Integration of Ion Exchange—AOP—Biological System for the Treatment of Real Textile Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Real Textile Wastewater Sample
2.3. Treatment with Ion Exchange Resins
2.4. Bicarbonate-Activated Peroxide (BAP)—Catalytic Tests
2.5. Aerobic Biological System (AES)—Assay Using Zahn–Wellens Test
2.6. Phytotoxicity Tests
3. Results and Discussion
3.1. Physicochemical Characterization of the Real Textile Wastewater
3.2. Treatment of Textile Wastewater Using Ion Exchange Resins
3.3. Oxidation Tests Using H2O2-Activated NaHCO3—Experimental Design
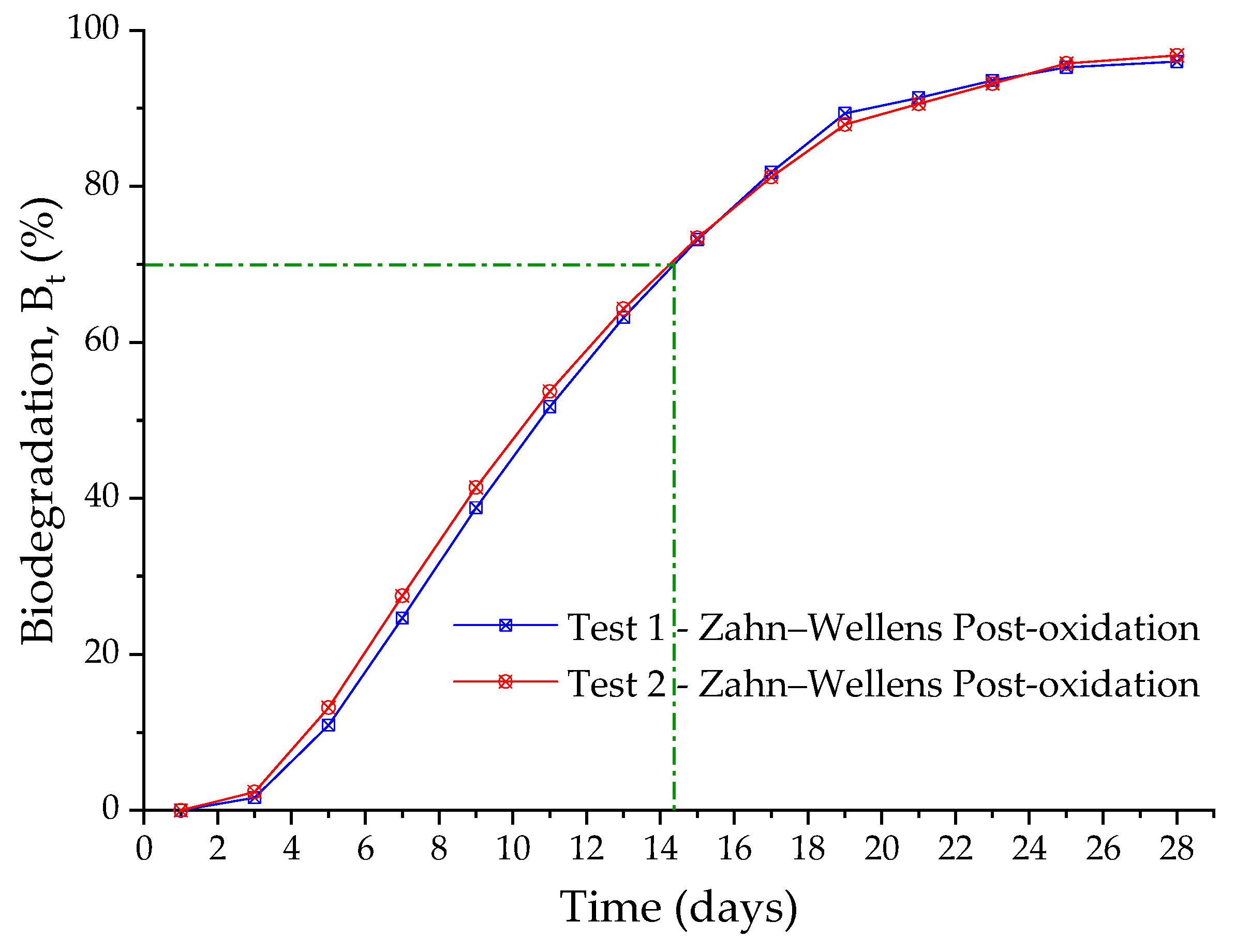
3.4. Aerobic Biological System (AES)
3.5. Effect of RTWW Treatments on Phytotoxicity
3.6. Improvement in the Biodegradability of Real Textile Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senthil Kumar, P.; Grace Pavithra, K. Chapter 2—Water and Textiles. In Water in Textiles and Fashion. Consumption, Footprint, and Life Cycle Assessment, 1st ed.; Muthu, S.S., Ed.; Woodhead Publishing (Elsevier Ltd.): Sawston, UK, 2019; pp. 21–40. [Google Scholar]
- Singha, K.; Maity, S.; Pandit, P. Chapter 1—Water footprint applications in textile sector: An overview. In Green Chemistry for Sustainable Textiles, 1st ed.; Ibrahim, N., Hussain, C.M., Eds.; Woodhead Publishing (Elsevier Ltd.): Sawston, UK, 2021; pp. 1–16. [Google Scholar]
- Kasavan, S.; Yusoff, S.; Guan, N.C.; Zaman, N.S.K.; Fakri, M.F.R. Global trends of textile waste research from 2005 to 2020 using bibliometric analysis. Environ. Sci. Pollut. Res. 2021, 28, 44780–44794. [Google Scholar] [CrossRef]
- Jahan, N.; Tahmid, M.; Shoronika, A.Z.; Fariha, A.; Roy, H.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. A comprehensive review on the sustainable treatment of textile wastewater: Zero liquid discharge and resource recovery perspectives. Sustainability 2022, 14, 15398. [Google Scholar] [CrossRef]
- Dhruv Patel, D.; Bhatt, S. Environmental pollution, toxicity profile, and physico-chemical and biotechnological approaches for treatment of textile wastewater. Biotechnol. Genet. Eng. Rev. 2022, 38, 33–86. [Google Scholar] [CrossRef] [PubMed]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Ravindran, V.; Gopinath, A.; Kumar, M.S. Emerging technologies for mixed industrial wastewater treatment in developing countries: An overview. Environ. Qual. Manag. 2022, 31, 121–141. [Google Scholar] [CrossRef]
- Clifford, D.; Subramonian, S.; Sorg, T.J. Water treatment processes. III. Removing dissolved inorganic contaminants from water. Environ. Sci. Technol. 1986, 20, 1072–1080. [Google Scholar] [CrossRef]
- Helfferich, F.G. Ion Exchange; Dover Publications Inc.: New York, NY, USA, 1995; p. 640. [Google Scholar]
- Islam, M.T.; Al Mamun, M.A.; Halim, A.F.M.F.; Peila, R.; Sanchez Ramirez, D.O. Current trends in textile wastewater treatment—Bibliometric review. Environ. Sci. Pollut. Res. 2024, 31, 19166–19184. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Henao-Aguirre, P.A.; Marín-Flórez, A.; Arredondo-López, S.M.; Sanabria-González, N.R. Bibliometric analysis of advanced oxidation processes (AOPs) in wastewater treatment: Global and Ibero-American research trends. Environ. Sci. Pollut. Res. 2021, 28, 23791–23811. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Saeed, M.U.; Hussain, N.; Sumrin, A.; Shahbaz, A.; Noor, S.; Bilal, M.; Aleya, L.; Iqbal, H.M.N. Microbial bioremediation strategies with wastewater treatment potentialities—A review. Sci. Total Environ. 2022, 818, 151754. [Google Scholar] [CrossRef]
- Babu Ponnusami, A.; Sinha, S.; Ashokan, H.; V Paul, M.; Hariharan, S.P.; Arun, J.; Gopinath, K.P.; Hoang Le, Q.; Pugazhendhi, A. Advanced oxidation process (AOP) combined biological process for wastewater treatment: A review on advancements, feasibility and practicability of combined techniques. Environ. Res. 2023, 237, 116944. [Google Scholar] [CrossRef] [PubMed]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Jeyakumar, R.B.; Somanathan, A. Challenges and emerging trends in advanced oxidation technologies and integration of advanced oxidation processes with biological processes for wastewater treatment. Sustainability 2023, 15, 4235. [Google Scholar] [CrossRef]
- Pan, H.; Gao, Y.; Li, N.; Zhou, Y.; Lin, Q.; Jiang, J. Recent advances in bicarbonate-activated hydrogen peroxide system for water treatment. Chem. Eng. J. 2021, 408, 127332. [Google Scholar] [CrossRef]
- Jawad, A.; Chen, Z.; Yin, G. Bicarbonate activation of hydrogen peroxide: A new emerging technology for wastewater treatment. Chin. J. Catal. 2016, 37, 810–825. [Google Scholar] [CrossRef]
- Urbina-Suarez, N.A.; Rivera-Caicedo, C.; González-Delgado, Á.D.; Barajas-Solano, A.F.; Machuca-Martínez, F. Bicarbonate-hydrogen peroxide system for treating dyeing wastewater: Degradation of organic pollutants and color removal. Toxics 2023, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Macías-Quiroga, I.F.; Rengifo-Herrera, J.A.; Arredondo-López, S.M.; Marín-Flórez, A.; Sanabria-González, N.R. Research trends on pillared interlayered clays (PILCs) used as catalysts in environmental and chemical processes: Bibliometric analysis. Sci. World J. 2022, 2022, 5728678. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Pineda, F.J.; Macías-Quiroga, I.F.; Hinojosa-Zambrano, D.F.; Rivera-Giraldo, J.D.; Ocampo-Serna, D.M.; Sanabria-González, N.R. Treatment of textile wastewater using the Co(II)/NaHCO3/H2O2 oxidation system. Heliyon 2023, 9, e22444. [Google Scholar] [CrossRef] [PubMed]
- Marín-González, N.; Giraldo-Loaiza, C.; Macías-Quiroga, I.F.; Rivera-Giraldo, J.D.; Cardona-Castaño, J.A.; Sanabria-González, N.R. Oxidation of Allura Red AC using the NaHCO3-activated H2O2 system catalyzed with cobalt supported on Al-PILC. ChemEngineering 2024, 8, 14. [Google Scholar] [CrossRef]
- Mora-Bonilla, K.Y.; Macías-Quiroga, I.F.; Sanabria-González, N.R.; Dávila-Arias, M.T. Bicarbonate–activated hydrogen peroxide for an azo dye degradation: Experimental design. ChemEngineering 2023, 7, 86. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Pérez-Flórez, A.; Arcila, J.S.; Giraldo-Goméz, G.I.; Sanabria-Gonzalez, N.R. Synthesis and characterization of Co/Al-PILCs for the oxidation of an azo dye using the bicarbonate-activated hydrogen peroxide system. Catal. Lett. 2022, 152, 1905–1916. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Rojas-Méndez, E.F.; Giraldo-Gómez, G.I.; Sanabria-González, N.R. Experimental data of a catalytic decolorization of Ponceau 4R dye using the cobalt (II)/NaHCO3/H2O2 system in aqueous solution. Data Brief. 2020, 30, 105463. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Q.; Wu, M.Y.; Li, R.; Deng, S.H.; Lee, B.C.Y.; Ong, S.L.; Hu, J.Y. Potential of combined advanced oxidation—Biological process for cost-effective organic matters removal in reverse osmosis concentrate produced from industrial wastewater reclamation: Screening of AOP pre-treatment technologies. Chem. Eng. J. 2020, 389, 123419. [Google Scholar] [CrossRef]
- Dobrosz-Gómez, I.; Quintero-Arias, J.-D.; Gómez-García, M.-Á. Fenton advanced oxidation process for the treatment of industrial textile wastewater highly polluted with acid-black 194 dye. Case Stud. Chem. Environ. Eng. 2024, 9, 100672. [Google Scholar] [CrossRef]
- Dobrosz-Gómez, I.; Quintero-Arias, J.-D.; Gómez-García, M.-Á. Coagulation-flocculation-Fenton-neutralization sequential process for the treatment of industrial effluent polluted with AB194 dye. Case Stud. Chem. Environ. Eng. 2024, 9, 100720. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Bajpai, S.; Gupta, S.K.; Dey, A.; Jha, M.K.; Bajpai, V.; Joshi, S.; Gupta, A. Application of central composite design approach for removal of chromium (VI) from aqueous solution using weakly anionic resin: Modeling, optimization, and study of interactive variables. J. Hazard. Mater. 2012, 227–228, 436–444. [Google Scholar] [CrossRef]
- Janighorban, M.; Rasouli, N.; Sohrabi, N.; Ghaedi, M. Use of central composite design and surface modeling for cadmium (II) ions removal from aqueous environment using novel modified layer double hydroxide. Asian J. Green Chem. 2021, 5, 151–175. [Google Scholar]
- OECD. Guidelines for the Testing of Chemicals, Section 3: Environmental Fate and Behaviour. Test No. 302B: Inherent Biodegradability: Zahn-Wellens/EVPA Test; OECD Publishing: Paris, France, 1992; pp. 1–8. [Google Scholar]
- Yang, M.; Wu, B.; Li, Q.; Xiong, X.; Zhang, H.; Tian, Y.; Xie, J.; Huang, P.; Tan, S.; Wang, G.; et al. Feasibility of the UV/AA process as a pretreatment approach for bioremediation of dye-laden wastewater. Chemosphere 2018, 194, 488–494. [Google Scholar] [CrossRef]
- Mascolo, G.; Balest, L.; Cassano, D.; Laera, G.; Lopez, A.; Pollice, A.; Salerno, C. Biodegradability of pharmaceutical industrial wastewater and formation of recalcitrant organic compounds during aerobic biological treatment. Bioresour. Technol. 2010, 101, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Park, J.; Kumar Pandey, L.; Choi, S.; Lee, H.; De Saeger, J.; Depuydt, S.; Han, T. Testing the toxicity of metals, phenol, effluents, and receiving waters by root elongation in Lactuca sativa L. Ecotoxicol. Environ. Saf. 2018, 149, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Neves, L.; Beber de Souza, J.; de Souza Vidal, C.M.; Herbert, L.T.; de Souza, K.V.; Geronazzo Martins, K.; Young, B.J. Phytotoxicity indexes and removal of color, COD, phenols and ISA from pulp and paper mill wastewater post-treated by UV/H2O2 and photo-Fenton. Ecotoxicol. Environ. Saf. 2020, 202, 110939. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Protocols for Short Term Toxicity Screening of Hazardous Waste Sites; US Environmetal Protection Agency: Chicago, IL, USA, 1996.
- Gálvez, A.; López-Galindo, A.; Peña, A. Effect of different surfactants on germination and root elongation of two horticultural crops: Implications for seed coating. N. Z. J. Crop Hortic. Sci. 2019, 47, 83–98. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Quan, Y.; Yin, Y.; Zheng, S. Effect of long-term organic removal on ion exchange properties and performance during sewage tertiary treatment by conventional anion exchange resins. Chemosphere 2015, 136, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Devaisy, S.; Kandasamy, J.; Aryal, R.; Johir, M.A.H.; Ratnaweera, H.; Vigneswaran, S. Removal of organics with ion-exchange resins (IEX) from reverse osmosis concentrate. Membranes 2023, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Majumdar, P. Application of response surface methodology for optimization of heavy metal biosorption using surfactant modified chitosan bead. J. Chem. Eng. 2011, 175, 376–387. [Google Scholar]
- Masouleh, S.Y.; Mozaffarian, M.; Dabir, B.; Ramezani, S.F. COD and ammonia removal from landfill leachate by UV/PMS/Fe2+ process: ANN/RSM modeling and optimization. Process Saf. Environ. Prot. 2022, 159, 716–726. [Google Scholar] [CrossRef]
- Esfandiaribayat, M.; Binazadeh, M.; Sabbaghi, S.; Mohammadi, M.; Ghaedi, S.; Rajabi, H. Tetracycline removal from wastewater via g-C3N4 loaded RSM-CCD-optimised hybrid photocatalytic membrane reactor. Sci. Rep. 2024, 14, 1163. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Abdirova, P.; Kanafina, D.; Arkhangelsky, E.; Kyzas, G.Z.; Poulopoulos, S.G. UV and Zero-Valent Iron (ZVI) Activated Continuous Flow Persulfate Oxidation of Municipal Wastewater. Catalysts 2023, 13, 25. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Dargahi, A.; Shabanloo, A.; Nasab, H.Z.; Vaziri, Y.; Ansari, A. Electrochemical degradation of methylene blue dye using a graphite doped PbO2 anode: Optimization of operational parameters, degradation pathway and improving the biodegradability of textile wastewater. Arab. J. Chem. 2020, 13, 6847–6864. [Google Scholar] [CrossRef]
- Ferreira, A.; Melkonyan, L.; Carapinha, S.; Ribeiro, B.; Figueiredo, D.; Avetisova, G.; Gouveia, L. Biostimulant and biopesticide potential of microalgae growing in piggery wastewater. Environ. Adv. 2021, 4, 100062. [Google Scholar] [CrossRef]
- Siles-Castellano, A.B.; López, M.J.; López-González, J.A.; Suárez-Estrella, F.; Jurado, M.M.; Estrella-González, M.J.; Moreno, J. Comparative analysis of phytotoxicity and compost quality in industrial composting facilities processing different organic wastes. J. Clean. Prod. 2020, 252, 119820. [Google Scholar] [CrossRef]
- Emino, E.R.; Warman, P.R. Biological assay for compost quality. Compost Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]
- Venegas, M.; Leiva, A.M.; Vidal, G. Influence of anaerobic digestion with pretreatment on the phytotoxicity of sewage sludge. Water Air Soil Poll. 2018, 229, 381. [Google Scholar] [CrossRef]
- Kumar Jaiswal, V.; Dutta Gupta, A.; Verma, V.; Sharan Singh, R. Degradation of p-cresol in the presence of UV light driven in an integrated system containing photocatalytic and packed bed biofilm reactor. Bioresour. Technol. 2023, 387, 129706. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Anotai, J.; Singhadech, S.; Lu, M.C. Enhancement of biodegradability of o-toluidine effluents by electro-assisted photo-Fenton treatment. Process Saf. Environ. Prot. 2017, 106, 60–67. [Google Scholar] [CrossRef]
- Dhanke, P.; Wagh, S. Treatment of vegetable oil refinery wastewater with biodegradability index improvement. Mater. Today Proc. 2020, 27, 181–187. [Google Scholar] [CrossRef]



| Resin | Functional Group | Resin Matrix | Exchange Capacity | Mean Particle Size (mm) | Density (g/mL) | pH Range |
|---|---|---|---|---|---|---|
| Lewatit® MonoPlus S 108 Strongly acidic | Sulfonic acid | Styrene–DVB copolymer | 2.2 eq/L | 0.62 ± 0.05 | 1.30 | 2–14 |
| Lewatit® MonoPlus M 500 Strongly basic | Quaternary ammonium type 1 | Styrene–DVB copolymer | 1.3 eq/L | 0.62 ± 0.05 | 1.08 | 0–12 |
| Factor | Name | Units | Coded Value | ||||
|---|---|---|---|---|---|---|---|
| −1.4142 | −1 | 0 | +1 | +1.4142 | |||
| X1 | H2O2 | mM | 108.3 | 150 | 300 | 450 | 491.7 |
| X2 | NaHCO3 | mM | 28.9 | 40 | 80 | 120 | 131.1 |
| Parameter | RTWW | ||
|---|---|---|---|
| CF | CF–IE SAC Resin | CF–IE SBA Resin | |
| pH | 8.9 ± 0.1 | 1.6 ± 0.1 | 7.8 ± 0.1 |
| Conductivity (μS/cm) | 7040 ± 99 | 4892 ± 64 | 61.6 ± 1.0 |
| Total hardness (mg/L) | 1170 ± 62 | <1.0 | <1.0 |
| Sulfates (mg/L) | 2679 ± 52 | 2679 ± 52 | <2.1 |
| Chloride (mg/L) | 137 ± 2.0 | 137 ± 2 | 18.1 ± 1.2 |
| Total alkalinity (mg/L) | 93 ± 1 | <10.0 | <10.0 |
| COD (mg/L) | 1428 ± 84 | 917 ± 42 | 826 ± 50 |
| BOD5 (mg/L) | 397 ± 31 | 302 ± 23 | |
| TOC, mg/L | 1832 ± 63 | 1755 ± 57 | 1625 ± 98 |
| Acid Black 194, (mg/L) | 29.6 ± 1.6 | 29.6 ± 1.6 | 0.16 ± 0.08 |
| Apparent color, (Pt-Co) | 2360 ± 35 | 780 ± 18 | 14 ± 1 |
| Cation Exchange Resin | Anion Exchange Resin | ||||
|---|---|---|---|---|---|
| SAC Volume (mL) | Hardness (mg/L) | SBA Volume (mL) | SO42− (mg/L) | Cl− (mg/L) | NA–194 (mg/L) |
| 0.0 | 1170 | 0.0 | 2679 | 137 | 29.6 |
| 1.0 | 81.8 | 2.5 | 309.5 | 82.4 | 12.0 |
| 2.0 | 25.0 | 5.0 | 23.1 | 73.6 | 2.8 |
| 3.0 | 22.4 | 9.0 | 5.0 | 48.9 | 2.6 |
| 4.0 | 15.0 | 11.0 | 4.6 | 36.5 | 1.2 |
| 5.0 | <1.0 | 14.0 | <2.1 | 18.1 | 0.16 |
| Cation Exchange 25 mL SAC Resin | Anion Exchange 70 mL SBA Resin | ||||
|---|---|---|---|---|---|
| Time (min) | Hardness (mg/L) | Time (min) | SO42− (mg/L) | Cl− (mg/L) | NA–194 (mg/L) |
| 0.0 | 1170 | 0.0 | 2679 | 137 | 29.6 |
| 1.0 | 160 | 1.0 | 118 | 120.2 | 5.85 |
| 5.0 | 45 | 5.0 | 5.0 | 89.0 | 3.53 |
| 15.0 | 3.4 | 15.0 | 4.7 | 60.3 | 2.32 |
| 30.0 | 1.2 | 30.0 | <2.1 | 42.4 | 1.48 |
| 60.0 | <1.0 | 60.0 | <2.1 | 21.6 | 0.77 |
| 120.0 | <1.0 | 120.0 | <2.1 | 18.1 | 0.16 |
| Std | Run | Factors Values | Response Variables—Removal | ||||
|---|---|---|---|---|---|---|---|
| Codified | Experimental (mM) | TOC (%) | COD (%) | ||||
| X1 | X2 | X1 | X2 | Y1 | Y2 | ||
| 9 | 1 | 0 | −α | 300 | 28.9 | 4.67 | 25.62 |
| 20 | 2 | 0 | −α | 300 | 28.9 | 4.77 | 23.61 |
| 14 | 3 | −1 | +1 | 150 | 120 | 6.97 | 17.57 |
| 5 | 4 | +α | 0 | 491.7 | 80 | 6.96 | 36.85 |
| 11 | 5 | 0 | 491.7 | 80 | 7.50 | 39.94 | |
| 8 | 6 | 0 | −α | 300 | 28.9 | 4.78 | 26.35 |
| 18 | 7 | 0 | 0 | 300 | 80 | 13.22 | 38.58 |
| 25 | 8 | 0 | 0 | 300 | 80 | 12.99 | 37.87 |
| 23 | 9 | 0 | 0 | 300 | 80 | 13.16 | 38.12 |
| 16 | 10 | +α | 0 | 491.7 | 80 | 7.03 | 38.94 |
| 17 | 11 | 0 | 0 | 300 | 80 | 12.31 | 37.67 |
| 2 | 12 | +1 | +1 | 450 | 120 | 14.58 | 42.17 |
| 10 | 13 | +1 | −1 | 450 | 40 | 0.56 | 31.97 |
| 21 | 14 | −α | 0 | 108.3 | 80 | 4.59 | 10.02 |
| 1 | 15 | −α | 0 | 108.3 | 80 | 4.75 | 12.77 |
| 15 | 16 | 0 | +α | 300 | 131.1 | 15.03 | 31.21 |
| 22 | 17 | −1 | −1 | 150 | 40 | 5.28 | 11.01 |
| 13 | 18 | 0 | +α | 300 | 131.1 | 15.67 | 33.28 |
| 6 | 19 | +1 | −1 | 450 | 40 | 0.50 | 33.52 |
| 3 | 20 | 0 | 0 | 300 | 80 | 13.29 | 37.25 |
| 7 | 21 | −1 | +1 | 150 | 120 | 7.82 | 16.04 |
| 24 | 22 | 0 | +α | 300 | 131.1 | 15.07 | 34.79 |
| 19 | 23 | −1 | −1 | 150 | 40 | 5.25 | 13.87 |
| 12 | 24 | −α | 0 | 108.3 | 80 | 4.58 | 11.74 |
| 4 | 25 | +1 | +1 | 450 | 120 | 15.04 | 41.87 |
| Parameter | Sum of Squares | Degrees Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 559.28 | 5 | 111.86 | 996.72 | <0.0001 a |
| X1 | 12.7 | 1 | 12.70 | 113.15 | <0.0001 a |
| X2 | 300.54 | 1 | 300.54 | 2678.06 | <0.0001 a |
| X1X2 | 73.81 | 1 | 73.81 | 657.71 | <0.0001 a |
| X12 | 172.23 | 1 | 172.23 | 1534.68 | <0.0001 a |
| X22 | 29.49 | 1 | 29.49 | 262.79 | <0.0001 a |
| Residual | 2.13 | 19 | 0.11 | ||
| Lack of fit | 0.57 | 3 | 0.19 | 1.96 | 0.1602 b |
| Pure error | 1.56 | 16 | 0.097 | ||
| Cor total | 561.42 | 24 | |||
| Parameter | Sum of Squares | Degrees Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 2885.15 | 5 | 577.03 | 297.24 | <0.0001 a |
| X1 | 2132.20 | 1 | 2132.20 | 1098.33 | <0.0001 a |
| X2 | 186.19 | 1 | 186.19 | 95.91 | <0.0001 a |
| X1X2 | 12.05 | 1 | 12.05 | 6.21 | <0.0001 a |
| X12 | 516.19 | 1 | 516.19 | 265.90 | <0.0001 a |
| X22 | 225.62 | 1 | 225.62 | 116.22 | <0.0001 a |
| Residual | 36.88 | 19 | 1.94 | ||
| Lack of fit | 10.07 | 3 | 3.36 | 2.00 | 0.1542 b |
| Pure error | 26.81 | 16 | 1.68 | ||
| Cor total | 2922.03 | 24 | |||
| Conditions | TOC Removal (%) | COD Removal (%) | ||
|---|---|---|---|---|
| Predic. | Exper. | Predic. | Exper. | |
| [H2O2] = 393.3 mM [NaHCO3] = 114.3 mM | 15.67 | 14.77 | 41.44 | 38.86 |
| [H2O2] = 150 mM [NaHCO3] = 100 mM | 7.93 | 7.81 | 19.09 | 19.40 |
| [H2O2] = 300 mM [NaHCO3] = 100 mM | 14.57 | 14.17 | 38.06 | 36.91 |
| Sample/Treatment | RSG (%) | RRG (%) | GI (%) | Interpretation [50,51,52] |
|---|---|---|---|---|
| Control (Distilled water) | 100 | 100 | 100 | GI ≥ 80, no-phytotoxic |
| RTWW–CF | 0 | 0 | 0 | GI = 0, phytotoxic that inhibits germination |
| RTWW–CF–IE | 30.8 ± 6.7 | 14.1 ± 4.9 | 4.2 ± 1.5 | GI ≤ 50%: highly phytotoxic |
| RTWW–CF–IE–BAP | 61.5 ± 6.7 | 84.5 ± 16.9 | 52.0 ± 11.3 | GI between 50–80%, moderately phytotoxic |
| RTWW–CF–IE–BAP–ABS | 100 | 233.8 ± 12.9 | 233.7 ± 18.7 | IG ≥ 100%, phytonutrient or phytostimulant effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo-Loaiza, C.; Salazar-Loaiza, A.M.; Sandoval-Barrera, M.A.; Macías-Quiroga, I.F.; Ocampo-Serna, D.M.; Sanabria-González, N.R. Integration of Ion Exchange—AOP—Biological System for the Treatment of Real Textile Wastewater. ChemEngineering 2024, 8, 76. https://doi.org/10.3390/chemengineering8040076
Giraldo-Loaiza C, Salazar-Loaiza AM, Sandoval-Barrera MA, Macías-Quiroga IF, Ocampo-Serna DM, Sanabria-González NR. Integration of Ion Exchange—AOP—Biological System for the Treatment of Real Textile Wastewater. ChemEngineering. 2024; 8(4):76. https://doi.org/10.3390/chemengineering8040076
Chicago/Turabian StyleGiraldo-Loaiza, Camila, Aura M. Salazar-Loaiza, María A. Sandoval-Barrera, Iván F. Macías-Quiroga, Diana M. Ocampo-Serna, and Nancy R. Sanabria-González. 2024. "Integration of Ion Exchange—AOP—Biological System for the Treatment of Real Textile Wastewater" ChemEngineering 8, no. 4: 76. https://doi.org/10.3390/chemengineering8040076
APA StyleGiraldo-Loaiza, C., Salazar-Loaiza, A. M., Sandoval-Barrera, M. A., Macías-Quiroga, I. F., Ocampo-Serna, D. M., & Sanabria-González, N. R. (2024). Integration of Ion Exchange—AOP—Biological System for the Treatment of Real Textile Wastewater. ChemEngineering, 8(4), 76. https://doi.org/10.3390/chemengineering8040076






