Process Optimization and Biotransformation of Ferulic Acid to Vanillin in a Low-Cost Nitrogen Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Inoculum Preparation
2.3. Medium Composition for Vanillin Production
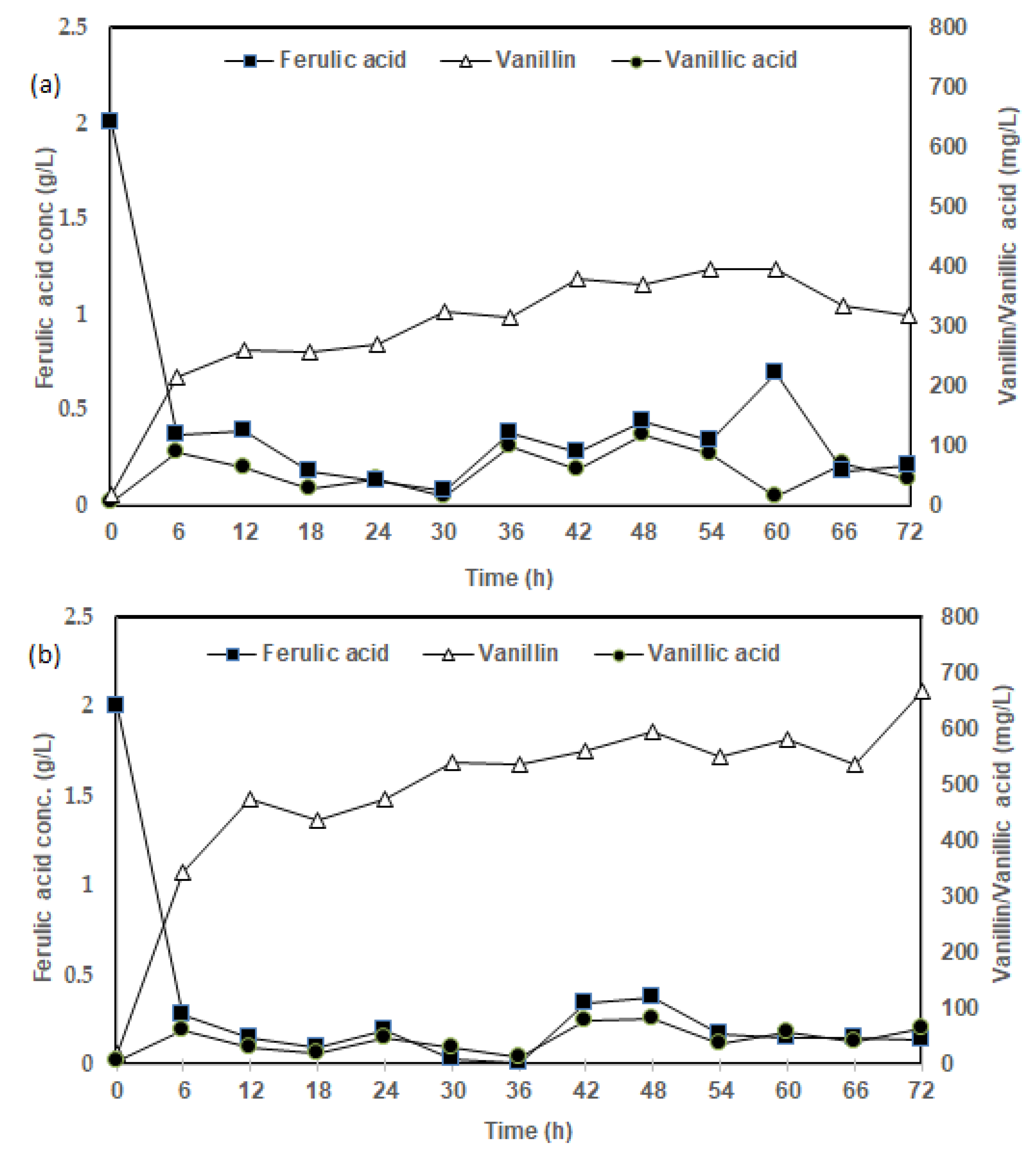
2.4. Batch Fermentation Study
2.5. Vanillin Production Experimental Design by Box–Behnken Using DOE
2.6. Statistical Analysis
2.7. Colorimetric Evaluation of Ferulic and Vanillic Acid
2.8. Measurement of Vanillin Colorimetric Method
3. Results
3.1. Influence of Media Formulation on FA Bioconversion to Vanillin
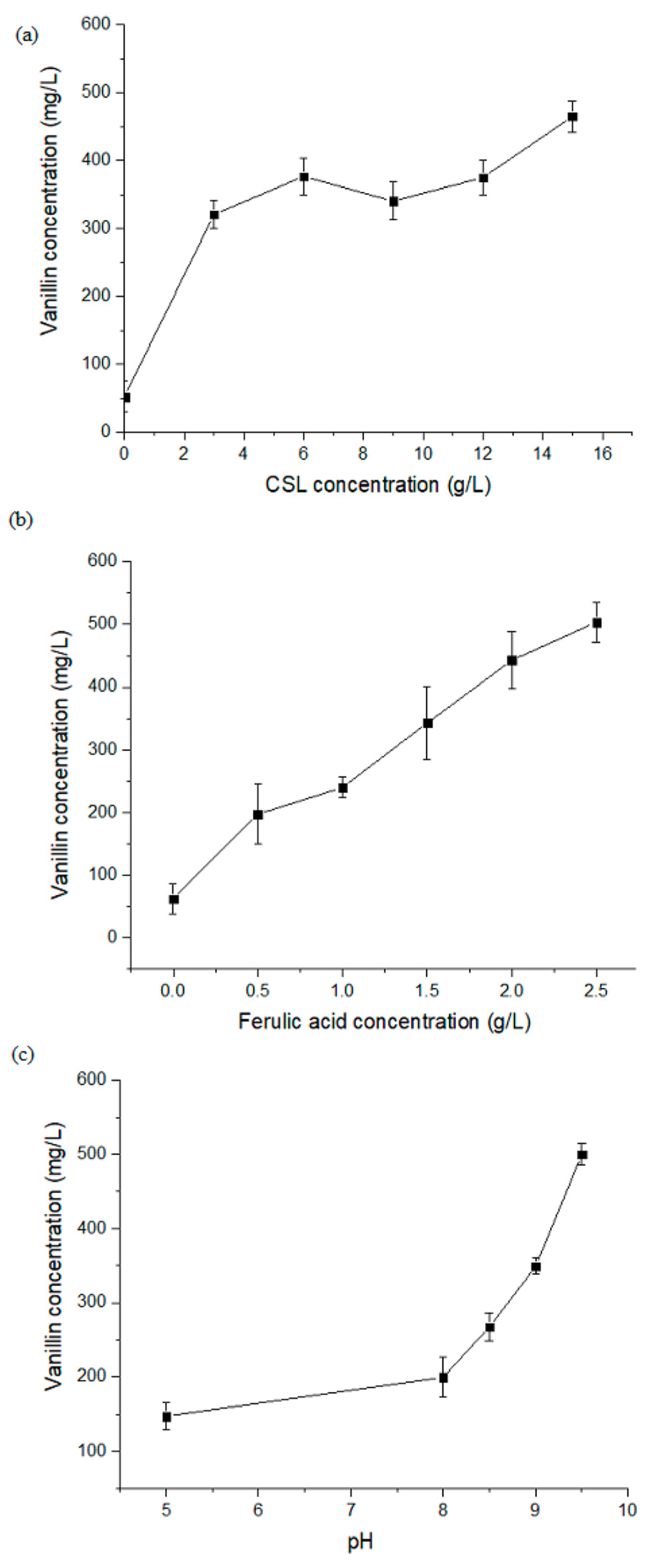
3.2. Influence of CSL Concentration on FA Bioconversion to Vanillin
3.3. Influence of FA Concentration on Vanillin Production
3.4. Influence of pH on FA Bioconversion to Vanillin
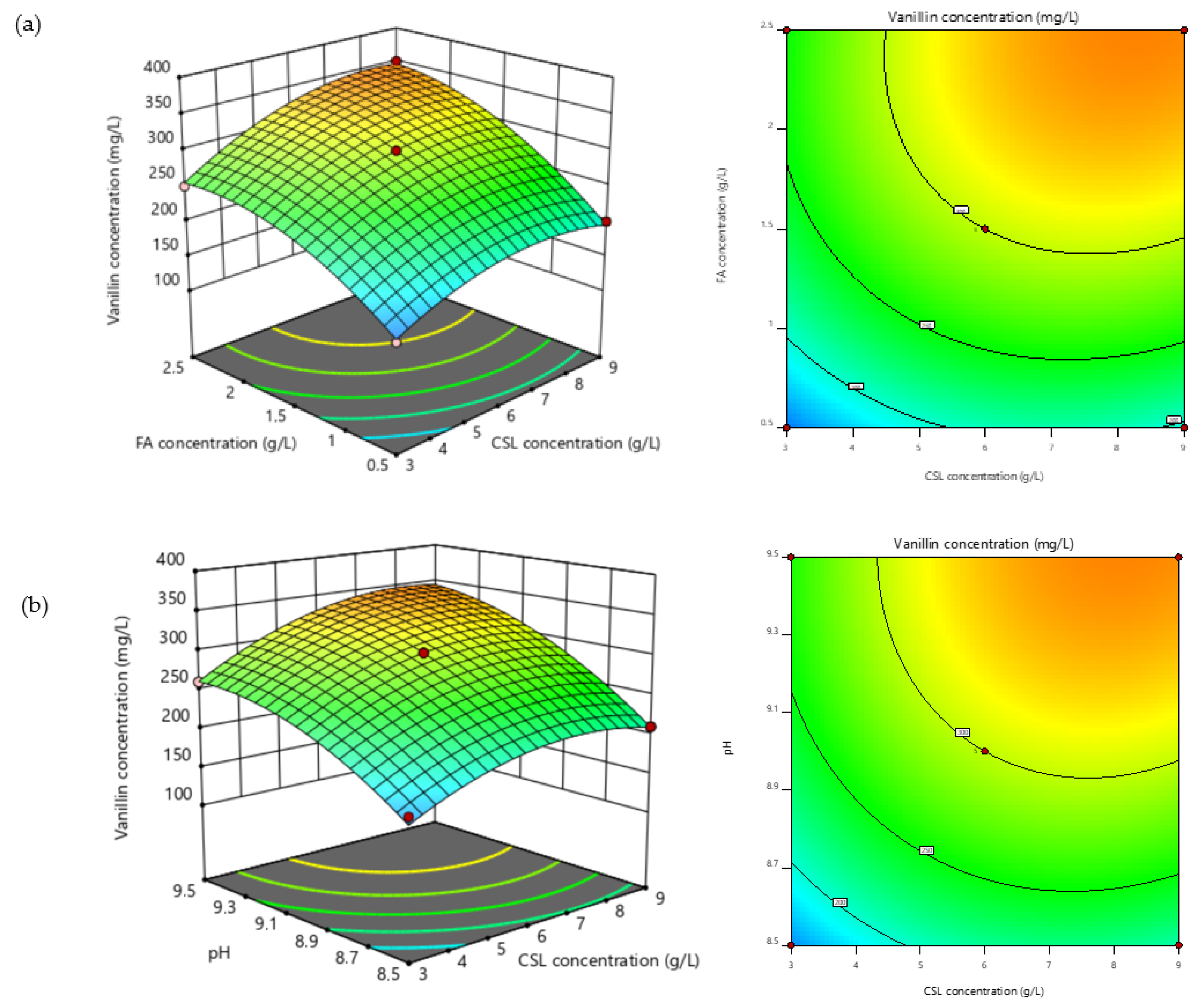
3.5. Modeling and Optimization of Vanillin Production
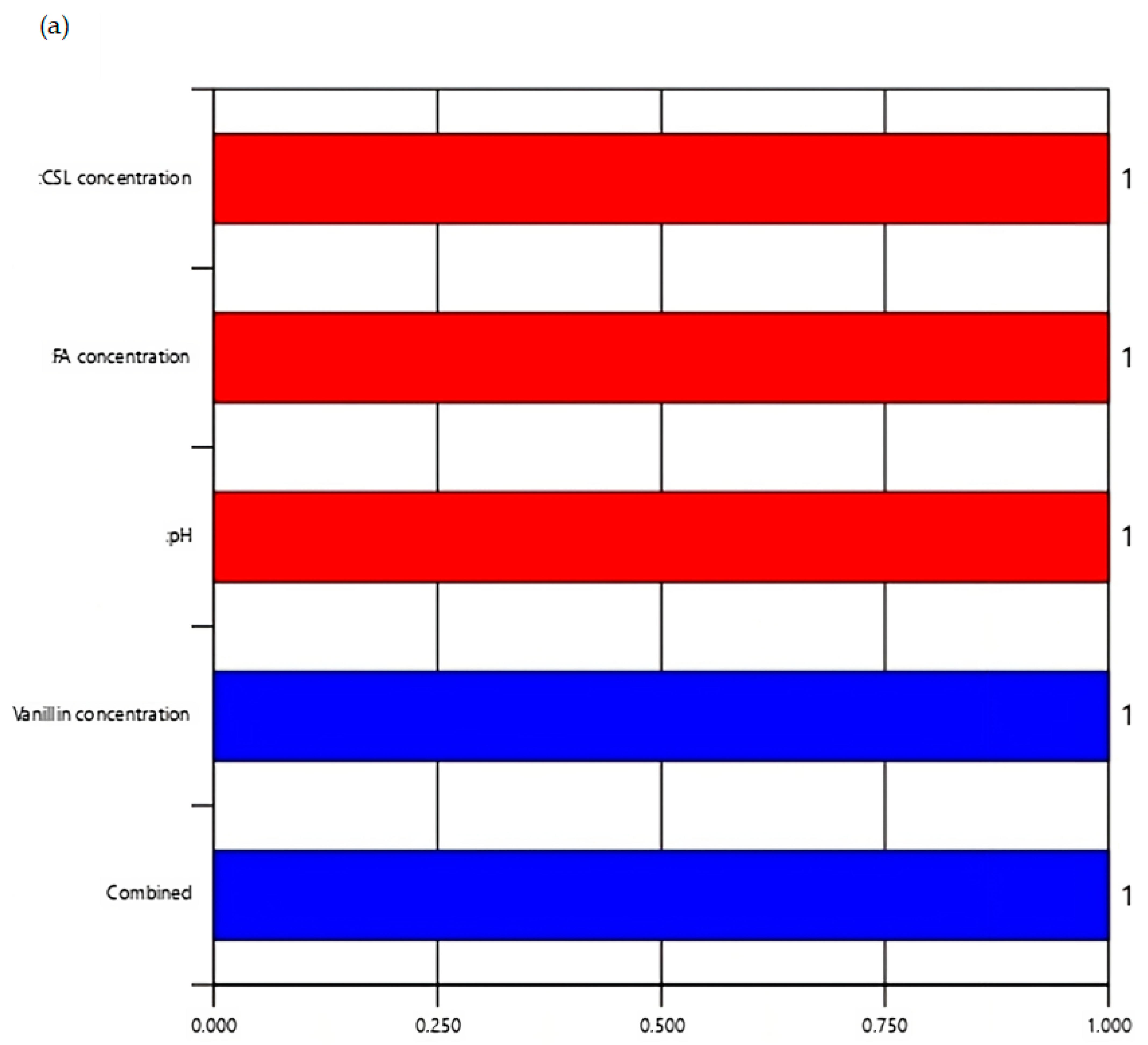
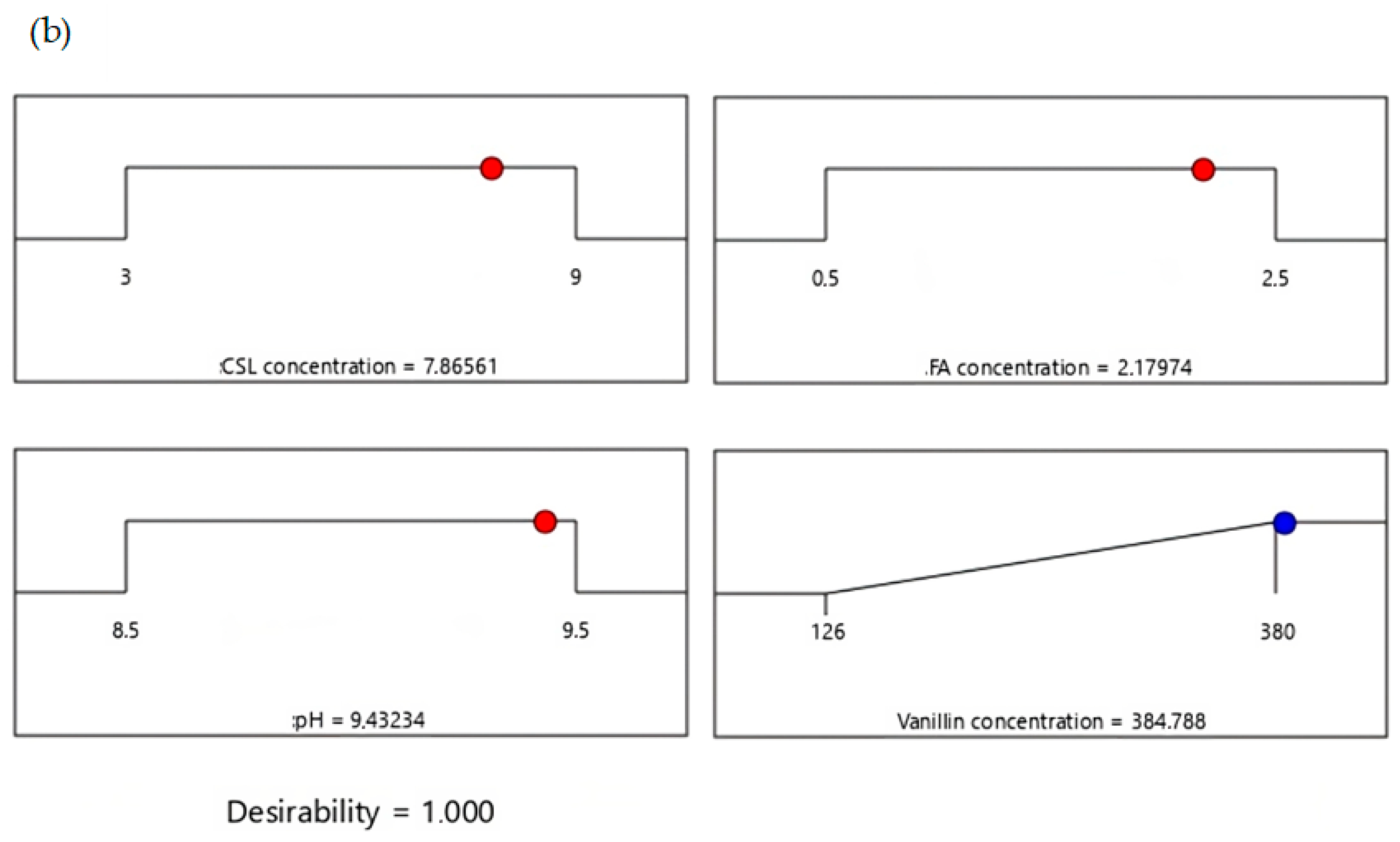
3.6. Numerical Optimization of Vanillin Using Desirability Function
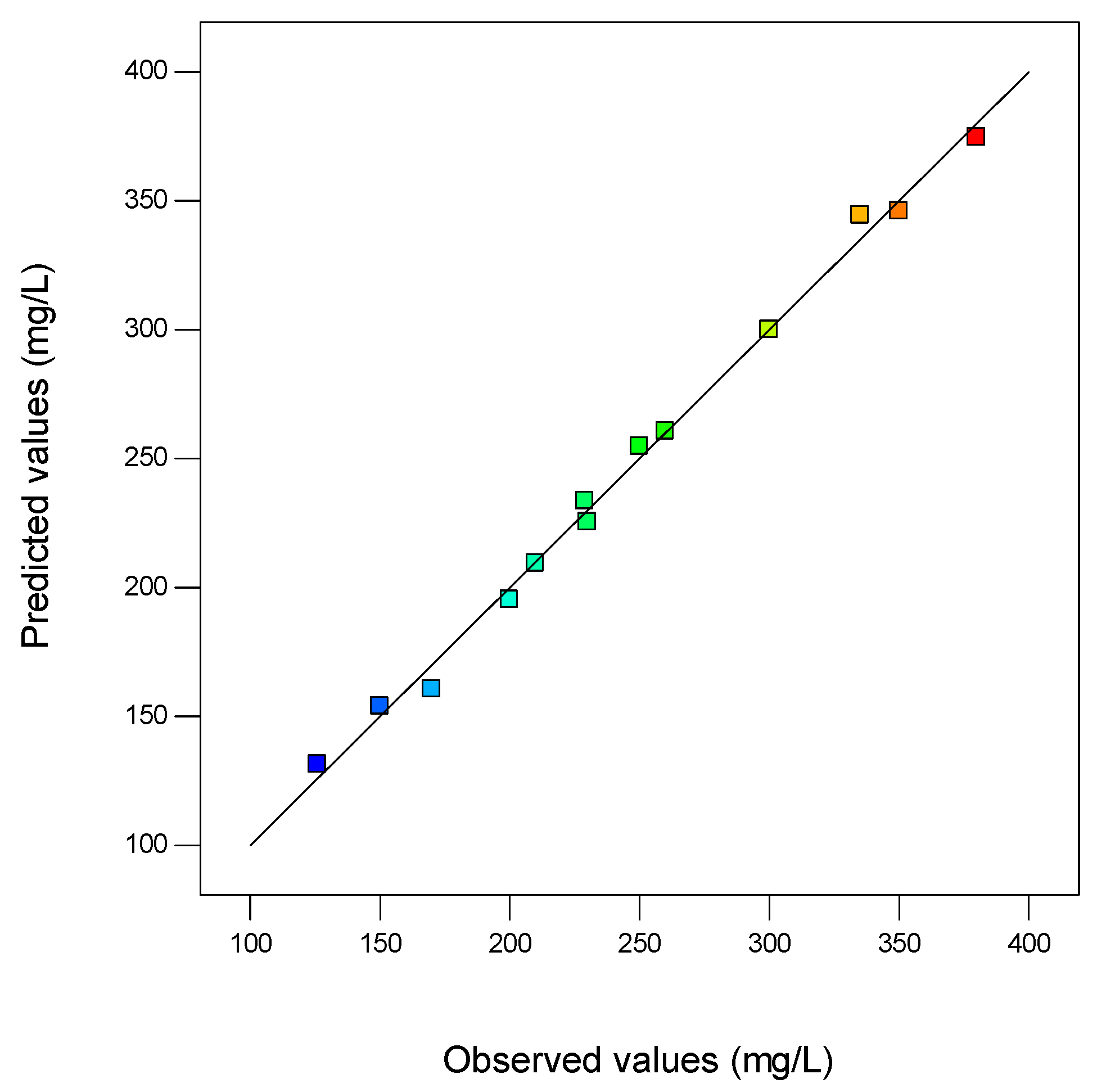
3.7. Validation Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Yan, L.; Zhang, S.; Wu, Z.; Li, S.; Yan, X.; Wang, N.; Liang, N.; Li, H. Optimizing bioconversion of ferulic acid to vanillin by Bacillus subtilis in the stirred packed reactor using Box-Behnken design and desirability function. Food Sci. Biotechnol. 2017, 26, 143–152. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Rossato, L.A.; Strini, A.; Serra, S. From Waste to Value: Recent Insights into Producing Vanillin from Lignin. Molecules 2024, 29, 442. [Google Scholar] [CrossRef] [PubMed]
- Nayak, J.; Basu, A.; Dey, P.; Kumar, R.; Upadhaya, A.; Ghosh, S.; Bishayee, B.; Mishra, S.R.; Tripathy, S.K.; Banerjee, S.; et al. Transformation of agro-biomass into vanillin through novel membrane integrated value-addition process: A state-of-art review. Biomass Convers. Biorefinery 2023, 13, 14317–14340. [Google Scholar] [CrossRef]
- Mehmood, T.; Saeed, S.; Hussain, N.; Waseem, R. Biotransformation of wheat straw into biovanillin by solid-state fermentation and optimization of conditions parameters through response surface methodology. Biomass Convers. Biorefinery 2024, 14, 7569–7578. [Google Scholar] [CrossRef]
- Tilay, A.; Bule, M.; Annapure, U. Production of biovanillin by one-step biotransformation using fungus Pycnoporous cinnabarinus. J. Agric. Food Chem. 2010, 58, 4401–4405. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liu, L.; Zhao, S.; Huang, Z.; Li, C.; Jiang, S.; Li, Q.; Gu, P. Biosynthesis of vanillin by different microorganisms: A review. World J. Microbiol. Biotechnol. 2022, 38, 40. [Google Scholar] [CrossRef]
- Di Gioia, D.; Luziatelli, F.; Negroni, A.; Ficca, A.G.; Fava, F.; Ruzzi, M. Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. J. Biotechnol. 2011, 156, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.I.F.; Rodrigues Pinto, P.C.; Loureiro, J.M.; Rodrigues, A.E. Recovery of vanillin and syringaldehyde from lignin oxidation: A review of separation and purification processes. Sep. Purif. Rev. 2016, 45, 227–259. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V. Role of Microbial Fermentation in the Bio-Production of Food Aroma Compounds from Vegetable Waste. Fermentation 2024, 10, 132. [Google Scholar] [CrossRef]
- Yan, L.; Chen, P.; Zhang, S.; Li, S.; Yan, X.; Wang, N.; Liang, N.; Li, H. Biotransformation of ferulic acid to vanillin in the packed bed-stirred fermentors. Sci. Rep. 2016, 6, 34644. [Google Scholar] [CrossRef]
- Paz, A.; Outeiriño, D.; Pinheiro de Souza Oliveira, R.; Domínguez, J.M. Fed-batch production of vanillin by Bacillus aryabhattai BA03. New Biotechnol. 2018, 40, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Prasath, C.S.; Sivadas, C.A.; Chandran, C.H.; Suchithra, T. Precision fermentation of sustainable products in the food industry. In Entrepreneurship with Microorganisms; Elsevier: Amsterdam, The Netherlands, 2024; pp. 163–177. [Google Scholar]
- Kaur, B.; Chakraborty, D. Statistical media and process optimization for biotransformation of rice bran to vanillin using Pediococcus acidilactici. Indian J. Exp. Biol. 2013, 51, 935–943. [Google Scholar] [PubMed]
- Xiao, Z.; Liu, P.; Qin, J.Y.; Xu, P. Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl. Microbiol. Biotechnol. 2007, 74, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yan, L.; Wu, Z.; Li, S.; Bai, Z.; Yan, X.; Wang, N.; Liang, N.; Li, H. A microbial transformation using Bacillus subtilis B7-S to produce natural vanillin from ferulic acid. Sci. Rep. 2016, 6, 20400. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.; Kareparamban, J.; Nikam, P.; Kadam, V. Spectrophotometric estimation of ferulic acid from Ferula asafoetida by Folin-ciocalteu’s reagent. Pharm. Sin. 2012, 3, 680–684. [Google Scholar]
- Wu, Y.-T.; Feng, M.; Ding, W.-W.; Tang, X.-Y.; Zhong, Y.-H.; Xiao, Z.-Y. Preparation of vanillin by bioconversion in a silicon rubber membrane bioreactor. Biochem. Eng. J. 2008, 41, 193–197. [Google Scholar] [CrossRef]
- Rana, R.; Mathur, A.; Jain, C.; Sharma, S.; Mathur, G. Microbial production of vanillin. Int. J. Biotechnol. Bioeng. Res. 2013, 4, 227–234. [Google Scholar]
- Wahjudi, S.M.W.; Petrzik, T.; Oudenne, F.; Lera Calvo, C.; Büchs, J. Unraveling the potential and constraints associated with corn steep liquor as a nutrient source for industrial fermentations. Biotechnol. Prog. 2023, 39, e3386. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, A.E.; Musonge, P. Valorization of Corn Steep Liquor for Improved Value-added Products: A Review. Recent Innov. Chem. Eng. Former. Recent Pat. Chem. Eng. 2024, 17, 26–43. [Google Scholar] [CrossRef]
- Taiwo, A.; Madzimbamuto, T.; Ojumu, T. Optimization of Corn Steep Liquor Dosage and Other Fermentation Parameters for Ethanol Production by Saccharomyces cerevisiae Type 1 and Anchor Instant Yeast. Energies 2018, 11, 1740. [Google Scholar] [CrossRef]
- Daneshi, A.; Younesi, H.; Ghasempouri, S.M.; Sharifzadeh, M. Production of poly-3-hydroxybutyrate by Cupriavidus necator from corn syrup: Statistical modeling and optimization of biomass yield and volumetric productivity. J. Chem. Technol. Biotechnol. 2010, 85, 1528–1539. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Musonge, P. Comparative evaluation of bioethanol fermentation process parameters using RSM, ANN and ANFIS. Biofuels Bioprod. Biorefining 2023, 17, 961–975. [Google Scholar] [CrossRef]
- Mourabet, M.; El Rhilassi, A.; El Boujaady, H.; Bennani-Ziatni, M.; El Hamri, R.; Taitai, A. Removal of fluoride from aqueous solution by adsorption on Apatitic tricalcium phosphate using Box–Behnken design and desirability function. Appl. Surf. Sci. 2012, 258, 4402–4410. [Google Scholar] [CrossRef]
- Betiku, E.; Odude, V.O.; Ishola, N.B.; Bamimore, A.; Osunleke, A.S.; Okeleye, A.A. Predictive capability evaluation of RSM, ANFIS and ANN: A case of reduction of high free fatty acid of palm kernel oil via esterification process. Energy Convers. Manag. 2016, 124, 219–230. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Vecino, X.; Barbosa-Pereira, L.; Moldes, A.; Cruz, J. A multifunctional extract from corn steep liquor: Antioxidant and surfactant activities. Food Funct. 2016, 7, 3724–3732. [Google Scholar] [CrossRef]
- Boateng, I.D. Application of graphical optimization, desirability, and multiple response functions in the extraction of food bioactive compounds. Food Eng. Rev. 2023, 15, 309–328. [Google Scholar] [CrossRef]
- Jimidar, M.; Bourguignon, B.; Massart, D. Application of Derringer’s desirability function for the selection of optimum separation conditions in capillary zone electrophoresis. J. Chromatogr. A 1996, 740, 109–117. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Karmakar, B.; Vohra, R.; Nandanwar, H.; Sharma, P.; Gupta, K.; Sobti, R. Rapid degradation of ferulic acid via 4-vinylguaiacol and vanillin by a newly isolated strain of Bacillus coagulans. J. Biotechnol. 2000, 80, 195–202. [Google Scholar] [CrossRef]
- Tan, J.; Jahim, J.; Wu, T.; Harun, S.; Mumtaz, T. Use of corn steep liquor as an economical nitrogen source for biosuccinic acid production by Actinobacillus succinogenes. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2016; Volume 36, p. 012058. [Google Scholar]

| Parameters | Notation | Range of Values Coded | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Corn steep liquor (g/L) | X1 | 3 | 6 | 9 |
| Ferulic acid (g/L) | X2 | 0.5 | 1.5 | 2.5 |
| pH | X3 | 8.5 | 9.0 | 9.5 |
| Experiment Order | CSL | FA | pH | Observed Vanillin | Predicted Vanillin | Residual |
|---|---|---|---|---|---|---|
| (g/L) | (g/L) | (mg/L) | (mg/L) | |||
| 1 | 3 | 1.5 | 9.5 | 260.00 | 260.62 | −0.62 |
| 2 | 6 | 1.5 | 9 | 300.00 | 300.00 | 0.00 |
| 3 | 3 | 0.5 | 9 | 150.00 | 154.00 | −4.00 |
| 4 | 6 | 0.5 | 9.5 | 230.00 | 225.37 | 4.63 |
| 5 | 3 | 1.5 | 8.5 | 170.00 | 160.62 | 9.38 |
| 6 | 6 | 1.5 | 9 | 300.00 | 300.00 | 0.00 |
| 7 | 6 | 2.5 | 9.5 | 380.00 | 374.63 | 5.38 |
| 8 | 9 | 2.5 | 9 | 350.00 | 346.00 | 4.00 |
| 9 | 6 | 1.5 | 9 | 300.00 | 300.00 | 0.00 |
| 10 | 9 | 1.5 | 8.5 | 210.00 | 209.37 | 0.63 |
| 11 | 3 | 2.5 | 9 | 250.00 | 254.75 | −4.75 |
| 12 | 9 | 0.5 | 9 | 200.00 | 195.25 | 4.75 |
| 13 | 6 | 0.5 | 8.5 | 126.00 | 131.37 | −5.37 |
| 14 | 6 | 2.5 | 8.5 | 229.00 | 233.63 | −4.63 |
| 15 | 9 | 1.5 | 9.5 | 335.00 | 344.37 | −9.37 |
| 16 | 6 | 1.5 | 9 | 300.00 | 300.00 | 0.00 |
| 17 | 6 | 1.5 | 9 | 300.00 | 300.00 | 0.00 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 81,934.8 | 9 | 9103.9 | 179.9 | <0.0001 |
| X1-CSL | 8778.1 | 1 | 8778.1 | 173.5 | <0.0001 |
| X2-FA | 31,626.1 | 1 | 31,626.1 | 624.9 | <0.0001 |
| X3-pH | 27,612.5 | 1 | 27,612.5 | 545.6 | <0.0001 |
| CSL.FA | 625.0 | 1 | 625.0 | 12.4 | 0.0098 |
| CSL.pH | 306.3 | 1 | 306.3 | 6.1 | 0.0435 |
| FA.pH | 552.3 | 1 | 552.3 | 10.9 | 0.0131 |
| CSL2 | 3789.5 | 1 | 3789.5 | 74.9 | <0.0001 |
| FA2 | 4447.4 | 1 | 4447.4 | 87.9 | <0.0001 |
| pH2 | 2901.3 | 1 | 2901.3 | 57.3 | 0.0001 |
| Residual | 354.3 | 7 | 50.6 | ||
| Lack of Fit | 354.3 | 3 | 118.1 | ||
| Pure Error | 0.0 | 4 | 0.0 | Pred-R2 | 0.931 |
| Cor Total | 82,289.06 | 16 | Adeq precision | 44.583 | |
| SD | 7.11 | R2 | 0.995 | ||
| % CV | 2.75 | Adjusted-R2 | 0.990 |
| FA (g/L) | Organism | Vanillin (mg/L) | Percentage Molar Yield | Nitrogen Source | Author |
|---|---|---|---|---|---|
| 0.6 | B. subtilis B7–S | 0.16 | 0.03 | Peptone and yeast extract | [15] |
| 1.0 | Bacillus coagulans | 16.8 | 2.14 | Yeast extract | [30] |
| 1.0 | B. aryabhattai BA03 | 147.1 | 18.81 | Yeast and beef extract | [11] |
| 1.0 | Pycnoporous cinnabarinus | 126 | 16.08 | Corn steep liquor | [5] |
| 2.33 | Bacillus amyloliquefaciens subsp. | 386 | 21.14 | Corn steep liquor | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taiwo, A.E.; Madzimbamuto, T.N.; Ojumu, T.V. Process Optimization and Biotransformation of Ferulic Acid to Vanillin in a Low-Cost Nitrogen Source. ChemEngineering 2024, 8, 68. https://doi.org/10.3390/chemengineering8040068
Taiwo AE, Madzimbamuto TN, Ojumu TV. Process Optimization and Biotransformation of Ferulic Acid to Vanillin in a Low-Cost Nitrogen Source. ChemEngineering. 2024; 8(4):68. https://doi.org/10.3390/chemengineering8040068
Chicago/Turabian StyleTaiwo, Abiola Ezekiel, Tafirenyika Nyamayaro Madzimbamuto, and Tunde Victor Ojumu. 2024. "Process Optimization and Biotransformation of Ferulic Acid to Vanillin in a Low-Cost Nitrogen Source" ChemEngineering 8, no. 4: 68. https://doi.org/10.3390/chemengineering8040068





