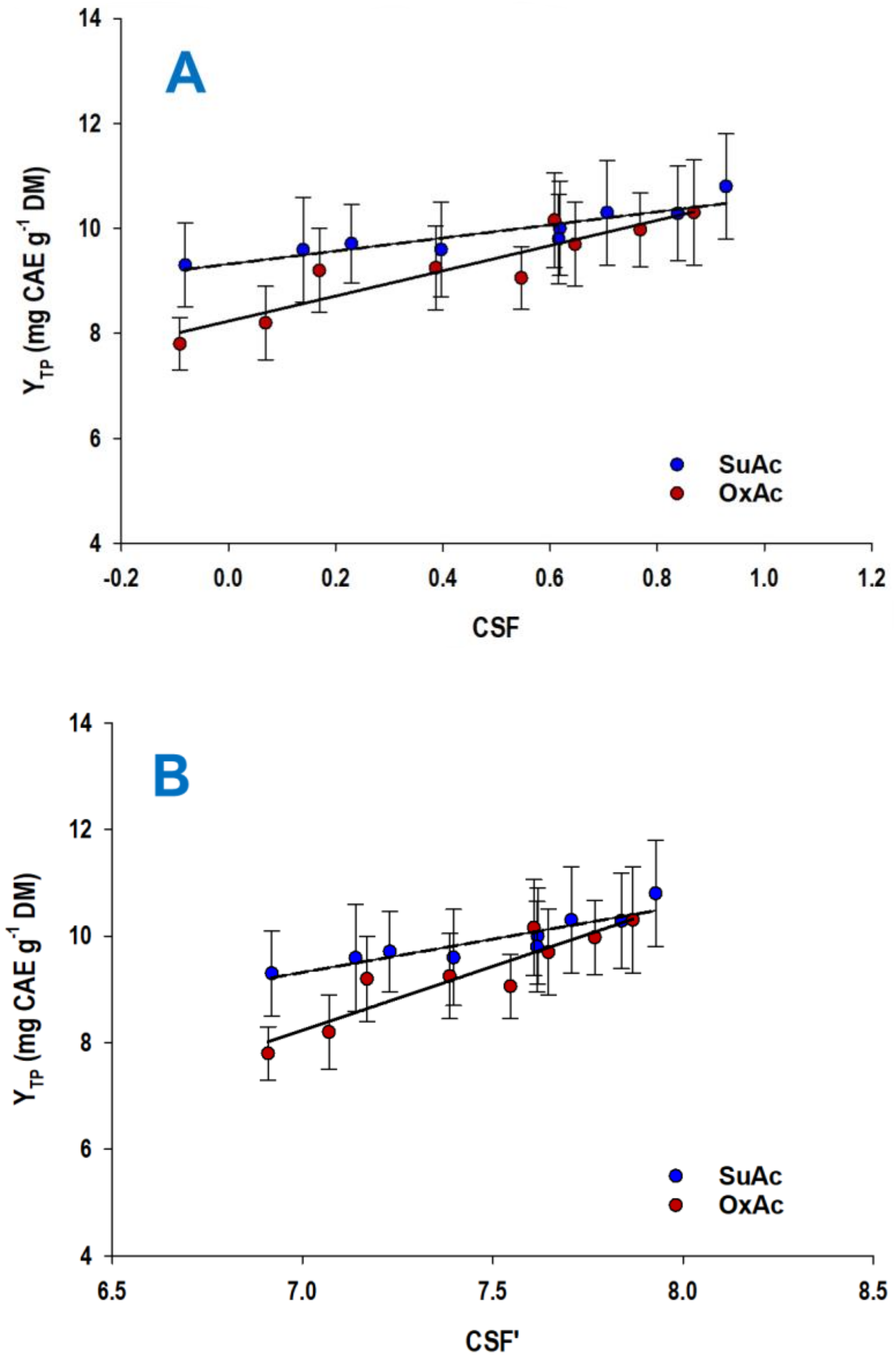
3.2. Effect of Acid Catalyst—Treatment Severity
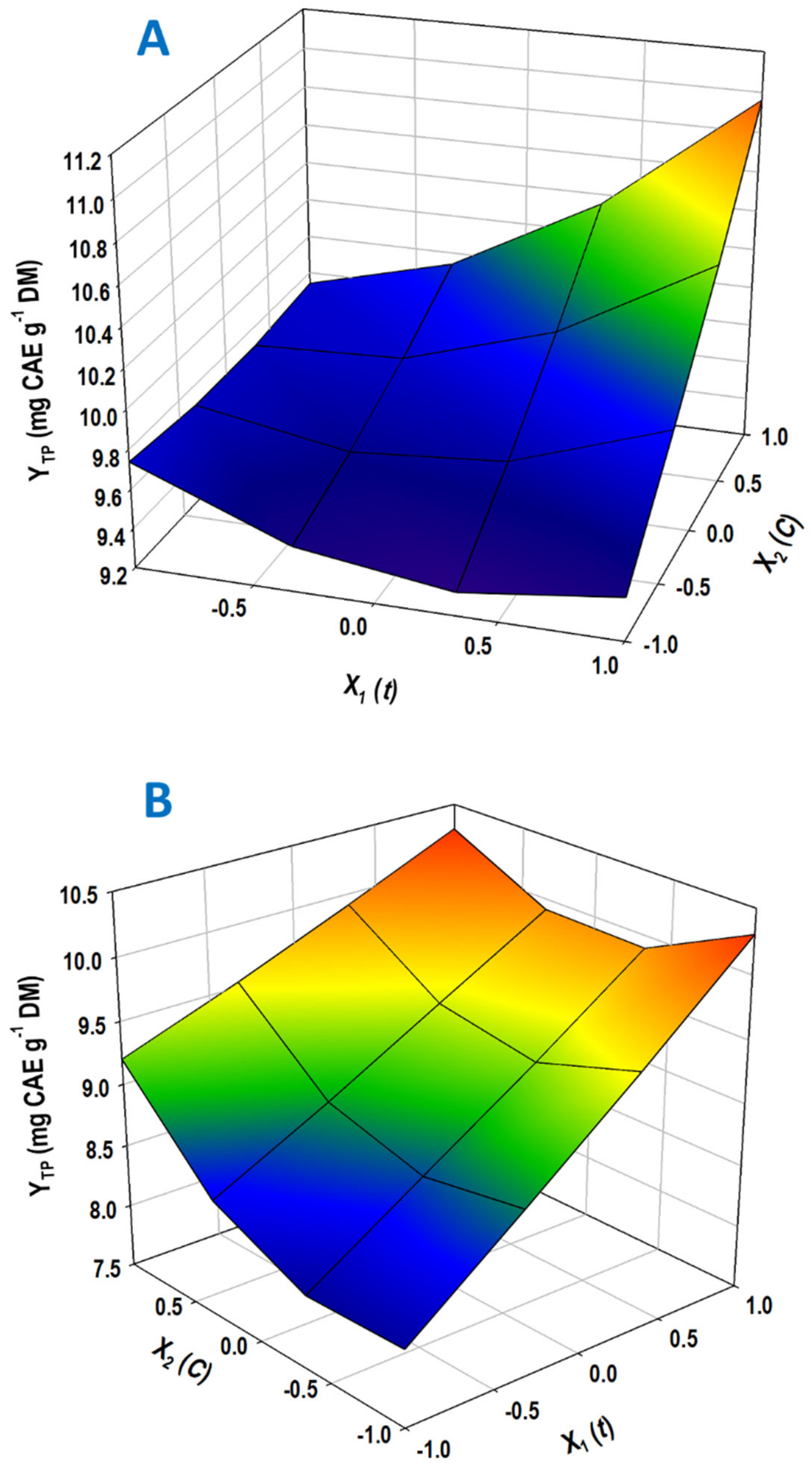
3.3. Treatment Optimization by Response Surface
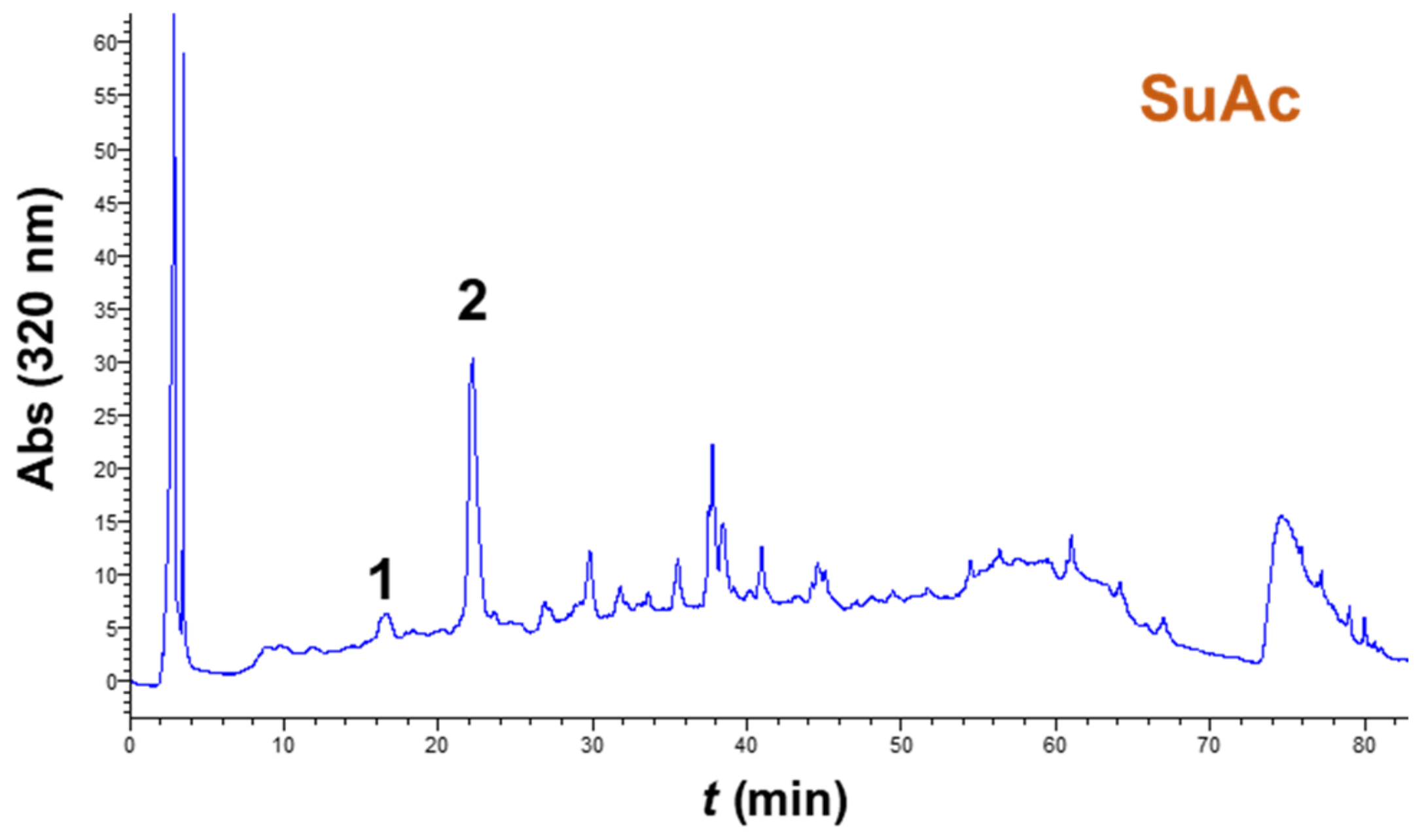
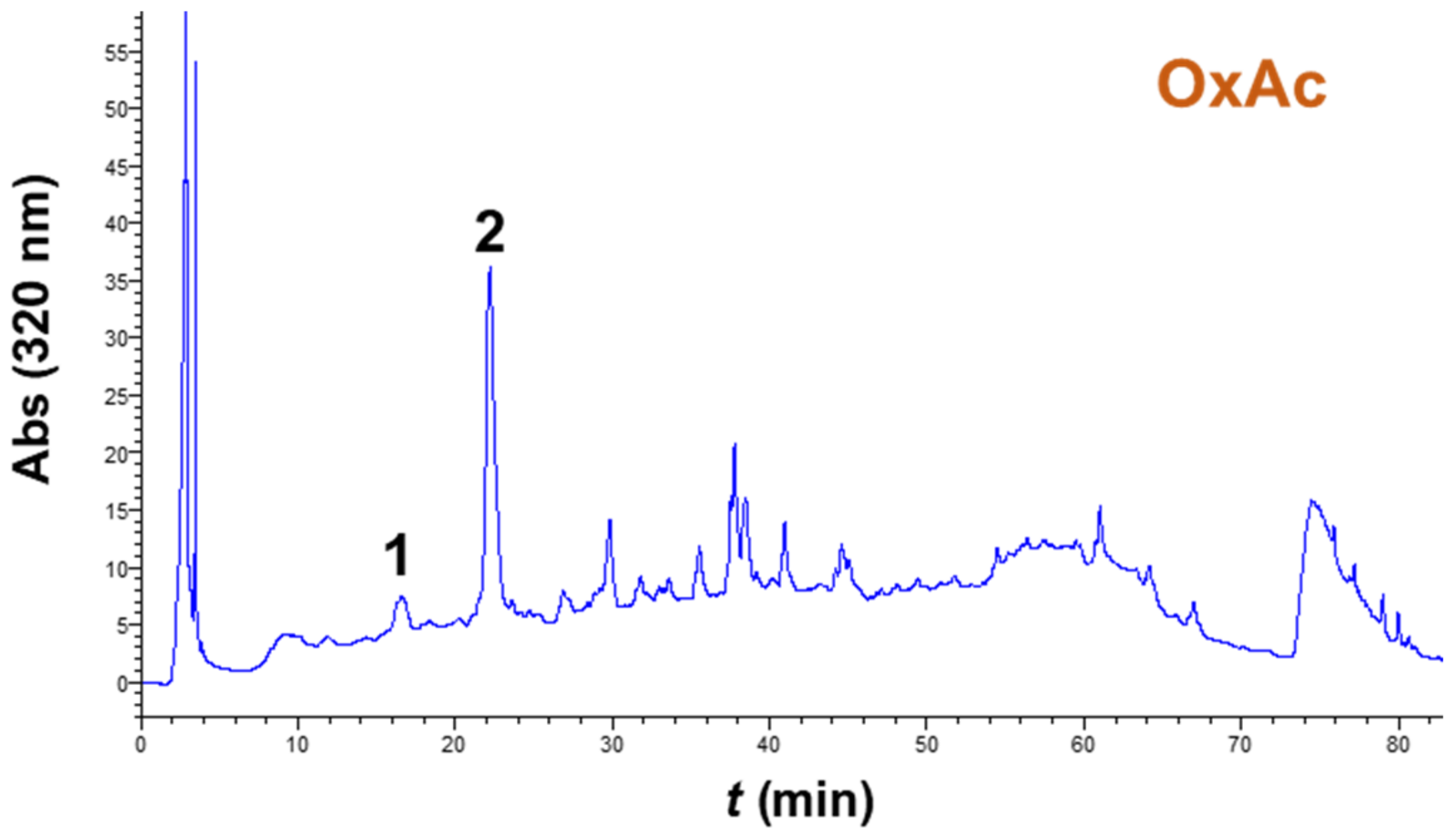
3.4. Extract Composition and Antioxidant Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAE | caffeic acid equivalent (mg g−1 DM) |
| COxAc | oxalic acid concentration (% w/v) |
| CSS | coffee silverskin |
| CSF | combined severity factor (dimensionless) |
| CSF′ | alternative combined severity factor (dimensionless) |
| CSuAc | sulfuric acid concentration (% w/v) |
| SF | severity factor (dimensionless) |
| T | temperature (°C) |
| t | time (min) |
References
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.-H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—A critical review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [PubMed]
- Monteiro, A.R.; Battisti, A.P.; Valencia, G.A.; de Andrade, C.J. The Production of High-Added-Value Bioproducts from Non-Conventional Biomasses: An Overview. Biomass 2023, 3, 9. [Google Scholar] [CrossRef]
- Serna-Jiménez, J.A.; Siles, J.A.; de los Ángeles Martín, M.; Chica, A.F. A Review on the Applications of Coffee Waste Derived from Primary Processing: Strategies for Revalorization. Processes 2022, 10, 2436. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Cho, E.-J.; Maskey, S.; Nguyen, D.-T.; Bae, H.-J. Value-Added Products from Coffee Waste: A Review. Molecules 2023, 28, 3562. [Google Scholar] [PubMed]
- Bessada, S.M.; Alves, R.C.; PP Oliveira, M.B. Coffee silverskin: A review on potential cosmetic applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids—Occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar]
- Bondam, A.F.; da Silveira, D.D.; dos Santos, J.P.; Hoffmann, J.F. Phenolic compounds from coffee by-products: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 123, 172–186. [Google Scholar] [CrossRef]
- Borand, M.N.; Karaosmanoğlu, F. Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: A review. J. Renew. Sustain. Energy 2018, 10, 033104. [Google Scholar] [CrossRef]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels Bioprod. Biorefining 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Ravindran, R.; Desmond, C.; Jaiswal, S.; Jaiswal, A.K. Optimisation of organosolv pretreatment for the extraction of polyphenols from spent coffee waste and subsequent recovery of fermentable sugars. Bioresour. Technol. Rep. 2018, 3, 7–14. [Google Scholar] [CrossRef]
- Lee, J.-W.; Jeffries, T.W. Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresour. Technol. 2011, 102, 5884–5890. [Google Scholar] [CrossRef] [PubMed]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Sidiras, D.; Politi, D.; Giakoumakis, G.; Salapa, I. Simulation and optimization of organosolv based lignocellulosic biomass refinery: A review. Bioresour. Technol. 2022, 343, 126158. [Google Scholar]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity–relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I.; Makris, D.P. β-Cyclodextrin-aided aqueous extraction of antioxidant polyphenols from peppermint (Mentha × piperita L.). Oxygen 2022, 2, 29. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Development of a low-temperature and high-performance green extraction process for the recovery of polyphenolic phytochemicals from waste potato peels using hydroxypropyl β-cyclodextrin. Appl. Sci. 2020, 10, 3611. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass Convers. Biorefining 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar]
- Papadaki, E.S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P.; Makris, D.P. Polyphenol Release from Wheat Bran Using Ethanol-Based Organosolv Treatment and Acid/Alkaline Catalysis: Process Modeling Based on Severity and Response Surface Optimization. Antioxidants 2022, 11, 2457. [Google Scholar] [CrossRef] [PubMed]
- Pazo-Cepeda, V.; Benito-Román, Ó.; Navarrete, A.; Alonso, E. Valorization of wheat bran: Ferulic acid recovery using pressurized aqueous ethanol solutions. Waste Biomass Valorization 2020, 11, 4701–4710. [Google Scholar] [CrossRef]
- Bozinou, E.; Palaiogiannis, D.; Athanasiadis, V.; Chatzilazarou, A.; Lalas, S.I.; Makris, D.P. Glycerol-Based Deep Eutectic Solvents for Simultaneous Organosolv Treatment/Extraction: High-Performance Recovery of Antioxidant Polyphenols from Onion Solid Wastes. Sustainability 2022, 14, 15715. [Google Scholar] [CrossRef]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed electric field assisted extraction of bioactive compounds from cocoa bean shell and coffee silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Vimercati, W.C.; da Silva Araújo, C.; Macedo, L.L.; Pimenta, C.J. Optimal extraction condition for the recovery of bioactive compounds and antioxidants from coffee silverskin. J. Food Process Eng. 2022, 45, e14009. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Zhao, M.; Senthamaraikannan, R.; Padamati, R.B.; Sun, D.W.; Tiwari, B.K. Green extraction of soluble dietary fibre from coffee silverskin: Impact of ultrasound/microwave-assisted extraction. Int. J. Food Sci. Technol. 2020, 55, 2242–2250. [Google Scholar] [CrossRef]
- Conde, T.; Mussatto, S.I. Isolation of polyphenols from spent coffee grounds and silverskin by mild hydrothermal pretreatment. Prep. Biochem. Biotechnol. 2016, 46, 406–409. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem. 2012, 135, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Ginting, A.R.; Kit, T.; Mingvanish, W.; Thanasupsin, S.P. Valorization of Coffee Silverskin through Subcritical Water Extraction: An Optimization Based on T-CQA Using Response Surface Methodology. Sustainability 2022, 14, 8435. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical composition, antioxidant and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Regazzoni, L.; Saligari, F.; Marinello, C.; Rossoni, G.; Aldini, G.; Carini, M.; Orioli, M. Coffee silver skin as a source of polyphenols: High resolution mass spectrometric profiling of components and antioxidant activity. J. Funct. Foods 2016, 20, 472–485. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In vitro bioaccessibility and antioxidant activity of coffee silverskin polyphenolic extract and characterization of bioactive compounds using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef]
- Choueiri, L.; Chedea, V.S.; Calokerinos, A.; Kefalas, P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC–MS. Food Chem. 2012, 133, 1039–1044. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]


| Treatment Variables | Codes | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| t (min) | X1 | 60 | 180 | 300 |
| CSuAc (%, w/v) | X2 | 0.5 | 1.0 | 1.5 |
| COxAc (%, w/v) | 4 | 8 | 12 | |
| CSuAc (% w/v) | t (min) | CSF | CSF′ | YTP (mg CAE g−1 DM) |
|---|---|---|---|---|
| 0.5 | 60 | −0.08 a | 6.92 a | 9.31 a |
| 180 | 0.40 c | 7.40 c | 9.60 c | |
| 300 | 0.62 c | 7.62 c | 10.02 c | |
| 1.0 | 60 | 0.14 a | 7.14 a | 9.59 c |
| 180 | 0.62 c | 7.62 c | 9.80 c | |
| 300 | 0.84 b | 7.84 b | 10.29 b | |
| 1.5 | 60 | 0.23 a | 7.23 a | 9.71 c |
| 180 | 0.71 c | 7.71 c | 10.33 b | |
| 300 | 0.93 b | 7.93 b | 10.84 b |
| COxAc (% w/v) | t (min) | CSF | CSF′ | YTP (mg CAE g−1 DM) |
|---|---|---|---|---|
| 4 | 60 | −0.09 a | 6.91 a | 7.80 a |
| 180 | 0.39 c | 7.39 c | 9.25 c | |
| 300 | 0.61 c | 7.61 c | 10.16 b | |
| 8 | 60 | 0.07 a | 7.07 a | 8.20 a |
| 180 | 0.55 c | 7.55 c | 9.06 c | |
| 300 | 0.77 b | 7.77 b | 9.98 b | |
| 12 | 60 | 0.17 a | 7.17 a | 9.20 c |
| 180 | 0.65 c | 7.65 c | 9.70 c | |
| 300 | 0.87 b | 7.87 b | 10.30 b |
| Design Point | Independent Variables | Response (YTP, mg CAE g−1 DM) | ||||
|---|---|---|---|---|---|---|
| X1 (t) | X2 (C) | SuAc | OxAc | |||
| Measured | Predicted | Measured | Predicted | |||
| 1 | −1 | −1 | 9.87 | 9.74 | 8.19 | 8.12 |
| 2 | −1 | 1 | 9.71 | 9.74 | 9.42 | 9.20 |
| 3 | 1 | −1 | 9.43 | 9.42 | 10.16 | 10.30 |
| 4 | 1 | 1 | 10.81 | 10.96 | 10.31 | 10.30 |
| 5 | −1 | 0 | 9.59 | 9.69 | 7.90 | 8.19 |
| 6 | 1 | 0 | 10.29 | 10.14 | 9.98 | 9.84 |
| 7 | 0 | −1 | 9.20 | 9.34 | 9.25 | 9.18 |
| 8 | 0 | 1 | 10.32 | 10.11 | 9.52 | 9.72 |
| 9 | 0 | 0 | 9.80 | 9.68 | 9.06 | 8.99 |
| 10 | 0 | 0 | 9.51 | 9.68 | 8.90 | 8.99 |
| 11 | 0 | 0 | 9.72 | 9.68 | 9.15 | 8.99 |
| Sample | nCGA (μg g−1 DM) | CGA (μg g−1 DM) | Total (μg g−1 DM) | AAR (μmol DPPH g−1 DM) | PR (μmol AAE g−1 DM) |
|---|---|---|---|---|---|
| SuAc | 37.56 ± 3.50 | 157.49 ± 12.40 | 195.05 | 75.93 ± 5.44 | 40.94 ± 2.32 |
| OxAc | 44.61 ± 3.88 | 179.59 ± 14.32 | 224.20 | 86.14 ± 6.02 | 32.02 ± 2.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smyrnakis, G.; Stamoulis, G.; Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Lalas, S.I.; Makris, D.P. Recovery of Polyphenolic Antioxidants from Coffee Silverskin Using Acid-Catalyzed Ethanol Organosolv Treatment. ChemEngineering 2023, 7, 72. https://doi.org/10.3390/chemengineering7040072
Smyrnakis G, Stamoulis G, Palaiogiannis D, Chatzimitakos T, Athanasiadis V, Lalas SI, Makris DP. Recovery of Polyphenolic Antioxidants from Coffee Silverskin Using Acid-Catalyzed Ethanol Organosolv Treatment. ChemEngineering. 2023; 7(4):72. https://doi.org/10.3390/chemengineering7040072
Chicago/Turabian StyleSmyrnakis, George, George Stamoulis, Dimitrios Palaiogiannis, Theodoros Chatzimitakos, Vassilis Athanasiadis, Stavros I. Lalas, and Dimitris P. Makris. 2023. "Recovery of Polyphenolic Antioxidants from Coffee Silverskin Using Acid-Catalyzed Ethanol Organosolv Treatment" ChemEngineering 7, no. 4: 72. https://doi.org/10.3390/chemengineering7040072
APA StyleSmyrnakis, G., Stamoulis, G., Palaiogiannis, D., Chatzimitakos, T., Athanasiadis, V., Lalas, S. I., & Makris, D. P. (2023). Recovery of Polyphenolic Antioxidants from Coffee Silverskin Using Acid-Catalyzed Ethanol Organosolv Treatment. ChemEngineering, 7(4), 72. https://doi.org/10.3390/chemengineering7040072