Arsenic in Water: Understanding the Chemistry, Health Implications, Quantification and Removal Strategies
Abstract
:1. Introduction
2. Prevalence of Arsenic Contamination
3. Behaviour of Arsenic in Water
4. Arsenic Implications on Human Health
5. Techniques Used for Arsenic Quantification in Water
6. Techniques Used for Arsenic Mitigation from Water
6.1. Oxidation Method
6.2. Precipitation Method
6.3. Membrane Technologies
6.4. Adsorption
6.5. Social Mitigation
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Katherine, A.J.; Jaymie, R.M.; Jerome, O.N. Arsenic. In International Encyclopedia of Public Health; Academic Press: Oxford, UK, 2017; pp. 170–175. [Google Scholar] [CrossRef]
- Adriano, D.C. Arsenic. In Trace Elements in Terrestrial Environments; Springer: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Parascandola, J. King of Poisons: A History of Arsenic; Potomac Books, Inc.: Sterling, VA, USA, 2012. [Google Scholar]
- Jing, C.; Meng, X.; Calvache, E.; Jiang, G. Remediation of organic and inorganic arsenic contaminated groundwater using a nanocrystalline TiO2-based adsorbent. Environ. Pollut. 2009, 157, 2514–2519. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- Rajakovic, L.; Rajakovic-Ognjanovic, V. Arsenic in water: Determination and removal. In Arsenic: Analytical Toxicological Studies; IntechOpen: London, UK, 2018; pp. 9–24. [Google Scholar]
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution: A Global Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 94. [Google Scholar]
- Ravenscroft, P. Predicting the global distribution of arsenic pollution in groundwater. In Proceedings of the Royal Geographical Society Annual International Conference, London, UK, 28–31 August 2007. [Google Scholar]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation-A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef]
- Ahmad, A.; Bhattacharya, P. Arsenic in drinking water: Is 10 μg/L a safe limit? Curr. Pollut. Rep. 2019, 5, 1–3. [Google Scholar] [CrossRef]
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic pollution sources. Rev. Environ. Contam. Toxicol. 2009, 197, 17–60. [Google Scholar]
- Amini, M.; Abbaspour, K.C.; Berg, M.; Winkel, L.; Hug, S.J.; Hoehn, E.; Yang, H.; Johnson, C.A. Statistical Modeling of Global Geogenic Arsenic Contamination in Groundwater. Environ. Sci. Technol. 2008, 42, 3669–3675. [Google Scholar] [CrossRef]
- Ali, W.; Mushtaq, N.; Javed, T.; Zhang, H.; Ali, K.; Rasool, A.; Farooqi, A. Vertical mixing with return irrigation water the cause of arsenic enrichment in groundwater of district Larkana Sindh, Pakistan. Environ. Pollut. 2019, 245, 77–88. [Google Scholar] [CrossRef]
- Ranjan, A. Spatial analysis of arsenic contamination of groundwater around the world and India. Int. J. Innov. Stud. Sociol. Humanit. 2019, 4, 6–15. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; Nirwan, J.S.; Smith, A.M.; Timmins, P.; Conway, B.R.; Ghori, M.U. Raft-forming polysaccharides for the treatment of gastroesophageal reflux disease (GORD): Systematic review. J. Appl. Polym. Sci. 2019, 136, 48012. [Google Scholar] [CrossRef]
- Nirwan, J.S.; Hasan, S.S.; Babar ZU, D.; Conway, B.R.; Ghori, M.U. Global prevalence and risk factors of gastro-oesophageal reflux disease (GORD): Systematic review with meta-analysis. Sci. Rep. 2020, 10, 5814. [Google Scholar] [CrossRef] [PubMed]
- Khizer, Z.; Sadia, A.; Sharma, R.; Farhaj, S.; Nirwan, J.S.; Kakadia, P.G.; Hussain, T.; Yousaf, A.M.; Shahzad, Y.; Conway, B.R.; et al. Drug delivery approaches for managing overactive bladder (OAB): A systematic review. Pharmaceuticals 2021, 14, 409. [Google Scholar] [CrossRef]
- Farhaj, S.; Agbotui, T.L.; Nirwan, J.S.; Mahmood, Q.; Yousaf, A.M.; Hussain, T.; Shahzad, Y.; Khan, N.; Conway, B.R.; Ghori, M.U. Carbohydrate Polymer-Based Targeted Pharmaceutical Formulations for Colorectal Cancer: Systematic Review of the Literature. Polysaccharides 2022, 3, 692–714. [Google Scholar] [CrossRef]
- Hasan, S.S.; Zaidi ST, R.; Nirwan, J.S.; Ghori, M.U.; Javid, F.; Ahmadi, K.; Babar ZU, D. Use of central nervous system (CNS) medicines in aged care homes: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1292. [Google Scholar] [CrossRef] [PubMed]
- Alhasso, B.; Ghori, M.U.; Conway, B.R. Systematic review on the effectiveness of essential and carrier oils as skin penetration enhancers in pharmaceutical formulations. Sci. Pharm. 2022, 90, 14. [Google Scholar] [CrossRef]
- Rosenboom, J.W. Not Just Red or Green: An Analysis of Arsenic Data from 15 Upazilas in Bangladesh; Government of the People’s Republic of Bangladesh, Ministry of Local Government, Rural Development, and Co-operatives, Department of Public Health & Engg., Arsenic Policy Support Unit.: Dhaka, Bangladesh, 2004. [Google Scholar]
- Chakraborti, D.; Rahman, M.M.; Das, B.; Murrill, M.; Dey, S.; Mukherjee, S.C.; Dhar, R.K.; Biswas, B.K.; Chowdhury, U.K.; Roy, S. Status of groundwater arsenic contamination in Bangladesh: A 14-year study report. Water Res. 2010, 44, 5789–5802. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.M.; Bhattacharya, P.; Hasan, M.A.; Akhter, S.H.; Alam, S.M.; Bhuyian, M.H.; Imam, M.B.; Khan, A.A.; Sracek, O. Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: An overview. Appl. Geochem. 2004, 19, 181–200. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mandal, B.K.; Chowdhury, T.R.; Sengupta, M.K.; Chowdhury, U.K.; Lodh, D.; Chanda, C.R.; Basu, G.K.; Mukherjee, S.C.; Saha, K.C. Arsenic groundwater contamination and sufferings of people in North 24-Parganas, one of the nine arsenic affected districts of West Bengal, India. J. Environ. Sci. Health Part A 2003, 38, 25–59. [Google Scholar] [CrossRef]
- Edmunds, W.; Ahmed, K.; Whitehead, P. A review of arsenic and its impacts in groundwater of the Ganges–Brahmaputra–Meghna delta, Bangladesh. Environ. Sci. Process. Impacts 2015, 17, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Sengupta, M.K.; Hossain, M.A.; Ahamed, S.; Das, B.; Nayak, B.; Lodh, D.; Rahman, M.M.; Chakraborti, D. Arsenic Contamination in Groundwater: A Global Perspective with Emphasis on the Asian Scenario. J. Health Popul. Nutr. 2006, 24, 142–163. [Google Scholar] [PubMed]
- Mitsunobu, S.; Hamanura, N.; Kataoka, T.; Shiraishi, F. Arsenic attenuation in geothermal streamwater coupled with biogenic arsenic (III) oxidation. Appl. Geochem. 2013, 35, 154–160. [Google Scholar] [CrossRef]
- Maity, J.P.; Nath, B.; Chen, C.-Y.; Bhattacharya, P.; Sracek, O.; Bundschuh, J.; Kar, S.; Thunvik, R.; Chatterjee, D.; Ahmed, K.M. Arsenic-enriched groundwaters of India, Bangladesh and Taiwan—Comparison of hydrochemical characteristics and mobility constraints. J. Environ. Sci. Health Part A 2011, 46, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Costa, M. Arsenic: A global environmental challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Baig, J.A.; Kazi, T.G.; Arain, M.B.; Afridi, H.I.; Kandhro, G.A.; Sarfraz, R.A.; Jamal, M.K.; Shah, A.Q. Evaluation of arsenic and other physico-chemical parameters of surface and ground water of Jamshoro, Pakistan. J. Hazard. Mater. 2009, 166, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Maity, J.P.; Jean, J.-S.; Li, Z.; Kar, S.; Sracek, O.; Yang, H.-J.; Chen, C.-Y.; Reza, A.S.; Bundschuh, J. The geochemical characteristics of the mud liquids in the Wushanting and Hsiaokunshui Mud Volcano region in southern Taiwan: Implications of humic substances for binding and mobilization of arsenic. J. Geochem. Explor. 2013, 128, 62–71. [Google Scholar] [CrossRef]
- Ali, W.; Rasool, A.; Junaid, M.; Zhang, H. A comprehensive review on current status, mechanism, and possible sources of arsenic contamination in groundwater: A global perspective with prominence of Pakistan scenario. Environ. Geochem. Health 2019, 41, 737–760. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Farooqi, A.; Hussain, K.; Haider, N. Evaluation of industrial based adsorbents for simultaneous removal of arsenic and fluoride from drinking water. J. Clean. Prod. 2015, 87, 882–896. [Google Scholar] [CrossRef]
- Brahman, K.D.; Kazi, T.G.; Afridi, H.I.; Naseem, S.; Arain, S.S.; Ullah, N. Evaluation of high levels of fluoride, arsenic species and other physicochemical parameters in underground water of two sub districts of Tharparkar, Pakistan: A multivariate study. Water Res. 2013, 47, 1005–1020. [Google Scholar] [CrossRef]
- Fatmi, Z.; Azam, I.; Ahmed, F.; Kazi, A.; Gill, A.B.; Kadir, M.M.; Ahmed, M.; Ara, N.; Janjua, N.Z.; Core Group for Arsenic Mitigation in Pakistan. Health burden of skin lesions at low arsenic exposure through groundwater in Pakistan. Is river the source? Environ. Res. 2009, 109, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Stengel, C.; Trang, P.T.K.; Viet, P.H.; Sampson, M.L.; Leng, M.; Samreth, S.; Fredericks, D. Magnitude of arsenic pollution in the Mekong and Red River Deltas—Cambodia and Vietnam. Sci. Total Environ. 2007, 372, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, S.; Angeloni, J.; Watkins, R. Arsenic-rich groundwater in an urban area experiencing drought and increasing population density, Perth, Australia. Appl. Geochem. 2006, 21, 83–97. [Google Scholar] [CrossRef]
- Romić, Ž.; Habuda-Stanić, M.; Kalajdžić, B.; Kuleš, M. Arsenic distribution, concentration and speciation in groundwater of the Osijek area, eastern Croatia. Appl. Geochem. 2011, 26, 37–44. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M.; Bundschuh, J.; Maity, J.P.; Bhattacharya, P. Natural arsenic in global groundwaters: Distribution and geochemical triggers for mobilization. Curr. Pollut. Rep. 2016, 2, 68–89. [Google Scholar] [CrossRef]
- Donahue, R.; Hendry, M. Geochemistry of arsenic in uranium mine mill tailings, Saskatchewan, Canada. Appl. Geochem. 2003, 18, 1733–1750. [Google Scholar] [CrossRef]
- Bondu, R.; Cloutier, V.; Rosa, E.; Benzaazoua, M. Mobility and speciation of geogenic arsenic in bedrock groundwater from the Canadian Shield in western Quebec, Canada. Sci. Total Environ. 2017, 574, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Worldwide Occurrences of Arsenic in Ground Water. Science 2002, 296, 2143–2145. [Google Scholar] [CrossRef]
- Buamah, R.; Petrusevski, B.; Schippers, J. Presence of arsenic, iron and manganese in groundwater within the gold-belt zone of Ghana. J. Water Supply Res. Technol.—AQUA 2008, 57, 519–529. [Google Scholar] [CrossRef]
- Smedley, P.; Edmunds, W.; Pelig-Ba, K. Mobility of arsenic in groundwater in the Obuasi gold-mining area of Ghana: Some implications for human health. Geol. Soc. Lond. Spec. Publ. 1996, 113, 163–181. [Google Scholar] [CrossRef]
- Bowell, R. Sorption of arsenic by iron oxides and oxyhydroxides in soils. Appl. Geochem. 1994, 9, 279–286. [Google Scholar] [CrossRef]
- Hough, R.L.; Fletcher, T.; Leonardi, G.S.; Goessler, W.; Gnagnarella, P.; Clemens, F.; Gurzau, E.; Koppova, K.; Rudnai, P.; Kumar, R. Lifetime exposure to arsenic in residential drinking water in Central Europe. Int. Arch. Occup. Environ. Health 2010, 83, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, G.; Vahter, M.; Clemens, F.; Goessler, W.; Gurzau, E.; Hemminki, K.; Hough, R.; Koppova, K.; Kumar, R.; Rudnai, P. Inorganic arsenic and basal cell carcinoma in areas of Hungary, Romania, and Slovakia: A case–control study. Environ. Health Perspect. 2012, 120, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Rowland, H.A.; Omoregie, E.O.; Millot, R.; Jimenez, C.; Mertens, J.; Baciu, C.; Hug, S.J.; Berg, M. Geochemistry and arsenic behaviour in groundwater resources of the Pannonian Basin (Hungary and Romania). Appl. Geochem. 2011, 26, 1–17. [Google Scholar] [CrossRef]
- Schwenzer, S.P.; Tommaseo, C.E.; Kersten, M.; Kirnbauer, T. Speciation and oxidation kinetics of arsenic in the thermal springs of Wiesbaden spa, Germany. Fresenius’ J. Anal. Chem. 2001, 371, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, G.; Udluft, P. Natural arsenic in Triassic rocks: A source of drinking-water contamination in Bavaria, Germany. Hydrogeol. J. 1999, 7, 468–476. [Google Scholar] [CrossRef]
- Murcott, S. Arsenic Contamination in the World; IWA Publishing: London, UK, 2012. [Google Scholar]
- Berg, M.; Tran, H.C.; Nguyen, T.C.; Pham, H.V.; Schertenleib, R.; Giger, W. Arsenic contamination of groundwater and drinking water in Vietnam: A human health threat. Environ. Sci. Technol. 2001, 35, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Aiuppa, A.; D’Alessandro, W.; Federico, C.; Palumbo, B.; Valenza, M. The aquatic geochemistry of arsenic in volcanic groundwaters from southern Italy. Appl. Geochem. 2003, 18, 1283–1296. [Google Scholar] [CrossRef]
- Middleton, D.; Watts, M.; Hamilton, E.; Ander, E.; Close, R.; Exley, K.; Crabbe, H.; Leonardi, G.; Fletcher, T.; Polya, D. Urinary arsenic profiles reveal exposures to inorganic arsenic from private drinking water supplies in Cornwall, UK. Sci. Rep. 2016, 6, 25656. [Google Scholar] [CrossRef]
- Millward, G.E.; Kitts, H.J.; Ebdon, L.; Allen, J.I.; Morris, A.W. Arsenic in the Thames Plume, UK. Mar. Environ. Res. 1997, 44, 51–67. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Mukherjee, A.B.; Bundschuh, J.; Zevenhoven, R.; Loeppert, R.H. Arsenic in Soil and Groundwater Environment: Biogeochemical Interactions, Health Effects and Remediation; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- de Figueiredo, B.R.; Borba, R.P.; Angélica, R.S. Arsenic occurrence in Brazil and human exposure. Environ. Geochem. Health 2007, 29, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Lillo, J.; Sahún, B. Naturally occurring arsenic in groundwater and identification of the geochemical sources in the Duero Cenozoic Basin, Spain. Environ. Geol. 2006, 50, 1151–1170. [Google Scholar] [CrossRef]
- Garcia-Sanchez, A.; Moyano, A.; Mayorga, P. High arsenic contents in groundwater of central Spain. Environ. Geol. 2005, 47, 847–854. [Google Scholar] [CrossRef]
- Queirolo, F.; Stegen, S.; Mondaca, J.; Cortés, R.; Rojas, R.; Contreras, C.; Munoz, L.; Schwuger, M.; Ostapczuk, P. Total arsenic, lead, cadmium, copper, and zinc in some salt rivers in the northern Andes of Antofagasta, Chile. Sci. Total Environ. 2000, 255, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Goswami, R.; Patel, A.K.; Srivastava, M.; Das, N. Scenario, perspectives and mechanism of arsenic and fluoride Co-occurrence in the groundwater: A review. Chemosphere 2020, 249, 126126. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Huyck, H.L. An overview of the abundance, relative mobility, bioavailability, and human toxicity of metals. Environ. Geochem. Miner. Depos. 1999, 6, 29–70. [Google Scholar]
- Grönwall, J.; Danert, K. Regarding groundwater and drinking water access through a human rights lens: Self-Supply as a norm. Water 2020, 12, 419. [Google Scholar] [CrossRef]
- Francesconi, K.A.; Kuehnelt, D. Arsenic Compounds in the environment. In Environmental Chemistry of Arsenic; Marcel Dekker: New York, NY, USA, 2001; pp. 51–94. [Google Scholar]
- Smedley, P.L. Sources and distribution of arsenic in groundwater and aquifers. In Arsenic in Groundwater: A World Problem; IAH: Utrecht, The Netherlands, 2008. [Google Scholar]
- Lengke, M.F.; Sanpawanitchakit, C.; Tempel, R.N. The oxidation and dissolution of arsenic-bearing sulfides. Can. Mineral. 2009, 47, 593–613. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, Y.; Gao, Z.; Gammons, C.H.; Li, D. Rates of arsenopyrite oxidation by oxygen and Fe (III) at pH 1.8–12.6 and 15–45 C. Environ. Sci. Technol. 2007, 41, 6460–6464. [Google Scholar] [CrossRef]
- Faiz, F.; Qiao, J.-q.; Lian, H.-z.; Mao, L.; Cui, X.-b. A combination approach using two functionalized magnetic nanoparticles for speciation analysis of inorganic arsenic. Talanta 2022, 237, 122939. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.; Goodale, B.; Raab, A.; Schwerdtle, T.; Reimer, K.; Conklin, S.; Karagas, M.R.; Francesconi, K.A. Human exposure to organic arsenic species from seafood. Sci. Total Environ. 2017, 580, 266–282. [Google Scholar] [CrossRef]
- McLean, J.E.; Bledsoe, B.E. Ground Water Issue; United States Environmental Protection Agency: Washington, DC, USA, 1992; pp. 5–92. [Google Scholar]
- Marinho, B.A.; Cristóvão, R.O.; Boaventura, R.A.; Vilar, V.J. As (III) and Cr (VI) oxyanion removal from water by advanced oxidation/reduction processes—A review. Environ. Sci. Pollut. Res. 2019, 26, 2203–2227. [Google Scholar] [CrossRef]
- Wang, Z.; Bush, R.T.; Sullivan, L.A.; Liu, J. Simultaneous redox conversion of chromium (VI) and arsenic (III) under acidic conditions. Environ. Sci. Technol. 2013, 47, 6486–6492. [Google Scholar] [CrossRef]
- Smedley, P.; Nicolli, H.; Macdonald, D.; Barros, A.; Tullio, J. Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259–284. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U.; Shikha. Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef]
- IARC. Arsenic, metals, fibres and dusts. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100 C. A review of human carcinogens; pp. 453–454. [Google Scholar]
- Campbell, K.M.; Nordstrom, D.K. Arsenic speciation and sorption in natural environments. Rev. Mineral. Geochem. 2014, 79, 185–216. [Google Scholar] [CrossRef]
- Rakhunde, R.; Jasudkar, D.; Deshpande, L.; Juneja, H.; Labhasetwar, P. Health effects and significance of arsenic speciation in water. Int. J. Environ. Sci. Res. 2012, 1, 92–96. [Google Scholar]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Somanna, Y.; Kim, H. Source, distribution, toxicity and remediation of arsenic in the environment–a review. Int. J. Appl. Environ. Sci. 2016, 11, 559–581. [Google Scholar]
- Cassone, G.; Chillé, D.; Foti, C.; Giuffré, O.; Ponterio, R.C.; Sponer, J.; Saija, F. Stability of hydrolytic arsenic species in aqueous solutions: As3+ vs. As5+. Phys. Chem. Chem. Phys. 2018, 20, 23272–23280. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Kumar, S.; Singh, V. Occurrence, behaviour and speciation of arsenic in groundwater. Curr. Sci. 2004, 86, 281–284. [Google Scholar]
- Frith, J. Arsenic-the “poison of kings” and the “saviour of syphilis”. J. Mil. Veterans Health 2013, 21, 11–17. [Google Scholar]
- Afaj, A. IARC Monograph on the Evaluation of Carcinogenic Risk to Humans, Volume 95: Household Use of Solid Fuels and High-Temperature Frying. 2011. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 5 October 2021).
- Minichilli, F.; Bianchi, F.; Ronchi, A.M.; Gorini, F.; Bustaffa, E. Urinary Arsenic in Human Samples from Areas Characterized by Natural or Anthropogenic Pollution in Italy. Int. J. Environ. Res. Public Health 2018, 15, 299. [Google Scholar] [CrossRef] [PubMed]
- McCarty, K.M.; Hanh, H.T.; Kim, K.-W. Arsenic geochemistry and human health in South East Asia. Rev. Environ. Health 2011, 26, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Jean, J.-S.; Kar, S. Bioaccessibility and health risk assessment of arsenic in arsenic-enriched soils, Central India. Ecotoxicol. Environ. Saf. 2013, 92, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kumar, D.; Sahu, A.P. Arsenic in the environment: Effects on human health and possible prevention. J. Environ. Biol. 2007, 28, 359. [Google Scholar]
- Chen, S.-J.; Yan, X.-J.; Chen, Z. Arsenic in Tissues, Organs, and Cells. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 135–138. [Google Scholar]
- Rom, W.N.; Markowitz, S.B. Environmental and Occupational Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- IRAC. Arsenic, metals, fibres, and dusts. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Arsenic and arsenic compounds; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100 C. A review of human carcinogens; pp. 41–94. [Google Scholar]
- Ahmad, S.A.; Khan, M.H.; Haque, M. Arsenic contamination in groundwater in Bangladesh: Implications and challenges for healthcare policy. Risk Manag. Healthc. Policy 2018, 11, 251–261. [Google Scholar] [CrossRef]
- Rasheed, H.; Kay, P.; Slack, R.; Gong, Y.Y. Assessment of arsenic species in human hair, toenail and urine and their association with water and staple food. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 624–632. [Google Scholar] [CrossRef]
- Farmer, J.G.; Johnson, L.R. Assessment of occupational exposure to inorganic arsenic based on urinary concentrations and speciation of arsenic. Br. J. Ind. Med. 1990, 47, 342–348. [Google Scholar] [CrossRef]
- Brodin, M.B. Andrews’ Diseases of the Skin: Clinical Dermatology. JAMA 1990, 264, 1045–1046. [Google Scholar] [CrossRef]
- Chen, Y.; Graziano, J.H.; Parvez, F.; Liu, M.; Slavkovich, V.; Kalra, T.; Argos, M.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ 2011, 342, d2431. [Google Scholar] [CrossRef]
- Flora, S.J.S. Handbook of Arsenic Toxicology; Academic Press: Oxford, UK, 2014. [Google Scholar]
- Bjørklund, G.; Oliinyk, P.; Lysiuk, R.; Rahaman, M.S.; Antonyak, H.; Lozynska, I.; Lenchyk, L.; Peana, M. Arsenic intoxication: General aspects and chelating agents. Arch. Toxicol. 2020, 94, 1879–1897. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; De Silva, P.M.C. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Gatti, M.F.; Gagliardi, T.; Cuccaro, F.; De Maria, L.; Caputi, A.; Quarato, M.; Baldassarre, A. Environmental exposure to arsenic and chromium in an industrial area. Environ. Sci. Pollut. Res. 2017, 24, 11528–11535. [Google Scholar] [CrossRef]
- Smith, A.H.; Marshall, G.; Roh, T.; Ferreccio, C.; Liaw, J.; Steinmaus, C. Lung, Bladder, and Kidney Cancer Mortality 40 Years After Arsenic Exposure Reduction. JNCI J. Natl. Cancer Inst. 2017, 110, 241–249. [Google Scholar] [CrossRef]
- Hopenhayn-Rich, C.; Biggs, M.L.; Smith, A.H. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Int. J. Epidemiol. 1998, 27, 561–569. [Google Scholar] [CrossRef]
- Kurokawa, M.; Ogata, K.; Idemori, M.; Tsumori, S.; Miyaguni, H.; Inoue, S.; Hotta, N. Investigation of skin manifestations of arsenicism due to intake ofarsenic-contaminated groundwater in residents of Samta, Jessore, Bangladesh. Arch. Dermatol. 2001, 137, 102–103. [Google Scholar] [PubMed]
- Chen, C.J.; Kuo, T.L.; Wu, M.M. Arsenic and cancers. Lancet 1988, 1, 414–415. [Google Scholar] [CrossRef]
- Smith, A.H.; Goycolea, M.; Haque, R.; Biggs, M.L. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am. J. Epidemiol. 1998, 147, 660–669. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Chou, H.-Y.; The, H.-W.; Chen, C.-M.; Chen, C.-J. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 2003, 24, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Calderon, J.; Navarro, M.; Jimenez-Capdeville, M.; Santos-Diaz, M.; Golden, A.; Rodriguez-Leyva, I.; Borja-Aburto, V.; Dıaz-Barriga, F. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ. Res. 2001, 85, 69–76. [Google Scholar] [CrossRef]
- Kirkley, A.G.; Carmean, C.M.; Ruiz, D.; Ye, H.; Regnier, S.M.; Poudel, A.; Hara, M.; Kamau, W.; Johnson, D.N.; Roberts, A.A.; et al. Arsenic exposure induces glucose intolerance and alters global energy metabolism. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 314, R294–R303. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Hsiao, C.K.; Chen, C.-L.; Hsu, L.-I.; Chiou, H.-Y.; Chen, S.-Y.; Hsueh, Y.-M.; Wu, M.-M.; Chen, C.-J. A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol. Appl. Pharmacol. 2007, 222, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Chen, Y.; Parvez, F.; Zablotska, L.; Argos, M.; Hussain, I.; Momotaj, H.; Levy, D.; Cheng, Z.; Slavkovich, V. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: Baseline results from the Health Effects of Arsenic Longitudinal Study. Am. J. Epidemiol. 2006, 163, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, D.; Mukherjee, S.C.; Pati, S.; Sengupta, M.K.; Rahman, M.M.; Chowdhury, U.K.; Lodh, D.; Chanda, C.R.; Chakraborti, A.K.; Basu, G.K. Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: A future danger? Environ. Health Perspect. 2003, 111, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Sohel, N.; Vahter, M.; Ali, M.; Rahman, M.; Rahman, A.; Streatfield, P.K.; Kanaroglou, P.S.; Persson, L.Å. Spatial patterns of fetal loss and infant death in an arsenic-affected area in Bangladesh. Int. J. Health Geogr. 2010, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Le, X.C.; Lu, X.; Li, X.-F. Peer reviewed: Arsenic speciation. Anal. Chem. 2004, 76, 26A–33A. [Google Scholar] [CrossRef]
- Komorowicz, I.; Barałkiewicz, D. Determination of total arsenic and arsenic species in drinking water, surface water, wastewater, and snow from Wielkopolska, Kujawy-Pomerania, and Lower Silesia provinces, Poland. Environ. Monit. Assess. 2016, 188, 504. [Google Scholar] [CrossRef] [PubMed]
- Ardini, F.; Dan, G.; Grotti, M. Arsenic speciation analysis of environmental samples. J. Anal. At. Spectrom. 2020, 35, 215–237. [Google Scholar] [CrossRef]
- Luong, J.H.; Lam, E.; Male, K.B. Recent advances in electrochemical detection of arsenic in drinking and ground waters. Anal. Methods 2014, 6, 6157–6169. [Google Scholar] [CrossRef]
- Chen, M.-L.; Ma, L.-Y.; Chen, X.-W. New procedures for arsenic speciation: A review. Talanta 2014, 125, 78–86. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Huang, X.-J. Voltammetric determination of inorganic arsenic. TrAC Trends Anal. Chem. 2014, 60, 25–35. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, H.; Kim, Y.-T.; Yoon, H.-O. Development of a simultaneous analytical method to determine arsenic speciation using HPLC-ICP-MS: Arsenate, arsenite, monomethylarsonic acid, dimethylarsinic acid, dimethyldithioarsinic acid, and dimethylmonothioarsinic acid. Microchem. J. 2017, 134, 295–300. [Google Scholar] [CrossRef]
- Terlecka, E. Arsenic speciation analysis in water samples: A review of the hyphenated techniques. Environ. Monit. Assess. 2005, 107, 259–284. [Google Scholar] [CrossRef]
- Kolya, H.; Hashitsume, K.; Kang, C.-W. Recent Advances in Colorimetric Detection of Arsenic Using Metal-Based Nanoparticles. Toxics 2021, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ariza, J.L.; Lorenzo, F.; García-Barrera, T. Comparative study of atomic fluorescence spectroscopy and inductively coupled plasma mass spectrometry for mercury and arsenic multispeciation. Anal. Bioanal. Chem. 2005, 382, 485–492. [Google Scholar] [CrossRef]
- Komorowicz, I.; Barałkiewicz, D. Arsenic and its speciation in water samples by high performance liquid chromatography inductively coupled plasma mass spectrometry—Last decade review. Talanta 2011, 84, 247–261. [Google Scholar] [CrossRef]
- Müller, K.; Ciminelli, V.S.; Dantas, M.S.S.; Willscher, S. A comparative study of As (III) and As (V) in aqueous solutions and adsorbed on iron oxy-hydroxides by Raman spectroscopy. Water Res. 2010, 44, 5660–5672. [Google Scholar] [CrossRef]
- Sounderajan, S.; Udas, A.; Venkataramani, B. Characterization of arsenic (V) and arsenic (III) in water samples using ammonium molybdate and estimation by graphite furnace atomic absorption spectroscopy. J. Hazard. Mater. 2007, 149, 238–242. [Google Scholar] [CrossRef]
- Barros, H.; Parra, L.-M.M.; Bennun, L.; Greaves, E.D. Determination of arsenic in water samples by Total Reflection X-Ray Fluorescence using pre-concentration with alumina. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 489–492. [Google Scholar] [CrossRef]
- Spanu, D.; Monticelli, D.; Rampazzi, L.; Dossi, C.; Recchia, S. Introducing Frontal Chromatography–Inductively Coupled Plasma-Mass Spectrometry as a Fast Method for Speciation Analysis: The Case of Inorganic Arsenic. Anal. Chem. 2019, 91, 13810–13817. [Google Scholar] [CrossRef]
- Shahlaei, M.; Pourhossein, A. Determination of Arsenic in Drinking Water Samples by Electrothermal Atomic Absorption Spectrometry after Preconcentration Using the Biomass of Aspergillus niger Loaded on Activated Charcoal. J. Chem. 2014, 2014, 912619. [Google Scholar] [CrossRef]
- Ronkart, S.N.; Laurent, V.; Carbonnelle, P.; Mabon, N.; Copin, A.; Barthélemy, J.-P. Speciation of five arsenic species (arsenite, arsenate, MMAAV, DMAAV and AsBet) in different kind of water by HPLC-ICP-MS. Chemosphere 2007, 66, 738–745. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Zachariadis, G.A.; Stratis, J.A. Determination of arsenic (III) and total inorganic arsenic in water samples using an on-line sequential insertion system and hydride generation atomic absorption spectrometry. Anal. Chim. Acta 2005, 547, 237–242. [Google Scholar] [CrossRef]
- Mulvihill, M.; Tao, A.; Benjauthrit, K.; Arnold, J.; Yang, P. Surface-enhanced Raman spectroscopy for trace arsenic detection in contaminated water. Angew. Chem. Int. Ed. 2008, 47, 6456–6460. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Gu, X.; Bao, J.; Yang, H.; Sun, L. Laser-induced breakdown spectroscopy application in environmental monitoring of water quality: A review. Environ. Monit. Assess. 2014, 186, 8969–8980. [Google Scholar] [CrossRef]
- Yu, X.; Liu, C.; Guo, Y.; Deng, T. Speciation analysis of trace arsenic, mercury, selenium and antimony in environmental and biological samples based on hyphenated techniques. Molecules 2019, 24, 926. [Google Scholar] [CrossRef]
- Mattusch, J.; Wennrich, R.; Schmidt, A.-C.; Reisser, W. Determination of arsenic species in water, soils and plants. Fresenius’ J. Anal. Chem. 2000, 366, 200–203. [Google Scholar] [CrossRef]
- Arain, M.; Kazi, T.; Baig, J.; Jamali, M.; Afridi, H.; Shah, A.; Jalbani, N.; Sarfraz, R. Determination of arsenic levels in lake water, sediment, and foodstuff from selected area of Sindh, Pakistan: Estimation of daily dietary intake. Food Chem. Toxicol. 2009, 47, 242–248. [Google Scholar] [CrossRef]
- Polya, D.A.; Watts, M.J. Sampling and analysis for monitoring arsenic in drinking water. In Best Practice Guide on the Control of Arsenic in Drinking Water; IWA Publishing: London, UK, 2017. [Google Scholar]
- Guo, Z.; Yang, M.; Huang, X.-J. Recent developments in electrochemical determination of arsenic. Curr. Opin. Electrochem. 2017, 3, 130–136. [Google Scholar] [CrossRef]
- Reid, M.S.; Hoy, K.S.; Schofield, J.R.; Uppal, J.S.; Lin, Y.; Lu, X.; Peng, H.; Le, X.C. Arsenic speciation analysis: A review with an emphasis on chromatographic separations. TrAC Trends Anal. Chem. 2020, 123, 115770. [Google Scholar] [CrossRef]
- Quináia, S.P.; Rollemberg, M.d.C.E. Selective reduction of arsenic species by hydride generation-atomic absorption spectrometry. Part 2-sample storage and arsenic determination in natural waters. J. Braz. Chem. Soc. 2001, 12, 37–41. [Google Scholar] [CrossRef]
- Anezaki, K.; Nukatsuka, I.; OHZEKI, K. Determination of arsenic (III) and total arsenic (III, V) in water samples by resin suspension graphite furnace atomic absorption spectrometry. Anal. Sci. 1999, 15, 829–834. [Google Scholar] [CrossRef]
- Du, J.; Cui, J.; Jing, C. Rapid in situ identification of arsenic species using a portable Fe3O4@Ag SERS sensor. Chem. Commun. 2014, 50, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-J.; Hao, J.; Xu, Z.; Meng, X. Surface-enhanced Raman scattering for arsenate detection on multilayer silver nanofilms. Anal. Chim. Acta 2011, 692, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, M.; Matys, S.; Raff, J.; Pompe, W. Colorimetric As (V) detection based on S-layer functionalized gold nanoparticles. Talanta 2015, 144, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Boruah, B.S.; Biswas, R.; Deb, P. A green colorimetric approach towards detection of arsenic (III): A pervasive environmental pollutant. Opt. Laser Technol. 2019, 111, 825–829. [Google Scholar] [CrossRef]
- Forzani, E.S.; Foley, K.; Westerhoff, P.; Tao, N. Detection of arsenic in groundwater using a surface plasmon resonance sensor. Sens. Actuators B Chem. 2007, 123, 82–88. [Google Scholar] [CrossRef]
- Parsons, C.; Grabulosa, E.M.; Pili, E.; Floor, G.H.; Roman-Ross, G.; Charlet, L. Quantification of trace arsenic in soils by field-portable X-ray fluorescence spectrometry: Considerations for sample preparation and measurement conditions. J. Hazard. Mater. 2013, 262, 1213–1222. [Google Scholar] [CrossRef]
- Hueber, D.M.; Winefordner, J.D. A flowing electrolytic hydride generator for continuous sample introduction in atomic spectrometry. Anal. Chim. Acta 1995, 316, 129–144. [Google Scholar] [CrossRef]
- Rajaković, L.V.; Todorović, Ž.; Rajaković-Ognjanović, V.N.; Onjia, A.E. Analytical methods for arsenic speciation analysis. J. Serbian Chem. Soc. 2013, 78, 1461–1479. [Google Scholar] [CrossRef]
- Sugár, É.; Tatár, E.; Záray, G.; Mihucz, V.G. Field separation-based speciation analysis of inorganic arsenic in public well water in Hungary. Microchem. J. 2013, 107, 131–135. [Google Scholar] [CrossRef]
- Hua-Ming, G.; Chun-Hua, L. Separation of inorganic arsenic species from aqueous solution by anion exchange column and its application in study of arsenic removal. Chin. J. Anal. Chem. 2012, 40, 1092–1097. [Google Scholar]
- Issa, N.B.; Rajaković-Ognjanović, V.N.; Jovanović, B.M.; Rajaković, L.V. Determination of inorganic arsenic species in natural waters—Benefits of separation and preconcentration on ion exchange and hybrid resins. Anal. Chim. Acta 2010, 673, 185–193. [Google Scholar] [CrossRef]
- Escudero, L.B.; Martinis, E.M.; Olsina, R.A.; Wuilloud, R.G. Arsenic speciation analysis in mono-varietal wines by on-line ionic liquid-based dispersive liquid–liquid microextraction. Food Chem. 2013, 138, 484–490. [Google Scholar] [CrossRef]
- Chen, D.; Huang, C.; He, M.; Hu, B. Separation and preconcentration of inorganic arsenic species in natural water samples with 3-(2-aminoethylamino) propyltrimethoxysilane modified ordered mesoporous silica micro-column and their determination by inductively coupled plasma optical emission spectrometry. J. Hazard. Mater. 2009, 164, 1146–1151. [Google Scholar] [CrossRef]
- Chen, S.; Zhan, X.; Lu, D.; Liu, C.; Zhu, L. Speciation analysis of inorganic arsenic in natural water by carbon nanofibers separation and inductively coupled plasma mass spectrometry determination. Anal. Chim. Acta 2009, 634, 192–196. [Google Scholar] [CrossRef]
- Tuzen, M.; Çıtak, D.; Mendil, D.; Soylak, M. Arsenic speciation in natural water samples by coprecipitation-hydride generation atomic absorption spectrometry combination. Talanta 2009, 78, 52–56. [Google Scholar] [CrossRef]
- Chen, M.; Lin, Y.; Gu, C.; Wang, J. Arsenic sorption and speciation with branch-polyethyleneimine modified carbon nanotubes with detection by atomic fluorescence spectrometry. Talanta 2013, 104, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Huang, Z.; Fu, J.; Kuang, J.; Hu, C. Preconcentration of ultra-trace arsenic with nanometre-sized TiO 2 colloid and determination by AFS with slurry sampling. Anal. Methods 2010, 2, 1140–1143. [Google Scholar] [CrossRef]
- Myong-Il, A.; Chen, M.-L.; Wang, J.-H. Analysis of Arsenic Speciation by Coupling Fe3O4 Nanoparticles Separation with Slurry Sampling and Hydride Generation Atomic Fluorescence Spectrometry. Chin. J. Anal. Chem. 2013, 41, 105–109. [Google Scholar]
- Chen, M.-L.; Gu, C.-B.; Yang, T.; Sun, Y.; Wang, J.-H. A green sorbent of esterified egg-shell membrane for highly selective uptake of arsenate and speciation of inorganic arsenic. Talanta 2013, 116, 688–694. [Google Scholar] [CrossRef]
- Wang, J.; Tao, H.; Lu, T.; Wu, Y. Adsorption enhanced the oxidase-mimicking catalytic activity of octahedral-shape Mn3O4 nanoparticles as a novel colorimetric chemosensor for ultrasensitive and selective detection of arsenic. J. Colloid Interface Sci. 2021, 584, 114–124. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, M.-L.; Chen, X.-W.; Wang, J.-H.; Hirano, Y.; Sakamoto, H.; Shirasaki, T. Arsenic preconcentration via solid phase extraction and speciation by HPLC-gradient hydride generation atomic absorption spectrometry. J. Anal. At. Spectrom. 2011, 26, 133–140. [Google Scholar] [CrossRef]
- Reddy, R.R.; Rodriguez, G.D.; Webster, T.M.; Abedin, M.J.; Karim, M.R.; Raskin, L.; Hayes, K.F. Evaluation of arsenic field test kits for drinking water: Recommendations for improvement and implications for arsenic affected regions such as Bangladesh. Water Res. 2020, 170, 115325. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Khan, S.; Patti, A.F. Arsenic contamination of groundwater and its implications for drinking water quality and human health in under-developed countries and remote communities—A Review. Appl. Sci. 2021, 11, 1926. [Google Scholar] [CrossRef]
- Shabbir, Z.; Shahid, M.; Khalid, S.; Khalid, S.; Imran, M.; Qureshi, M.I.; Niazi, N.K. Use of agricultural bio-wastes to remove arsenic from contaminated water. Environ. Geochem. Health 2020, 45, 5703–5712. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for arsenic removal from water: Current status and future perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.I.; Naushad, M.; Chaudhry, S.A. Promising prospects of nanomaterials for arsenic water remediation: A comprehensive review. Process Saf. Environ. Prot. 2019, 126, 60–97. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, H.; Teng, H.; Sun, Y.; Guo, J.; Xie, W.; Yang, Q.; Chen, W. Chemical coagulation process for the removal of heavy metals from water: A review. Desalination Water Treat. 2016, 57, 1733–1748. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H. Arsenic—A review. Part II: Oxidation of arsenic and its removal in water treatment. Acta Hydrochim. Hydrobiol. 2003, 31, 97–107. [Google Scholar] [CrossRef]
- Perez, S.; Ho, S.W.L.; Felix, R.M. Electrochemical oxidation of arsenites, by an anode of reticulated glassy carbon as previous step for removal. ECS Trans. 2007, 3, 61. [Google Scholar] [CrossRef]
- Dodd, M.C.; Vu, N.D.; Ammann, A.; Le, V.C.; Kissner, R.; Pham, H.V.; Cao, T.H.; Berg, M.; Von Gunten, U. Kinetics and mechanistic aspects of As (III) oxidation by aqueous chlorine, chloramines, and ozone: Relevance to drinking water treatment. Environ. Sci. Technol. 2006, 40, 3285–3292. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Du, J.; Meng, X.; Sun, Y.; Sun, B.; Hu, Q. Application of titanium dioxide in arsenic removal from water: A review. J. Hazard. Mater. 2012, 215–216, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zazouli, M.A.; Kalankesh, L.R. Removal of precursors and disinfection by-products (DBPs) by membrane filtration from water; a review. J. Environ. Health Sci. Eng. 2017, 15, 25. [Google Scholar] [CrossRef]
- Driehaus, W.; Seith, R.; Jekel, M. Oxidation of arsenate (III) with manganese oxides in water treatment. Water Res. 1995, 29, 297–305. [Google Scholar] [CrossRef]
- Kartinen, E.O.; Martin, C.J. An overview of arsenic removal processes. Desalination 1995, 103, 79–88. [Google Scholar] [CrossRef]
- Kim, M.-J.; Nriagu, J. Oxidation of arsenite in groundwater using ozone and oxygen. Sci. Total Environ. 2000, 247, 71–79. [Google Scholar] [CrossRef]
- Sorlini, S.; Gialdini, F. Conventional oxidation treatments for the removal of arsenic with chlorine dioxide, hypochlorite, potassium permanganate and monochloramine. Water Res. 2010, 44, 5653–5659. [Google Scholar] [CrossRef]
- Pettine, M.; Campanella, L.; Millero, F.J. Arsenite oxidation by H2O2 in aqueous solutions. Geochim. Cosmochim. Acta 1999, 63, 2727–2735. [Google Scholar] [CrossRef]
- Hu, C.; Liu, H.; Chen, G.; Jefferson, W.A.; Qu, J. As(III) Oxidation by Active Chlorine and Subsequent Removal of As(V) by Al13 Polymer Coagulation Using a Novel Dual Function Reagent. Environ. Sci. Technol. 2012, 46, 6776–6782. [Google Scholar] [CrossRef]
- Luong, V.T.; Cañas Kurz, E.E.; Hellriegel, U.; Luu, T.L.; Hoinkis, J.; Bundschuh, J. Iron-based subsurface arsenic removal technologies by aeration: A review of the current state and future prospects. Water Res. 2018, 133, 110–122. [Google Scholar] [CrossRef]
- Ryu, J.; Monllor-Satoca, D.; Kim, D.-h.; Yeo, J.; Choi, W. Photooxidation of arsenite under 254 nm irradiation with a quantum yield higher than unity. Environ. Sci. Technol. 2013, 47, 9381–9387. [Google Scholar] [CrossRef]
- Sorlini, S.; Gialdini, F.; Stefan, M. UV/H2O2 oxidation of arsenic and terbuthylazine in drinking water. Environ. Monit. Assess. 2014, 186, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Wegelin, M.; Gechter, D.; Hug, S.; Mahmud, A.; Motaleb, A. SORAS—A simple arsenic removal process. In Proceedings of the 26th WEDC International Conference: Water, Sanitation and Hygiene—Challenges of the Millennium, Dhaka, Bangladesh, 5–9 November 2000. [Google Scholar]
- Fazi, S.; Amalfitano, S.; Casentini, B.; Davolos, D.; Pietrangeli, B.; Crognale, S.; Lotti, F.; Rossetti, S. Arsenic removal from naturally contaminated waters: A review of methods combining chemical and biological treatments. Rend. Lincei 2016, 27, 51–58. [Google Scholar] [CrossRef]
- Gihring, T.M.; Druschel, G.K.; McCleskey, R.B.; Hamers, R.J.; Banfield, J.F. Rapid arsenite oxidation by Thermus aquaticus and Thermus thermophilus: Field and laboratory investigations. Environ. Sci. Technol. 2001, 35, 3857–3862. [Google Scholar] [CrossRef]
- Cai, G.; Tian, Y.; Li, D.; Zhang, J.; Li, L.; Wang, Q.; Sun, H.; Zhang, H.; Wang, P. Self-enhanced and efficient removal of As (III) from water using Fe–Cu–Mn composite oxide under visible-light irradiation: Synergistic oxidation and mechanisms. J. Hazard. Mater. 2022, 422, 126908. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, W.; Wang, Z.; Yin, K.; Chen, T.; Liu, C. Enhanced removal of As (III) by heterogeneous catalytic oxidation of As (III) on Fe-biochar fibers with H2O2 and hydroxylamine. Chem. Eng. J. 2022, 428, 131200. [Google Scholar] [CrossRef]
- García, A.; Rosales, M.; Thomas, M.; Golemme, G. Arsenic photocatalytic oxidation over TiO2-loaded SBA-15. J. Environ. Chem. Eng. 2021, 9, 106443. [Google Scholar] [CrossRef]
- Leiva-Aravena, E.; Vera, M.A.; Nerenberg, R.; Leiva, E.D.; Vargas, I.T. Biofilm formation of Ancylobacter sp. TS-1 on different granular materials and its ability for chemolithoautotrophic As (III)-oxidation at high concentrations. J. Hazard. Mater. 2022, 421, 126733. [Google Scholar] [CrossRef]
- Yuan, L.; Wen, J.; Xue, Z.; Li, Y.; Yang, C.; Yin, X. Microscopic investigation into remediation of cadmium and arsenite Co-contamination in aqueous solution by Fe-Mn-incorporated titanosilicate. Sep. Purif. Technol. 2021, 279, 119809. [Google Scholar] [CrossRef]
- Parsania, S.; Mohammadi, P.; Soudi, M.R. Biotransformation and removal of arsenic oxyanions by Alishewanella agri PMS5 in biofilm and planktonic states. Chemosphere 2021, 284, 131336. [Google Scholar] [CrossRef]
- Su, J.; Lyu, T.; Cooper, M.; Mortimer, R.J.; Pan, G. Efficient arsenic removal by a bifunctional heterogeneous catalyst through simultaneous hydrogen peroxide (H2O2) catalytic oxidation and adsorption. J. Clean. Prod. 2021, 325, 129329. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Zhang, C.; Dai, M.; Zhang, Z.; Xi, Y.; Quan, B.; Lu, S.; Liu, Y. Degrading arsanilic acid and adsorbing the released inorganic arsenic simultaneously in aqueous media with CuFe2O4 activating peroxymonosulfate system: Factors, performance, and mechanism. Chem. Eng. J. 2021, 424, 128537. [Google Scholar] [CrossRef]
- Zeng, Y.; Fang, G.; Fu, Q.; Dionysiou, D.D.; Wang, X.; Gao, J.; Zhou, D.; Wang, Y. Photochemical characterization of paddy water during rice cultivation: Formation of reactive intermediates for As (III) oxidation. Water Res. 2021, 206, 117721. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, Y.; Xie, X.; Gan, Y.; Su, C.; Pi, K.; Finfrock, Y.Z.; Liu, P. The dual role of oxygen in redox-mediated removal of aqueous arsenic (III/V) by Fe-modified biochar. Bioresour. Technol. 2021, 340, 125674. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, M.; Zhang, J.; Lyu, T.; Cooper, M.; Pan, G. Reducing arsenic toxicity using the interfacial oxygen nanobubble technology for sediment remediation. Water Res. 2021, 205, 117657. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, K.; Wang, F.; Sun, S.; Li, D.; Zhang, J. Adsorption of As (III) from aqueous solutions using MnO2 strengthened WTRs-chitosan beads made by homogenous method with freeze-drying. React. Funct. Polym. 2021, 167, 105016. [Google Scholar] [CrossRef]
- Luo, T.; Xu, J.; Li, J.; Wu, F.; Zhou, D. Strengthening arsenite oxidation in water using metal-free ultrasonic activation of sulfite. Chemosphere 2021, 281, 130860. [Google Scholar] [CrossRef]
- Saleh, S.; Mohammadnejad, S.; Khorgooei, H.; Otadi, M. Photooxidation/adsorption of arsenic (III) in aqueous solution over bentonite/chitosan/TiO2 heterostructured catalyst. Chemosphere 2021, 280, 130583. [Google Scholar] [CrossRef] [PubMed]
- Harper, T.R.; Kingham, N.W. Removal of arsenic from wastewater using chemical precipitation methods. Water Environ. Res. 1992, 64, 200–203. [Google Scholar] [CrossRef]
- McNeill, L.S.; Edwards, M. Arsenic removal during precipitative softening. J. Environ. Eng. 1997, 123, 453–460. [Google Scholar] [CrossRef]
- Fields, K.A.; Chen, A.H.; Wang, L. Arsenic Removal from Drinking Water by Coagulation/Filtration and Lime Softening Plants; National Risk Management Research Laboratory, Office of Research and Development: Washington, DC, USA, 2000. [Google Scholar]
- Hering, J.G.; Chen, P.-Y.; Wilkie, J.A.; Elimelech, M. Arsenic removal from drinking water during coagulation. J. Environ. Eng. 1997, 123, 800–807. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak KP, Y.; Wu, T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Mustereț, C.P.; Morosanu, I.; Ciobanu, R.; Plavan, O.; Gherghel, A.; Al-Refai, M.; Roman, I.; Teodosiu, C. Assessment of Coagulation–Flocculation Process Efficiency for the Natural Organic Matter Removal in Drinking Water Treatment. Water 2021, 13, 3073. [Google Scholar] [CrossRef]
- Tubić, A.; Dalmacija, B.; Agbaba, J.; Ivančev-Tumbas, I.; Klašnja, M.; Dalmacija, M. Tracking disinfection by-products and arsenic removal during various drinking water treatment trains. Water Sci. Technol. 2010, 61, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Sancha, A.M. Review of coagulation technology for removal of arsenic: Case of Chile. J. Health Popul. Nutr. 2006, 24, 267–272. [Google Scholar] [PubMed]
- Khan, M.; Yamamoto, K.; Ahmed, M. A low cost technique of arsenic removal from drinking water by coagulation using ferric chloride salt and alum. Water Sci. Technol. Water Supply 2002, 2, 281–288. [Google Scholar] [CrossRef]
- Lakshmanan, D.; Clifford, D.; Samanta, G. Arsenic removal by coagulation with aluminum, iron, titanium, and zirconium. J.-Am. Water Work. Assoc. 2008, 100, 76–88. [Google Scholar] [CrossRef]
- Hussain, S.; Awad, J.; Sarkar, B.; Chow, C.W.K.; Duan, J.; van Leeuwen, J. Coagulation of dissolved organic matter in surface water by novel titanium (III) chloride: Mechanistic surface chemical and spectroscopic characterisation. Sep. Purif. Technol. 2019, 213, 213–223. [Google Scholar] [CrossRef]
- Baskan, M.B.; Pala, A. Determination of arsenic removal efficiency by ferric ions using response surface methodology. J. Hazard. Mater. 2009, 166, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, G.; Xiong, X.; Guan, X.; Li, L.; Bao, H. Enhanced arsenite removal from water by Ti(SO4)2 coagulation. Water Res. 2013, 47, 4340–4348. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, Á.; Rodríguez, J.F.; Castañeda, L.F.; Nava, J.L.; Coreño, O.; Carreño, G. Abatement of As and hydrated silica from natural groundwater by electrocoagulation in a continuous plant having an electrolyzer and a flocculator-settler. Sep. Purif. Technol. 2022, 281, 119895. [Google Scholar] [CrossRef]
- Kumar, I.; Quaff, A. Comparative study on the effectiveness of natural coagulant aids and commercial coagulant: Removal of arsenic from water. Int. J. Environ. Sci. Technol. 2019, 16, 5989–5994. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Akram, M.; Khan, S.; Park, D.R.; Yeom, I.T. Interaction of arsenic species with organic ligands: Competitive removal from water by coagulation-flocculation-sedimentation (C/F/S). Molecules 2019, 24, 1619. [Google Scholar] [CrossRef]
- Chiavola, A.; D’Amato, E.; Sirini, P.; Caretti, C.; Gori, R. Arsenic removal from a highly contaminated groundwater by a combined coagulation-filtration-adsorption process. Water Air Soil Pollut. 2019, 230, 87. [Google Scholar] [CrossRef]
- Li, Y.; Bland, G.D.; Yan, W. Enhanced arsenite removal through surface-catalyzed oxidative coagulation treatment. Chemosphere 2016, 150, 650–658. [Google Scholar] [CrossRef]
- Fox, D.I.; Stebbins, D.M.; Alcantar, N.A. Combining ferric salt and cactus mucilage for arsenic removal from water. Environ. Sci. Technol. 2016, 50, 2507–2513. [Google Scholar] [CrossRef]
- Mackie, A.; Laliberté, M.; Walsh, M. Comparison of single and two-stage ballasted flocculation processes for enhanced removal of arsenic from mine water. J. Environ. Eng. 2016, 142, 04015062. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Nujić, M.; Romić, Ž.; Lončarić, A.; Ergović Ravančić, M.; Kralj, E. Arsenic preoxidation and its removal from groundwater using iron coagulants. Desalination Water Treat. 2015, 56, 2105–2113. [Google Scholar] [CrossRef]
- Cui, J.; Jing, C.; Che, D.; Zhang, J.; Duan, S. Groundwater arsenic removal by coagulation using ferric (III) sulfate and polyferric sulfate: A comparative and mechanistic study. J. Environ. Sci. 2015, 32, 42–53. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Yang, X.; Huang, Z.; Wang, S.; Wang, C.; Wei, Q.; Zhang, G.; Xiao, J. Arsenic removal by coagulation process and the field expanding experiments for Yangzonghai Lake. Huan Jing Ke Xue Huanjing Kexue 2015, 36, 202–208. [Google Scholar]
- Ali, I.; Gupta, V.K.; Khan, T.A.; Asim, M. Removal of arsenate from aqueous solution by electro-coagulation method using Al-Fe electrodes. Int. J. Electrochem. Sci. 2012, 7, 1898–1907. [Google Scholar] [CrossRef]
- Chen, G.-X.; Hu, C.-Z.; Zhu, L.-F.; Tong, H.-Q. Influencing factors and mechanism of arsenic removal during the aluminum coagulation process. Huan Jing Ke Xue Huanjing Kexue 2013, 34, 1386–1391. [Google Scholar]
- Litter, M.I.; Alarcón-Herrera, M.T.; Arenas, M.J.; Armienta, M.A.; Avilés, M.; Cáceres, R.E.; Cipriani, H.N.; Cornejo, L.; Dias, L.E.; Cirelli, A.F. Small-scale and household methods to remove arsenic from water for drinking purposes in Latin America. Sci. Total Environ. 2012, 429, 107–122. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Mulder, M.; Mulder, J. Basic Principles of Membrane Technology; Springer Science & Business Media: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Beolchini, F.; Pagnanelli, F.; De Michelis, I.; Vegliò, F. Micellar Enhanced Ultrafiltration for Arsenic(V) Removal: Effect of Main Operating Conditions and Dynamic Modelling. Environ. Sci. Technol. 2006, 40, 2746–2752. [Google Scholar] [CrossRef]
- Richards, L.A. The Removal of Inorganic Contaminants Using Nanofiltration and Reverse Osmosis; Heriot-Watt University: Edinburgh, UK, 2012. [Google Scholar]
- Cirillo, A.I.; Tomaiuolo, G.; Guido, S. Membrane Fouling Phenomena in Microfluidic Systems: From Technical Challenges to Scientific Opportunities. Micromachines 2021, 12, 820. [Google Scholar] [CrossRef]
- Waypa, J.J.; Elimelech, M.; Hering, J.G. Arsenic removal by RO and NF membranes. J.-Am. Water Work. Assoc. 1997, 89, 102–114. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Polya, D.; Jovanovic, D. Best Practice Guide on the Control of Arsenic in Drinking Water; IWA Publishing: London, UK, 2017. [Google Scholar]
- Mruthunjayappa, M.H.; Kotrappanavar, N.S.; Mondal, D. Bioinspired engineering protein nanofibrils-based multilayered self-cleaning membranes for universal water purification. J. Hazard. Mater. 2022, 424, 127561. [Google Scholar] [CrossRef]
- Selvan, B.K.; Thiyagarajan, K.; Das, S.; Jaya, N.; Jabasingh, S.A.; Saravanan, P.; Rajasimman, M.; Vasseghian, Y. Synthesis and characterization of nano zerovalent iron-kaolin clay (nZVI-Kaol) composite polyethersulfone (PES) membrane for the efficacious As2O3 removal from potable water samples. Chemosphere 2022, 288, 132405. [Google Scholar] [CrossRef]
- Santoro, S.; Timpano, P.; Avci, A.H.; Argurio, P.; Chidichimo, F.; De Biase, M.; Straface, S.; Curcio, E. An integrated membrane distillation, photocatalysis and polyelectrolyte-enhanced ultrafiltration process for arsenic remediation at point-of-use. Desalination 2021, 520, 115378. [Google Scholar] [CrossRef]
- Kumar, M.; Isloor, A.M.; Todeti, S.R.; Ismail, A.F.; Farnood, R. Hydrophilic nano-aluminum oxide containing polyphenylsulfone hollow fiber membranes for the extraction of arsenic (As-V) from drinking water. J. Water Process Eng. 2021, 44, 102357. [Google Scholar] [CrossRef]
- Yadav, P.; Farnood, R.; Kumar, V. HMO-incorporated electrospun nanofiber recyclable membranes: Characterization and adsorptive performance for Pb (II) and As (V). J. Environ. Chem. Eng. 2021, 9, 106507. [Google Scholar] [CrossRef]
- Suren, S.; Ampronpong, W.; Pancharoen, U.; Maneeintr, K. The elimination of trace arsenic via hollow fiber supported liquid membrane: Experiment and mathematical model. Sci. Rep. 2021, 11, 11790. [Google Scholar] [CrossRef]
- Usman, M.; Katsoyiannis, I.; Rodrigues, J.H.; Ernst, M. Arsenate removal from drinking water using by-products from conventional iron oxyhydroxides production as adsorbents coupled with submerged microfiltration unit. Environ. Sci. Pollut. Res. 2021, 28, 59063–59075. [Google Scholar] [CrossRef]
- Ma, S.; Yang, F.; Chen, X.; Khor, C.M.; Jung, B.; Iddya, A.; Sant, G.; Jassby, D. Removal of As (III) by Electrically Conducting Ultrafiltration Membranes. Water Res. 2021, 204, 117592. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; de Paula, E.C.; de Souza Santos, L.V.; Amaral, M.C.S. Recycled reverse osmosis membrane combined with pre-oxidation for improved arsenic removal from high turbidity waters and retrofit of conventional drinking water treatment process. J. Clean. Prod. 2021, 312, 127859. [Google Scholar] [CrossRef]
- Dela Peña, E.M.B.; Araño, K.; Dela Cruz, M.L.; de Yro, P.A.; Diaz, L.J.L. The design of a bench-scale adsorbent column based on nanoclay-loaded electrospun fiber membrane for the removal of arsenic in wastewater. Water Environ. J. 2021, 35, 937–942. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; de Souza Santos, L.V.; Amaral, M.C.S. Dead-end ultrafiltration as a cost-effective strategy for improving arsenic removal from high turbidity waters in conventional drinking water facilities. Chem. Eng. J. 2021, 417, 128132. [Google Scholar] [CrossRef]
- Usman, M.; Belkasmi, A.I.; Kastoyiannis, I.A.; Ernst, M. Pre-deposited dynamic membrane adsorber formed of microscale conventional iron oxide-based adsorbents to remove arsenic from water: Application study and mathematical modeling. J. Chem. Technol. Biotechnol. 2021, 96, 1504–1514. [Google Scholar] [CrossRef]
- Jarma, Y.A.; Karaoğlu, A.; Tekin, Ö.; Baba, A.; Ökten, H.E.; Tomaszewska, B.; Bostancı, K.; Arda, M.; Kabay, N. Assessment of different nanofiltration and reverse osmosis membranes for simultaneous removal of arsenic and boron from spent geothermal water. J. Hazard. Mater. 2021, 405, 124129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Isloor, A.M.; Todeti, S.R.; Nagaraja, H.; Ismail, A.F.; Susanti, R. Effect of binary zinc-magnesium oxides on polyphenylsulfone/cellulose acetate derivatives hollow fiber membranes for the decontamination of arsenic from drinking water. Chem. Eng. J. 2021, 405, 126809. [Google Scholar] [CrossRef]
- García-García, J.J.; Gómez-Espinosa, R.M.; Rangel, R.N.; Romero, R.R.; Morales, G.R. New material for arsenic (V) removal based on chitosan supported onto modified polypropylene membrane. Environ. Sci. Pollut. Res. 2022, 29, 1909–1916. [Google Scholar] [CrossRef]
- Marino, T.; Figoli, A. Arsenic removal by liquid membranes. Membranes 2015, 5, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Aragon, M.J.; Everett, R.L.; Siegel, M.D.; Aragon, A.R.; Kottenstette, R.J.; Holub, W.E., Jr.; Wright, J.L.; Dwyer, B.P. Arsenic Pilot Plant Operation and Results: Anthony, New Mexico; Sandia National Laboratories: Albuquerque, NM, USA, 2007. [Google Scholar]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Amy, G.L. Adsorbent Treatment Technologies for Arsenic Removal; American Water Works Association: Denver, CO, USA, 2005. [Google Scholar]
- Goldberg, S. Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci. Soc. Am. J. 2002, 66, 413–421. [Google Scholar] [CrossRef]
- Hilbrandt, I.; Lehmann, V.; Zietzschmann, F.; Ruhl, A.S.; Jekel, M. Quantification and isotherm modelling of competitive phosphate and silicate adsorption onto micro-sized granular ferric hydroxide. RSC Adv. 2019, 9, 23642–23651. [Google Scholar] [CrossRef]
- Ahmad, A.; Rutten, S.; Eikelboom, M.; de Waal, L.; Bruning, H.; Bhattacharya, P.; van der Wal, A. Impact of phosphate, silicate and natural organic matter on the size of Fe(III) precipitates and arsenate co-precipitation efficiency in calcium containing water. Sep. Purif. Technol. 2020, 235, 116117. [Google Scholar] [CrossRef]
- SunBaek, B.; XiaoGuang, M. A review of arsenic interactions with anions and iron hydroxides. Environ. Eng. Res. 2004, 9, 184–192. [Google Scholar]
- Lenoble, V.; Bouras, O.; Deluchat, V.; Serpaud, B.; Bollinger, J.-C. Arsenic Adsorption onto Pillared Clays and Iron Oxides. J. Colloid Interface Sci. 2002, 255, 52–58. [Google Scholar] [CrossRef]
- Genç-Fuhrman, H.; Bregnhøj, H.; McConchie, D. Arsenate removal from water using sand–red mud columns. Water Res. 2005, 39, 2944–2954. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Beker, U.; Cumbal, L.; Duranoglu, D.; Kucuk, I.; Sengupta, A.K. Preparation of Fe oxide nanoparticles for environmental applications: Arsenic removal. Environ. Geochem. Health 2010, 32, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-based nanocomposite materials for arsenic removal from water: A review. Heliyon 2019, 5, e01577. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fields, K.A.; Chen, A.S. Arsenic Removal from Drinking Water by Ion Exchange and Activated Alumina Plants; National Risk Management Research Laboratory, Office of Research and Development: Washington, DC, USA, 2000. [Google Scholar]
- Greenleaf, J.E.; Lin, J.c.; Sengupta, A.K. Two novel applications of ion exchange fibers: Arsenic removal and chemical-free softening of hard water. Environ. Prog. 2006, 25, 300–311. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Mohapatra, D.; Mishra, D.; Chaudhury, G.R.; Das, R. Arsenic (V) adsorption mechanism using kaolinite, montmorillonite and illite from aqueous medium. J. Environ. Sci. Health Part A 2007, 42, 463–469. [Google Scholar] [CrossRef]
- Liu, B.; Kim, K.-H.; Kumar, V.; Kim, S. A review of functional sorbents for adsorptive removal of arsenic ions in aqueous systems. J. Hazard. Mater. 2020, 388, 121815. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Pintor, A.; Boaventura, R.A.; Botelho, C. Current Trends of Arsenic Adsorption in Continuous Mode: Literature Review and Future Perspectives. Sustainability 2021, 13, 1186. [Google Scholar] [CrossRef]
- Zeng, H.; Arashiro, M.; Giammar, D.E. Effects of water chemistry and flow rate on arsenate removal by adsorption to an iron oxide-based sorbent. Water Res. 2008, 42, 4629–4636. [Google Scholar] [CrossRef]
- Sweetman, M.J.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.E.; Hayball, J.D. Activated carbon, carbon nanotubes and graphene: Materials and composites for advanced water purification. C 2017, 3, 18. [Google Scholar] [CrossRef]
- Pena, M.E.; Korfiatis, G.P.; Patel, M.; Lippincott, L.; Meng, X. Adsorption of As(V) and As(III) by nanocrystalline titanium dioxide. Water Res. 2005, 39, 2327–2337. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Nujić, M. Arsenic removal by nanoparticles: A review. Environ. Sci. Pollut. Res. 2015, 22, 8094–8123. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Bezbaruah, A.N. Comparative study of arsenic removal by iron-based nanomaterials: Potential candidates for field applications. Sci. Total Environ. 2021, 764, 142914. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Luan, Z.; Ding, J.; Di, Z.; Li, Y.; Tian, B. Ceria nanoparticles supported on carbon nanotubes for the removal of arsenate from water. Mater. Lett. 2005, 59, 399–403. [Google Scholar] [CrossRef]
- Cui, H.; Li, Q.; Gao, S.; Shang, J.K. Strong adsorption of arsenic species by amorphous zirconium oxide nanoparticles. J. Ind. Eng. Chem. 2012, 18, 1418–1427. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Abbas, S.H.; Ismail, I.M.; Mostafa, T.M.; Sulaymon, A.H. Biosorption of heavy metals: A review. J. Chem. Sci. Technol. 2014, 3, 74–102. [Google Scholar]
- Ahemad, M.; Kibret, M. Recent trends in microbial biosorption of heavy metals: A review. Biochem. Mol. Biol. 2013, 1, 19–26. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Chowdhury, I.R.; Kabir, F.; Mazumder, M.A.J.; Zahir, M.; Alhooshani, K. Alginate-based biotechnology: A review on the arsenic removal technologies and future possibilities. J. Water Supply Res. Technol.-Aqua 2019, 68, 369–389. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A review of biochar-based sorbents for separation of heavy metals from water. Int. J. Phytoremediation 2020, 22, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Mustafiz, S.; Islam, M.; Bjorndalen, N.; Rahaman, M.; Chaalal, O. A comprehensive approach for modeling sorption of lead and cobalt ions through fish scales as an adsorbent. Chem. Eng. Commun. 2006, 193, 580–605. [Google Scholar] [CrossRef]
- Ranjan, D.; Talat, M.; Hasan, S. Rice polish: An alternative to conventional adsorbents for treating arsenic bearing water by up-flow column method. Ind. Eng. Chem. Res. 2009, 48, 10180–10185. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, M.; Wu, W.; Jin, W. Synthesis of the cotton cellulose based Fe (III)-loaded adsorbent for arsenic (V) removal from drinking water. Desalination 2009, 249, 1006–1011. [Google Scholar] [CrossRef]
- Jaiswal, V.; Saxena, S.; Kaur, I.; Dubey, P.; Nand, S.; Naseem, M.; Singh, S.B.; Srivastava, P.K.; Barik, S.K. Application of four novel fungal strains to remove arsenic from contaminated water in batch and column modes. J. Hazard. Mater. 2018, 356, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Morrison, G.; Perrusquia, G.; Gutierrez, M.; Aguilera, A.; Cano-Aguilera, I.; Gardea-Torresdey, J. Characteristics of arsenic adsorption to sorghum biomass. J. Hazard. Mater. 2007, 145, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yu, Y.; Yan, L.; Yan, W.; Jing, C. Asenic removal from groundwater using granular chitosan-titanium adsorbent. J. Environ. Sci. 2022, 112, 202–209. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.; Zhao, L. Application of magnetic chitosan nanocomposites modified by graphene oxide and polyethyleneimine for removal of toxic heavy metals and dyes from water. Int. J. Biol. Macromol. 2021, 192, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Sun, S.; Yu, Y.; Zhang, J.; Li, D. Column studies on the adsorption of As (V) by granular chitosan adsorbent prepared with backwashing iron-containing sludge. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127247. [Google Scholar] [CrossRef]
- Burillo, J.C.; Ballinas, L.; Burillo, G.; Guerrero-Lestarjette, E.; Lardizabal-Gutierrez, D.; Silva-Hidalgo, H. Chitosan hydrogel synthesis to remove arsenic and fluoride ions from groundwater. J. Hazard. Mater. 2021, 417, 126070. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, T.; Lv, L.; Chen, Y.; Tang, W.; Tang, S. Functionalization of chitosan by grafting sulfhydryl groups to intensify the adsorption of arsenite from water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126601. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; El Ibrahimi, B.; Abou Oualid, H.; Kassab, Z.; Quintal-Franco, C.; Giácoman-Vallejos, G.; Gamero-Melo, P. Iron-zirconium microwave-assisted modification of small-pore zeolite W and its alginate composites for enhanced aqueous removal of As (V) ions: Experimental and theoretical studies. Chem. Eng. J. 2021, 421, 129909. [Google Scholar] [CrossRef]
- Lilhare, S.; Mathew, S.B.; Singh, A.K.; Carabineiro, S.A. Calcium Alginate Beads with Entrapped Iron Oxide Magnetic Nanoparticles Functionalized with Methionine—A Versatile Adsorbent for Arsenic Removal. Nanomaterials 2021, 11, 1345. [Google Scholar] [CrossRef]
- Biftu, W.K.; Kunta, R. Iron-alginate beads doped with green synthesised ‘nano-CeO2-ZrO2’ as an effective adsorbent for removal of highly toxic Arsenic-ions from polluted water. Int. J. Environ. Anal. Chem. 2021, 103, 1490–1508. [Google Scholar] [CrossRef]
- Naga Babu, A.; Raja Sree, T.; Srinivasa Reddy, D.; Suresh Kumar, G.; Krishna Mohan, G. Experimental and statistical analysis of As (III) adsorption from contaminated water using activated red mud doped calcium-alginate beads. Environ. Technol. 2021, 42, 1810–1825. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ni, F.; Cui, A.; Chen, X.; Deng, S.; Shen, F.; Huang, C.; Yang, G.; Song, C.; Zhang, J. New insight into adsorption and co-adsorption of arsenic and tetracycline using a Y-immobilized graphene oxide-alginate hydrogel: Adsorption behaviours and mechanisms. Sci. Total Environ. 2020, 701, 134363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, A.; Chauhan, K.; Gupta, R.; Ahn, J.-H.; Chauhan, G.S. Removal of As (V) from water by pectin based active hydrogels following geochemical approach. Bioresour. Technol. 2009, 100, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Karmakar, M.; Chattopadhyay, P.K.; Halder, A.; Singha, N.R. Scale-up one-pot synthesis of waste collagen and apple pomace pectin incorporated pentapolymer biocomposites: Roles of waste collagen for elevations of properties and unary/ternary removals of Ti (IV), As (V), and V (V). J. Hazard. Mater. 2021, 409, 124873. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Ghimire, K.N.; Zhu, Y.; Yano, M.; Makino, K.; Miyajima, T. Effective use of orange juice residue for removing heavy and radioactive metals from environments. Geosystem Eng. 2002, 5, 31–37. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Yan, J.; Wang, T.; Xie, X.; Yang, S. Efficient removal arsenate from water by biochar-loaded Ce3+-enriched ultra-fine ceria nanoparticles through adsorption-precipitation. Sci. Total Environ. 2021, 794, 148691. [Google Scholar] [CrossRef] [PubMed]
- Wrighton-Araneda, K.; Ortega, D.E.; Cortes-Arriagada, D. Effective Removal of Water-Soluble Methylated Arsenic Contaminants with Phosphorene Oxide Nanoflakes: A DFT Study. J. Mol. Liq. 2021, 341, 117423. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.; Liu, X.; Bai, S.; Lin, H.; Wang, D. Reduction and removal of As (V) in aqueous solution by biochar derived from nano zero-valent-iron (nZVI) and sewage sludge. Chemosphere 2021, 277, 130273. [Google Scholar] [CrossRef]
- Yin, S.; Cheng, C.-L.; Parsons, J.; Mao, Y.; Kang, J.; Kim, J. Evaluation of arsenic sorption performance using dendritic anatase and polycrystalline rutile nano-TiO2 for environmental applications. Int. J. Environ. Sci. Technol. 2021, 18, 2113–2124. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Anang, E.; Li, C.; Fan, X. Enhanced As (III) sequestration using nanoscale zero-valent iron modified by combination of loading and sulfidation: Characterizations, performance, kinetics and mechanism. Water Sci. Technol. 2021, 83, 2886–2900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Li, G.; Du, W.; Lu, J.; Song, J.; Yang, Q.; Li, X.; Xu, H.; He, X. Simultaneous adsorption and oxidation of para arsanilic acid by a highly efficient nanostructured Fe-Ti-Mn composite oxide. Chem. Eng. J. 2021, 407, 127142. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, Z.; Liang, Z.; Liu, J.; Shi, W.; Cui, F. Efficient degradation of p-arsanilic acid with arsenic adsorption by magnetic CuO-Fe3O4 nanoparticles under visible light irradiation. Chem. Eng. J. 2018, 334, 1527–1536. [Google Scholar] [CrossRef]
- Zeng, H.; Zhai, L.; Qiao, T.; Zhang, J.; Li, D. Removal of As (V) by a core-shell magnetic nanoparticles synthesized with iron-containing water treatment residuals. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 627, 127074. [Google Scholar] [CrossRef]
- Morales-Amaya, C.G.; Alarcón-Herrera, M.T.; Astudillo-Sánchez, P.D.; Lozano-Morales, S.A.; Licea-Jiménez, L.; Reynoso-Cuevas, L. Ferrous Magnetic Nanoparticles for Arsenic Removal from Groundwater. Water 2021, 13, 2511. [Google Scholar] [CrossRef]
- Shen, Z.; Jin, J.; Fu, J.; Yang, M.; Li, F. Anchoring Al-and/or Mg-oxides to magnetic biochars for Co-uptake of arsenate and fluoride from water. J. Environ. Manag. 2021, 293, 112898. [Google Scholar] [CrossRef]
- Huo, J.-B.; Yu, G. Mesoporous cerium oxide-anchored magnetic polyhedrons derived from MIL-100 (Fe) for enhanced removal of arsenite from aqueous solution. J. Hazard. Mater. 2021, 415, 125709. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, M.; Wei, T.; Nagasaka, T. Synergistic removal of As (V) from aqueous solution by nanozero valent iron loaded with zeolite 5A synthesized from fly ash. J. Hazard. Mater. 2022, 424, 127428. [Google Scholar] [CrossRef] [PubMed]
- Negroiu, M.; Țurcanu, A.A.; Matei, E.; Râpă, M.; Covaliu, C.I.; Predescu, A.M.; Pantilimon, C.M.; Coman, G.; Predescu, C. Novel Adsorbent Based on Banana Peel Waste for Removal of Heavy Metal Ions from Synthetic Solutions. Materials 2021, 14, 3946. [Google Scholar] [CrossRef]
- Khownpurk, P.; Chandra-Ambhorn, W. Removal of As (III) from aqueous solution by the oyster shell powder–treated rice husk ash composite (OS-TRHA) pellet. J. Chin. Inst. Eng. 2019, 42, 411–419. [Google Scholar] [CrossRef]
- Minzatu, V.; Davidescu, C.M.; Negrea, A.; Ciopec, M.; Negrea, P.; Duteanu, N.; Motoc, M.; Velimirovici, D. Biopolymers-Carbon Sources for Composite Materials Used as Adsorbents for As (V). Mater. Plast. 2019, 56, 210–215. [Google Scholar] [CrossRef]
- Gabris, M.A.; Rezania, S.; Rafieizonooz, M.; Khankhaje, E.; Devanesan, S.; AlSalhi, M.S.; Aljaafreh, M.J.; Shadravan, A. Chitosan magnetic graphene grafted polyaniline doped with cobalt oxide for removal of Arsenic (V) from water. Environ. Res. 2022, 207, 112209. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.F.; Kamboh, M.A.; Nodeh, H.R.; Abd Halim, S.N.B.; Mohamad, S. Synthesis of piperazine functionalized magnetic sporopollenin: A new organic-inorganic hybrid material for the removal of lead (II) and arsenic (III) from aqueous solution. Environ. Sci. Pollut. Res. 2017, 24, 21846–21858. [Google Scholar] [CrossRef] [PubMed]
- Jumah, M.N.B.; Eid, M.H.; AL-Huqail, A.A.; Mohammad, M.A.; Bin-Murdhi, N.S.; Abu-Taweel, G.M.; Altoom, N.; Allam, A.A.; AbuKhadra, M.R. Enhanced remediation of As (V) and Hg (II) ions from aqueous environments using β-cyclodextrin/MCM-48 composite: Batch and column studies. J. Water Process Eng. 2021, 42, 102118. [Google Scholar] [CrossRef]
- Jagirani, M.S.; Balouch, A.; Mahesar, S.A.; Kumar, A.; Mustafai, F.A.; Bhanger, M.I. Preparation of novel arsenic-imprinted polymer for the selective extraction and enhanced adsorption of toxic As3+ ions from the aqueous environment. Polym. Bull. 2020, 77, 5261–5279. [Google Scholar] [CrossRef]
- Argos, M.; Ahsan, H.; Graziano, J.H. Arsenic and human health: Epidemiologic progress and public health implications. Rev. Environ. Health 2012, 27, 191–195. [Google Scholar] [CrossRef]
- Howard, G. Arsenic, Drinking-Water and Health Risk Substitution in Arsenic Mitigation: A Discussion Paper; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Krupoff, M.; Mobarak, A.M.; Geen, A.v. Evaluating strategies to reduce arsenic poisoning in South Asia: A view from the social sciences. Asian Dev. Rev. 2020, 37, 21–44. [Google Scholar] [CrossRef]
- Marshall, G.; Ferreccio, C.; Yuan, Y.; Bates, M.N.; Steinmaus, C.; Selvin, S.; Liaw, J.; Smith, A.H. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J. Natl. Cancer Inst. 2007, 99, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.I.; López, J.F.; Vivaldi, B.; Coz, F. Long-term impact of arsenic in drinking water on bladder cancer health care and mortality rates 20 years after end of exposure. J. Urol. 2012, 187, 856–861. [Google Scholar] [CrossRef]
- Sanyal, T.; Bhattacharjee, P.; Paul, S. Recent advances in arsenic research: Significance of differential susceptibility and sustainable strategies for mitigation. Front. Public Health 2020, 8, 464. [Google Scholar] [CrossRef]
- Pfaff, A.; Schoenfeld Walker, A.; Ahmed, K.M.; van Geen, A. Reduction in exposure to arsenic from drinking well-water in Bangladesh limited by insufficient testing and awareness. J. Water Sanit. Hyg. Dev. 2017, 7, 331–339. [Google Scholar] [CrossRef]
- Barnwal, P.; van Geen, A.; von der Goltz, J.; Singh, C.K. Demand for environmental quality information and household response: Evidence from well-water arsenic testing. J. Environ. Econ. Manag. 2017, 86, 160–192. [Google Scholar] [CrossRef]
- George, C.M.; Inauen, J.; Rahman, S.M.; Zheng, Y. The effectiveness of educational interventions to enhance the adoption of fee-based arsenic testing in Bangladesh: A cluster randomized controlled trial. Am. J. Trop. Med. Hyg. 2013, 89, 138–144. [Google Scholar] [CrossRef]
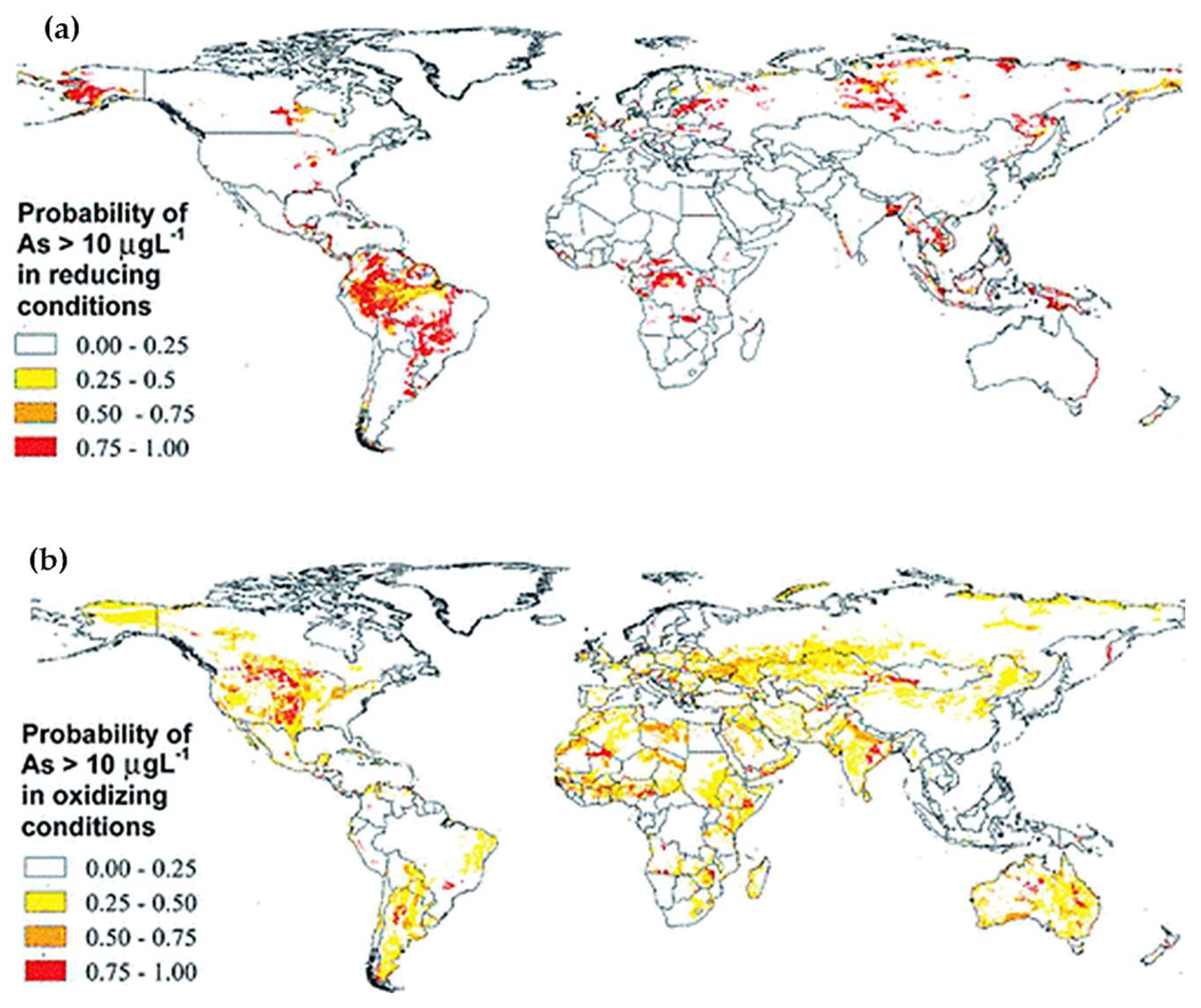
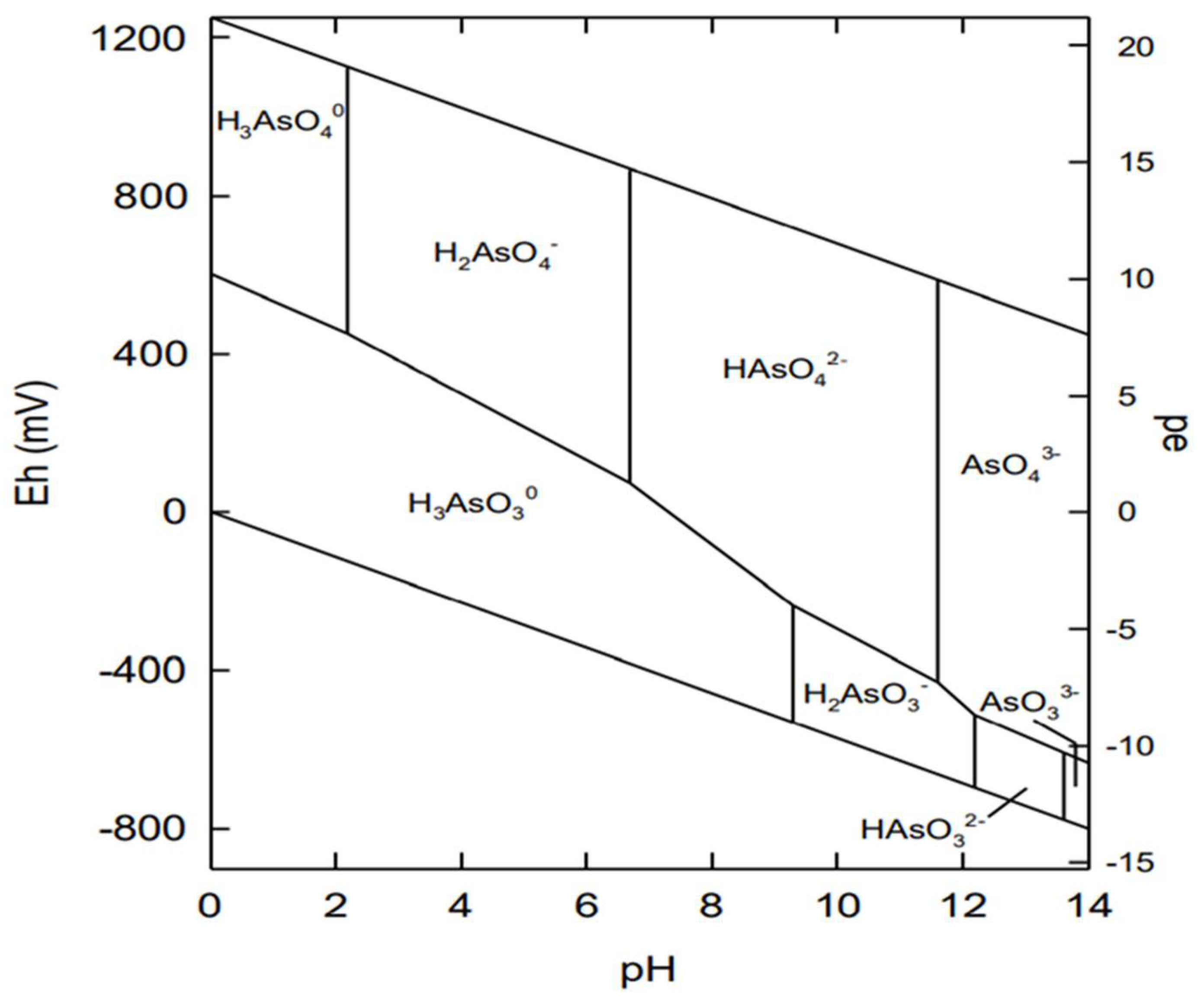

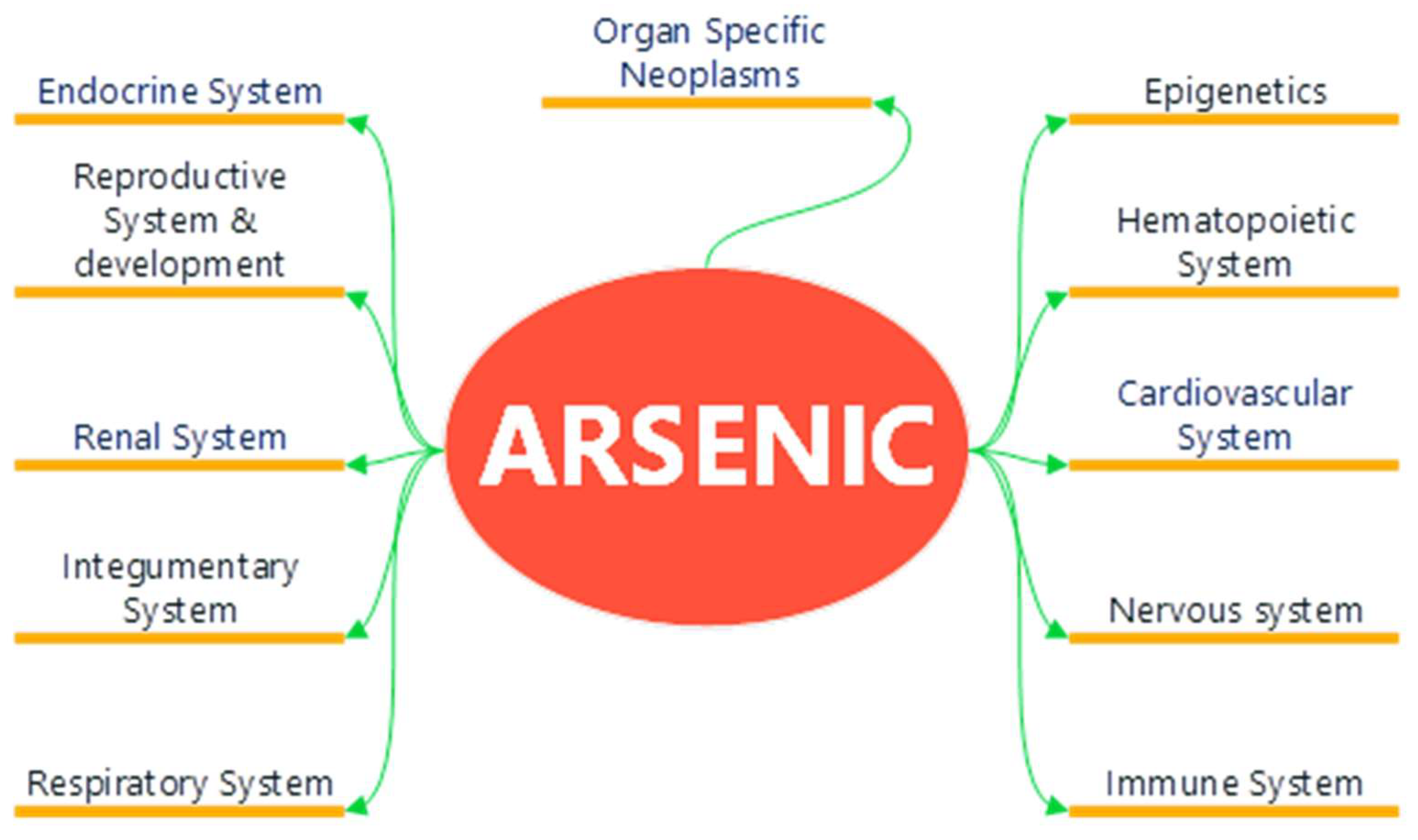

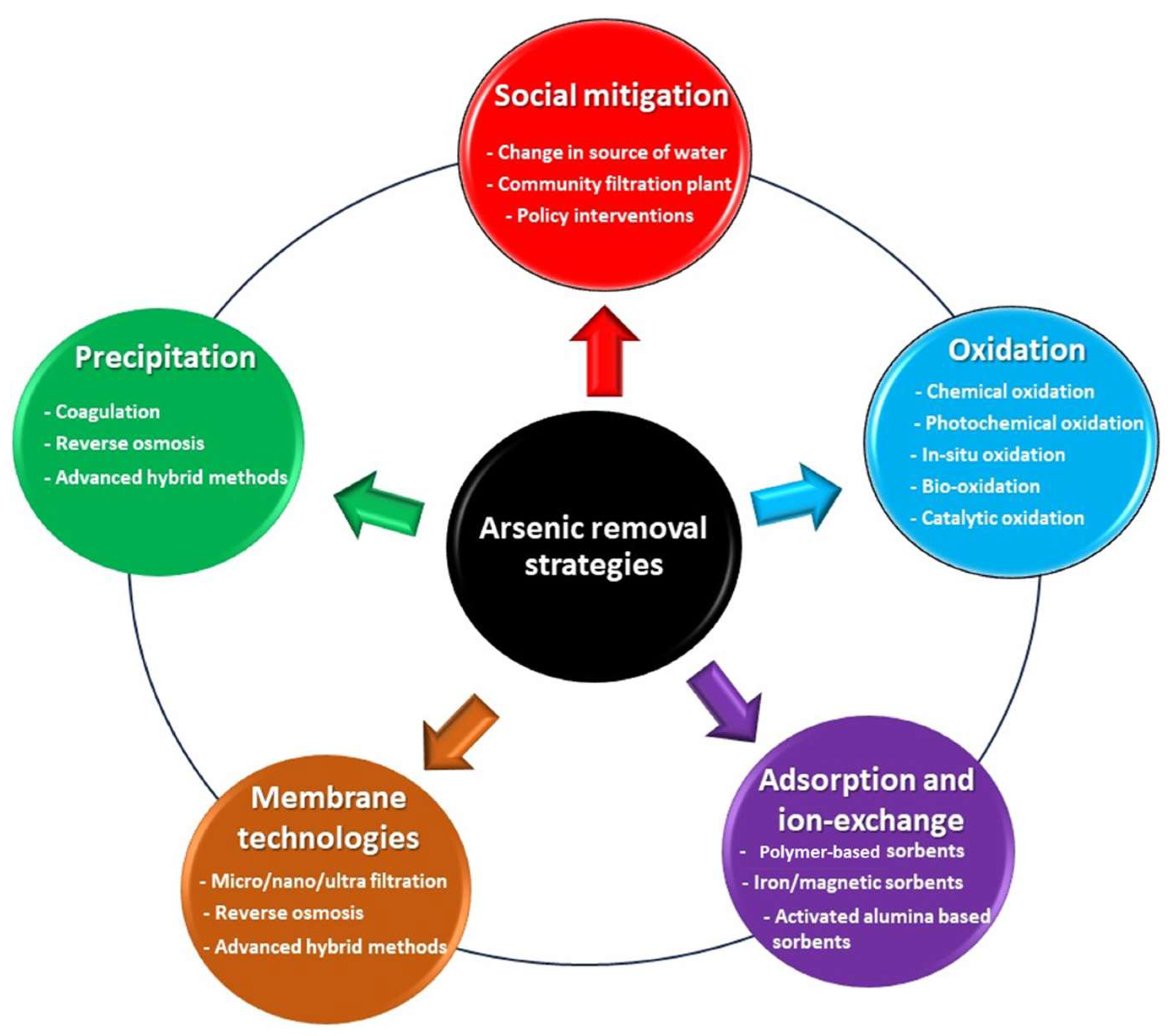



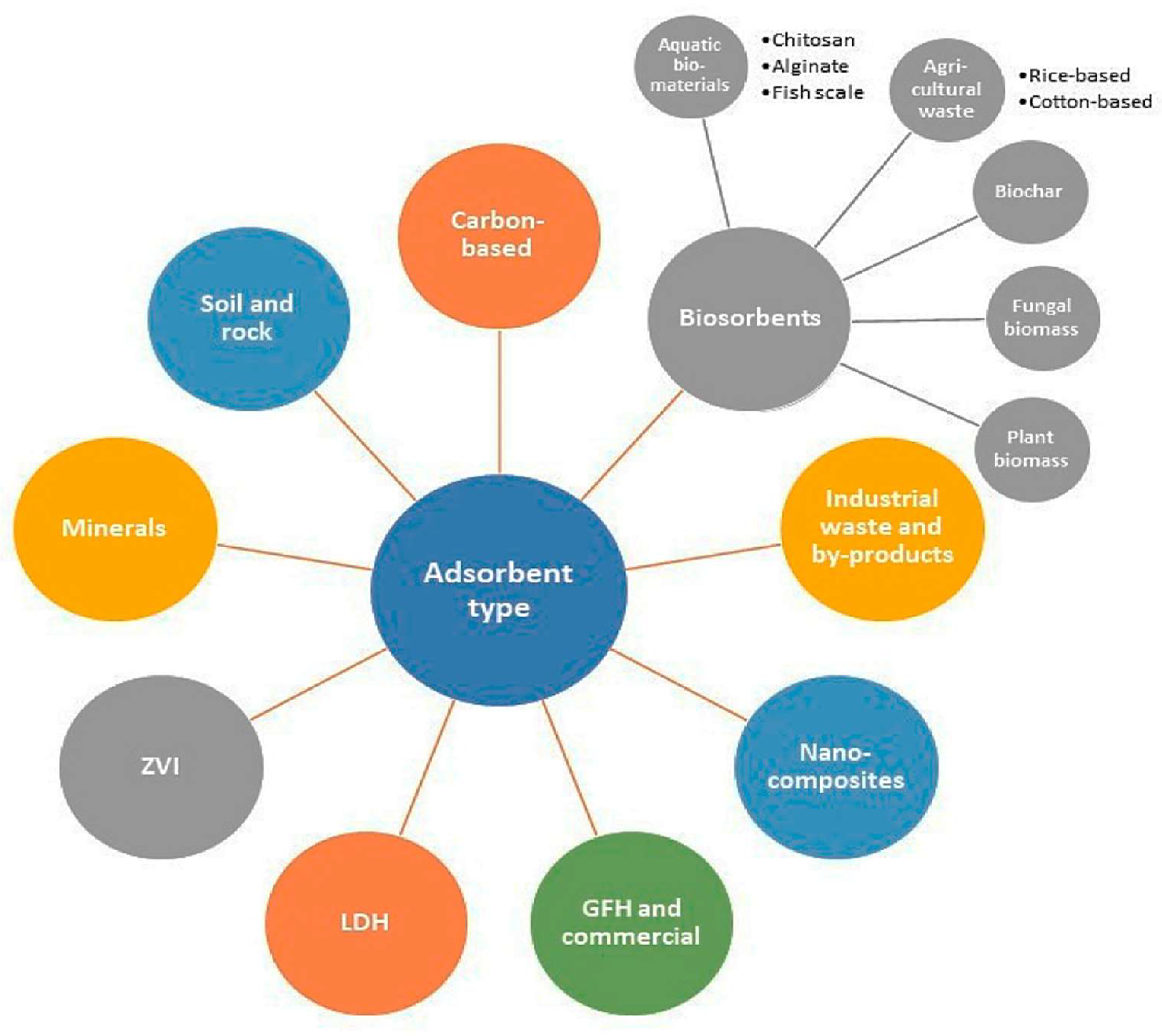
| Country/Region | Districts/Provinces | Contamination Source | Concentration [As] μg/L | Population at Risk (Million) | References |
|---|---|---|---|---|---|
| Bangladesh | 61 * | Flood plain, deltaic sediments, alluvial aquifers and organic matter | 2–4730 | 85 | [24,25,26,27,28] |
| Japan | 3 * | [As] sulfide from industrial activities, holocene coastal sands, volcanic activity and quaternary alluvium aquifer | 1–25,700 | *** | [29,30] |
| Taiwan | 4 * | Fine-grained fluvial deposits, aquifers and aquitards and black shales | 2.5–1820 | 0.1–0.2 | [31,32,33,34] |
| Pakistan | 27 * | Dissolution of sedimentary deposits and minerals, especially iron oxyhydroxides | 3.0–2580 | 13 | [33,35,36,37,38] |
| Cambodia | 1 ** | Ferrous sediments | 1–1610 | 2.4 | [39] |
| Australia | 4 * | Gold mining, iron oxyhydroxides and iron hydro-oxides | 5–300,000 | 0.001976 | [10,40] |
| Eastern Croatia | 1 * | Sediments | 27 | 0.12 | [41] |
| India | 20 * | Alluvial, deltaic and igneous sediments | 1–3880 | 50 | [10,42] |
| Canada | 5 ** | Thermal springs, volcanic rocks, uranium mines and sediments | 1.5–100,000 | minimal | [11,43,44] |
| Ghana | 5 * | Gold mining | <1–4500 | <0.1 | [45] |
| Thailand | 1 * | Mining and dredged alluvium | 1–5000 | 0.0015 | [46,47,48,49] |
| Argentina | 4 * | Volcanic ash and thermal springs | 10–2000 | 2 | [42,46] |
| Slovakia | 2 * | Aquifer sediments | 37–39 | 1.1 **** | [50,51] |
| Mexico | 3 * | Volcanic sediments and mining | 8–620 | 0.4 | [11,46] |
| New Zealand | 4 * | Volcanic activities | 21–8500 | minimal | [10] |
| Romania and Hungary | 2 * | Sediments | 0.5–240 | 0.4 | [11,46,52] |
| Germany | 1 * | Sandstone and alluvium sediments | 10–150 | minimal | [53,54] |
| Afghanistan | 1 * | Sediments | 10–500 | 0.5 | [29,55] |
| Vietnam | 2 * | Pleistocene and Holocene sediments | 1–3050 | 0.5–10 | [55,56] |
| Italy | 3 * | Volcanic aquifers | 0.1–6940 | 1 ***** | [57] |
| UK | 4 ** | Mining, limestone, sandstone, estuarine alluvium, alluvial or glacial aquifers | <1–355 | 0.56 | [58,59] |
| Greece | 1 * | Mine tailings | 10,000 | *** | [55,60] |
| Brazil | 1 * | Gold mining | 0.4–350 | *** | [29,55,61] |
| Spain | 1 * | Alluvial sediments | <1–100 | >0.05 | [11,62,63] |
| Chile | 1 * | Volcanogenic sediments, thermal springs and mining | 100–1000 | 0.5 | [64] |
| Ores | Mineral Type | Chemical Formula | [As] Concentration Range (g/kg) |
|---|---|---|---|
| Marcasite | Sulfide minerals | FeS2 | 0.02–276 |
| Pyrite | Sulfide minerals | FeS2 | 0.1–120 |
| Maghemite | Oxide Minerals | γ-Fe2O3 | Up to 186 |
| Iron (III) oxyhydroxide | Oxide Minerals | FeO(OH) | Up to 76 |
| Haematite | Oxide Minerals | Fe2O3 | Up to 29 |
| Sphalerite | Sulfide minerals | (Zn,Fe)S | 0.005–17 |
| Galena | Sulfide minerals | PbS | 0.005–10 |
| Chalcopyrite | Sulfide minerals | CuFeS2 | 0.010–5 |
| Jarosite | Sulphate minerals | KFe3(SO4)2(OH)6 | 0.034–1 |
| Apatite | Phosphate minerals | Ca10(PO4)6(OH,F,Cl)2 | <0.001–1 |
| Pyrrhotite | Sulfide minerals | FeS | 0.005–0.1 |
| Halite | Halide Minerals | NaCl | <0.003–0.03 |
| Fluorite | Halide Minerals | CaF2 | <0.02 |
| Magnetite | Oxide Minerals | Fe3O4 | 0.027–0.041 |
| Barite | Sulphate minerals | BaSO4 | <0.001–0.012 |
| Dolomite | Carbonate minerals | CaMg(CO3)2 | <0.003 |
| Siderite | Carbonate minerals | FeCO3 | <0.003 |
| Amphibole | Silicate minerals | X7Si8O22(OH)2 | 0.0011–0.0023 |
| Feldspar | Silicate minerals | KAlSi3O8–NaAlSi3O8–CaAl2Si2O8 | <0.00001–0.0021 |
| Biotite | Silicate minerals | K3AlSi3O10 2 | 0.0014 |
| Quartz | Silicate minerals | SiO2 | 0.0004–0.0013 |
| Calcite | Carbonate minerals | CaCO3 | 0.001–0.008 |
| Gypsum/anhydrite | Sulphate minerals | CaCO4 | <0.001–0.006 |
| Pyroxene | Silicate minerals | XYZrOu | 0.00005–0.0008 |
| Name | Family | Synonyms | Structural Formula | Chemical Formula | Solid State Structure | Comments |
|---|---|---|---|---|---|---|
| Arsenilic acid | Organic | Arsonic acid, (4-aminophenyl)- | 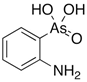 | C6H8AsNO3 | 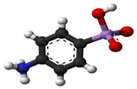 | a, b, c, d and n are applicable. |
| Arsenic | Inorganic | Metallic arsenic |  | [As] |  | c is applicable. |
| Arsenic(V) pentoxide | Inorganic | Arsenic oxide [As2O5] |  | As2O5 |  | a and c are applicable. |
| Arsenic(III) sulfide | Inorganic | Arsenic sulfide [As2S3] |  | As2S3 |  | c and e are applicable. |
| Arsenic(III) trichloride | Inorganic | Arsenic chloride [AsCl3] |  | AsCl3 |  | c and f are applicable. |
| Arsenic(III) trioxide | Inorganic | Arsenic oxide [As2O3] |  | As2O3 |  | a and b are applicable. |
| Arsenobetaine | Organic | Arsonium, (carboxymethyl) trimethyl-, hydroxide, inner salt; 2-(trimethylarsonio)acetate |  | C5H11AsO2 |  | d, b, c and a are applicable. |
| Arsine | Inorganic | Arsenic hydride |  | AsH3 |  | g is applicable. |
| Calcium arsenate | Inorganic | Arsenic acid [H3AsO4] calcium salt (2:3) | 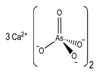 | (AsO4)2.3Ca | 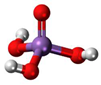 | a, c and h are applicable. |
| Dimethylarsinic acid | Organic | Cacodylic acid |  | C2H7AsO2 | 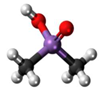 | b, a, c and d are applicable. |
| Metal (ME)/Lead arsenate/Me = Na, Ca, K, Mg, Al, Mn, Cu, Co, Cd, Fe, Ni, Pb, Sr, Zn | Inorganic | Arsenic acid [H3AsO4], Me/Pb (2+) salt (1:1) |  | HAsO4.Pb/ME |  | j, a, c and b are applicable. |
| Methanearsonic acid, disodium salt | Organic | Arsonic acid, methyl-, disodium salt | 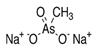 | CH3AsO3.Na2 |  | I, k, d, a and c are applicable. |
| Methanearsonic acid, monosodium salt | Organic | Arsonic acid, methyl-, monosodium salt |  | CH4AsO3.Na |  | I, k, d, a and c are applicable. |
| Potassium arsenate | Inorganic | Arsenic acid [H3AsO4], dipotassium salt |  | H2AsO4.K |  | l, a and c are applicable. |
| Potassium arsenite | Inorganic | Arsenous acid, potassium salt |  | AsO2.K |  | l, a and c are applicable. |
| Sodium arsenate | Inorganic | Arsenic acid, [H3AsO4], monosodium salt |  | H2AsO4.Na | 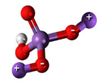 | m, a, and c are applicable. |
| Sodium arsenite | Inorganic | Arsenous acid, sodium salt | 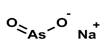 | AsO3.Na |  | a, c and k are applicable. |
| Sodium cacodylate | Organic | Arsenic acid, dimethyl-, sodium salt | 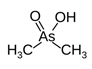 | C2H6AsO2.Na | 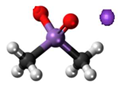 | a, b, c, d and k are applicable. |
| Sample Type | Acceptable Range [As] | Units | Reference |
|---|---|---|---|
| Urine | 0.005–0.04 | mg/day | [90] |
| Nails | 0.43–1.08 | mg/kg | [88] |
| Hair | 1 | mg/kg | [91] |
| [As] Species | Methodology | Technique Description | Detection Limit (μg/L) | Reference |
|---|---|---|---|---|
| DMA, As(III) and As(V) | HG-ASS | NaBH4 was used as a reductant in a separate reduction media. River water samples were analysed using HG-ASS. | 600, 1100 and 500 | [142] |
| Total As and As(III) | GF-AAS | A sample preconcentration method for As(III) by APDC and ion exchange resin followed with detection by GF-AAS. | 0.001 | [143] |
| As(III) | SERS | Fe3O4@Ag was used as a magnetic substrate. EXAFS T spectroscopy was used to characterise the molecular structure of As on Fe3O4@Ag followed by SERS. | 10 | [144] |
| As(V) | SERS S | SERS quantification analysis technique deployed for [As(V)] using multi-layer silver nanofilms deposited on glass slides as SERS-active substrates by an electroless deposition process. | ∼5 | [145] |
| As(V) | UV/vis Spectrometry | S–layer protein functionalized AuNPs reacted with As species, which changed the colour of AuNPs, detected by UV/vis spectrometry. | 240 | [146] |
| As(III) | UV/vis Spectrometry | Spectrometry method deployed with receptor as glucose in AuNPs platform. | 0.53 | [147] |
| Total As | SPRS B | Sensor probes of thiol-containing organic compounds using a SPRS method to detect As. | 10 | [148] |
| As(III), As(V), DMAA, MMAA and AsBet | HPLC-ICP-MS | The method involved Ion Chromatography hyphenated to an inductively coupled plasma mass spectrometer. | 0.02 | [132] |
| Total As | FP-XRF C | X-ray tube-based FP-XRF methodology was used to determine low concentrations of As. | <0.02 | [149] |
| Total As | ICP-AES | Electrolytic reduction was used as an alternate hydride generation method in atomic spectrometry for sample introduction. As detected by Flame atomic absorption spectrometry. | 0.7 | [150] |
| Total As | HPLC-ICP-MS | Species of As separated using an isocratic elute and identified by ESI-MS U. | ~0.1 | [122,151] |
| Total As | ICP-SF-MS | SPE F cartridges packed with anion exchange resin (modified conventional sorbent) before detection by ICP-SF-MS. | 0.06 | [152,153] |
| Total As, As(III) and As(V) | HG-AAS | SPE protocol with dual sorbent followed. SBAE G resin and hydrate iron (III) oxide particle-integrated HY resin cartridges were used before detection by HG-AAS. | 0.24 I | [154] |
| As(III) | ETAAS J | Based on IL V dispersive microextraction technique implemented in a flow analysis system followed by ETAAS detection. | 0.05 | [155] |
| As(V) | ICP-OES K | After pre-treatment of the sample with mesoporous silica, total iAs is quantified by oxidation (by KMnO4) of As(III) to As(V) and followed by ICP-OES and the As(III) measurement is found by the subtraction of As(V) from total As. | 0.05 | [156] |
| As(III) and As(V) | ICP-MS | Pre-treatment of water sample by SPE procedure with CNFs L modified with APDC M and then analysed with ICP-MS. | 0.0045 and 0.24 | [157] |
| Total As | HG-AAS | HG-AAS was adopted after pre-treatment of the sample with cellulose fibre coated with Y(OH)3 precipitate layer as adsorbent. | 0.012 | [158] |
| As(V) | AFS P | MWCNTs N modified with BPEI O used as a sorbent for pre-treatment of water sample followed by sequential injection technique and then analysed by AFS. | 0.014 | [159] |
| As (Ultra Trace) | AFS | Nano-TiO2 colloid was used to pre-treat the sample. A slurry sampling technique was adopted, followed by AFS detection. | 0.01 | [160] |
| As(III) | HG-AFS | Pre-treatment of the sample using Fe3O4 nanoparticles and SPE procedure was achieved, followed by HG-AFS method for detection. | 0.0135 | [161] |
| Total As and As(V) | HG-AFS | Methyl esterified egg-shell membrane was used to pre-treat the sample, followed by detection using a HG-AFS method. | 0.015 | [162] |
| Colorimetric Chemo-sensor | Mn3O4 nanoparticles were used as absorbents. Adsorption of [As] changed the surface morphology of Mn3O4 and the colour to yellow. This property led to the development of a novel colourimetric chemosensor method for [As] detection. | 1.32 | [163] | |
| Total As | ASV W | ASV involving the reduction of As3+ to As0, followed by stripping or oxidation to As3+ or As5+ species using electrodes modified by nanoparticles (carbonaceous nanomaterials). | 0.05 | [119] |
| As(III), As(V), MMA and DMA | HPLC-GHG-AAS A1 | As species analysis by interfacing solid phase preconcentration-LC X separation-GHG Y-QFAAS Z. | 0.019, 0.33, 0.39, 0.62 | [164] |
| Test Kit | Manufacturer | No. of Test in Box | Reaction Time per Test (Hr) | Methodology | Concentration Intervals on Colour Chart (μ/L) | Results |
|---|---|---|---|---|---|---|
| Merck | Merck, Darmstadt, Germany | 100 | 0.35 | Colorimetric | 5, 10, 25, 50, 100, 250, 500 | Either accurate or precise, but not both. |
| Quick II | Industrial Test Systems, Rock Hill, USA | 200 | 0.25 | Colorimetric | <1, 2, 3, 4, 5, 6, 7, 8, 10, 13, 20, 25, 30, 40, >50, >80, >120, >160 | Accurate and precise estimates of [As]. |
| Econo-Quick II | Industrial Test Systems, Rock Hill, USA | 50 | 0.25 | Colorimetric | <2, 4, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 100, >150, >300 | Neither accurate nor precise estimate of [As]. |
| LaMotte | LaMotte, Chestertown, USA | 50 | 0.25 | Colorimetric | <4, 4, 8, 10, 12, 14, 16, 20, 25, 30, 50, 85, 100, 150, 175, 200, 300, 400 | Accurate and precise estimates of As. |
| Wagtech | Palintest, Gateshead, UK | 200 | 0.35 | Colorimetric | <10, 20–40, 50, 60–80, 100, 100–200, 200–300, 300–400, 400–500 | Either accurate or precise, but not both. |
| Quick | Industrial Test Systems, Rock Hill, USA | 100 | 0.25 | Colorimetric | 0, 5, 10, 20, 30, 40, 50, 60, 80, 100, 150, 200, 250, 300, 400, 500, >500 | Either accurate or precise, but not both. |
| Econo-Quick | Industrial Test Systems, Rock Hill, USA | 300 | 0.25 | Colorimetric | 0, 10, 25, 50, 100, 200, 300, 500, 1000 | Either accurate or precise, but not both. |
| Hach EZ | Hach, Loveland, USA | 100 | 0.35 | Colorimetric | 0, 10, 25, 50, 250, 500 | Neither accurate nor precise estimate of [As]. |
| Oxidizing Agent | Chemical Formula | pH | Initial Conc. (μg/L) | Results | Reference |
|---|---|---|---|---|---|
| Ozone and Oxygen | O3 and O2 | 7.6–8.5 | 46–62 | Oxidation of As(III) to As(V) was fast (4 min) with O3 and slow (2–5 days and 4–9 days, respectively) with pure O2 and air. | [178] |
| Chlorine dioxide | ClO2 | 5.7–6–7 and 8 | 300 | The 90% oxidation (maximum) of As(III) to As(V) yield was achieved with CIO2 to As(III) concentration ratio of 3 at a contact time of 6 days. | [179] |
| Hydrogen peroxide | H2O2 | 7.5–10.3 | 50 | Oxidation efficiency was found to increase with an increase in pH from 7.5 to 10.3. | [180] |
| Monochloramine | NH2Cl | 5.7–6–7 and 8 | 50 | A 100% oxidation yield was achieved using an oxidant-to-concentration ratio of 3 and with contact times of 2 days. | [179] |
| Hypochlorite | ClO- | 5.7–6–7 and 8 | 50 and 300 | An oxidant to As(III) ratio of 3 resulted in 100% oxidation of As(III) to As(V) after 5 min of contact time for all the pH conditions. | [179] |
| Chlorine | Cl2 | 8.3 | 300 | As(III) was oxidized to As(V) with a stoichiometric ratio of 0.99 at the fastest rate. | [181] |
| Potassium permanganate | KMnO4 | 5.7–6–7 and 8 | 50 | An oxidant to As(III) ratio of 1–2 resulted in 100% oxidation of As(III) to As(V) after 1 min for all the pH conditions studied. | [179] |
| Water Type | Materials | Method | Study Characteristics | Reference |
|---|---|---|---|---|
| Deionized water | Fe–Cu–Mn composite oxide (FCMOx) | Synergistic oxidation and ultrasonic coprecipitation method | FCMOx adsorbent was used to separate As from aqueous solutions. FCMOx showed efficient results for photocatalytic oxidation with the use of UV irradiation. The combination of adsorption and synergistic oxidation improved the removal efficiency of As(III). | [188] |
| Deionized water | Fe-biochar fibres, H2O2 and Hydroxylamine (HA). | Oxidation and adsorption | Fe-biochar fibres integrated with H2O2 and Hydroxylamine (HA) were used to prepare a heterogenous material which showed efficient and rapid result for the chemical oxidation of As(III) to As(V) followed by absorption of As(V) by Fe-biochar. | [189] |
| Distilled water | Santa Barbara Amorphous-15 (SBA-15) and TiO2. | Photocatalytic oxidation and adsorption | Impregnation was performed to produce Ti-SBA-15. This novel material was an excellent photocatalyst at all pHs with 98% efficiency. | [190] |
| Distilled water | Ancylobacter sp. TS-1, zeolite, polypropylene, graphite and sand. | Biological oxidation | Ancylobacter sp. TS-1 (biomaterial) was used to make films on all materials mentioned excluding polypropylene. Film of Ancylobacter sp. TS-1 on graphite material was the most rapid oxidant of As(III) to As(V). | [191] |
| Milli-Q water | Fe-Mn-incorporated titanosilicate material (ETFMS-10). | Adsorption and oxidation | ETFMS-10 was prepared by hydrothermal method. The material was used to oxidize and adsorb As with efficiency of 70%. | [192] |
| Gold mine effluent. | Alishewanella agri strain (PMS5). | Bio-Oxidation | Microorganisms (PMS5) showed an ability to reduce As(V) and oxidize As(III) with efficiency of 75.5–94.7% and 8%, respectively. | [193] |
| Ultrapure water. | Manganese-doped Lanthanum oxycarbonate (MnL) and H2O2. | Oxidation and adsorption | MnL catalysed H2O2 oxidation of As(III) to As(V) studied. Efficient oxidation and adsorption results were seen over a wide range of pHs. | [194] |
| Milli-Q water | CuFe2O4 activating peroxymonosulfate. | Oxidation and adsorption | A novel material was introduced for the efficient oxidation and adsorption of As/As(III). | [195] |
| Paddy rice water | Reactive intermediates (RIs) from paddy water. | Oxidation | RIs improved the oxidation rate of As(III) to As(V) by 1.8–4.1-fold in paddy water compared to surface water with oxygen during a dark reaction. | [196] |
| Deionized water | Fe-modified biochar (FeBC) | Oxidation and adsorption. | With the use of FeBC, the efficiency of [As] removal increased from 99.2% and 86.4%, respectively, for As(V) and As(III) in the absence of 02, to >99.9% in the presence of O2. | [197] |
| Sediment water | O2 nanobubbles | Bio oxidation and air oxidation | With the application of O2 nanobubbles to sediment water sample with algal-induced hypoxia, the concentration of [As] in the sample rose to <10 μg/L from 23.2 μg/L as nanobubbles catalysed the oxidation of As(III) to As(V) (65–75%) and methylated [As] (10–15%). | [198] |
| Deionized water | molybdenum-disulfide/iron-oxide (MoS2/FeOx@BC) | Oxidation and adsorption | MoS2/FeOx@BC oxidize the As(III) to As(V) and then adsorb with greater efficiency as compared to MoS2/BC and FeOx@BC. | [198] |
| Ultrapure water | WTRs-chitosan beads (WCB) and Mn-WCB | Oxidation and adsorption | Results suggest that oxidation ability improved; hence, adsorption ability of WCB improved with MnO2 added to WCB. | [199] |
| Ultrapure water | Sulfite | Oxidation | A novel sulfite activation method using ultrasound for achieving an improved oxidation rate of As(III) compared to oxidation supported by ultrasound (US) showed a 2.9% rise in As(III) oxidation rate at 7 pH. | [200] |
| Distilled water | Bentonite/chitosan/titania (BT/CS-TiO2). | Photooxidation and adsorption | BT/CS-TiO2 was prepared and then tested under UV, which produced 97% oxidation of As(III) to As(V). | [201] |
| Coagulant | Chemical Formula | pH | Initial Conc. of As (μg/L) | Results | Reference |
|---|---|---|---|---|---|
| Ferric chloride | FeCl3 | 8.0–8.4 | 400–600 | The technology was deployed at a large scale in northern Chile for provision of drinking water. The result shows reduction in As concentration from 400 μg/L to 10 μg/L. | [207] |
| Alum | X Al(SO4)2·12H2O | 7 | 2000 | The results showed that at 125 mg/L and 100 mg/L doses of alum at pH 7, the removal efficiency of As was between 82 and 86%. | [210] |
| Zirconium (iv) Chloride | ZrCl4 | 7.5 | 50 | A total of 55% removal of [As(V)] was observed with 2 mg/L does of coagulant. The efficiency is found directly proportional at pH range 6.5–8.5, whereas removal of As(III) only found as 8% irrelevant to pH. | [211] |
| Titanium (iii) chloride | TiCl3 | 7.5 | 50 | With 2 mg/L dose of coagulant, 32% of [As(III)] and 75% of [As(V)] removal achieved. The process remained pH dependant. | [211] |
| Titanium (iv) chloride | TiCl4 | 7.5 | 50 | A total of 55% removal of As(V) was observed on distilled water sample with 2 mg/L dose of coagulant. The efficiency is directly proportional at the pH range 6.5–8.5, whereas removal of As(III) was only found as 26% irrelevant to pH. When tested on wastewater, the results showed that coagulant performs excellently in waters with high DOC and low alkalinity. | [211,212] |
| Titanium(IV) oxychloride | Cl2OTi | 7.5 | 50 | With 2 mg/L of coagulant dose, 37% and 20% removal efficiency were achieved for [As(V)] and As(III). The methods remained pH dependent. | [211] |
| zirconium oxychloride | Cl2H2OZr | 7.5 | 50 | With a 2 mg/L dosage of coagulant, 59% and 8% removal efficiency was achieved for As(V) and As(III). | [211] |
| Ferric Sulphate | Fe2(SO4)3 | 7 | 500 and 1000 | Dosages of 32 mg/L (1000 μg/L initial concentration) and 28 mg/L (500 μg/L initial concentration) of coagulant give 100% results. | [213] |
| Titanium (IV) Sulphate | TiOSO4 | 7 | 1000 | Removal of 90% of As(III) was achieved with 25 mg/L dosage of coagulant. | [214] |
| Water Type | Materials | Method | Study Characteristics | Reference |
|---|---|---|---|---|
| Groundwater | Aluminium sacrificial anodes | Electrocoagulation (EC) and flocculation | Hydrated silica and aluminium react in an EC system to form aluminosilicates, and As becomes adsorbed on newly formed compound. The result showed achievement of As concentration in water less than 10 ppm. | [215] |
| Tap water | Cellulose and chitosan-based natural biopolymer and ferric chloride (FeCl3) | Coagulation, flocculation and adsorption | In the comparative study, initial concentration of 2 mg/L removed with efficiency of 69.25% using FeCl3 as coagulant, whereas the use of cellulose and chitosan-based ferric chloride FeCl3 has enhanced the removal efficiency to 84.62%. | [216] |
| Deionized water | Hydrophilic ligands and FeCl3 | Coagulation and flocculation | Hydrophilic ligands were introduced for the separation of As from water. It is noted that smaller doses of coagulant (Fecl3) are required to achieve the higher As(III,V) removal, comparatively. | [217] |
| Groundwater | Mining drainage effluent and exchange resin with iron oxides. | Coagulation, flocculation, and adsorption | To achieve high removal rate and low cost, coagulation, flocculation, and adsorption were combined to test in comparison with other conventional systems, and the new system has produced better results. | [218] |
| Milli-Q purified water | Fe(II), H2O2 and [Fe(III)] | Coagulation | Introduction of H2O2 and Fe(II) to conventional iron-based Fe(III) coagulation was studied, and it was found that addition of H2O2 significantly enhanced the performance efficiency of removal of As from water. | [219] |
| Deionized water | Cactus mucilage and Ferric [Fe(III)] Salt. | Coagulation and flocculation | The addition of mucilage treatment (flocs) to the conventional system of coagulation enhanced As removal efficiency and removed 75–96% of As in 30 min of contact time. | [220] |
| Mine water | Ferric sulphate [(Fe2(SO4)3] | Coagulation, lime precipitation, ballasted flocculation, and sedimentation | Fe2(SO4)3 used for single-stage and two-stage coagulation, lime precipitation, ballasted flocculation, and sedimentation was compared, and found the two-stage system is more efficient. | [221] |
| Groundwaters | Coagulants: FeCl3, Fe2(SO4)3, FeSO4, and oxidants: H2O2 and KMnO4 | Oxidation, coagulation and flocculation | Different oxidants and coagulants were used to treat [As] bearing groundwater, and 97% removal efficiency is achieved with the initial concentration of 204 μg/L. | [222] |
| Groundwater | Ferric(III) sulfate (FS) and Polyferric sulfate (PFS) | Coagulation and flocculation and sand filtration | FS and PFS coagulants were compared using microscopic techniques to analyse the surface complexes of Fe flocs and two-bucket systems, combining coagulation and sand filtration. PFS is found superior. | [223] |
| Lake water | Ferric chloride | Facile remediation strategy by coagulation process | The coagulant was directly sprayed on the lake affected with As without any pre-treatment, and a removal efficiency of 95.1–96.7% was achieved. | [224] |
| Millipore water | Iron Cathode and Aluminium anode | Electrocoagulation (EC) | EC method used with iron cathode and aluminium anode to check removal efficiency. At pH 5–8, 3 volts and 12 min processing time, initial concentration of 2–5 mgL−1 conc. of As(V) was brought down to <10 ppm. | [225] |
| Deionized water | Aluminium chloride (AlCl3) and polyaluminium chloride (PACl) | Coagulation | Alum coagulants (AlCl3 and PACl) were investigated for removal efficiencies of As(III) and As(V). Both were found not impressive for the removal of As(III), whereas they showed 100% removal efficiency for As(V). | [226] |
| Deionized water | Multiple-Review | Coagulation/flocculation and adsorption | A review of small-scale and household technologies using coagulation/flocculation and adsorption methods were deployable in rural/underdeveloped areas with the least cost. | [227] |
| Raw water | Sodium hypochlorite (NaClO) and FeCl3 | Oxidation and coagulation-flocculation | Enhanced coagulation in an integrated efficient-whirling clarifier was performed with FeCl3 to achieve the result of 10 µg/L from the initial 100 µg/L concentration. | [181] |
| Water Type | Materials | Method | Study Characteristics | Reference |
|---|---|---|---|---|
| Deionized Water | Silk nanofibrils | Membrane filtration | Silk nanofibrils-based, multi-layered, novel material membranes were prepared. The system produced a flux of >890 L/m2/h (high) and >89% of rejection of pollutants, including As. The membrane was also found to avoid fouling. | [236] |
| Deionized Water | Nano zerovalent iron-kaolin clay (nZVI-Kaol) | Membrane filtration | Membranes were prepared with nZVI-Kaol clay in different compositions and tested for As(III) removal from water. Greater than 50% removal efficiency for As2O3 was achieved. | [237] |
| Freshwater | Chelating polymer | Membrane distillation (MD), photocatalytic oxidation and polymer-enhanced ultrafiltration (PEUF) | MD deployed with an efficiency of 98.8% from an initial contraction of 0.059–5 mg/L. In addition, MD retentate was applied with the photocatalytic process for the oxidation of As(III) to As(V), which was subsequently removed by PEUF with 98.2% efficiency. | [238] |
| Deionized Water | Nano-aluminium oxide and polyphenylsulfone (PPSU) | Membrane filtration | Ultra-membrane containing nano-aluminium oxide with PPSU prepared. A membrane fabricated with 1.5% w/w e nano-aluminium oxide in PPSU removed 98.41% of As(V). | [239] |
| Deionized Water | Hydrous manganese oxide (HMO) nano clay and polyacrylonitrile (PAN) nanofibers | Electrospinning technique (ET) and membrane filtration | A membrane was fabricated with ET by impregnating HMO into PAN forming nanofiber membrane. The novel absorbent showed high efficiency for As(V) uptake and was found reusable after cycles. | [240] |
| Synthetic wastewater | Microporous polypropylene fibres. | Membrane filtration | A hollow fibre-supported liquid membrane (HFSLM) was used to mitigate As ions from water. The results demonstrated that mitigation can be conducted up to the discharge limit of wastewater standards of Thailand (250 ppb). | [241] |
| Deionized Water | Micro tetravalent manganese feroxyhyte (μTMF) and micro granular ferric hydroxide (μGFH). | Adsorption and membrane filtration | μTMF and μGFH, by-products of manufacturing granular absorbents, were tested with hybrid submerged microfiltration membrane adsorption and adsorption kinetic method. μTMF is found better in mitigating As(V) from modelled water with shorter residence time (~3 h) and greater efficiency (1.4-fold) compared to μGFH. | [242] |
| Tap water | Hydrophilic nickel-carbon nanotubes. | Membrane filtration | Cathode penitential using hydrophilic nickel–carbon nanotubes deployed to enhance the performance of UF membranes to mitigate As(III), which has lower removal efficiency when mitigated in water by NF and RO. A significant increase in As(III) rejection in water is monitored. | [243] |
| Surface water | NaOCl | Membrane filtration | Membrane fouling is a significant challenge in the wastewater industry. The RO membrane is recycled by oxidation and converted into a UF membrane. Application of pe-oxidation to feed was also made, and As was later removed by UF. Economic and environmental benefits are highlighted. | [244] |
| Waste-water | Polycaprolactone and iron- intercalated montmorillonite filler. | Membrane filtration and adsorption | In the batch scale study, a membrane fabricated with polycaprolactone matrix and iron-intercalated montmorillonite filler was studied for As removal and found that the membrane is a promising alternative for As removal from wastewater. | [245] |
| Surface water | Zeeweed UF membrane by Suez Water Technologies and Solutions. | Oxidation, coagulation, flocculation, sedimentation and membrane filtration | Dead-end UF incorporated with conventional water treatment systems comprising oxidation, coagulation, flocculation and sedimentation systems. The threshold value for As in water was achieved. | [246] |
| Deionized water | μTMF and μGFH | Adsorption and membrane filtration | MF membrane was modified by depositing iron oxide adsorptive materials on the membrane. The membrane was tested by application study and mathematical modelling on water, and this procedure may increase the life of the membrane filtration cycle for As(V) removal with other advantages. | [247] |
| Geothermal water | Commercial membranes; CK- NF, XLE BWRO, Osmonics and NF90 and spiral wound membranes; BW30, TR-BE-BW and TR-NE90-NF | Membrane filtration | The various membranes of NF and RO were tested for the removal of As from geothermal water on a mini-pilot scale (membranes; BW30, TR-BE-BW and TR-NE90-NF) and cross-flow flat-sheet membrane testing unit (commercial membranes; CK-NF, XLE BWRO, Osmonics and NF90). Maximum As removal efficiency of 99% with XLE BWRO commercial membrane was achieved. Removal efficiency of 90% was performed on all membranes tested on the mini-pilot scale. | [248] |
| Deionized water | Zinc-magnesium oxides and cellulose | Membrane filtration | Hollow membranes with polyphenylsulfone/cellulose acetate derivatives and zinc–magnesium oxide combinations were fabricated and tested. Membrane prepared with novel binary effect showed increase As retention efficiency. | [249] |
| Deionized water | Chitosan and Polypropylene | Membrane filtration | A total of 75% removal efficiency was achieved when tested for removal of As(V) after the fabrication of membrane from newly synthesised material (modified polypropylene with chitosan). | [250] |
| Water Type | Materials | Method | Study Characteristics | Reference |
|---|---|---|---|---|
| Groundwater | Chitosan and titanium oxysulfate (TiOSO4) | Adsorption | Using the sol-gel method, a novel granular adsorbent is fabricated with titanium and chitosan, which is reasonably amorphous for adsorption As(V). Small-scale column tests results reveal that at pH range 8.0–8.5, 165.6 μg/L of initial concentration was brought down to <10 μg/L at 126-bed volume with adsorption capacity of novel adsorbent around 1.22 mg/g at effluent equilibrium (1370 BV). The adsorbent is found to have good prospects compared to other adsorbents, with a capacity of 70% after four cycles. | [291] |
| Ultrapure water | Chitosan, graphene oxide and polyethyleneimine | Adsorption | Combining chitosan, graphene oxide and polyethyleneimine, a new magnetic novel absorbent formed abbreviated as MCS/GO-PEI. A Langmuir isotherm model showed that the new material was a single-layer absorbent. Adsorption equilibrium was achieved in 8 min for As at pH 7. The adsorbent has shown reasonable characteristics for reusability. | [292] |
| Deionized water. | Iron-manganese treatment sludge and chitosan | Adsorption | Iron-manganese treatment by-product (backwashing sludge) in powered form converted to granular with the combination of chitosan. Langmuir model gave an adsorption capacity of 14.95 mg/g for As(V), followed by a filtration test in the plexiglass column. With a bed depth of 15 cm, total sorption (10.755 mg) and unit sorption capacity (0.779 mg/g) were calculated. Compared with when bed depth was 60 cm, the total and unit adsorption was calculated as 20.405 mg and 0.370 mg/g, respectively. At a bed height of 30 cm, EBCT is given 9 min and a breakthrough time of 13 h. After regeneration of the material for adsorption, a removal efficiency of 96% for As(V) also showed potential for reuse. | [293] |
| Groundwater | Chitosan and N-vinylcaprolactam/N-N-dimethylacrylamide (net-CS)-g-NVCL/DMAAm hydrogels | Adsorption | A new novel material for adsorption of As from groundwater (pH range 7–8.3) formed with a grating of hydrogel on chitosan. Evaluation by isotherms and kinetics sorption tests was conducted. An adsorption capacity of 0.0022 mg/g was measured on the new sorbent after 50 h. Removal efficiency of 40% (isotherms) and 46% (kinetics sorption test) for As was achieved. | [294] |
| Ultra-pure water | Chitosan and 3-mercaptopropyl trimethoxysilane (C6H16O3SSi) | Adsorption | With the reaction of C6H16O3SSi and chitosan, a new chitosan-based material was formed. Removal efficiency for As(III) of 99% was achieved from the initial concentration of 10 mg/L at an extensive pH range (3–10). A maximum adsorption capacity of 21.01 mg/g is monitored at pH 7. With oxalic acid, the material can be regenerated with 80% [As] removal efficiency in 2 cycles. | [295] |
| Deionized water. | Coal fly ash, ZrOCl2 and FeCl3·6H2O | Adsorption | Zeolite W (ZW) (fly ash-based) was modified using microwaves, with the resultant material having iron and zirconium at 6.9 and 5.04% w/w, respectively. The modified material tested for adsorption of As(V) and achieved 99.87% removal efficiency at pH from 2 to 10. Maximum adsorption of 42.31 mg/g was attained at pH 2. | [296] |
| Double distilled water | Calcium alginate, Iron Oxide Magnetic Nanoparticles and methionine | Adsorption | Reacting calcium alginate with magnetic iron oxide nanoparticles functionalized with methionine formed a new bead absorbent. Laboratory-based tests confirmed that novel material can remove As(III) up to 99.56% at an equilibrium time of 1.83 h and pH 7.0–7.5. Langmuir isotherm calculation provided an adsorption capacity (qm) of 6.6533 mg/g. | [297] |
| Deionized water. | Seeds skin extract of Sapindus, CeO2-ZrO2 and Iron alginate | Adsorption | Nanooxides of CeO2-ZrO2 (mixed) formed. As stabilizing agent to control the size of nano mixed oxides, Sapindus plant seeds’ skin was extracted. These nano oxide particles are embedded in iron alginate to prepare beads. After the test of adsorption, the results gave an adsorption capacity of 140.5 mg/g and 153.25 mg/g for As(V) and As(III), respectively. | [298] |
| Wastewater | Waste red mud and calcium-alginate | Adsorption | Beads of novel material formed by doping red mud with calcium alginate. The material beads are tested to remove As(III) ions from wastewater and achieved results by reducing the concentration in effluent from 0.101 mg/L to 0.008 mg/L. A total of 1.807 mg/g is found to be peak adsorption capacity. The material has shown the potential to be used for the removal of As from water and wastewater. | [299] |
| Livestock farm wastewater | Yttrium (III) chloride hexahydrate, graphene oxide and alginate hydrogel | Adsorption | A new novel hydrogel was prepared by reacting graphene oxide, yttrium (III) chloride and alginate hydrogel and was tested by an adsorption experiment under room temperature. Maximum adsorption of As(V) was calculated as 273.39 mg/g, which is reported to be far higher than other absorbents available. | [300] |
| Distilled water | Pectin, Glutaraldehyde, N,N-methylene bisacrylamide, N,N,N, N-tetramethyl ethylenediamine and EGDMA | Adsorption | The pectin-based hydrogel was prepared, loaded with Fe(II) and tested to remove As(V) from water. Hydrogel was designed by crosslinking pectin in the presence of crosslinkers and 2-acrylamido-2-methylpropanesulphonic acid. Maximum uptake of As(V) was achieved at 35 °C temperature and 7.0–9.2 pH with 5% glutaraldehyde crosslinked hydrogels. | [301] |
| Distilled water. | Solid particulate waste collagen and APPN-g-pentapolymer (apple-based pectin) hydrogels | Adsorption | Collagenic fibres from tannery waste are incorporated into apple pomace pectin to form pectin-grafted penta-polymers created by one-pot facile polymerization of APPN and synthetic monomers. These polymers were tested for adsorption of As(V) and achieved 180.47 mg/g of As adsorption at three pH with a 25 mg dose of polymer. | [302] |
| Distilled water. | Waste orange juice residue | Adsorption | Pectin, cellulose and hemicellulose from waste orange juice were converted into acidic polysaccharides (pectic acid)/hydrogels by saponification and phosphorylation. Gels produced showed a strong affinity for iron ions and absorbed other metal ions (oxo-anions) such as As. | [303] |
| Deionized water | Steam-exploded straw and CeCl3 | Adsorption | Ce3+-based biochar-loaded nanoparticles formed as a novel adsorbent for the adsorption of As. The material was tested using spectroscopy detection technologies and batch experiments. The Langmuir model showed a sorption capacity of 219.8 mg/g for As(V) at room temperature with 5 pH. Therefore, a promising new material from As(V) absorption was invented. | [304] |
| Water | Phosphorene-based materials—Theoretical study | Adsorption | Calculation based upon density functional theory was performed to evaluate the nanoflakes’ phosphorene oxide (PO) ability to mitigate methylarsenicals from polluted water sources by using solid phase adsorption. The study was conducted to understand PO’s adsorption characteristics for peril methylarsenicals, providing a valued framework for future development. The study suggests PO is suitable for the function as it has a large surface area, no pre-oxidation required for the process, high adsorption capacity and easy recovery. | [305] |
| Deionized water. | Dry sewage sludge and nano-zero-valent iron | Adsorption | Sewage sludge and nano-zero-valent iron passed through the co-pyrolysis process to prepare biochar. Biochar was tested for As(V) adsorption with batch experiments with initial pH and concentration as 2 and 20 mg/L, respectively. The biochar dose was 10 g/L, at a temperature of 298 K and contact time of 24 h, As(V) removal efficiency was given as 99%. With the Freundlich model, the adsorption capacity (max) is calculated as 60.61 mg/g for As(V). | [306] |
| Deionized water | SiO2 and titanium chloride (TiCl3) | Adsorption | Two nanomaterials, aggregated dendritic anatase-titanium dioxide and aggregated dendritic anatase-titanium dioxide, reacted to the surface of SiO2 and were evaluated. As removal performance by both materials was tested under different conditions by laboratory batch experiments. The maximum removal efficiency of 95% was given by aggregated nanomaterial. | [307] |
| Deionized water | Attapulgite, sodium thiosulfate (Na2S2O3) and Ferrous sulfate heptahydrate (FeSO4·7H2O) | Adsorption | Nanoscale zero-valent iron supported by attapulgite and modified by sulfide to prepare a new nanomaterial for As(III) remediation in water. With support and modification, the specific surface area of nanomaterial increased to 46.04 m2/g from 19.61 m2/g. As a comparative result, the removal efficiency of As(III) increased to 65.1% from 51.4% at 20 min and 51.4% to 65.1% at 20 min. The increase in performance of nanomaterial is seen at a pH range of 3.0–6.0. A total of 193.8 mg/g is given as adsorption capacity (max). | [308] |
| Deionized water | Milli-Q water | Adsorption and photo-oxidation. | Composite oxide of nanostructure properties of iron, titanium, and manganese was prepared to treat the para-arsanilic acid. A total of 424.7 m2/g is calculated as the specific area of the new material. Adsorption capacity (maximum) is given as 45.6 mg/g. In addition to adsorption, the photo-oxidation of the pollutant from As(III) to As(V) is also performed in the system to achieve better results. | [309] |
| Ultrapure water | Ferric chloride, Ferrous chloride and Copper chloride | Adsorption and photo oxidation. | Magnetised CuO-Fe3O4 material deployed for photo-oxidation of p-arsanilic acid with visible light irradiation and subsequent removal by adsorption. The As is seen completely oxidized from As(III) to As(V) in 36 min, and adsorption by nano-CuO-Fe3O4 is also observed with efficiency of 95% at a pH range of 4 to 7. | [310] |
| Ultrapure water | Iron sludge | Adsorption | A novel nanomaterial prepared by Fe3O4@C magnetic nanoparticles of amorphous iron oxides from iron sludge for the removal of As(V) from water. BET analysis calculated the surface area as 122.7 m2/g. At a pH range of 3–9, the adsorption of As(V) is noted as high. The adsorption capacity (maximum) at room temperature is given as 13.47 mg/g, which was 3.29-fold higher than non-modified material (Fe3O4@C). | [311] |
| Deionized water | Fe (NO3)3•9H2O, MnSO4•H2O and CoSO4•7H2O | Adsorption | CoFe2O4 and MnFe2O4 nanoparticles are prepared with magnetic properties and tested to remove As. Freundlich model equation obeyed to show multilayer adsorption with adsorption capacity (maximum) as 230 mg/g for MnFe2O4 and 250 mg/g for CoFe2O4. | [312] |
| Milli-Q ultrapure water | Magnesium chloride hexahydrate, Aluminum chloride hexahydrate and Ferric chloride | Adsorption | Facile pyrolysis method was adopted to prepare four types of magnetic biochar, out of which one is prepared as magnetic biochar only, while the other three are anchored with Aluminium, manganese and both (Al and Mn) in addition. Upon testing, the optimal pH range was given as 4–6. Aluminium-based magnetic biochar outperformed all other comparisons for the co-up-take of As(V) and fluoride. From adsorption isotherm information, the adsorption capacity (maximum) is given as 34.45 mg/g for As(V) at a temperature of 10 °C and pH of 5. | [313] |
| Ultrapure water | Cerium oxide and polyhedrons | Adsorption | With the combination and application of calcination and impregnation, cerium oxide anchored on polyhedrons (from MIL-100(Fe)) to fabricate magnetic mesoporous nanocomposite was denoted as Fe2O3/CeO2-t with a unique octahedral nanostructure. Fe2O3, a conventional absorbent, when modified on a surface with CeO2 and tested as an absorbent of As(III), reduced the concentration to 10 µg/L from 180 µg/L in 20 min, which is 9-fold faster than the conventional comparison. By an isotherm method, the adsorption capacity (maximum) is 68.25 mg/g, which was higher than many similar absorbents. | [314] |
| Deionized water | Fly ash and ferrous sulfate heptahydrate | Adsorption | Zeolite 5A loaded on nanozero valent iron to form a new material. New material denoted as NZVI-5A and reported with a specific area of 238 m2/g with a removal capacity of 72.09 mg/g from the isotherm model. pH range was reported as 4–12, and the method was not reported as pH sensitive. Removal efficiencies remain unaffected by the co-existence of other pollutants. NZVI-5A zeolite worked with 84% removal efficiency after 5 cycles. | [315] |
| Deionized water | Sodium alginate, Chitosan and banana peels | Adsorption | Two adsorbents were prepared by combining alginate and chitosan separately with banana peel ash and biopolymeric matrix ash. Both sorbents were characterised and tested for the removal of heavy metals. The material formed with alginate was proven to be an excellent absorbent for heavy metals with 100% efficiency. | [316] |
| Deionized water | Oyster shell powder and rice husk ash | Adsorption | Rice husk ash (TRHA) and oyster shell (OS) powder are used to prepare pallets for use as adsorbent for As(III) removal from water samples. A ratio of 0.3:0.7 = TRHA: OS has been reported as the best ratio for removal with 26.2 mg/g as adsorption capacity with breaking. Further analysis concluded that CaO reacted with As(III), which was 25% by weight of the pellet while the remaining 75% is CaSiO3, which helps the pellet to keep in a stable position (avoiding cracking). | [317] |
| Bi-distilled water | Soluble starch, cellulose and iron chloride | Adsorption | Soluble starch and cellulose, used as carbon sources, and iron chloride, used as the source of iron, were deployed to make a few composite materials to test as sorbent for As(V) in water samples. The maximum adsorption capacity was measured as 280 µg As(V)/g of novel material. | [318] |
| Deionized water | Cobalt acetate, Aniline, Ferric chloride and Chitosan from shrimp | Adsorption | Magnetization and functionalization of biopolymer to prepare cobalt oxide doped magnetic-chitosan graphene oxide grafted with polyaniline. Take up of As(V) by new material tested by batch experiments and theoretical investigation. An adsorption capacity of 90.91 mg/g was given at 7 pH—the highest removal efficacy of 89% given at 50 min of interaction. | [319] |
| Deionized water | 1-(2-Hydroxyethyl) piperazine, 3-cyanopropyltriethoxysilan, Sporopollenin and Ferric chloride | Adsorption | With 1-(2-hydroxyethyl) piperazine, the sporopollenin from Lycopodium clavatum was magnetized/functionalized. The novel bio-polymer-based sorbent was used to test in batch experiments for the adsorption of As(III) and Pb(II). Maximum adsorption of 69.85 mg/g was calculated for As(III). | [320] |
| Distilled water | Cetyltrimethylammonium bromide surfactant, tetraethylorthosilicate and β-cyclodextrin polymer | Adsorption | A combination of β-cyclodextrin polymer and mesoporous silica was performed to prepare a hybrid structure for the mitigation of As(V) and Hg (II). Maximum adsorption capacity calculated as 265.6 mg/g. As per data, Langmuir equilibrium is followed to attain a monolayered homogenous absorbent. Column test (fixed bed) achieved a removal efficiency of 72.8% for As (V). | [321] |
| Canal water | Arsenic, 2-hydroxyethyl methacrylate and 4-vinyl pyridine | Adsorption | As-imprinted polymer prepared by polymerization and precipitation using 2-hydroxyethyl methacrylate and 4-vinyl pyridine. The absorbent is tested for the adsorption of As(III) in aqueous environments. Adsorption capacity (maximum) calculated as 106.3 mg/g. A total of 99% removal efficiency achieved for removal of As(III) by the polymer as adsorbent. | [322] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, M.M.; Hussain, S.; Du, C.; Conway, B.R.; Ghori, M.U. Arsenic in Water: Understanding the Chemistry, Health Implications, Quantification and Removal Strategies. ChemEngineering 2024, 8, 78. https://doi.org/10.3390/chemengineering8040078
Chaudhary MM, Hussain S, Du C, Conway BR, Ghori MU. Arsenic in Water: Understanding the Chemistry, Health Implications, Quantification and Removal Strategies. ChemEngineering. 2024; 8(4):78. https://doi.org/10.3390/chemengineering8040078
Chicago/Turabian StyleChaudhary, Muhammad Murtaza, Saqib Hussain, Chenyu Du, Barbara R. Conway, and Muhammad Usman Ghori. 2024. "Arsenic in Water: Understanding the Chemistry, Health Implications, Quantification and Removal Strategies" ChemEngineering 8, no. 4: 78. https://doi.org/10.3390/chemengineering8040078






