Supramolecular Sensing Platforms: Techniques for In Vitro Biosensing
Abstract
1. Introduction
1.1. Supramolecular Assemblies and Their Scope for Sensing
1.2. Importance of In Vitro Sensing
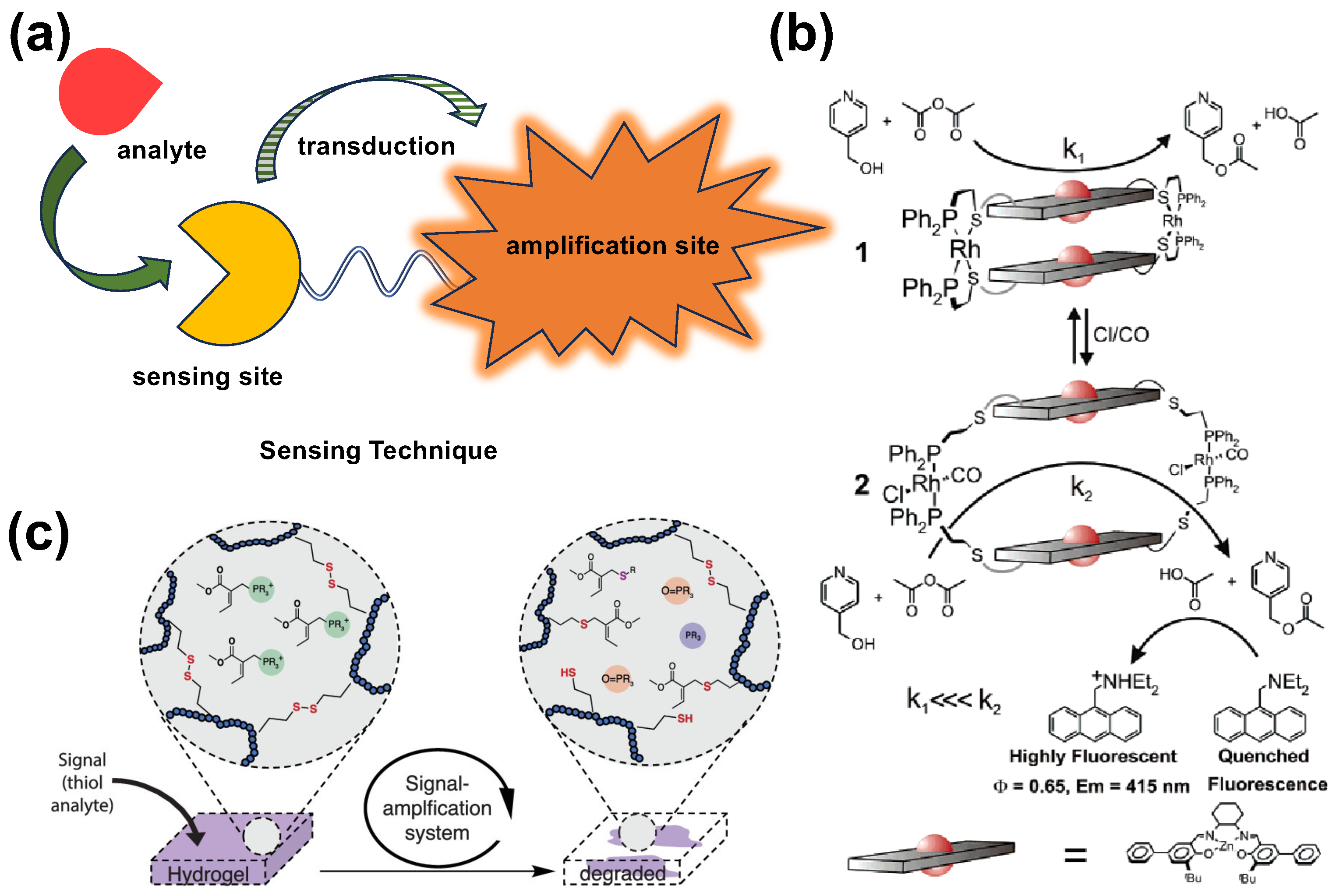
2. Small Molecule Sensing
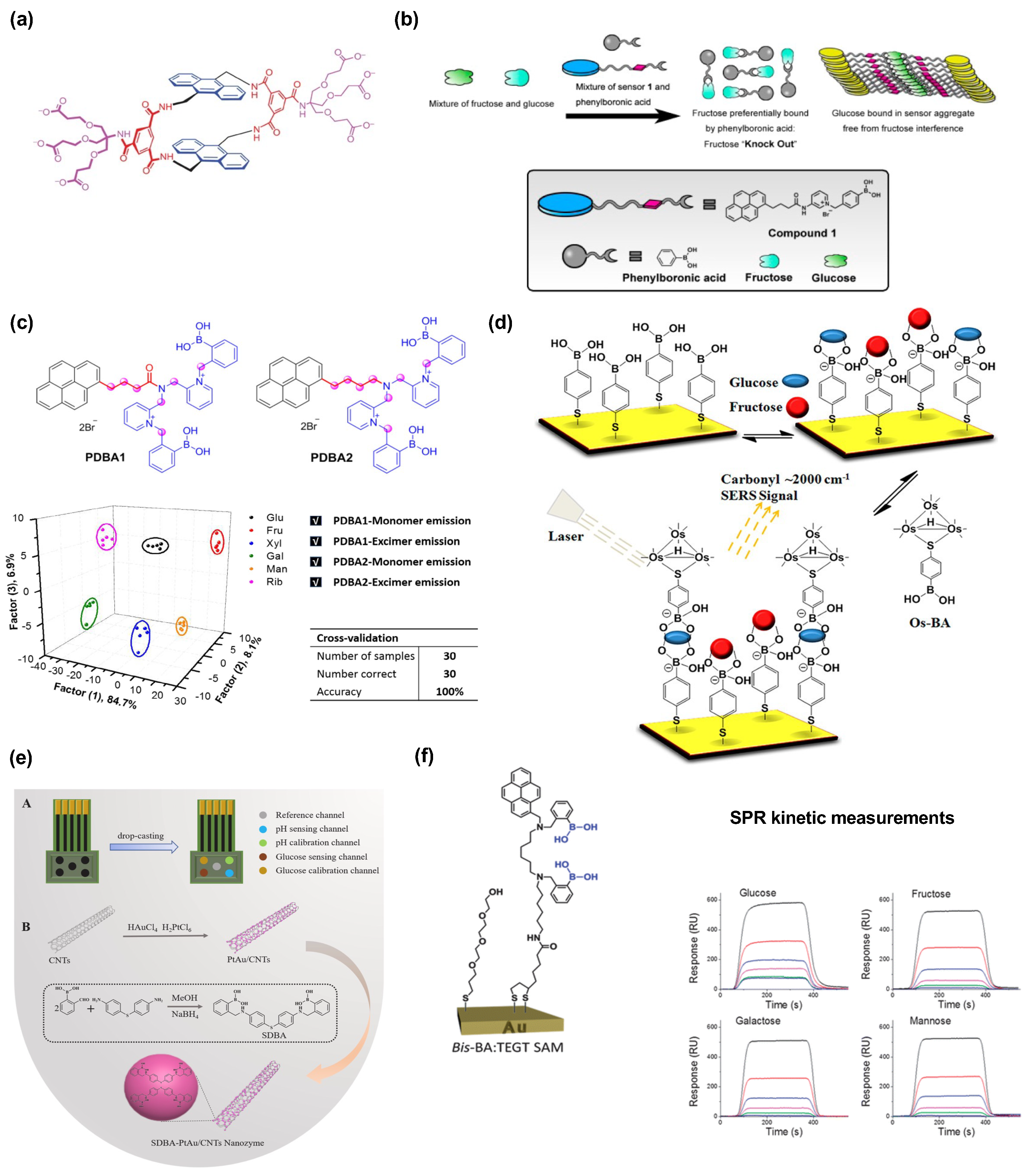
2.1. Glucose and Saccharide Sensing
2.2. Hydrogen Peroxide (H2O2) Sensing
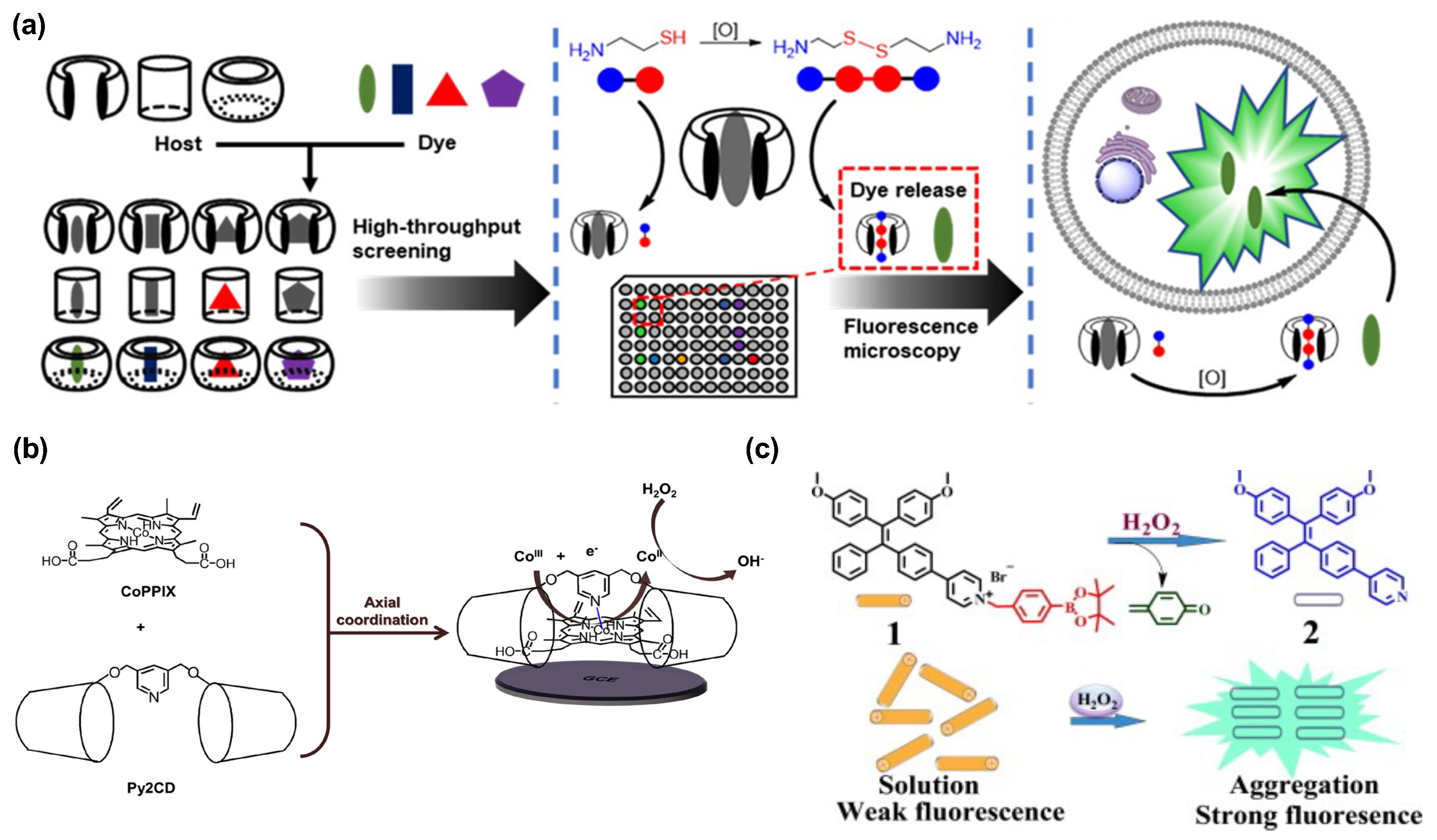
2.3. Metal Ions
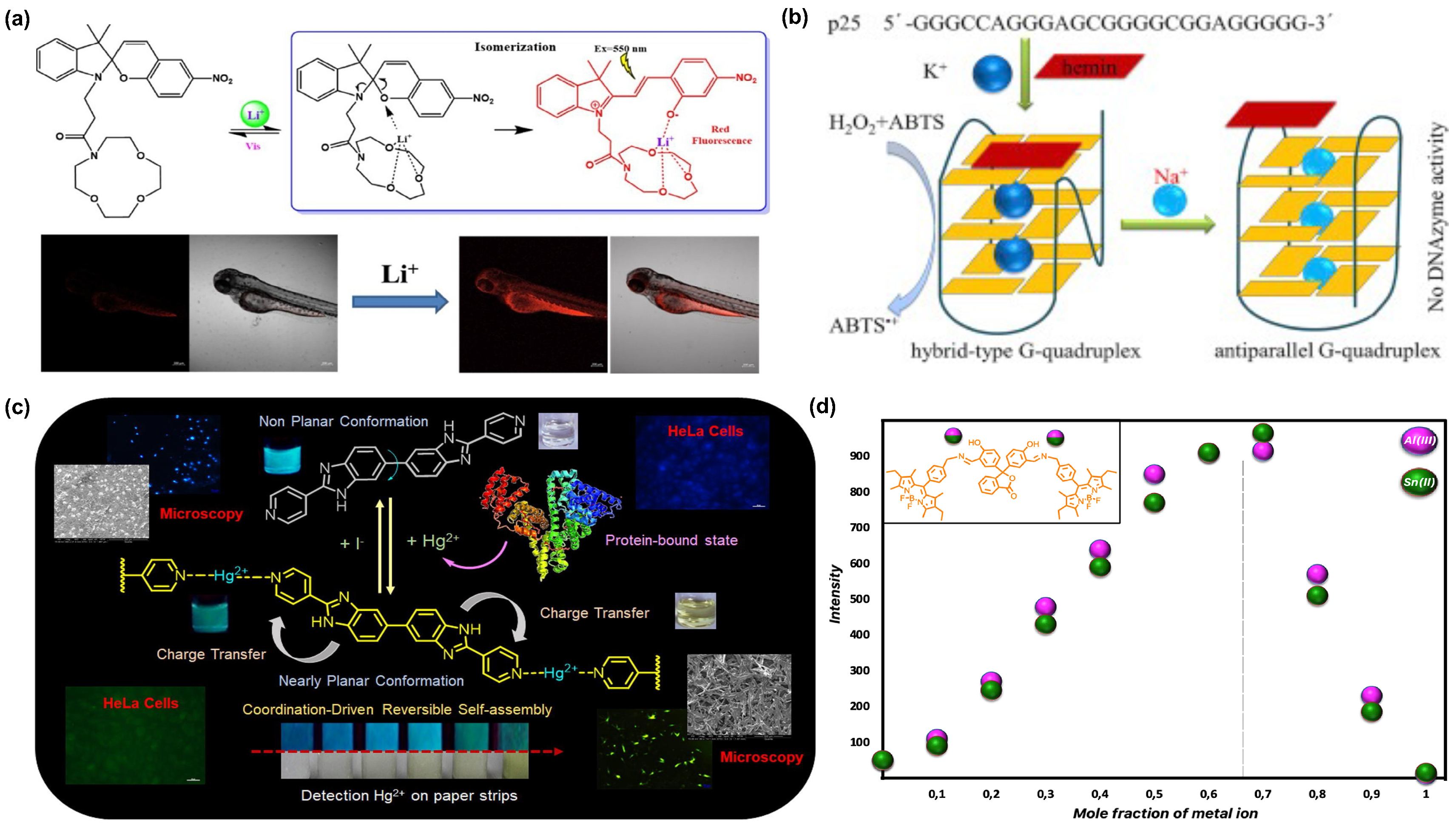
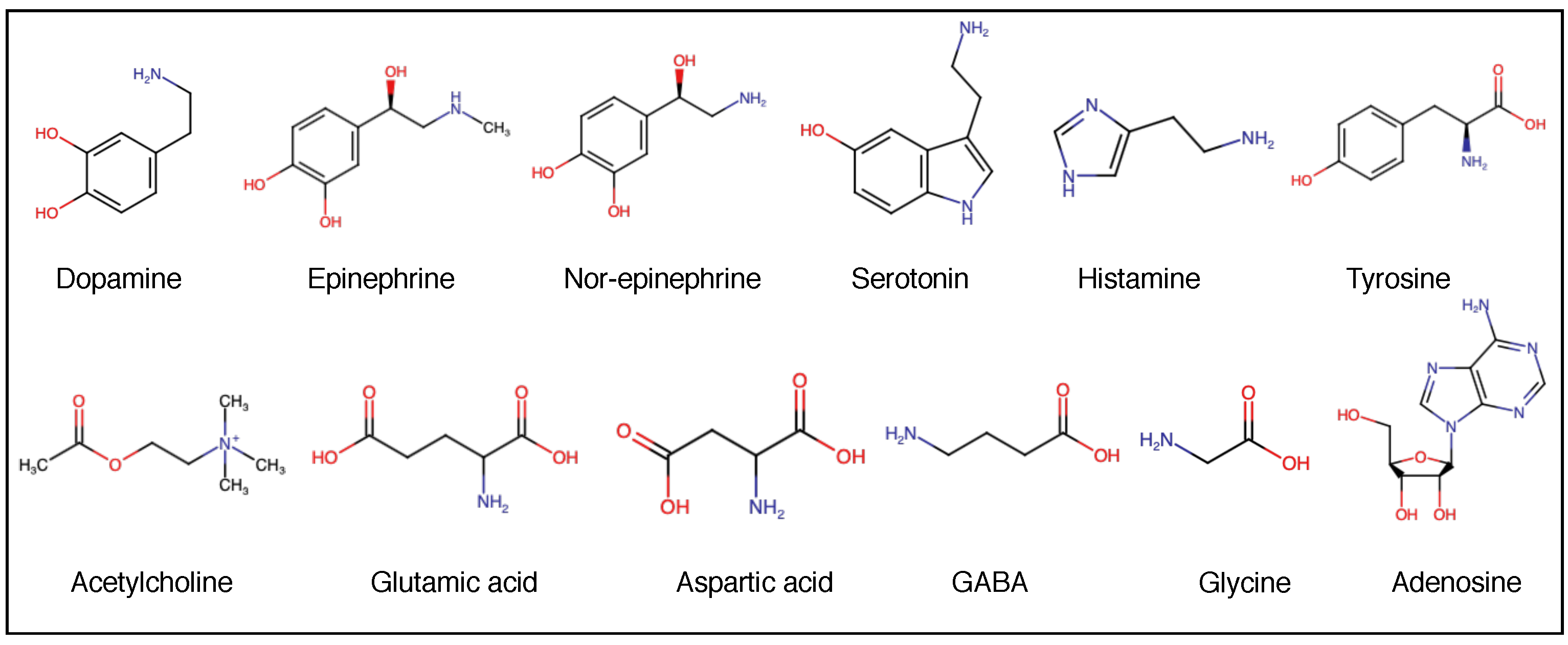
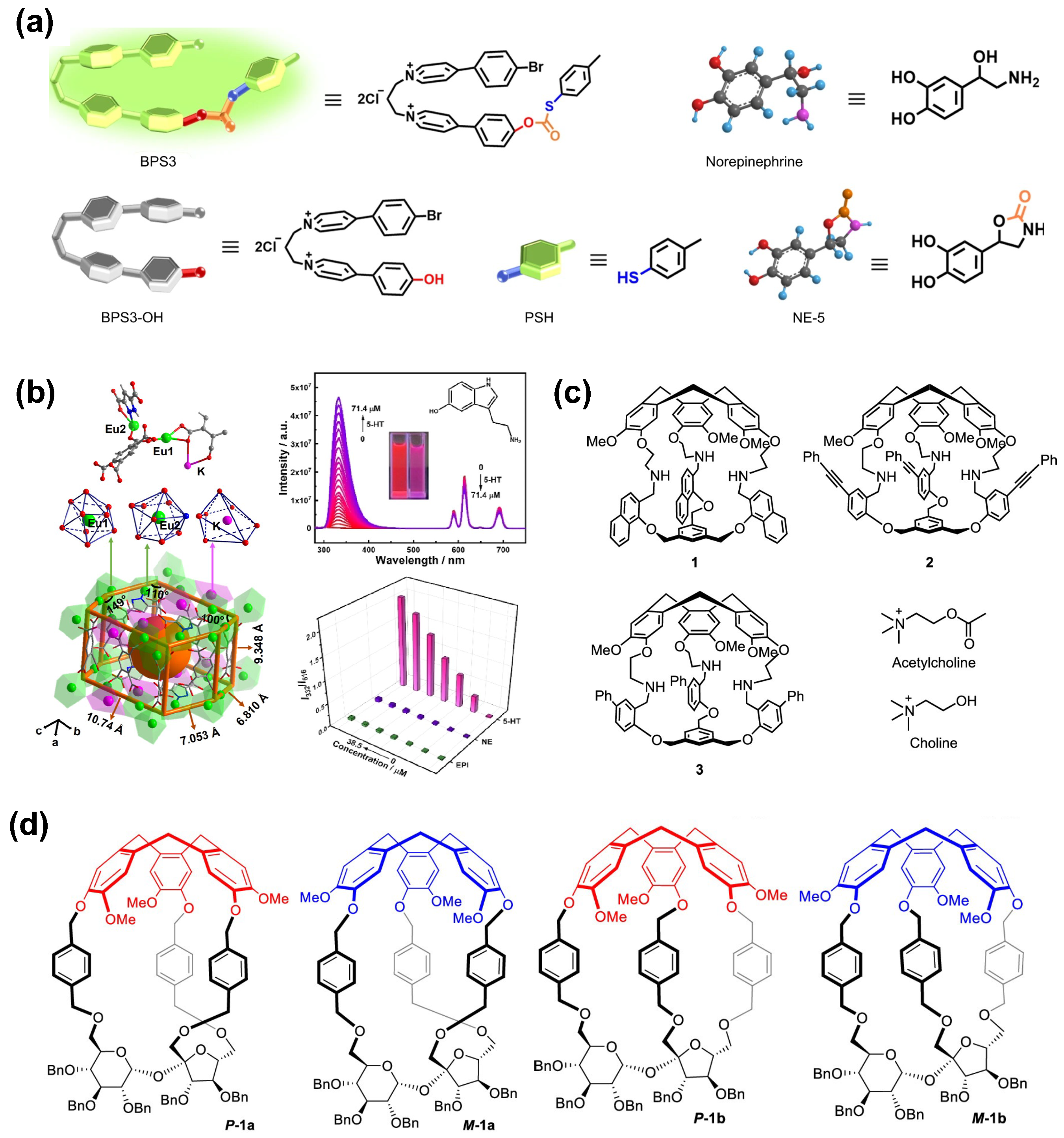
2.4. Neurotransmitters
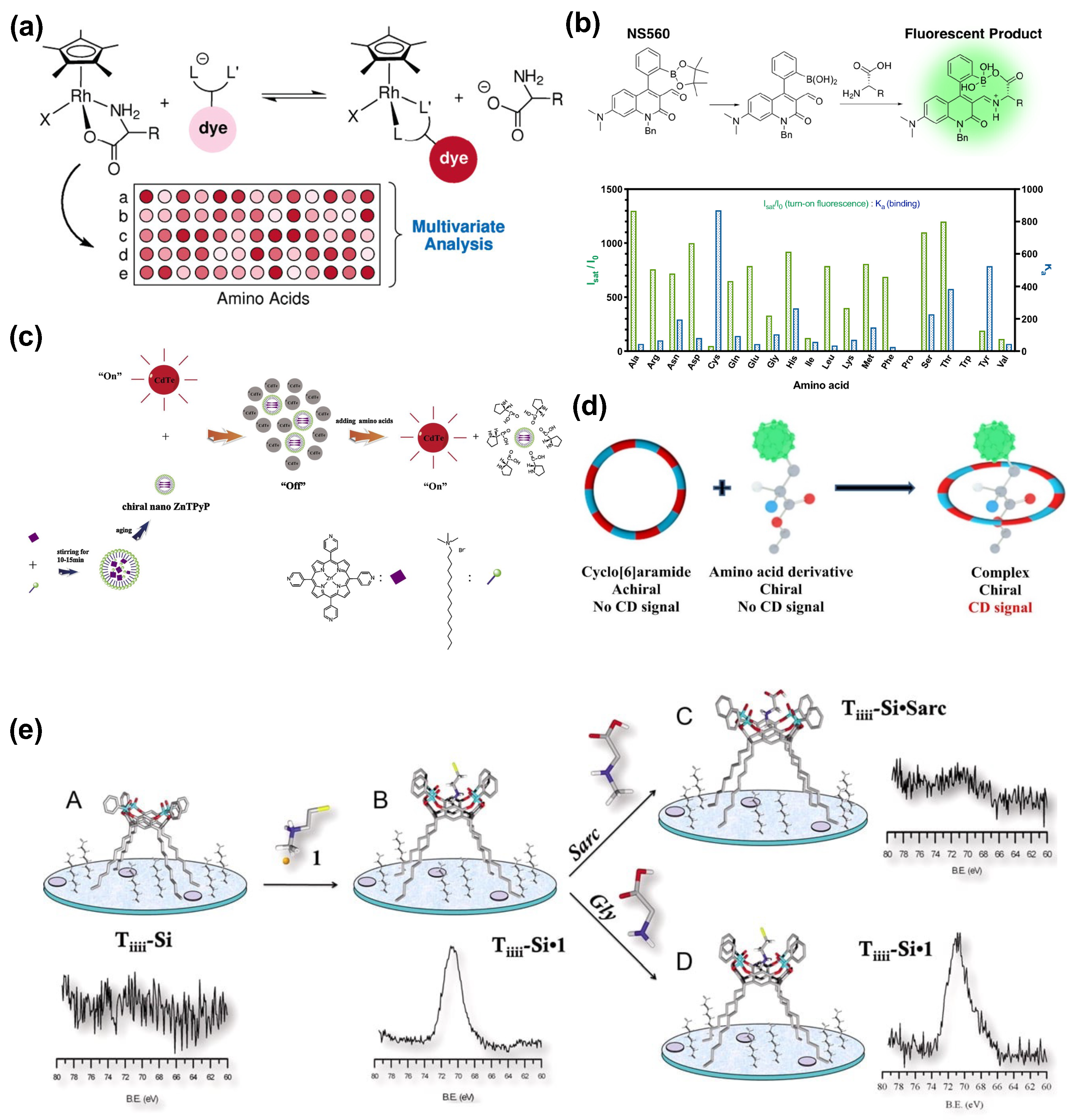
2.5. Amino Acids
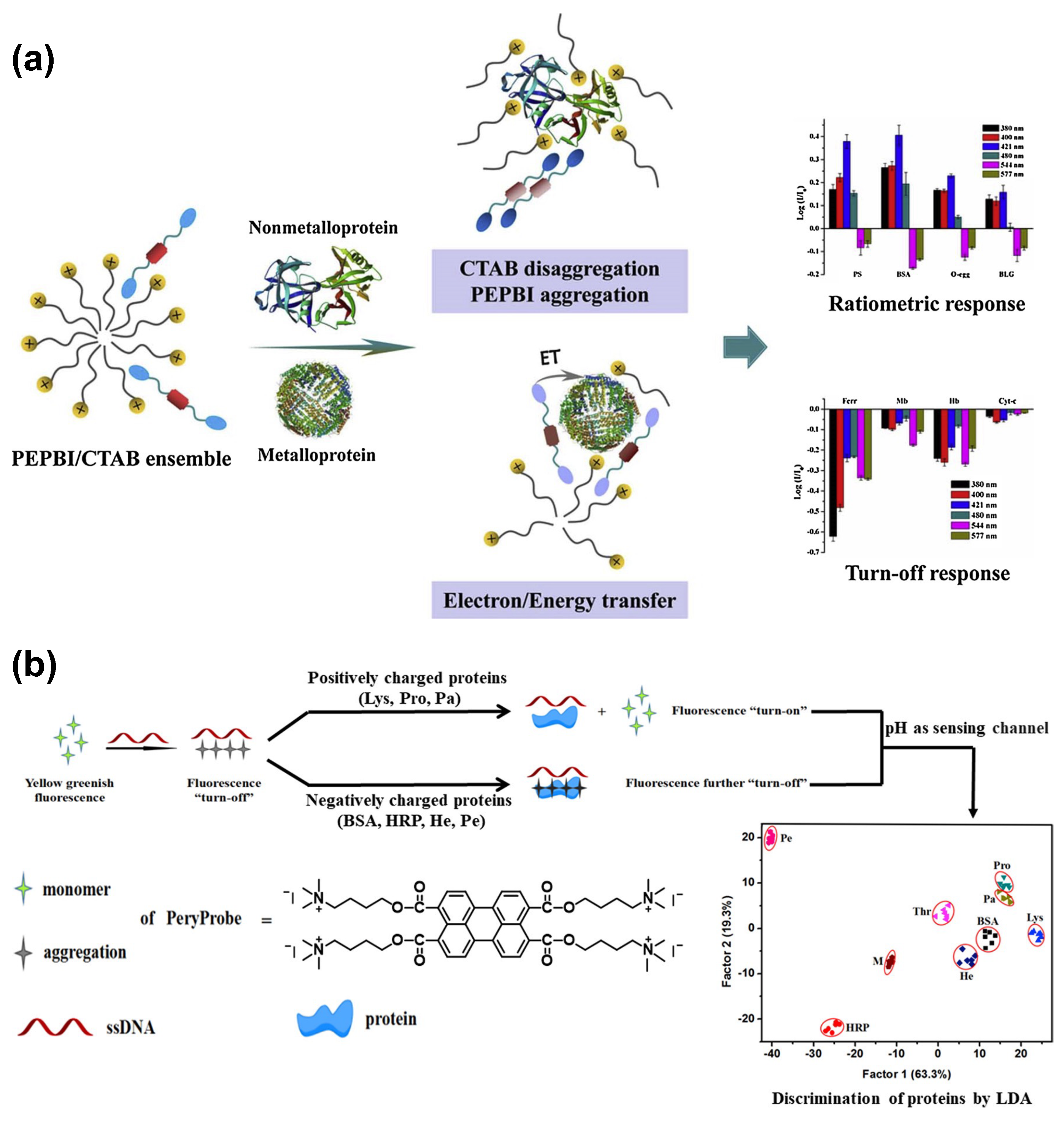
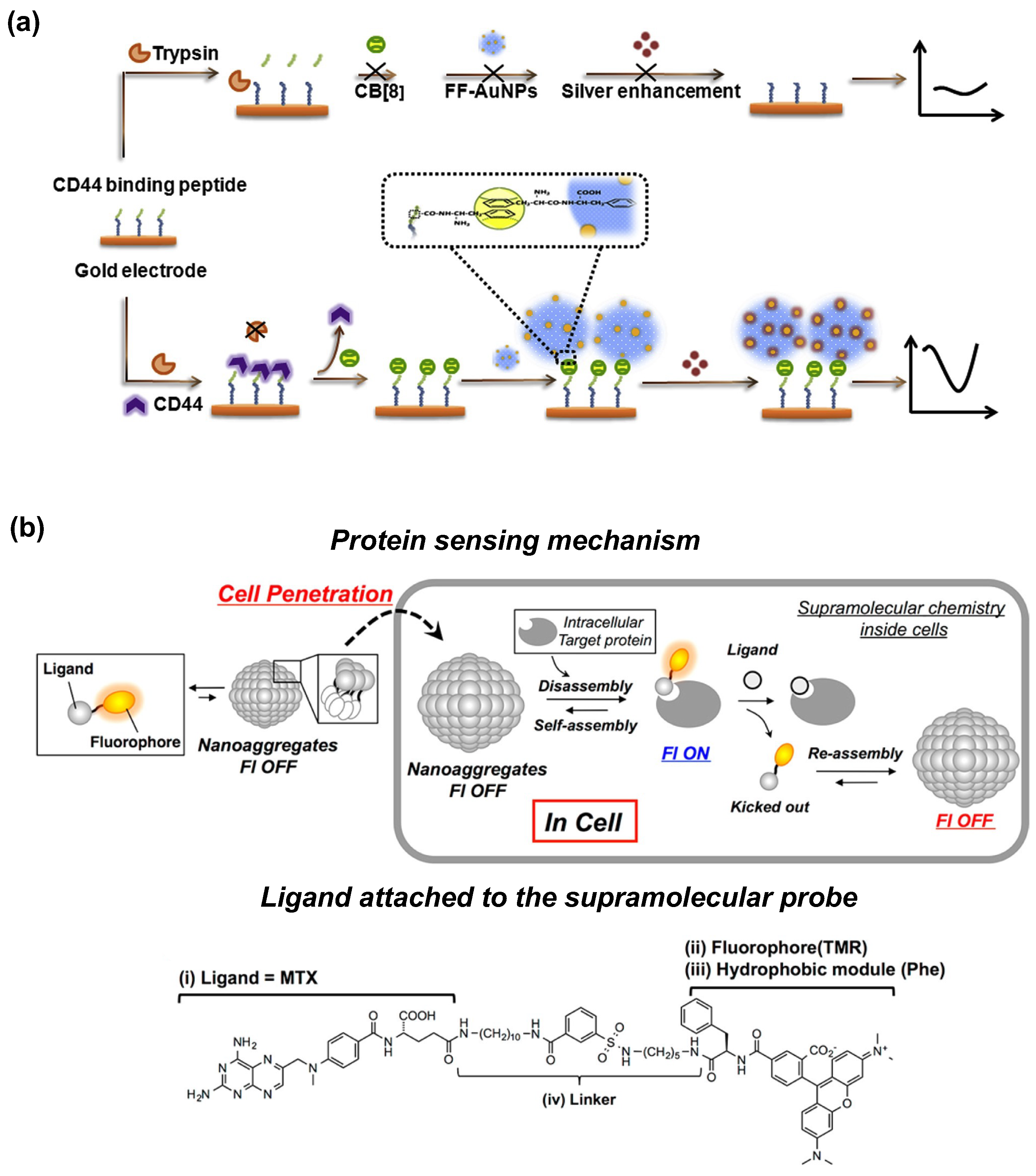
3. Protein Sensing
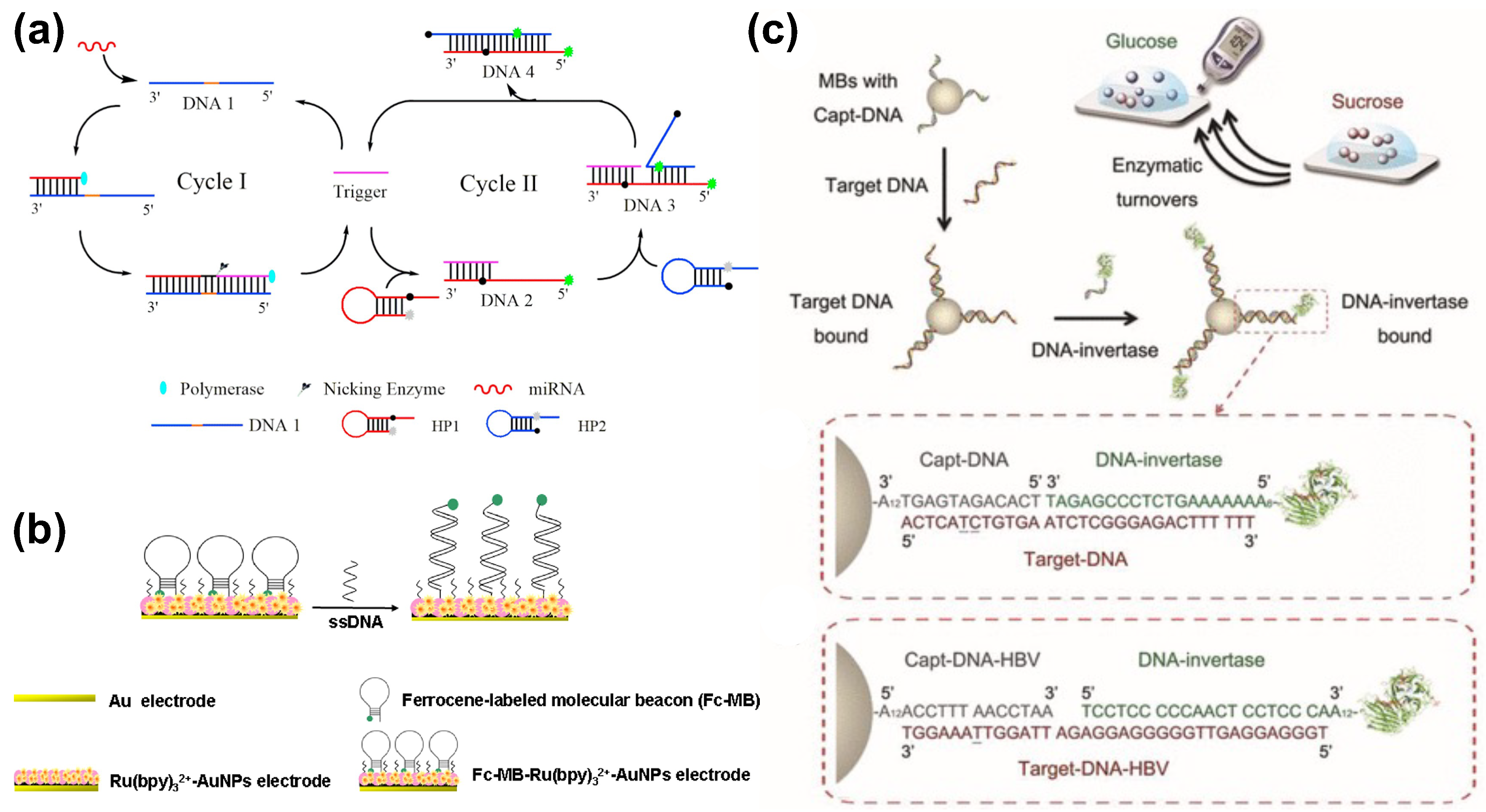
4. Nucleic Acid Sensing
5. Comparison of Different Methodologies
6. Scope and Future Prospect
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIEE | aggregation-induced enhancement of emission |
| AuNP | gold nanoparticle |
| α-HL | α-hemolysin |
| ABOs | amyloid beta oligos |
| Aβ | amyloid beta |
| ALG | acropora like gold |
| β-CD | β-cyclodextrin |
| BACE1 | beta-secretase 1 |
| BODIPY | 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene |
| CCDP | circular common target molecule (non-nucleic acid strand)-displacement polymerization |
| CSCs | cancer stem cells |
| CSF | cerebro-spinal fluid |
| CTAB | Cetyltrimethylammonium bromide |
| Ch | choline |
| CV | cyclic voltammetry |
| ConA | concanavalin A |
| DNA | deoxyribonucleic acid |
| DTAB | dodecyltrimethylammonium bromide |
| DNBS | 2,4-dinitrobenzenesulfonyl |
| DOPA | dopamine |
| DPV | differential pulse voltammetry |
| EXPAR | exponential isothermal amplification |
| FAM | 6-carboxyfluorescein |
| FRET | fluorescence resonance energy transfer |
| GCE | glassy carbon electrode |
| GCEs | glassy carbon electrodes |
| HSP | heat shock protein |
| 5-HT | 5- hydroxytryptamine |
| HP | hairpin probe |
| LD | Linear dichroism |
| LSPR | Localized Surface Plasmon Resonance |
| MOFs | Molecular organic frameworks |
| MWCNTs | Multi-walled carbon nanotubes |
| NIR | Near-infrared |
| OTAB | octadecyl-trimethylammonium bromide |
| PGM | personal glucose meter |
| PNA | peptide nucleic acid |
| PAMAM | poly(amidoamine) |
| PD-PAINT | proximity-dependent point accumulation imaging in nanoscale topography |
| PMP22 | Peripheral myelin protein 22 |
| PCR | polymerase chain reaction |
| PCN-222 | porphyrin-based metal-organic framework |
| PEPBI | bispyrene modified perylene |
| PET | photoinduced electron transfer |
| QDs | quantum dots |
| rGO | reduced graphene oxide |
| ROX | carboxy-X-rhodamine |
| SPCEs | screen-printed carbon electrodes |
| Tb-MOF | terbium-molecular organic framework |
| TPE | tetraphenylethylene |
| UPCs | up-conversion phosphor nanoparticles |
| 3WJ | three-way junction |
| XPS | X-ray photoelectron spectroscopy |
References
- Govaerts, M.J.; Van der Vleuten, C.P.; Schuwirth, L.W.; Muijtjens, A.M. Broadening perspectives on clinical performance assessment: Rethinking the nature of in-training assessment. Adv. Health Sci. Educ. 2007, 12, 239–260. [Google Scholar] [CrossRef]
- Gellman, S.H. Introduction: Molecular recognition. Chem. Rev. 1997, 97, 1231–1232. [Google Scholar] [CrossRef]
- Fischer, E. Influence of the configuration on the effect of the enzymes. Rep. Ger. Chem. Soc. 1894, 27, 2985–2993. [Google Scholar]
- Cram, D.J. The design of molecular hosts, guests, and their complexes (Nobel lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1009–1020. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular chemistry—Scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Pedersen, C.J. The discovery of crown ethers (Noble Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027. [Google Scholar] [CrossRef]
- Van Noorden, R.; Castelvecchi, D. World’s tiniest machines win chemistry Nobel. Nature 2016, 538, 152–153. [Google Scholar] [CrossRef]
- Sauvage, J.P. Transition metal-containing rotaxanes and catenanes in motion: Toward molecular machines and motors. Accounts Chem. Res. 1998, 31, 611–619. [Google Scholar] [CrossRef]
- Brough, B.; Northrop, B.H.; Schmidt, J.J.; Tseng, H.R.; Houk, K.N.; Stoddart, J.F.; Ho, C.M. Evaluation of synthetic linear motor-molecule actuation energetics. Proc. Natl. Acad. Sci. USA 2006, 103, 8583–8588. [Google Scholar] [CrossRef]
- Koumura, N.; Zijlstra, R.W.; van Delden, R.A.; Harada, N.; Feringa, B.L. Light-driven monodirectional molecular rotor. Nature 1999, 401, 152–155. [Google Scholar] [CrossRef]
- Browne, W.R.; Feringa, B.L. Making molecular machines work. Nat. Nanotechnol. 2006, 1, 25–35. [Google Scholar] [CrossRef]
- Davis, J.T. G-quartets 40 years later: From 5’-GMP to molecular biology and supramolecular chemistry. Angew. Chem. Int. Ed. Engl. 2004, 43, 668–698. [Google Scholar] [CrossRef]
- Guschlbauer, W.; Chantot, J.F.; Thiele, D. Four-stranded nucleic acid structures 25 years later: From guanosine gels to telomer DNA. J. Biomol. Struct. Dyn. 1990, 8, 491–511. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Kudr, J.; Nejdl, L.; Maskova, D.; Kizek, R.; Adam, V. G-quadruplexes as sensing probes. Molecules 2013, 18, 14760–14779. [Google Scholar] [CrossRef]
- Zhang, J.X.; Hoshino, K. Molecular Sensors and Nanodevices: Principles, Designs and Applications in Biomedical Engineering; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Guo, C.; Sedgwick, A.C.; Hirao, T.; Sessler, J.L. Supramolecular Fluorescent Sensors: An Historical Overview and Update. Coord. Chem. Rev. 2021, 427, 213560. [Google Scholar] [CrossRef]
- Gianneschi, N.C.; Nguyen, S.T.; Mirkin, C.A. Signal amplification and detection via a supramolecular allosteric catalyst. J. Am. Chem. Soc. 2005, 127, 1644–1645. [Google Scholar] [CrossRef]
- Klemm, B.; Roshanasan, A.; Piergentili, I.; van Esch, J.H.; Eelkema, R. Naked-eye thiol analyte detection via self-propagating, amplified reaction cycle. J. Am. Chem. Soc. 2023, 145, 21222–21230. [Google Scholar] [CrossRef]
- Goswami, A.; Saha, S.; Biswas, P.K.; Schmittel, M. (Nano)mechanical motion triggered by metal coordination: From functional devices to networked multicomponent catalytic machinery. Chem. Rev. 2019, 120, 125–199. [Google Scholar] [CrossRef]
- Ercolani, G. Assessment of Cooperativity in Self-Assembly. J. Am. Chem. Soc. 2003, 125, 16097–16103. [Google Scholar] [CrossRef] [PubMed]
- Hembury, G.A.; Borovkov, V.V.; Inoue, Y. Chirality-Sensing Supramolecular Systems. Chem. Rev. 2008, 108, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and Their Role in Improving the Solubility, Biocompatibility, Stability, Bioavailability, Detection, and Transport of Biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef]
- Notestein, J.M.; Katz, A.; Iglesia, E. Energetics of small molecule and water complexation in hydrophobic calixarene cavities. Langmuir 2006, 22, 4004–4014. [Google Scholar] [CrossRef]
- Men, G.; Han, W.; Chen, C.; Liang, C.; Jiang, S. A cyanide-sensing detector in aqueous solution based on anion-π interaction-driven electron transfer. Analyst 2019, 144, 2226–2230. [Google Scholar] [CrossRef]
- Lou, X.; Lafleur, R.P.M.; Leenders, C.M.A.; Schoenmakers, S.M.C.; Matsumoto, N.M.; Baker, M.B.; van Dongen, J.L.J.; Palmans, A.R.A.; Meijer, E.W. Dynamic diversity of synthetic supramolecular polymers in water as revealed by hydrogen/deuterium exchange. Nat. Commun. 2017, 8, 15420. [Google Scholar] [CrossRef]
- Banno, T.; Sawada, D.; Toyota, T. Construction of Supramolecular Systems That Achieve Lifelike Functions. Materials 2022, 15, 2391. [Google Scholar] [CrossRef]
- Nguyen, R.; Allouche, L.; Buhler, E.; Giuseppone, N. Dynamic combinatorial evolution within self-replicating supramolecular assemblies. Angew. Chem. Int. Ed. Engl. 2009, 48, 1093–1096. [Google Scholar] [CrossRef]
- Giuseppone, N.; Lehn, J.M. Electric-field modulation of component exchange in constitutional dynamic liquid crystals. Angew. Chem. Int. Ed. Engl. 2006, 45, 4619–4624. [Google Scholar] [CrossRef]
- Cho, Y.; Christoff-Tempesta, T.; Kaser, S.J.; Ortony, J.H. Dynamics in supramolecular nanomaterials. Soft Matter. 2021, 17, 5850–5863. [Google Scholar] [CrossRef]
- Chen, H.; Tong, K. The Contributions of Supramolecular Kinetics to Dynamics of Supramolecular Polymers. ChemPlusChem 2022, 87, e202200279. [Google Scholar] [CrossRef]
- Otto, S.; Severin, K. Dynamic Combinatorial Libraries for the Development of Synthetic Receptors and Sensors. In Creative Chemical Sensor Systems; Springer: Berlin/Heidelberg, Germany, 2007; pp. 267–288. [Google Scholar] [CrossRef]
- Harrison, E.E.; Carpenter, B.A.; St Louis, L.E.; Mullins, A.G.; Waters, M.L. Development of “Imprint-and-Report” Dynamic Combinatorial Libraries for Differential Sensing Applications. J. Am. Chem. Soc. 2021, 143, 14845–14854. [Google Scholar] [CrossRef]
- Javanbakht, S.; Darvishi, S.; Dorchei, F.; Hosseini-Ghalehno, M.; Dehghani, M.; Pooresmaeil, M.; Suzuki, Y.; Ul Ain, Q.; Ruiz Rubio, L.; Shaabani, A.; et al. Cyclodextrin Host–Guest Recognition in Glucose-Monitoring Sensors. ACS Omega 2023, 8, 33202–33228. [Google Scholar] [CrossRef]
- Nan, K.; Jiang, Y.N.; Li, M.; Wang, B. Recent Progress in Diboronic-Acid-Based Glucose Sensors. Biosensors 2023, 13, 618. [Google Scholar] [CrossRef]
- Schenning, A.P.H.J.; Meijer, E.W. Supramolecular electronics; nanowires from self-assembled π-conjugated systems. Chem. Commun. 2005, 26, 3245–3258. [Google Scholar] [CrossRef]
- Baeg, K.; Binda, M.; Natali, D.; Caironi, M.; Noh, Y. Organic light detectors: Photodiodes and phototransistors. Adv. Mater. 2013, 25, 4267–4295. [Google Scholar] [CrossRef]
- O’Donnell, A.; Salimi, S.; Hart, L.; Babra, T.; Greenland, B.; Hayes, W. Applications of supramolecular polymer networks. React. Funct. Polym. 2022, 172, 105209. [Google Scholar] [CrossRef]
- Clarke, S.F.; Foster, J.R. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br. J. Biomed. Sci. 2012, 69, 83–93. [Google Scholar] [CrossRef]
- Ahmad, A.; Imran, M.; Ahsan, H. Biomarkers as Biomedical Bioindicators: Approaches and Techniques for the Detection, Analysis, and Validation of Novel Biomarkers of Diseases. Pharmaceutics 2023, 15, 1630. [Google Scholar] [CrossRef]
- Aronson, J.K.; Ferner, R.E. Biomarkers—A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9.23.1–9.23.17. [Google Scholar] [CrossRef]
- Hussein, A.A.; Forouzanfar, T.; Bloemena, E.; de Visscher, J.; Brakenhoff, R.H.; Leemans, C.R.; Helder, M.N. A review of the most promising biomarkers for early diagnosis and prognosis prediction of tongue squamous cell carcinoma. Br. J. Cancer 2018, 119, 724–736. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef]
- Liu, D.; Evans, T.; Zhang, F. Applications and advances of metabolite biosensors for metabolic engineering. Metab. Eng. 2015, 31, 35–43. [Google Scholar] [CrossRef]
- Giuliano, K.A.; Taylor, D.L. Fluorescent-protein biosensors: New tools for drug discovery. Trends Biotechnol. 1998, 16, 135–140. [Google Scholar] [CrossRef]
- Moon, J.H.; MacLean, P.; McDaniel, W.; Hancock, L.F. Conjugated polymer nanoparticles for biochemical protein kinase assay. Chem. Commun. 2007, 46, 4910–4912. [Google Scholar] [CrossRef]
- Kong, L.Z.; Kim, S.M.; Wang, C.; Lee, S.Y.; Oh, S.C.; Lee, S.; Jo, S.; Kim, T.D. Understanding nucleic acid sensing and its therapeutic applications. Exp. Mol. Med. 2023, 55, 2320–2331. [Google Scholar] [CrossRef]
- Poole, L.; Hackett, R.A.; Panagi, L.; Steptoe, A. Subjective wellbeing as a determinant of glycated hemoglobin in older adults: Longitudinal findings from the English Longitudinal Study of Ageing. Psychol. Med. 2020, 50, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; James, T.D. Glucose sensing in supramolecular chemistry. Chem. Rev. 2015, 115, 8001–8037. [Google Scholar] [CrossRef]
- James, T.D.; Phillips, M.D.; Shinkai, S. Boronic Acids in Saccharide Recognition; Royal Society of Chemistry: London, UK, 2007. [Google Scholar]
- Barwell, N.P.; Crump, M.P.; Davis, A.P. A synthetic lectin for b-glucosyl. Angew. Chem. Int. Ed. 2009, 48, 7673–7676. [Google Scholar] [CrossRef]
- Ke, C.; Destecroix, H.; Crump, M.P.; Davis, A.P. A simple and accessible synthetic lectin for glucose recognition and sensing. Nat. Chem. 2012, 4, 718–723. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Y.; Wang, J.; Zhou, X.; Liu, Z. A new biosensor for glucose determination in serum based on up-converting fluorescence resonance energy transfer. Biosens. Bioelectron. 2011, 28, 414–420. [Google Scholar] [CrossRef]
- Melavanki, R.; Kusanur, R.; Sadasivuni, K.K.; Singh, D.; Patil, N. Investigation of interaction between boronic acids and sugar: Effect of structural change of sugars on binding affinity using steady state and time resolved fluorescence spectroscopy and molecular docking. Heliyon 2020, 6, e05081. [Google Scholar] [CrossRef]
- Cao, H.; Diaz, D.I.; DiCesare, N.; Lakowicz, J.R.; Heagy, M.D. Monoboronic acid sensor that displays anomalous fluorescence sensitivity to glucose. Org. Lett. 2002, 4, 1503–1505. [Google Scholar] [CrossRef]
- Cao, Z.; Nandhikonda, P.; Heagy, M.D. Highly water-soluble monoboronic acid probes that show optical sensitivity to glucose based on 4-sulfo-1, 8-naphthalic anhydride. J. Org. Chem. 2009, 74, 3544–3546. [Google Scholar] [CrossRef]
- Trupp, S.; Schweitzer, A.; Mohr, G.J. A fluorescent water-soluble naphthalimide-based receptor for saccharides with highest sensitivity in the physiological pH range. Org. Biomol. Chem. 2006, 4, 2965–2968. [Google Scholar] [CrossRef]
- Sun, X.; James, T.D.; Anslyn, E.V. Arresting “loose bolt” internal conversion from- B(OH)2 groups is the mechanism for emission turn-on in ortho-aminomethylphenylboronic acid-based saccharide sensors. J. Am. Chem. Soc. 2018, 140, 2348–2354. [Google Scholar] [CrossRef]
- James, T.D.; Sandanayake, K.S.; Shinkai, S. A glucose-selective molecular fluorescence sensor. Angew. Chem. Int. Ed. Engl. 1994, 33, 2207–2209. [Google Scholar] [CrossRef]
- James, T.D.; Sandanayake, K.S.; Iguchi, R.; Shinkai, S. Novel saccharide-photoinduced electron transfer sensors based on the interaction of boronic acid and amine. J. Am. Chem. Soc. 1995, 117, 8982–8987. [Google Scholar] [CrossRef]
- Wu, X.; Lin, L.R.; Huang, Y.J.; Li, Z.; Jiang, Y.B. A 2: 2 stilbeneboronic acid–γ-cyclodextrin fluorescent ensemble highly selective for glucose in aqueous solutions. Chem. Commun. 2012, 48, 4362–4364. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Fang, W.; Yang, L.; Hu, Q.; Wang, Z.; Sun, J.Z.; Tang, B.Z. Sugar-based aggregation-induced emission luminogens: Design, structures, and applications. Chem. Rev. 2020, 120, 4534–4577. [Google Scholar] [CrossRef]
- Lee, G.; Park, J.; Jang, S.H.; Lee, S.Y.; Seong, J.; Jung, J.W.; Kim, K.; Hwang, T.G.; Choi, J. Synthesis and characterization of diketopyrrolopyrrole-based aggregation-induced emission nanoparticles for bioimaging. Molecules 2022, 27, 2984. [Google Scholar] [CrossRef]
- Zalmi, G.A.; Jadhav, R.W.; Mirgane, H.A.; Bhosale, S.V. Recent advances in aggregation-induced emission active materials for sensing of biologically important molecules and drug delivery system. Molecules 2021, 27, 150. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Ouyang, W.J.; Wu, X.; Li, Z.; Fossey, J.S.; James, T.D.; Jiang, Y.B. Glucose sensing via aggregation and the use of “knock-out” binding to improve selectivity. J. Am. Chem. Soc. 2013, 135, 1700–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.t.; Wang, S.; Xing, G.w. Aggregates-based boronlectins with pyrene as fluorophore: Multichannel discriminative sensing of monosaccharides and their applications. ACS Appl. Mater. Interfaces 2016, 8, 12007–12017. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Cheng, H.; Chen, W.H.; Cheng, S.X.; Zhuo, R.X.; Zhang, X.Z. In situ recognition of cell-surface glycans and targeted imaging of cancer cells. Sci. Rep. 2013, 3, 2679. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, R.; Zhao, X.; Ma, Y.; Ren, L.; Ren, Y.; Chen, G.; Ye, D.; Wu, J.; Hu, X.; et al. Reversible recognition-based boronic acid probes for glucose detection in live cells and zebrafish. J. Am. Chem. Soc. 2023, 145, 8408–8416. [Google Scholar] [CrossRef] [PubMed]
- Stuart, D.A.; Yonzon, C.R.; Zhang, X.; Lyandres, O.; Shah, N.C.; Glucksberg, M.R.; Walsh, J.T.; Van Duyne, R.P. Glucose sensing using near-infrared surface-enhanced Raman spectroscopy: Gold surfaces, 10-day stability, and improved accuracy. Anal. Chem. 2005, 77, 4013–4019. [Google Scholar] [CrossRef] [PubMed]
- Elfeky, S.A.; D’Hooge, F.; Poncel, L.; Chen, W.; Perera, S.P.; van den Elsen, J.M.; James, T.D.; Jenkins, A.T.A.; Cameron, P.J.; Fossey, J.S. A surface plasmon enhanced fluorescence sensor platform. New J. Chem. 2009, 33, 1466–1469. [Google Scholar] [CrossRef]
- Kong, K.V.; Lam, Z.; Lau, W.K.O.; Leong, W.K.; Olivo, M. A transition metal carbonyl probe for use in a highly specific and sensitive SERS-based assay for glucose. J. Am. Chem. Soc. 2013, 135, 18028–18031. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, H.; Park, C.S.; Yim, H.S.; Kim, D.; Lee, S.; Lee, Y. Diboronic-Acid-Based electrochemical sensor for enzyme-free selective and sensitive glucose detection. Biosensors 2023, 13, 248. [Google Scholar] [CrossRef]
- Adil, L.R.; Parui, R.; Khatun, M.N.; Chanu, M.A.; Li, L.; Wang, S.; Iyer, P.K. Nanomaterials for sensors: Synthesis and applications. In Advanced Nanomaterials for Point of Care Diagnosis and Therapy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 121–168. [Google Scholar]
- Wang, F.; Zhang, J.; Zhang, M.; Xu, C.; Cheng, S.; Wang, Q.; Zhang, F.; He, X.; He, P. A multi-calibration potentiometric sensing array based on diboronic acid-PtAu/CNTs nanozyme for home monitoring of urine glucose. Anal. Chim. Acta 2023, 1237, 340598. [Google Scholar] [CrossRef]
- Stephenson-Brown, A.; Wang, H.C.; Iqbal, P.; Preece, J.A.; Long, Y.; Fossey, J.S.; James, T.D.; Mendes, P.M. Glucose selective surface plasmon resonance-based bis-boronic acid sensor. Analyst 2013, 138, 7140–7145. [Google Scholar] [CrossRef] [PubMed]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Noninvasive continuous monitoring of physiological glucose using a monosaccharide-sensing contact lens. Anal. Chem. 2004, 76, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Glucose sensing with phenylboronic acid functionalized hydrogel-based optical diffusers. ACS Nano 2018, 12, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Ng, S.C.; Hsu, J.Y.; Liu, H.; Chen, C.J.; Huang, C.Y.; Kuo, W.W. Galangin reverses H2O2-induced dermal fibroblast senescence via SIRT1-PGC-1α/NRF2 signaling. Int. J. Mol. Sci. 2022, 23, 1387. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.H.; Kader, K.N. Mechanisms of H2O2-induced oxidative stress in endothelial cells exposed to physiologic shear stress. Asaio J. 2007, 53, 17–22. [Google Scholar] [CrossRef]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to cause oxidative stress, the catalase issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Supramolecular self-healing materials from non-covalent cross-linking host–guest interactions. Chem. Commun. 2020, 56, 4381–4395. [Google Scholar] [CrossRef]
- Kang, Y.; Ju, X.; Ding, L.S.; Zhang, S.; Li, B.J. Reactive oxygen species and glutathione dual redox-responsive supramolecular assemblies with controllable release capability. ACS Appl. Mater. Interfaces 2017, 9, 4475–4484. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Qian, C.; Lu, Y.; Kahkoska, A.R.; Xie, Z.; Jing, X.; Buse, J.B.; Gu, Z. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano 2017, 11, 613–620. [Google Scholar] [CrossRef]
- Lee, D.; Khaja, S.; Velasquez-Castano, J.C.; Dasari, M.; Sun, C.; Petros, J.; Taylor, W.R.; Murthy, N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007, 6, 765–769. [Google Scholar] [CrossRef]
- Jiang, S.; Yang, J.; Ling, L.; Wang, S.; Ma, D. Supramolecular fluorescent probes for the detection of reactive oxygen species discovered via high-throughput screening. Anal. Chem. 2022, 94, 5634–5641. [Google Scholar] [CrossRef]
- Peng, L.; Feng, A.; Huo, M.; Yuan, J. Ferrocene-based supramolecular structures and their applications in electrochemical responsive systems. Chem. Commun. 2014, 50, 13005–13014. [Google Scholar] [CrossRef]
- Falcone, N.; Basak, S.; Dong, B.; Syed, J.; Ferranco, A.; Lough, A.; She, Z.; Kraatz, H.B. A ferrocene–tryptophan conjugate: The role of the indolic nitrogen in supramolecular assembly. ChemPlusChem 2017, 82, 1282–1289. [Google Scholar] [CrossRef]
- Falcone, N.; Kraatz, H.B. Ferrocene Peptide-based Supramolecular Gels: Current Trends and Applications. In Advances in Bioorganometallic Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 57–74. [Google Scholar]
- Menger, F.; Sherrod, M. Docking calculations on ferrocene complexation with cyclodextrins. J. Am. Chem. Soc. 1988, 110, 8606–8611. [Google Scholar] [CrossRef]
- Luong, J.H.; Brown, R.S.; Schmidt, P.M. Characterization of interacting ferrocene–cyclodextrin systems and their role in mediated biosensors. J. Mol. Recognit. 1995, 8, 132–138. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, L.; Liu, F.; Astruc, D.; Gu, H. Supramolecular redox-responsive ferrocene hydrogels and microgels. Coord. Chem. Rev. 2020, 419, 213406. [Google Scholar] [CrossRef]
- Casas-Solvas, J.M.; Ortiz-Salmerón, E.; Fernández, I.; García-Fuentes, L.; Santoyo-González, F.; Vargas-Berenguel, A. Ferrocene–β-Cyclodextrin Conjugates: Synthesis, Supramolecular Behavior, and Use as Electrochemical Sensors. Chem. Eur. J. 2009, 15, 8146–8162. [Google Scholar] [CrossRef]
- Kasprzak, A.; Koszytkowska-Stawinska, M.; Nowicka, A.M.; Buchowicz, W.; Poplawska, M. Supramolecular interactions between β-cyclodextrin and the nucleobase derivatives of ferrocene. J. Org. Chem. 2019, 84, 15900–15914. [Google Scholar] [CrossRef]
- Ertas, N.A.; Kavak, E.; Salman, F.; Kazici, H.C.; Kivrak, H.; Kivrak, A. Synthesis of ferrocene based naphthoquinones and its application as novel non-enzymatic hydrogen peroxide. Electroanalysis 2020, 32, 1178–1185. [Google Scholar] [CrossRef]
- Fan, M.F.; Wang, H.M.; Nan, L.J.; Wang, A.J.; Luo, X.; Yuan, P.X.; Feng, J.J. The mimetic assembly of cobalt prot-porphyrin with cyclodextrin dimer and its application for H2O2 detection. Anal. Chim. Acta 2020, 1097, 78–84. [Google Scholar] [CrossRef]
- Liu, W.; Pan, H.; Liu, C.; Su, C.; Liu, W.; Wang, K.; Jiang, J. Ultrathin phthalocyanine-conjugated polymer nanosheet-based electrochemical platform for accurately detecting H2O2 in real time. ACS Appl. Mater. Interfaces 2019, 11, 11466–11473. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, P.; Zhou, S.; Chen, S.; Liang, Y.; Tian, F.; Zhou, J.; Huo, D.; Hou, C. Development of Au–Pd@ UiO-66-on-ZIF-L/CC as a self-supported electrochemical sensor for in situ monitoring of cellular hydrogen peroxide. J. Mater. Chem. 2021, 9, 9031–9040. [Google Scholar] [CrossRef]
- Dey, N.; Haynes, C.J. Supramolecular coordination complexes as optical biosensors. ChemPlusChem 2021, 86, 418–433. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, H.; Li, Y.; Zhang, Q.; Li, J.; Zhang, C.; Zhou, C.; Yu, C. Peroxidase activity of the coronene bisimide supramolecular architecture and its applications in colorimetric sensing of H2O2 and glucose. J. Mater. Chem. B 2017, 5, 6572–6578. [Google Scholar] [CrossRef]
- Hu, F.; Huang, Y.; Zhang, G.; Zhao, R.; Zhang, D. A highly selective fluorescence turn-on detection of hydrogen peroxide and D-glucose based on the aggregation/deaggregation of a modified tetraphenylethylene. Tetrahedron Lett. 2014, 55, 1471–1474. [Google Scholar] [CrossRef]
- Dutta, A.; Maitra, U. Naked-Eye Detection of Hydrogen Peroxide on Photoluminescent Paper Discs. ACS Sensors 2022, 7, 513–522. [Google Scholar] [CrossRef]
- Ren, C.; Chu, L.; Huang, F.; Yang, L.; Fan, H.; Liu, J.; Yang, C. A novel H2O2 responsive supramolecular hydrogel for controllable drug release. RSC Adv. 2017, 7, 1313–1317. [Google Scholar] [CrossRef]
- Chan, C.W.T.; Law, A.S.Y.; Yam, V.W.W. A Luminescence Assay in the Red for the Detection of Hydrogen Peroxide and Glucose Based on Metal Coordination Polyelectrolyte-Induced Supramolecular Self-Assembly of Alkynylplatinum (II) Complexes. Chem. Eur. J. 2023, 29, e202300203. [Google Scholar] [CrossRef]
- Anastassopoulou, J.; Theophanides, T. The role of metal ions in biological systems and medicine. In Bioinorganic Chemistry: An Inorganic Perspective of Life; Springer: Berlin/Heidelberg, Germany, 1995; pp. 209–218. [Google Scholar]
- Garg, A. Role of Metal ions in Biological System. Contemp. Adv. Sci. Technol. 2022, 2, 67. [Google Scholar]
- Kleczkowski, M.; Garncarz, M. The role of metal ions in biological oxidation-the past and the present. Pol. J. Vet. Sci. 2012, 15, 165–173. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H.; Sigel, R.K. Interrelations between Essential Metal Ions and Human Diseases; Springer: Berlin/Heidelberg, Germany, 2013; Volume 13. [Google Scholar]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar]
- Sigel, H.; McCormick, D.B. Discriminating behavior of metal ions and ligands with regard to their biological significance. Accounts Chem. Res. 1970, 3, 201–208. [Google Scholar] [CrossRef]
- Aich, P.; Dasgupta, D. Role of magnesium ion in mithramycin-DNA interaction: Binding of mithramycin-Mg2+ complexes with DNA. Biochemistry 1995, 34, 1376–1385. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, W.; Xue, Y.; Zhang, J. Fluorescent Sensors for Detecting and Imaging Metal Ions in Biological Systems: Recent Advances and Future Perspectives. Chemosensors 2023, 11, 226. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Bradshaw, J.S.; Izatt, R.M. Crown ethers: The search for selective ion ligating agents. Accounts Chem. Res. 1997, 30, 338–345. [Google Scholar] [CrossRef]
- Gokel, G.W. Crown ethers. In Encyclopedia of Supramolecular Chemistry-Two-Volume Set (Print); CRC Press: Boca Raton, FL, USA, 2013; pp. 326–333. [Google Scholar]
- Ullah, F.; Khan, T.A.; Iltaf, J.; Anwar, S.; Khan, M.F.A.; Khan, M.R.; Ullah, S.; Fayyaz ur Rehman, M.; Mustaqeem, M.; Kotwica-Mojzych, K.; et al. Heterocyclic crown ethers with potential biological and pharmacological properties: From synthesis to applications. Appl. Sci. 2022, 12, 1102. [Google Scholar] [CrossRef]
- Aprahamian, I. The future of molecular machines. ACS Cent. Sci. 2020, 6, 347–358. [Google Scholar] [CrossRef]
- Moulin, E.; Faour, L.; Carmona-Vargas, C.C.; Giuseppone, N. From molecular machines to stimuli-responsive materials. Adv. Mater. 2020, 32, 1906036. [Google Scholar] [CrossRef]
- Tsukube, H. Double armed crown ethers and armed macrocycles as a new series of metal-selective reagents: A review. Talanta 1993, 40, 1313–1324. [Google Scholar] [CrossRef]
- Yoshio, M.; Noguchi, H. Crown ethers for chemical analysis: A Review. Anal. Lett. 1982, 15, 1197–1276. [Google Scholar] [CrossRef]
- Nakamura, H.; Takagi, M.; Ueno, K. Photometric reagents for alkali metal ions, based on crown-ether complex formation—III 4-picrylaminobenzo-15-crown-5 derivatives. Talanta 1979, 26, 921–927. [Google Scholar] [CrossRef]
- Takagi, M.; Nakamura, H.; Ueno, K. A novel colorimetric reagent for potassium based on crown ether complex formation. Anal. Lett. 1977, 10, 1115–1122. [Google Scholar] [CrossRef]
- Stubing, D.; Heng, S.; Abell, A. Crowned spiropyran fluoroionophores with a carboxyl moiety for the selective detection of lithium ions. Org. Biomol. Chem. 2016, 14, 3752–3757. [Google Scholar] [CrossRef]
- Kang, J.; Li, E.; Cui, L.; Shao, Q.; Yin, C.; Cheng, F. Lithium ion specific fluorescent reversible extraction-release based on spiropyran isomerization combining crown ether coordination and its bioimaging. Sensors Actuators Chem. 2021, 327, 128941. [Google Scholar] [CrossRef]
- Gunnar, O.; Jens, U.; Qijin, C. Crown-Ether Derived Graphene Hybrid Composite for Membrane-Free Potentiometric Sensing of Alkali Metal Ions. ACS Appl. Mater. Interfaces 2016, 8, 37–41. [Google Scholar]
- Sun, H.; Chen, H.; Zhang, X.; Liu, Y.; Guan, A.; Li, Q.; Yang, Q.; Shi, Y.; Xu, S.; Tang, Y. Colorimetric detection of sodium ion in serum based on the G-quadruplex conformation related DNAzyme activity. Anal. Chim. Acta 2016, 912, 133–138. [Google Scholar] [CrossRef]
- Dey, N. Coordination-driven reversible supramolecular assembly formation at biological pH: Trace-level detection of Hg2+ and I− ions in real life samples. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2022, 267, 120447. [Google Scholar] [CrossRef]
- Gul, A.; Oguz, M.; Kursunlu, A.N.; Yilmaz, M. A novel colorimetric/fluorometric dual-channel sensor based on phenolphthalein and Bodipy for Sn (II) and Al (III) ions in half-aqueous medium and its applications in bioimaging. Dyes Pigments 2020, 176, 108221. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, R.; Feng, Y.; Liu, Z.; Huang, J.; Ma, C.; Wang, K. Functional nucleic acid-based fluorescent probes for metal ion detection. Coord. Chem. Rev. 2022, 459, 214453. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, J.; Yang, Z.; Mou, Q.; Ma, Y.; Xiong, Y.; Lu, Y. Functional DNA regulated CRISPR-Cas12a sensors for point-of-care diagnostics of non-nucleic-acid targets. J. Am. Chem. Soc. 2019, 142, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Bai, J.; Zhang, S.; Li, C. A guanosine-based 2-formylphenylborate ester hydrogel with high selectivity to K+ ions. RSC Adv. 2020, 10, 28536–28540. [Google Scholar] [CrossRef]
- Chitbankluai, K.; Thavarungkul, P.; Kanatharana, P.; Kaewpet, M.; Buranachai, C. Newly found K+-Thioflavin T competitive binding to DNA G-quadruplexes and the development of a label-free fluorescent biosensor with extra low detection limit for K+ determination in urine samples. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2022, 276, 121244. [Google Scholar] [CrossRef] [PubMed]
- Deore, P.S.; Manderville, R.A. Ratiometric fluorescent sensing of the parallel G-quadruplex produced by PS2. M: Implications for K+ detection. Analyst 2020, 145, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, J.; Liu, H.; Gan, J.; Chandrasekaran, A.R.; Sheng, J. Fluorescent Aptaswitch for Detection of Lead Ions. ACS Appl. Bio Mater. 2022, 5, 5089–5093. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Gao, T.; Zhang, T.; Xu, L.; Wang, B.; Wang, J.; Pei, R. Selection of DNA aptamers for the development of light-up biosensor to detect Pb (II). Sens. Actuators Chem. 2018, 254, 214–221. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, C.; Ma, T.; Liu, X.; Chen, Z.; Li, S.; Deng, Y. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J. Nanobiotechnol. 2021, 19, 166. [Google Scholar] [CrossRef]
- Zhou, W.; Ding, J.; Liu, J. A selective Na+ aptamer dissected by sensitized Tb3+ luminescence. ChemBioChem 2016, 17, 1563–1570. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J. An in Vitro–Selected DNAzyme Mutant Highly Specific for Na+ under Slightly Acidic Conditions. ChemBioChem 2019, 20, 537–542. [Google Scholar] [CrossRef]
- Zhou, W.; Saran, R.; Chen, Q.; Ding, J.; Liu, J. A new Na+-dependent RNA-cleaving DNAzyme with over 1000-fold rate acceleration by ethanol. ChemBioChem 2016, 17, 159–163. [Google Scholar] [CrossRef]
- Torabi, S.F.; Wu, P.; McGhee, C.E.; Chen, L.; Hwang, K.; Zheng, N.; Cheng, J.; Lu, Y. In vitro selection of a sodium-specific DNAzyme and its application in intracellular sensing. Proc. Natl. Acad. Sci. USA 2015, 112, 5903–5908. [Google Scholar] [CrossRef]
- McGhee, C.E.; Yang, Z.; Guo, W.; Wu, Y.; Lyu, M.; DeLong, C.J.; Hong, S.; Ma, Y.; McInnis, M.G.; O’Shea, K.S.; et al. DNAzyme-based lithium-selective imaging reveals higher lithium accumulation in bipolar disorder patient-derived neurons. ACS Cent. Sci. 2021, 7, 1809–1820. [Google Scholar] [CrossRef]
- Yang, C.; Yin, X.; Huan, S.Y.; Chen, L.; Hu, X.X.; Xiong, M.Y.; Chen, K.; Zhang, X.B. Two-photon DNAzyme–gold nanoparticle probe for imaging intracellular metal ions. Anal. Chem. 2018, 90, 3118–3123. [Google Scholar] [CrossRef]
- Yu, Q.; Shi, J.; Mudiyanselage, A.P.K.; Wu, R.; Zhao, B.; Zhou, M.; You, M. Genetically encoded RNA-based sensors for intracellular imaging of silver ions. Chem. Commun. 2019, 55, 707–710. [Google Scholar] [CrossRef]
- Huang, P.J.J.; Liu, J. An ultrasensitive light-up Cu2+ biosensor using a new DNAzyme cleaving a phosphorothioate-modified substrate. Anal. Chem. 2016, 88, 3341–3347. [Google Scholar] [CrossRef]
- Zhan, S.; Xu, H.; Zhang, W.; Zhan, X.; Wu, Y.; Wang, L.; Zhou, P. Sensitive fluorescent assay for copper (II) determination in aqueous solution using copper-specific ssDNA and Sybr Green I. Talanta 2015, 142, 176–182. [Google Scholar] [CrossRef]
- Saran, R.; Chen, Q.; Liu, J. Searching for a DNAzyme Version of the Leadzyme. J. Mol. Evol. 2015, 81, 235–244. [Google Scholar]
- Ren, W.; Huang, P.J.J.; de Rochambeau, D.; Moon, W.J.; Zhang, J.; Lyu, M.; Wang, S.; Sleiman, H.; Liu, J. Selection of a metal ligand modified DNAzyme for detecting Ni2+. Biosens. Bioelectron. 2020, 165, 112285. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Xu, S.; Li, C.; Dong, B. Recent progress in fluorescent probes for metal ion detection. Front. Chem. 2022, 10, 875241. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Yi, T.; Piao, Y.; Park, D.H.; Cui, L.; Cui, C. Heavy Metal Ions Trigger a Fluorescent Quenching in DNA–Organic Semiconductor Hybrid Assemblies. Polymers 2022, 14, 3591. [Google Scholar] [CrossRef]
- Madhu, S.; Sharma, D.K.; Basu, S.K.; Jadhav, S.; Chowdhury, A.; Ravikanth, M. Sensing Hg (II) in vitro and in vivo using a benzimidazole substituted BODIPY. Inorg. Chem. 2013, 52, 11136–11145. [Google Scholar] [CrossRef]
- Sprenger, T.; Schwarze, T.; Müller, H.; Sperlich, E.; Kelling, A.; Holdt, H.J.; Paul, J.; Martos Riaño, V.; Nazaré, M. BODIPY-Equipped Benzo-Crown-Ethers as Fluorescent Sensors for pH Independent Detection of Sodium and Potassium Ions. ChemPhotoChem 2023, 7, e202200270. [Google Scholar] [CrossRef]
- Lin, Q.; Gruskos, J.J.; Buccella, D. Bright, red emitting fluorescent sensor for intracellular imaging of Mg2+. Org. Biomol. Chem. 2016, 14, 11381–11388. [Google Scholar] [CrossRef]
- Kursunlu, A.N. A fluorescent “turn on” chemosensor based on Bodipy–anthraquinone for Al (III) ions: Synthesis and complexation/spectroscopic studies. RSC Adv. 2015, 5, 41025–41032. [Google Scholar] [CrossRef]
- Dodani, S.C.; He, Q.; Chang, C.J. A turn-on fluorescent sensor for detecting nickel in living cells. J. Am. Chem. Soc. 2009, 131, 18020–18021. [Google Scholar] [CrossRef]
- Sui, B.; Tang, S.; Liu, T.; Kim, B.; Belfield, K.D. Novel BODIPY-based fluorescence turn-on sensor for Fe3+ and its bioimaging application in living cells. ACS Appl. Mater. Interfaces 2014, 6, 18408–18412. [Google Scholar] [CrossRef]
- Xue, X.; Fang, H.; Chen, H.; Zhang, C.; Zhu, C.; Bai, Y.; He, W.; Guo, Z. In vivo fluorescence imaging for Cu2+ in live mice by a new NIR fluorescent sensor. Dyes Pigments 2016, 130, 116–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Niu, L.Y.; Yang, Q.Z.; Guan, Y.F.; Feng, L. An SPE-assisted BODIPY fluorometric paper sensor for the highly selective and sensitive determination of Cd2+ in complex sample: Rice. Analyst 2014, 139, 3146–3153. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, D.; Lu, Q.; Zhang, R.; Xia, J. Rational design of fluorescent chemosensor for Pd2+ based on the formation of cyclopalladated complex. Talanta 2023, 253, 123967. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, T.; Liu, H. Naked-eye chromogenic and fluorogenic chemosensor for mercury (II) ion based on substituted distyryl BODIPY complex. Dyes Pigments 2019, 165, 65–70. [Google Scholar] [CrossRef]
- Goshisht, M.K.; Patra, G.K.; Tripathi, N. Fluorescent Schiff base sensors as a versatile tool for metal ion detection: Strategies, mechanistic insights, and applications. Mater. Adv. 2022, 3, 2612–2669. [Google Scholar] [CrossRef]
- Ghorai, A.; Mondal, J.; Saha, R.; Bhattacharya, S.; Patra, G.K. A highly sensitive reversible fluorescent-colorimetric azino bis-Schiff base sensor for rapid detection of Pb2+ in aqueous media. Anal. Methods 2016, 8, 2032–2040. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Liu, S.; Zhang, L.; Wang, G. Synthesis and characterization of fire-safety PET by Schiff base with nitro group. Eur. Polym. J. 2021, 145, 110230. [Google Scholar] [CrossRef]
- Kumar, J.; Sarma, M.J.; Phukan, P.; Das, D.K. A new simple Schiff base fluorescence “on” sensor for Al3+ and its living cell imaging. Dalton Trans. 2015, 44, 4576–4581. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, J.; Park, S.; Jung, J.; Kim, D. A schiff base fluorescence enhancement probe for Fe(III) and its sensing applications in cancer cells. Sensors 2019, 19, 2500. [Google Scholar] [CrossRef] [PubMed]
- Pisagatti, I.; Crisafulli, D.; Pappalardo, A.; Sfrazzetto, G.T.; Notti, A.; Nastasi, F.; Parisi, M.; Micali, N.; Gattuso, G.; Villari, V. Photoinduced electron transfer in host-guest interactions of a viologen derivative with a didansyl-pillar [5] arene. Mater. Today Chem. 2022, 24, 100841. [Google Scholar] [CrossRef]
- Wang, P.; Wu, X.; Wu, J.; Liao, Y. Highly selective and sensitive peptide-based fluorescent chemosensor for detection of Zinc (II) ions in aqueous medium and living cells. J. Photochem. Photobiol. Chem. 2019, 382, 111929. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Zamani-Kalajahi, M. A turn-on/off fluorescent sensor based on nano-structured Mg-Al layered double hydroxide intercalated with salicylic acid for monitoring of ferric ion in human serum samples. Anal. Chim. Acta 2019, 1061, 152–160. [Google Scholar] [CrossRef]
- Zheng, W.; Li, H.; Chen, W.; Zhang, J.; Wang, N.; Guo, X.; Jiang, X. Rapid detection of copper in biological systems using click chemistry. Small 2018, 14, 1703857. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, G.; Lee, T.S. New fluorescent metal-ion detection using a paper-based sensor strip containing tethered rhodamine carbon nanodots. ACS Appl. Mater. Interfaces 2015, 7, 15649–15657. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, Y.G.; Kang, J.; Yang, S.H.; Kim, J.H.; Ghisaidoobe, A.B.T.; Kang, H.J.; Lee, S.R.; Lim, M.H.; Chung, S.J. Monitoring metal–amyloid-β complexation by a FRET-based probe: Design, detection, and inhibitor screening. Chem. Sci. 2019, 10, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Loewi, O. Über humorale übertragbarkeit der Herznervenwirkung. PflüGers Arch. Eur. J. Physiol. 1921, 189, 239–242. [Google Scholar] [CrossRef]
- Goldstein, D.S. Catecholamines: Bridging basic science with clinical medicine. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Seto, D.; Maki, T.; Soh, N.; Nakano, K.; Ishimatsu, R.; Imato, T. A simple and selective fluorometric assay for dopamine using a calcein blue–Fe2+ complex fluorophore. Talanta 2012, 94, 36–43. [Google Scholar] [CrossRef]
- Suzuki, Y. Design and synthesis of fluorescent reagents for selective detection of dopamine. Sensors Actuators Chem. 2017, 239, 383–389. [Google Scholar] [CrossRef]
- Ganguly, M.; Mondal, C.; Jana, J.; Pal, A.; Pal, T. Selective dopamine chemosensing using silver-enhanced fluorescence. Langmuir 2014, 30, 4120–4128. [Google Scholar] [CrossRef]
- Raymo, F.M.; Cejas, M.A. Supramolecular association of dopamine with immobilized fluorescent probes. Org. Lett. 2002, 4, 3183–3185. [Google Scholar] [CrossRef]
- Pizer, R.; Babcock, L. Mechanism of the complexation of boron acids with catechol and substituted catechols. Inorg. Chem. 1977, 16, 1677–1681. [Google Scholar] [CrossRef]
- Coskun, A.; Akkaya, E.U. Three-point recognition and selective fluorescence sensing of L-DOPA. Org. Lett. 2004, 6, 3107–3109. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Y.; Qin, Y. Polymeric membrane sensors with boronic acid functionalized boron dipyrromethene for selective measurement of dopamine. Sens. Actuators Chem. 2014, 191, 227–232. [Google Scholar] [CrossRef]
- Sangubotla, R.; Won, S.; Kim, J. Boronic acid-modified fluorescent sensor using coffee biowaste-based carbon dots for the detection of dopamine. J. Photochem. Photobiol. Chem. 2023, 438, 114542. [Google Scholar] [CrossRef]
- Rafiee, M.; Nematollahi, D. Electrochemical study of catechol–boric acid complexes. Electrochim. Acta 2008, 53, 2751–2756. [Google Scholar] [CrossRef]
- Rather, I.A.; Sofi, F.A.; Bhat, M.A.; Ali, R. Synthesis of novel one-walled meso-phenylboronic acid-functionalized calix [4] pyrrole: A highly sensitive electrochemical sensor for dopamine. ACS Omega 2022, 7, 15082–15089. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, J.; Wu, X.; Du, A. A sensitive fluorimetric method for the determination of epinephrine. J. Fluoresc. 2005, 15, 131–136. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, J.; Wu, X.; Mao, H. Study on the co-luminescence effect of Tb–Gd–epinephrine system and its application to the sensitive determination of epinephrine at nanomol level. Talanta 2007, 73, 227–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, B. A 2-in-1 multi-functional sensor for efficient epinephrine detection based on a cucurbit [7] uril functionalized lanthanide metal–organic framework and its intelligent application in a molecular robot. J. Mater. Chem. 2022, 10, 9326–9333. [Google Scholar] [CrossRef]
- Mao, L.; Han, Y.; Zhang, Q.W.; Tian, Y. Two-photon fluorescence imaging and specifically biosensing of norepinephrine on a 100-ms timescale. Nat. Commun. 2023, 14, 1419. [Google Scholar] [CrossRef]
- Min, H.; Sun, T.; Cui, W.; Han, Z.; Yao, P.; Cheng, P.; Shi, W. Cage-Based Metal–Organic Framework as an Artificial Energy Receptor for Highly Sensitive Detection of Serotonin. Inorg. Chem. 2023, 62, 8739–8745. [Google Scholar] [CrossRef]
- Long, A.; Antonetti, E.; Insuasty, A.; Pinet, S.; Gosse, I.; Robert, V.; Dutasta, J.P.; Martinez, A. Hemicryptophanes with improved fluorescent properties for the selective recognition of acetylcholine over choline. J. Org. Chem. 2020, 85, 6400–6407. [Google Scholar] [CrossRef]
- Szyszka, Ł.; Górecki, M.; Cmoch, P.; Jarosz, S. Fluorescent molecular cages with sucrose and cyclotriveratrylene units for the selective recognition of choline and acetylcholine. J. Org. Chem. 2021, 86, 5129–5141. [Google Scholar] [CrossRef]
- Schulze, P.; Schüttpelz, M.; Sauer, M.; Belder, D. Two-photon excited fluorescence detection at 420 nm for label-free detection of small aromatics and proteins in microchip electrophoresis. Lab. Chip. 2007, 7, 1841–1844. [Google Scholar] [CrossRef]
- Yoshitake, T.; Fujino, K.; Kehr, J.; Ishida, J.; Nohta, H.; Yamaguchi, M. Simultaneous determination of norepinephrine, serotonin, and 5-hydroxyindole-3-acetic acid in microdialysis samples from rat brain by microbore column liquid chromatography with fluorescence detection following derivatization with benzylamine. Anal. Biochem. 2003, 312, 125–133. [Google Scholar] [CrossRef]
- Kai, M.; Iida, H.; Nohta, H.; Lee, M.K.; Ohta, K. Fluorescence derivatizing procedure for 5-hydroxytryptamine and 5-hydroxyindoleacetic acid using 1, 2-diphenylethylenediamine reagent and their sensitive liquid chromatographic determination. J. Chromatogr. Biomed. Sci. Appl. 1998, 720, 25–31. [Google Scholar] [CrossRef]
- Peng, Q.; Jiang, C. A new spectrofluorimetric method for determination of trace amounts 5-hydroxytryptamine in human urine and serum. J. Fluoresc. 2007, 17, 339–343. [Google Scholar] [CrossRef]
- Yoshida, H.; Kido, F.; Yoshitake, M.; Todoroki, K.; Nohta, H.; Yamaguchi, M. Determination of catecholamines and indoleamines in human urine based on intramolecular excimer-forming derivatization and fluorescence detection. Anal. Sci. 2007, 23, 485–488. [Google Scholar] [CrossRef]
- Chandra, F.; Dutta, T.; Koner, A.L. Supramolecular encapsulation of a neurotransmitter serotonin by cucurbit [7] uril. Front. Chem. 2020, 8, 582757. [Google Scholar] [CrossRef]
- Xue, C.; Wang, X.; Zhu, W.; Han, Q.; Zhu, C.; Hong, J.; Zhou, X.; Jiang, H. Electrochemical serotonin sensing interface based on double-layered membrane of reduced graphene oxide/polyaniline nanocomposites and molecularly imprinted polymers embedded with gold nanoparticles. Sens. Actuators Chem. 2014, 196, 57–63. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Honarvarfard, E.; Baharifar, H.; Gholami, M.; Faridbod, F.; Larijani, B.; Faridi Majidi, R.; Khorramizadeh, M.R. Nanomaterial based electrochemical sensing of the biomarker serotonin: A comprehensive review. Microchim. Acta 2019, 186, 49. [Google Scholar] [CrossRef]
- Abbaspour, A.; Noori, A. A cyclodextrin host–guest recognition approach to an electrochemical sensor for simultaneous quantification of serotonin and dopamine. Biosens. Bioelectron. 2011, 26, 4674–4680. [Google Scholar] [CrossRef]
- Liang, W.; Rong, Y.; Fan, L.; Zhang, C.; Dong, W.; Li, J.; Niu, J.; Yang, C.; Shuang, S.; Dong, C.; et al. Simultaneous electrochemical sensing of serotonin, dopamine and ascorbic acid by using a nanocomposite prepared from reduced graphene oxide, Fe3O4 and hydroxypropyl-β-cyclodextrin. Microchim. Acta 2019, 186, 751. [Google Scholar] [CrossRef]
- Yoshitake, T.; Ichinose, F.; Yoshida, H.; Todoroki, K.i.; Kehr, J.; Inoue, O.; Nohta, H.; Yamaguchi, M. A sensitive and selective determination method of histamine by HPLC with intramolecular excimer-forming derivatization and fluorescence detection. Biomed. Chromatogr. 2003, 17, 509–516. [Google Scholar] [CrossRef]
- Nakano, T.; Todoroki, K.; Ishii, Y.; Miyauchi, C.; Palee, A.; Min, J.Z.; Inoue, K.; Suzuki, K.; Toyo’oka, T. An easy-to-use excimer fluorescence derivatization reagent, 2-chloro-4-methoxy-6-(4-(pyren-4-yl) butoxy)-1, 3, 5-triazine, for use in the highly sensitive and selective liquid chromatography analysis of histamine in Japanese soy sauces. Anal. Chim. Acta 2015, 880, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Dou, X.; Li, D.; Xu, S.; Zhang, J.; Ding, Z.; Xie, J. Recent Progress of Fluorescence Sensors for Histamine in Foods. Biosensors 2022, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Altun, Y.; Köseoĝlu, F. Stability of copper (II), nickel (II) and zinc (II) binary and ternary complexes of histidine, histamine and glycine in aqueous solution. J. Solut. Chem. 2005, 34, 213–231. [Google Scholar] [CrossRef]
- Ali, M.; Ramirez, P.; Duznovic, I.; Nasir, S.; Mafe, S.; Ensinger, W. Label-free histamine detection with nanofluidic diodes through metal ion displacement mechanism. Colloids Surfaces Biointerfaces 2017, 150, 201–208. [Google Scholar] [CrossRef]
- Sahudin, M.A.; Su’ait, M.S.; Tan, L.L.; Lee, Y.H.; Abd Karim, N.H. Zinc (II) salphen complex-based fluorescence optical sensor for biogenic amine detection. Anal. Bioanal. Chem. 2019, 411, 6449–6461. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.N.; Li, Z.Y.; Chan, W.H.; Lau, K.C.; Crossley, M.J. Appending zinc tetraphenylporphyrin with an amine receptor at β-pyrrolic carbon for designing a selective histamine chemosensor. Supramol. Chem. 2010, 22, 122–129. [Google Scholar] [CrossRef]
- Gu, X.; Wang, X. An overview of recent analysis and detection of acetylcholine. Anal. Biochem. 2021, 632, 114381. [Google Scholar] [CrossRef]
- He, S.B.; Wu, G.W.; Deng, H.H.; Liu, A.L.; Lin, X.H.; Xia, X.H.; Chen, W. Choline and acetylcholine detection based on peroxidase-like activity and protein antifouling property of platinum nanoparticles in bovine serum albumin scaffold. Biosens. Bioelectron. 2014, 62, 331–336. [Google Scholar] [CrossRef]
- Shadlaghani, A.; Farzaneh, M.; Kinser, D.; Reid, R.C. Direct electrochemical detection of glutamate, acetylcholine, choline, and adenosine using non-enzymatic electrodes. Sensors 2019, 19, 447. [Google Scholar] [CrossRef] [PubMed]
- Korbakov, N.; Timmerman, P.; Lidich, N.; Urbach, B.; Sa’ar, A.; Yitzchaik, S. Acetylcholine detection at micromolar concentrations with the use of an artificial receptor-based fluorescence switch. Langmuir 2008, 24, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tabaie, E.Z.; Hickey, B.L.; Gao, Z.; Raz, A.A.P.; Li, Z.; Wilson, E.H.; Hooley, R.J.; Zhong, W. Selective Molecular Recognition and Indicator Displacement Sensing of Neurotransmitters in Cellular Environments. ACS Sensors 2023, 8, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Martinez, A.; Dutasta, J.P. Emergence of hemicryptophanes: From synthesis to applications for recognition, molecular machines, and supramolecular catalysis. Chem. Rev. 2017, 117, 4900–4942. [Google Scholar] [CrossRef] [PubMed]
- Perraud, O.; Robert, V.; Martinez, A.; Dutasta, J.P. A designed cavity for zwitterionic species: Selective recognition of taurine in aqueous media. Chem. Eur. J. 2011, 48, 13405–13408. [Google Scholar] [CrossRef]
- Long, A.; Fantozzi, N.; Pinet, S.; Genin, E.; Pétuya, R.; Bégué, D.; Robert, V.; Dutasta, J.P.; Gosse, I.; Martinez, A. Selective recognition of acetylcholine over choline by a fluorescent cage. Org. Biomol. Chem. 2019, 17, 5253–5257. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Nakano, Y.; Koiso, K.; Nohta, H.; Ishida, J.; Yamaguchi, M. Liquid chromatographic determination of ornithine and lysine based on intramolecular excimer-forming fluorescence derivatization. Anal. Sci. 2001, 17, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Nan, C.G.; Ping, W.X.; Ping, D.J.; Qing, C.H. A study on electrochemistry of histidine and its metabolites based on the diazo coupling reaction. Talanta 1999, 49, 319–330. [Google Scholar] [CrossRef]
- Mackay, G.; Forrest, C.; Stoy, N.; Christofides, J.; Egerton, M.; Stone, T.; Darlington, L. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur. J. Neurol. 2006, 13, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; Morimoto, Y.; Martell, A.E. Infrared Spectra of Aqueous Solutions. I. Metal Chelate Compounds of Amino Acids1. J. Am. Chem. Soc. 1961, 83, 4528–4532. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, G.J.; Lee, S.A.; Kim, C. A colorimetric chemosensor for the sequential detection of copper ion and amino acids (cysteine and histidine) in aqueous solution. RSC Adv. 2015, 5, 31179–31188. [Google Scholar] [CrossRef]
- Buryak, A.; Severin, K. A chemosensor array for the colorimetric identification of 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 3700–3701. [Google Scholar] [CrossRef]
- Smith, M.R.; Zhang, L.; Jin, Y.; Yang, M.; Bade, A.; Gillis, K.D.; Jana, S.; Bypaneni, R.N.; Glass, T.E.; Lin, H. A turn-on fluorescent amino acid sensor reveals chloroquine’s effect on cellular amino acids via inhibiting Cathepsin L. ACS Cent. Sci. 2023, 9, 980–991. [Google Scholar] [CrossRef]
- Farinone, M.; Urbańska, K.; Pawlicki, M. BODIPY-and porphyrin-based sensors for recognition of amino acids and their derivatives. Molecules 2020, 25, 4523. [Google Scholar] [CrossRef]
- Shao, J.; Guo, H.; Ji, S.; Zhao, J. Styryl-BODIPY based red-emitting fluorescent OFF–ON molecular probe for specific detection of cysteine. Biosens. Bioelectron. 2011, 26, 3012–3017. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, X.; Li, C.; Xie, Y. A novel p-aminophenylthio-and cyano-substituted BODIPY as a fluorescence turn-on probe for distinguishing cysteine and homocysteine from glutathione. Dyes Pigments 2018, 148, 212–218. [Google Scholar] [CrossRef]
- Mizutani, T.; Ema, T.; Yoshida, T.; Kuroda, Y.; Ogoshi, H. Recognition of. alpha.-amino acid esters by zinc porphyrin derivatives via coordination and hydrogen bonding interactions. Evidence for two-point fixation from thermodynamic and induced circular dichroism spectroscopic studies. Inorg. Chem. 1993, 32, 2072–2077. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kato, Y.; Higashioji, T.; Hasegawa, J.y.; Kawanami, S.; Takahashi, M.; Shiraishi, N.; Tanabe, K.; Ogoshi, H. Chiral amino acid recognition by a porphyrin-based artificial receptor. J. Am. Chem. Soc. 1995, 117, 10950–10958. [Google Scholar] [CrossRef]
- Mizutani, T.; Ema, T.; Tomita, T.; Kuroda, Y.; Ogoshi, H. Design and synthesis of a trifunctional chiral porphyrin with C2 symmetry as a chiral recognition host for amino acid esters. J. Am. Chem. Soc. 1994, 116, 4240–4250. [Google Scholar] [CrossRef]
- Liang, Q.F.; Liu, J.J.; Chen, J. Sandwich structure of a ruthenium porphyrin and an amino acid hydrazide for probing molecular chirality by circular dichroism. Tetrahedron Lett. 2011, 52, 3987–3991. [Google Scholar] [CrossRef]
- Valderrey, V.; Aragay, G.; Ballester, P. Porphyrin tweezer receptors: Binding studies, conformational properties and applications. Coord. Chem. Rev. 2014, 258, 137–156. [Google Scholar] [CrossRef]
- Fu, H.; Hu, O.; Fan, Y.; Hu, Y.; Huang, J.; Wang, Z.; She, Y. Rational design of an “on-off-on” fluorescent assay for chiral amino acids based on quantum dots and nanoporphyrin. Sensors Actuators Chem. 2019, 287, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Ji, J.; Liu, Z.; Cai, Y.; Tang, J.; Shi, Y.; Yang, C.; Yuan, L. Chiroptical Sensing of Amino Acid Derivatives by Host–Guest Complexation with Cyclo [6] aramide. Molecules 2021, 26, 4064. [Google Scholar] [CrossRef]
- Biavardi, E.; Tudisco, C.; Maffei, F.; Motta, A.; Massera, C.; Condorelli, G.G.; Dalcanale, E. Exclusive recognition of sarcosine in water and urine by a cavitand-functionalized silicon surface. Proc. Natl. Acad. Sci. USA 2012, 109, 2263–2268. [Google Scholar] [CrossRef]
- Roy, M.N.; Ekka, D.; Saha, S.; Chandra Roy, M. Host–guest inclusion complexes of α and β-cyclodextrins with α-amino acids. RSC Adv. 2014, 4, 42383–42390. [Google Scholar] [CrossRef]
- Douteau-Guével, N.; Coleman, A.W.; Morel, J.P.; Morel-Desrosiers, N. Complexation of the basic amino acids lysine and arginine by three sulfonatocalix [n] arenes (n = 4, 6 and 8) in water: Microcalorimetric determination of the Gibbs energies, enthalpies and entropies of complexation. J. Chem. Soc. Perkin Trans. 2 1999, 3, 629–634. [Google Scholar] [CrossRef]
- Jiang, C.; Song, Z.; Fizir, M.; Yang, P.; Liu, M.; Dramou, P.; He, H. Host-guest interaction between cucurbit [6] uril and chain amino acids. Chem. Phys. Lett. 2021, 783, 139039. [Google Scholar] [CrossRef]
- Wei, T.B.; Chen, J.F.; Cheng, X.B.; Li, H.; Han, B.B.; Zhang, Y.M.; Yao, H.; Lin, Q. A novel functionalized pillar[5]arene-based selective amino acid sensor for l-tryptophan. Org. Chem. Front. 2017, 4, 210–213. [Google Scholar] [CrossRef]
- Bailey, D.M.; Hennig, A.; Uzunova, V.D.; Nau, W.M. Supramolecular tandem enzyme assays for multiparameter sensor arrays and enantiomeric excess determination of amino acids. Chem. Eur. J. 2008, 14, 6069–6077. [Google Scholar] [CrossRef]
- Jing, P.; Zhao, C.; Yin, Z.Z.; Yang, B.; Li, J.; Cai, W.; Kong, Y. An electrochemical chiral sensor based on competitive host–guest interaction for the discrimination of electroinactive amino acids. Analyst 2022, 147, 5068–5074. [Google Scholar] [CrossRef]
- Brancatelli, G.; Dalcanale, E.; Pinalli, R.; Geremia, S. Probing the structural determinants of amino acid recognition: X-ray studies of crystalline ditopic host-guest complexes of the positively charged amino acids, ARg, Lys, and His with a cavitand molecule. Molecules 2018, 23, 3368. [Google Scholar] [CrossRef]
- Goodnow, T.T.; Reddington, M.V.; Stoddart, J.F.; Kaifer, A.E. Cyclobis (paraquat-p-phenylene): A novel synthetic receptor for amino acids with electron-rich aromatic moieties. J. Am. Chem. Soc. 1991, 113, 4335–4337. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, H.; Dong, Y.; Zhao, Y.; Yu, Y.; Cao, L. Tetraphenylethene-based tetracationic cyclophanes and their selective recognition for amino acids and adenosine derivatives in water. Chem. Commun. 2019, 55, 2372–2375. [Google Scholar] [CrossRef]
- Wang, K.; Cui, J.H.; Xing, S.Y.; Ren, X.W. Selective recognition of acidic amino acids in water by calixpyridinium. Asian J. Org. Chem. 2017, 6, 1385–1389. [Google Scholar] [CrossRef]
- Alfonso, I.; Rebolledo, F.; Gotor, V. Optically active dioxatetraazamacrocycles: Chemoenzymatic syntheses and applications in chiral anion recognition. Chem. Eur. J. 2000, 6, 3331–3338. [Google Scholar] [CrossRef]
- Mandl, C.P.; König, B. Luminescent crown ether amino acids: Selective binding to N-terminal lysine in peptides. J. Org. Chem. 2005, 70, 670–674. [Google Scholar] [CrossRef]
- Fokkens, M.; Schrader, T.; Klärner, F.G. A molecular tweezer for lysine and arginine. J. Am. Chem. Soc. 2005, 127, 14415–14421. [Google Scholar] [CrossRef]
- Schmuck, C.; Geiger, L. Efficient complexation of N-acetyl amino acid carboxylates in water by an artificial receptor: Unexpected cooperativity in the binding of glutamate but not aspartate. J. Am. Chem. Soc. 2005, 127, 10486–10487. [Google Scholar] [CrossRef]
- Krainer, G.; Saar, K.L.; Arter, W.E.; Welsh, T.J.; Czekalska, M.A.; Jacquat, R.P.B.; Peter, Q.; Traberg, W.C.; Pujari, A.; Jayaram, A.K.; et al. Direct digital sensing of protein biomarkers in solution. Nat. Commun. 2023, 14, 653. [Google Scholar] [CrossRef]
- Wang, P.; Pei, H.; Wan, Y.; Li, J.; Zhu, X.; Su, Y.; Fan, C.; Huang, Q. Nanomechanical identification of proteins using microcantilever-based chemical sensors. Nanoscale 2012, 4, 6739–6742. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, L.; Huang, X.; Xin, Y.; Ding, L. Discrimination of metalloproteins by a mini sensor array based on bispyrene fluorophore/surfactant aggregate ensembles. ACS Appl. Mater. Interfaces 2016, 8, 35650–35659. [Google Scholar] [CrossRef]
- Hu, W.; Ding, L.; Cao, J.; Liu, L.; Wei, Y.; Fang, Y. Protein binding-induced surfactant aggregation variation: A new strategy of developing fluorescent aqueous sensor for proteins. ACS Appl. Mater. Interfaces 2015, 7, 4728–4736. [Google Scholar] [CrossRef]
- Bo, Y.; Fan, J.; Yan, S.; Ding, M.; Liu, J.; Peng, J.; Ding, L. Surfactant modulation effect on the fluorescence emission of a dual-fluorophore: Realizing a single discriminative sensor for identifying different proteins in aqueous solutions. Sensors Actuators Chem. 2019, 295, 168–178. [Google Scholar] [CrossRef]
- Li, P.; Luo, L.; Cheng, D.; Sun, Y.; Zhang, Y.; Liu, M.; Yao, S. Regulation of the Structure of Zirconium-Based Porphyrinic Metal–Organic Framework as Highly Electrochemiluminescence Sensing Platform for Thrombin. Anal. Chem. 2022, 94, 5707–5714. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Jia, L.; Chen, S.; Shen, Y. Ultrasensitive fluorescent detection of trypsin on the basis of surfactant–protamine assembly with tunable emission wavelength. RSC Adv. 2016, 6, 93551–93557. [Google Scholar] [CrossRef]
- Zhou, W.; Hou, J.; Li, Y.; Zhou, H.; Huang, H.; Zhang, L.; Nawaz, M.A.H.; Yu, C. Protein discrimination based on DNA induced perylene probe self-assembly. Talanta 2021, 224, 121897. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Chen, J.; Nie, L.; Nie, Z.; Yao, S. Sensitive Bifunctional Aptamer-Based Electrochemical Biosensor for Small Molecules and Protein. Anal. Chem. 2009, 81, 9972–9978. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; Sawma, P.; Duneau, J.P.; Khao, J.; Hénin, J.; Bagnard, D.; Sturgis, J. Single-spanning transmembrane domains in cell growth and cell-cell interactions: More than meets the eye? Cell Adhes. Migr. 2010, 4, 313–324. [Google Scholar] [CrossRef]
- Ambrosi, C.; Boassa, D.; Pranskevich, J.; Smock, A.; Oshima, A.; Xu, J.; Nicholson, B.J.; Sosinsky, G.E. Analysis of four connexin26 mutant gap junctions and hemichannels reveals variations in hexamer stability. Biophys. J. 2010, 98, 1809–1819. [Google Scholar] [CrossRef]
- Loo, T.W.; Clarke, D.M. The cystic fibrosis V232D mutation inhibits CFTR maturation by disrupting a hydrophobic pocket rather than formation of aberrant interhelical hydrogen bonds. Biochem. Pharmacol. 2014, 88, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Crawford, J.; Gianazza, E. Protein stains for proteomic applications: Which, when, why? Proteomics 2006, 6, 5385–5408. [Google Scholar] [CrossRef]
- Clowsley, A.H.; Kaufhold, W.T.; Lutz, T.; Meletiou, A.; Di Michele, L.; Soeller, C. Detecting nanoscale distribution of protein pairs by proximity-dependent super-resolution microscopy. J. Am. Chem. Soc. 2020, 142, 12069–12078. [Google Scholar] [CrossRef] [PubMed]
- Dey, J.; Roberts, A.; Mahari, S.; Gandhi, S.; Tripathi, P.P. Electrochemical detection of Alzheimer’s disease biomarker, β-secretase enzyme (BACE1), with one-step synthesized reduced graphene oxide. Front. Bioeng. Biotechnol. 2022, 10, 873811. [Google Scholar] [CrossRef] [PubMed]
- Razzino, C.A.; Serafín, V.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yáñez-Sedeño, P.; Campuzano, S.; et al. An electrochemical immunosensor using gold nanoparticles-PAMAM-nanostructured screen-printed carbon electrodes for tau protein determination in plasma and brain tissues from Alzheimer patients. Biosens. Bioelectron. 2020, 163, 112238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, Y.; Zhang, C.; Tian, X.; Ross, A.W.; Moir, R.D.; Sun, H.; Tanzi, R.E.; Moore, A.; Ran, C. Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 9734–9739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, Y.; Li, Z.; Tian, X.; Sun, H.; Liu, H.; Moore, A.; Ran, C. Design and synthesis of curcumin analogues for in vivo fluorescence imaging and inhibiting copper-induced cross-linking of amyloid beta species in Alzheimer’s disease. J. Am. Chem. Soc. 2013, 135, 16397–16409. [Google Scholar] [CrossRef] [PubMed]
- Law, A.S.Y.; Lee, L.C.C.; Yeung, M.C.L.; Lo, K.K.W.; Yam, V.W.W. Amyloid protein-induced supramolecular self-assembly of water-soluble platinum (II) complexes: A luminescence assay for amyloid fibrillation detection and inhibitor screening. J. Am. Chem. Soc. 2019, 141, 18570–18577. [Google Scholar] [CrossRef]
- Sun, L.; Zhong, Y.; Gui, J.; Wang, X.; Zhuang, X.; Weng, J. A hydrogel biosensor for high selective and sensitive detection of amyloid-beta oligomers. Int. J. Nanomed. 2018, 13, 843–856. [Google Scholar] [CrossRef]
- Khatri, A.; Punjabi, N.; Ghosh, D.; Maji, S.K.; Mukherji, S. Detection and differentiation of α-Synuclein monomer and fibril by chitosan film coated nanogold array on optical sensor platform. Sens. Actuators Chem. 2018, 255, 692–700. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Lim, C.T. Surface plasmon resonance assay for identification of small molecules capable of inhibiting Aβ aggregation. ACS Appl. Mater. Interfaces 2021, 13, 27845–27855. [Google Scholar] [CrossRef]
- Antman-Passig, M.; Wong, E.; Frost, G.R.; Cupo, C.; Shah, J.; Agustinus, A.; Chen, Z.; Mancinelli, C.; Kamel, M.; Li, T.; et al. Optical nanosensor for intracellular and intracranial detection of amyloid-beta. ACS Nano. 2022, 16, 7269–7283. [Google Scholar] [CrossRef] [PubMed]
- Kristofikova, Z.; Ripova, D.; Hegnerová, K.; Sirova, J.; Homola, J. Protein τ-mediated effects on rat hippocampal choline transporters CHT1 and τ-amyloid β interactions. Neurochem. Res. 2013, 38, 1949–1959. [Google Scholar] [CrossRef]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Poste, G.; Compton, C.C.; Barker, A.D. The national biomarker development alliance: Confronting the poor productivity of biomarker research and development. Expert Rev. Mol. Diagn. 2015, 15, 211–218. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, Y.; Cao, Y.; Chen, T.; Chen, X.; Mao, X.; Yin, Y.; Chen, G. Amplified electrochemical detection of surface biomarker in breast cancer stem cell using self-assembled supramolecular nanocomposites. Electrochim. Acta 2018, 283, 1072–1078. [Google Scholar] [CrossRef]
- Yoshii, T.; Mizusawa, K.; Takaoka, Y.; Hamachi, I. Intracellular protein-responsive supramolecules: Protein sensing and in-cell construction of inhibitor assay system. J. Am. Chem. Soc. 2014, 136, 16635–16642. [Google Scholar] [CrossRef]
- Xu, J.; Chen, W.; Shi, M.; Huang, Y.; Fang, L.; Zhao, S.; Yao, L.; Liang, H. An aptamer-based four-color fluorometic method for simultaneous determination and imaging of alpha-fetoprotein, vascular endothelial growth factor-165, carcinoembryonic antigen and human epidermal growth factor receptor 2 in living cells. Microchim. Acta 2019, 186, 204. [Google Scholar] [CrossRef]
- Qiu, L.P.; Wu, Z.S.; Shen, G.L.; Yu, R.Q. Highly Sensitive and Selective Bifunctional Oligonucleotide Probe for Homogeneous Parallel Fluorescence Detection of Protein and Nucleotide Sequence. Anal. Chem. 2011, 83, 3050–3057. [Google Scholar] [CrossRef] [PubMed]
- Negahdary, M.; Hirata, M.H.; Sakata, S.K.; Ciconelli, R.M.; Bastos, G.M.; Borges, J.B.; Thurow, H.S.; Junior, A.T.S.; Sampaio, M.F.; Guimarães, L.B.; et al. Sandwich-like electrochemical aptasensing of heat shock protein 70 kDa (HSP70): Application in diagnosis/prognosis of coronavirus disease 2019 (COVID-19). Anal. Chim. Acta 2023, 1242, 340716. [Google Scholar] [CrossRef]
- Lim, S.; Kuang, Y.; Ardoña, H.A.M. Evolution of supramolecular systems towards next-generation biosensors. Front. Chem. 2021, 9, 723111. [Google Scholar] [CrossRef]
- Law, A.S.Y.; Lee, L.C.C.; Lo, K.K.W.; Yam, V.W.W. Aggregation and supramolecular self-assembly of low-energy red luminescent alkynylplatinum (II) complexes for RNA detection, nucleolus imaging, and RNA synthesis inhibitor screening. J. Am. Chem. Soc. 2021, 143, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Krasheninina, O.; Lomzov, A.; Fishman, V.; Novopashina, D.; Venyaminova, A. Rational design and studies of excimer forming novel dual probes to target RNA. Bioorganic Med. Chem. 2017, 25, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Semikolenova, O.; Golyshev, V.; Kim, B.; Venyaminova, A.G.; Novopashina, D. New Two-Component Pyrene Probes Based on Oligo (2′-O-Methylribonucleotides) for microRNA Detection. Russ. J. Bioorganic Chem. 2021, 47, 432–440. [Google Scholar] [CrossRef]
- Astakhova, K.; Golovin, A.V.; Prokhorenko, I.A.; Ustinov, A.V.; Stepanova, I.A.; Zatsepin, T.S.; Korshun, V.A. Design of 2′-phenylethynylpyrene excimer forming DNA/RNA probes for homogeneous SNP detection: The attachment manner matters. Tetrahedron 2017, 73, 3220–3230. [Google Scholar] [CrossRef]
- Krasheninina, O.; Fishman, V.; Novopashina, D.; Venyaminova, A. 5′-Bispyrene molecular beacons for RNA detection. Russ. J. Bioorganic Chem. 2017, 43, 259–269. [Google Scholar] [CrossRef]
- Sethi, K.; Dailey, G.P.; Zahid, O.K.; Taylor, E.W.; Ruzicka, J.A.; Hall, A.R. Direct detection of conserved viral sequences and other nucleic acid motifs with solid-state nanopores. ACS Nano. 2021, 15, 8474–8483. [Google Scholar] [CrossRef]
- Qing, Y.; Ionescu, S.A.; Pulcu, G.S.; Bayley, H. Directional control of a processive molecular hopper. Science 2018, 361, 908–912. [Google Scholar] [CrossRef]
- Heerema, S.J.; Vicarelli, L.; Pud, S.; Schouten, R.N.; Zandbergen, H.W.; Dekker, C. Probing DNA translocations with inplane current signals in a graphene nanoribbon with a nanopore. ACS Nano. 2018, 12, 2623–2633. [Google Scholar] [CrossRef]
- Plesa, C.; Ruitenberg, J.W.; Witteveen, M.J.; Dekker, C. Detection of individual proteins bound along DNA using solid-state nanopores. Nano Lett. 2015, 15, 3153–3158. [Google Scholar] [CrossRef]
- Xue, L.; Cadinu, P.; Paulose Nadappuram, B.; Kang, M.; Ma, Y.; Korchev, Y.; Ivanov, A.P.; Edel, J.B. Gated single-molecule transport in double-barreled nanopores. ACS Appl. Mater. Interfaces 2018, 10, 38621–38629. [Google Scholar] [CrossRef]
- Lin, X.; Ivanov, A.P.; Edel, J.B. Selective single molecule nanopore sensing of proteins using DNA aptamer-functionalised gold nanoparticles. Chem. Sci. 2017, 8, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Akeson, M.; Branton, D.; Kasianowicz, J.J.; Brandin, E.; Deamer, D.W. Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophys. J. 1999, 77, 3227–3233. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.Z.; Pavlenok, M.; Derrington, I.M.; Niederweis, M.; Gundlach, J.H. Single-molecule DNA detection with an engineered MspA protein nanopore. Proc. Natl. Acad. Sci. USA 2008, 105, 20647–20652. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, A.H.; Derrington, I.M.; Brinkerhoff, H.; Langford, K.W.; Nova, I.C.; Samson, J.M.; Bartlett, J.J.; Pavlenok, M.; Gundlach, J.H. Detection and mapping of 5-methylcytosine and 5-hydroxymethylcytosine with nanopore MspA. Proc. Natl. Acad. Sci. USA 2013, 110, 18904–18909. [Google Scholar] [CrossRef]
- Asandei, A.; Mereuta, L.; Park, J.; Seo, C.H.; Park, Y.; Luchian, T. Nonfunctionalized PNAs as beacons for nucleic acid detection in a nanopore system. ACS Sens. 2019, 4, 1502–1507. [Google Scholar] [CrossRef]
- Chalupowicz, L.; Dombrovsky, A.; Gaba, V.; Luria, N.; Reuven, M.; Beerman, A.; Lachman, O.; Dror, O.; Nissan, G.; Manulis-Sasson, S. Diagnosis of plant diseases using the Nanopore sequencing platform. Plant Pathol. 2019, 68, 229–238. [Google Scholar] [CrossRef]
- Mereuta, L.; Asandei, A.; Dragomir, I.S.; Bucataru, I.C.; Park, J.; Seo, C.H.; Park, Y.; Luchian, T. Sequence-specific detection of single-stranded DNA with a gold nanoparticle-protein nanopore approach. Sci. Rep. 2020, 10, 11323. [Google Scholar] [CrossRef]
- Liu, H.; Tian, T.; Zhang, Y.; Ding, L.; Yu, J.; Yan, M. Sensitive and rapid detection of microRNAs using hairpin probes-mediated exponential isothermal amplification. Biosens. Bioelectron. 2017, 89, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, P.; Fang, Y. A solid-state electrochemiluminescence biosensing switch for detection of DNA hybridization based on ferrocene-labeled molecular beacon. J. Lumin. 2010, 130, 1481–1484. [Google Scholar] [CrossRef]
- Pavlov, V.; Shlyahovsky, B.; Willner, I. Fluorescence Detection of DNA by the Catalytic Activation of an Aptamer/Thrombin Complex. J. Am. Chem. Soc. 2005, 127, 6522–6523. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, Z.; Xue, N.; Cheng, Z.; Miao, X. A gold nanoparticle based fluorescent probe for simultaneous recognition of single-stranded DNA and double-stranded DNA. Microchim. Acta 2018, 185, 93. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, Y. Using Commercially Available Personal Glucose Meters for Portable Quantification of DNA. Anal. Chem. 2012, 84, 1975–1980. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.W.; Fan, W.; Li, X.; Liu, Y.; Li, Z.; Jiang, W.; Wu, J.; Wang, Z. Molecular carbons: How far can we go? ACS Nano. 2023, 17, 20734–20752. [Google Scholar] [CrossRef]
- Shen, W.; Li, G. Recent progress in liquid crystal-based smart windows: Materials, structures, and design. Laser Photonics Rev. 2023, 17, 2200207. [Google Scholar] [CrossRef]
- Sun, P.; Liu, D.; Zhu, F.; Yan, D. An efficient solid-solution crystalline organic light-emitting diode with deep-blue emission. Nat. Photonics 2023, 17, 264–272. [Google Scholar] [CrossRef]
- Qureshi, R.; Irfan, M.; Ali, H.; Khan, A.; Nittala, A.S.; Ali, S.; Shah, A.; Gondal, T.M.; Sadak, F.; Shah, Z.; et al. Artificial intelligence and biosensors in healthcare and its clinical relevance: A review. IEEE Access 2023, 11, 61600–61620. [Google Scholar] [CrossRef]
- Steiner, S.; Wolf, J.; Glatzel, S.; Andreou, A.; Granda, J.M.; Keenan, G.; Hinkley, T.; Aragon-Camarasa, G.; Kitson, P.J.; Angelone, D.; et al. Organic synthesis in a modular robotic system driven by a chemical programming language. Science 2019, 363, eaav2211. [Google Scholar] [CrossRef]


| Metal Ions | Optimum Level in Physiological System |
|---|---|
| Na+ | 135–145 mM (serum) |
| K+ | 3.5–5.4 mM (serum), 19–66 nM (urea) |
| Ca2+ | 10–6 M (intracellular), 10–3 M (extracellular fluid) |
| Mg2+ | 0.65–1.05 mM (serum) |
| Cu2+ | 1.4–2.1 mg/kg (adult human body) |
| Zn2+ | 12–16 μM (serum) |
| Fe3+ | 14–32 μM (serum) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahiri, H.; Basu, K. Supramolecular Sensing Platforms: Techniques for In Vitro Biosensing. ChemEngineering 2024, 8, 66. https://doi.org/10.3390/chemengineering8040066
Lahiri H, Basu K. Supramolecular Sensing Platforms: Techniques for In Vitro Biosensing. ChemEngineering. 2024; 8(4):66. https://doi.org/10.3390/chemengineering8040066
Chicago/Turabian StyleLahiri, Hiya, and Kingshuk Basu. 2024. "Supramolecular Sensing Platforms: Techniques for In Vitro Biosensing" ChemEngineering 8, no. 4: 66. https://doi.org/10.3390/chemengineering8040066
APA StyleLahiri, H., & Basu, K. (2024). Supramolecular Sensing Platforms: Techniques for In Vitro Biosensing. ChemEngineering, 8(4), 66. https://doi.org/10.3390/chemengineering8040066






