Reverse Polarity-Based Soil Electrokinetic Remediation: A Comprehensive Review of the Published Data during the Past 31 Years (1993–2023)
Abstract
1. Introduction
2. Data Collection Methodology
3. Utilizing the Reverse-Polarity Mode (RPM) during the SEKR
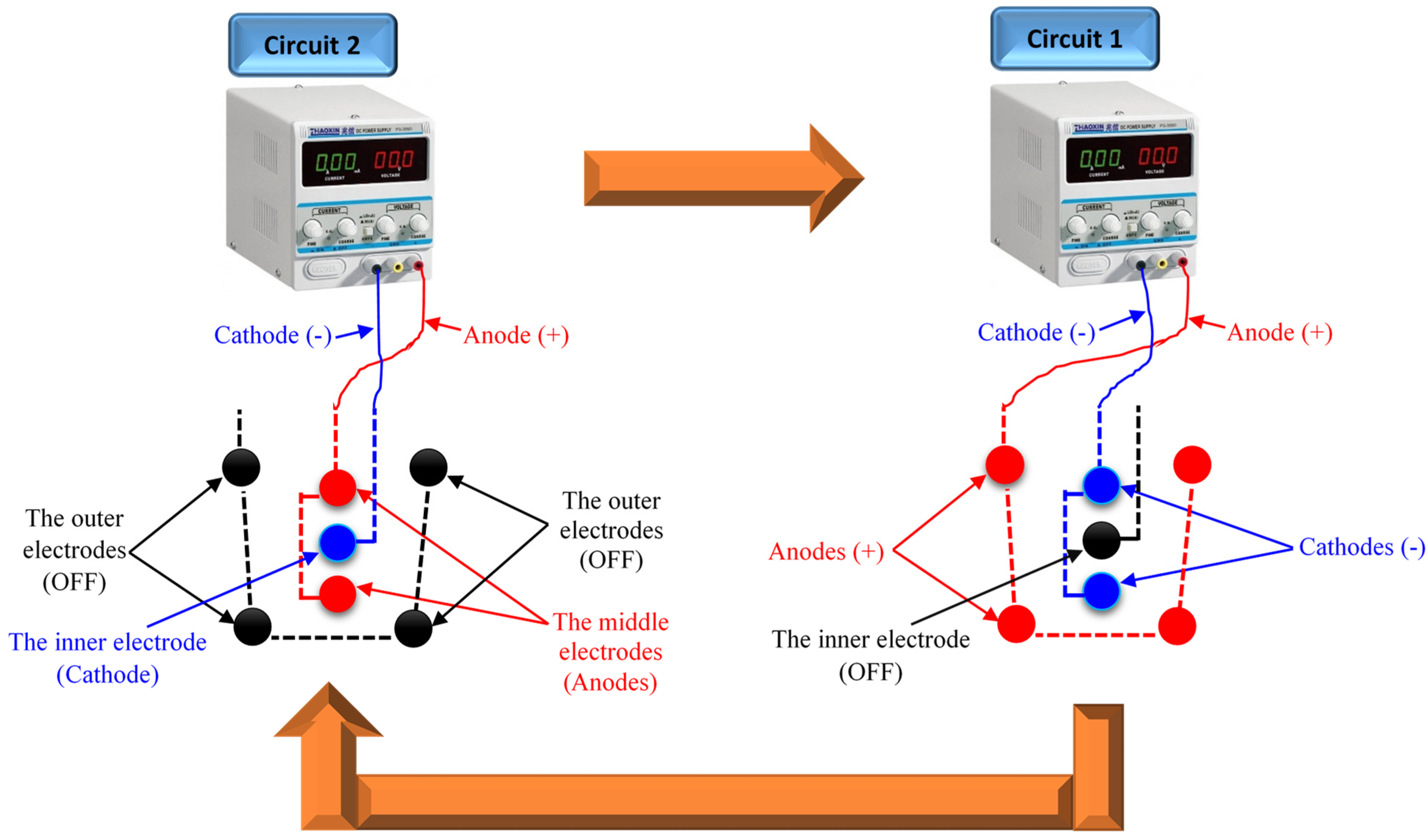
3.1. The SEK-RPM Connection Mechanisms
3.2. Applying the SEK-RPM to Remediate Soil Contaminated with Organic Pollutants
| No. | Organic Pollutant-Contaminated Soil | Applied Voltage | Reverse-Polarity Mode | Experimental Period | References |
|---|---|---|---|---|---|
| 1 | Diesel-contaminated soil |
|
|
| [76] |
| 2 | Diesel-contaminated soil |
|
|
| [66] |
| 3 | Diesel-contaminated soil |
|
|
| [67] |
| 4 | Total petroleum hydrocarbon-contaminated soil |
|
|
| [62] |
| 5 | Petroleum-contaminated soil |
|
|
| [77] |
| 6 | Polycyclic aromatic hydrocarbon (PAH)-contaminated soil |
|
|
| [48] |
| 7 | PAH-contaminated soil |
|
|
| [49] |
| 8 | PAH (pyrene)-contaminated soil |
|
|
| [78] |
| 9 | Pyrene-contaminated soil |
|
|
| [79] |
| 10 | Pyrene-contaminated soil |
|
|
| [80] |
| 11 | PAH-contaminated soil |
|
|
| [81] |
| 12 | Heavily PAH-contaminated soil |
|
|
| [64] |
| 13 | Soil contaminated with contaminants of emergent concern |
|
|
| [47] |
| 14 | Cycloparaffinic-contaminated soil |
|
|
| [68] |
| 15 | Chronically hydrocarbon-contaminated soil |
|
|
| [74] |
| 16 | PAH-contaminated soil |
|
|
| [72] |
| 17 | Crude oil-contaminated soil |
|
|
| [82] |
| 18 | Pentachlorophenol-contaminated soil |
|
|
| [61] |
| 19 | Pentachlorophenol-containing unsaturated soil |
|
|
| [83] |
| 20 | Phenanthrene-contaminated soil |
|
|
| [84] |
| 21 | Phenol-contaminated soil |
|
|
| [39] |
| 22 | Herbicide (oxyfluorfen)-contaminated soil |
|
|
| [85] |
| 23 | Oxyfluorfen-contaminated soil |
|
|
| [86] |
| 24 | Oxyfluorfen-contaminated soil |
|
|
| [87] |
| 25 | Oxyfluorfen-contaminated soil |
|
|
| [75] |
| 26 | Oxyfluorfen- and atrazine-contaminated soil |
|
|
| [88] |
| 27 | Organochlorine herbicide (clopyralid)-contaminated soil |
|
|
| [89] |
| 28 | Soils contaminated with herbicides (2,4 chlorsulfuron (CLSF) and dichlorophenoxyacetic acid (2,4-D)) |
|
|
| [46] |
| 29 | Antibiotic-contaminated soil |
|
|
| [69] |
| 30 | Antibiotic resistance in soil |
|
|
| [70] |
| 31 | Tetracycline-contaminated soil |
|
|
| [90] |
| 32 | Soil contaminated with herbicide |
|
|
| [91] |
| 33 | Phthalates ester-contaminated soil |
|
|
| [71] |
| 34 | Hexachlorocyclohexane-contaminated soil |
|
|
| [92] |
| 35 | Hexachlorocyclohexane-contaminated soil |
|
|
| [73] |
3.3. Applying the SEK-RPM to Remediate Soil Contaminated with Inorganic Pollutants
| No. | Inorganic Pollutant-Contaminated Soil | Applied Voltage | Reverse-Polarity Mode | Experimental Period | References |
|---|---|---|---|---|---|
| 1 | Cr-contaminated soil |
|
|
| [109] |
| 2 | Cr-contaminated aquifer |
|
|
| [110] |
| 3 | Cr-contaminated soil |
|
|
| [111] |
| 4 | Cr-contaminated soil |
|
|
| [55] |
| 5 | Cr-contaminated soil |
|
|
| [105] |
| 6 | Cr and Cd-containing abandoned industrial sites |
|
|
| [112] |
| 7 | Cd-contaminated soil |
|
|
| [104] |
| 8 | Cd-contaminated paddy soil |
|
|
| [53] |
| 9 | Cd-contaminated saline–sodic soil |
|
|
| [103] |
| 10 | Inorganic contaminants |
|
|
| [114] |
| 11 | Pb-contaminated soil |
|
|
| [107] |
| 12 | Pb-contaminated soil |
|
|
| [115] |
| 13 | Cu-contaminated mine tailings |
|
|
| [22] |
| 14 | Cu-contaminated soil |
|
|
| [106] |
| 15 | Heavy metal (Mn)-contaminated soil |
|
|
| [102] |
| 16 | Hg-contaminated clayey soil |
|
|
| [108] |
| 17 | Arsenic-contaminated soil |
|
|
| [116] |
| 18 | Heavy metal-contaminated mine tailings |
|
|
| [117] |
3.4. Simultaneous Removal of Organic and Inorganic Pollutants Using the SEKR-RPM
3.5. The SEKR-RPM Integrated with Phytoremediation
3.5.1. Removal of Inorganic Pollutants
3.5.2. Removal of Organic Pollutants
3.5.3. Simultaneous Removal of Inorganic and Organic Pollutants
3.6. The SEKR-RPM Integrated with Phytoremediation and Bioremediation Simultaneously
| No. | Pollutant-Contaminated Soil | Applied Voltage | Reverse-Polarity Mode | Experimental Period | References |
|---|---|---|---|---|---|
| 1 | Metal-contaminated soil |
|
|
| [20] |
| 2 | Heavy metal-contaminated soil |
|
|
| [121] |
| 3 | Uranium-contaminated soil |
|
|
| [119] |
| 4 | Cd-contaminated soil |
|
|
| [123] |
| 5 | Cd-contaminated soil |
|
|
| [54] |
| 6 | Pb-contaminated soil |
|
|
| [124] |
| 7 | Crude oil-contaminated soil |
|
|
| [19] |
| 8 | Petroleum-contaminated soils |
|
|
| [125] |
| 9 | Pesticide (atrazine)-contaminated soil |
|
|
| [126] |
| 10 | Atrazine-contaminated soil |
|
|
| [120] |
| 11 | PAH (phenanthrene and pyrene)-contaminated soil |
|
|
| [127] |
| 12 | Soils contaminated with inorganic (heavy metals) and organic (PAHs) pollutants |
|
|
| [51] |
3.7. The Utilization of the SEK-RPM during Soil Consolidation, Stabilization, and Sedimentation
3.8. The SEKR-RPM Integrated with Fenton Oxidation
3.9. Effect of the SEKR-RPM on Electrokinetic Operation Parameters and Soil Properties
4. Conclusions
- (a).
- Controlling the soil temperature, pH, and moisture values at desirable levels;
- (b).
- Reducing the large number of chemical additives;
- (c).
- High remediation efficiency;
- (d).
- Maintaining the indigenous fungal community’s appropriate diversity and abundance;
- (e).
- Stable and higher electric current passing, owing to avoiding a loss in soil–electrode contact;
- (f).
- Enhancing microbial growth;
- (g).
- Decreasing the electric field’s negative impact on degrading microorganisms;
- (h).
- Stimulating the contact between microorganisms, contaminants, and nutrients;
- (i).
- Enhancing the nutrient distribution and mitigating differences between remediation rates surrounding the anode and cathode;
- (j).
- Increased soil enzyme activity;
- (k).
- Smaller/optimal intervals can force the movement back and forth of the bacteria and phenol to primary positions;
- (l).
- Increasing reactive forms of heavy metals; however, decreasing residual forms;
- (m).
- Distributing ion-containing soil more uniformly;
- (n).
- Enhancing the Cr removal rate, particularly for Cr(III), which may be owing to improving the electric current and reducing the focusing phenomena;
- (o).
- Preventing Pb precipitation and the re-dissolution of precipitates;
- (p).
- Reducing polarization;
- (q).
- The RPM can completely remove the precipitated heavy metals located around the cathode;
- (r).
- Improving plant germination and growth, in addition to high biomass production and the removal of TPH;
- (s).
- The RPM can effectively participate in water evaporation flux reduction and mobilization;
- (t).
- Reducing the corrosion of electrodes and avoiding excessive desiccation;
- (u).
- Stimulating a more uniform distribution of shear strength and water content because of the enhanced pore pressure development and a more uniform effective stress elevation;
- (v).
- The occurrence of zeta potential reversal during the application of the RPM is negligible;
- (w).
- Avoiding the dryness of the adjacent anode, which ultimately causes cracks’ appearance.

- (a).
- Reducing the electroosmosis flow;
- (b).
- Relatively high energy consumption;
- (c).
- Reducing the diversity of soil microbes by prolonging the experimental period;
- (d).
- The RPM does not enhance the remediation of the contaminants of emergent concern;
- (e).
- The circulation of mixed electrolytes could control the soil pH well, rather than the application of the RPM;
- (f).
- The larger intervals may result in the accumulation of bacteria or phenol in a specific zone;
- (g).
- Cr accumulates in soil, which may be owing to the reciprocal migration of Cr;
- (h).
- in some cases, no significant increases in heavy metal uptake via plants were achieved;
- (i).
- Applying the RPM provides oxygen for a microbial community that may not be desirable for anaerobic bacteria;
- (j).
- Prolonging the RPM does not achieve the highest degradation of hexachlorobenzene.

Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.; Pu, M.; Li, H.; Lu, B.; Zhang, X.; Li, S.; Zhao, C.; Pu, W.; Liu, R.; Guo, K.; et al. Progress and prospects for remediation of soil potentially toxic elements pollution: A state-of-the-art review. Environ. Technol. Innov. 2024, 35, 103703. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Shi, C.; Tu, W.; Li, L.; Li, Q. Recent developments in modification of biochar and its application in soil pollution control and ecoregulation. Environ. Pollut. 2022, 313, 120184. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Ai, X.; Wang, B.; Bian, Y.; Wang, Y.; Hu, Z.; Zhang, Z. Single atom catalysts for organic pollutant degradation. J. Environ. Chem. Eng. 2023, 11, 110573. [Google Scholar] [CrossRef]
- Li, L.; Han, J.; Huang, X.; Qiu, S.; Liu, X.; Liu, L.; Zhao, M.; Qu, J.; Zou, J.; Zhang, J. Organic pollutants removal from aqueous solutions using metal-organic frameworks (MOFs) as adsorbents: A review. J. Environ. Chem. Eng. 2023, 11, 111217. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Q.; Yang, J.; Xing, Y.; Ling, W.; Liu, K.; Yang, Q.; Ma, H.; Pei, Z.; Wu, T.; et al. The fate of microplastic pollution in the Changjiang River estuary: A review. J. Clean. Prod. 2023, 425, 138970. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Iqbal, M.; Mehmood, K.; Li, Y.; Tang, Z.; Zhang, H. Challenges of fluoride pollution in environment: Mechanisms and pathological significance of toxicity—A review. Environ. Pollut. 2022, 304, 119241. [Google Scholar] [CrossRef] [PubMed]
- Diener, A.; Hartmann, P.; Urso, L.; Vives i Batlle, J.; Gonze, M.A.; Calmon, P.; Steiner, M. Approaches to modelling radioactive contaminations in forests—Overview and guidance. J. Environ. Radioact. 2017, 178–179, 203–211. [Google Scholar] [CrossRef]
- Li, C.Y.; He, R.; Tian, C.Y.; Song, J. Utilization of halophytes in saline agriculture and restoration of contaminated salinized soils from genes to ecosystem: Suaeda salsa as an example. Mar. Pollut. Bull. 2023, 197, 115728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Wang, X.; Jiao, W.; Zhang, M.; Ma, F. A review of functions and mechanisms of clay soil conditioners and catalysts in thermal remediation compared to emerging photo-thermal catalysis. J. Environ. Sci. 2025, 147, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, F.M.; Ganbat, N.; Altaee, A.; Samal, A.K.; Ibrar, I.; Zhou, J.L.; Sharif, A.O. Hybrid and enhanced electrokinetic system for soil remediation from heavy metals and organic matter. J. Environ. Sci. 2025, 147, 424–450. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Yao, B.; Zhou, Y.; Yang, J.; Zhi, D. Combination of electrochemical advanced oxidation and biotreatment for wastewater treatment and soil remediation. J. Environ. Sci. 2025, 150, 36–53. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Alhaj Hamoud, Y.; Sun, Q.; Sheteiwy, M.S.; AbdElgawad, H. Soil flushing coupled with aminated-nanocellulose/MOF hydrogel nanocomposites adsorbents: A novel sustainable remediation strategy for Cr(VI)-contaminated agricultural soils. Sep. Purif. Technol. 2025, 353, 128440. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, J.D.; Raimondo, E.E.; Saez, J.M.; Costa-Gutierrez, S.B.; Álvarez, A.; Benimeli, C.S.; Polti, M.A. The current approach to soil remediation: A review of physicochemical and biological technologies, and the potential of their strategic combination. J. Environ. Chem. Eng. 2022, 10, 107141. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Ismail, S.; Yossif, T.M.H.; Yassin, S.A.; Ali, M.E.A.; Habib, A.A.M.; Khalil, A.K.A.; Tag-Elden, M.A.; Emam, T.M.; Mahmoud, A.A.; et al. Comprehensive review of progress made in soil electrokinetic research during 1993–2020, part II. No.1: Materials additives for enhancing the intensification process during 2017–2020. S. Afr. J. Chem. Eng. 2023, 45, 182–200. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Ali, M.E.A.; Ismail, S.; Abd-Elmottaleb, O.; Kotp, Y.H.; Osman, M.A.; Hegab, R.H.; Habib, A.A.M.; Saudi, A.M.; Eissa, D.; et al. Comprehensive review of progress made in soil electrokinetic research during 1993–2020, Part I: Process design modifications with brief summaries of main output. S. Afr. J. Chem. Eng. 2023, 44, 156–256. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Cheng, F.; Guo, P.; Guo, S. Enhancement of electrokinetic-bioremediation by ryegrass: Sustainability of electrokinetic effect and improvement of n-hexadecane degradation. Environ. Res. 2020, 188, 109717. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tang, J.; Niu, Z.; Giesy, J.P. Interactions between electrokinetics and rhizoremediation on the remediation of crude oil-contaminated soil. Chemosphere 2019, 229, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Schlaak, M.; Siefert, E.; Lord, R.; Connolly, H. Influence of electrical fields (AC and DC) on phytoremediation of metal polluted soils with rapeseed (Brassica napus) and tobacco (Nicotiana tabacum). Chemosphere 2011, 83, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shady, A.; Yu, W. Recent advances in electrokinetic methods for soil remediation. A critical review of selected data for the period 2021–2022. Int. J. Electrochem. Sci. 2023, 18, 100234. [Google Scholar] [CrossRef]
- Rojo, A.; Hansen, H.K.; Agramonte, M. Electrokinetic remediation with high frequency sinusoidal electric fields. Sep. Purif. Technol. 2011, 79, 139–143. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Peng, C. New process for ex situ electrokinetic pollutant removal. I: Process evaluation. J. Ind. Eng. Chem. 2012, 18, 2162–2176. [Google Scholar] [CrossRef]
- Selim, E.-M.M.; Abou-Shady, A.; Ghoniem, A.E.; Abdelhamied, A.S. Enhancement of the electrokinetic removal of heavy metals, cations, anions, and other elements from soil by integrating PCPSS and a chelating agent. J. Taiwan Inst. Chem. Eng. 2022, 134, 104306. [Google Scholar] [CrossRef]
- Acar, Y.B.; Gale, R.J.; Alshawabkeh, A.N.; Marks, R.E.; Puppala, S.; Bricka, M.; Parker, R. Electrokinetic remediation: Basics and technology status. J. Hazard. Mater. 1995, 40, 117–137. [Google Scholar] [CrossRef]
- Acar, Y.B.; Alshawabkeh, A.N.; Gale, R.J. Fundamentals of extracting species from soils by electrokinetics. Waste Manag. 1993, 13, 141–151. [Google Scholar] [CrossRef]
- Acar, Y.B.; Alshawabkeh, A.N. Principles of electrokinetic remediation. Environ. Sci. Technol. 1993, 27, 2638–2647. [Google Scholar] [CrossRef]
- Probstein, R.F.; Hicks, R.E. Removal of contaminants from soils by electric fields. Science 1993, 260, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.P.; Probstein, R.F. Removal of contaminants from saturated clay by electroosmosis. Environ. Sci. Technol. 1993, 27, 283–291. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, M.; Chen, L.; Gong, Z.; Hu, J.; Ma, D. Electrokinetic remediation for the removal of heavy metals in soil: Limitations, solutions and prospection. Sci. Total Environ. 2023, 903, 165970. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, P.; Khare, A.; Patil, M.; Kumar, A.R. Roles of surfactant, oxidant and activator on enhanced electrokinetic-persulfate technique for the removal of hydrophobic organic compounds in soil: A review. J. Environ. Chem. Eng. 2023, 11, 109525. [Google Scholar] [CrossRef]
- Phulpoto, I.A.; Yu, Z.; Qazi, M.A.; Ndayisenga, F.; Yang, J. A comprehensive study on microbial-surfactants from bioproduction scale-up toward electrokinetics remediation of environmental pollutants: Challenges and perspectives. Chemosphere 2023, 311, 136979. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Wen, F.; Ren, Y.; Liu, G.; Jiang, Y.; Wang, Z.; Zhu, X. An overview of bioelectrokinetic and bioelectrochemical remediation of petroleum-contaminated soils. Environ. Sci. Ecotechnol. 2023, 16, 100278. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shady, A.; El-Araby, H. Electro-agric, a novel environmental engineering perspective to overcome the global water crisis via marginal water reuse. Nat. Hazards Res. 2021, 1, 202–226. [Google Scholar] [CrossRef]
- Keykha, H.A.; Huat, B.B.K.; Asadi, A. Electrokinetic stabilization of soft soil using carbonate-producing bacteria. Geotech. Geol. Eng. 2014, 32, 739–747. [Google Scholar] [CrossRef]
- Chung, H.I. Dewatering and decontamination of artificially contaminated sediments during electrokinetic sedimentation and remediation processes. KSCE J. Civ. Eng. 2006, 10, 181–187. [Google Scholar] [CrossRef]
- Peng, C.; Almeira, J.O.; Abou-Shady, A. Enhancement of ion migration in porous media by the use of varying electric fields. Sep. Purif. Technol. 2013, 118, 591–597. [Google Scholar] [CrossRef]
- Almeira, J.; Peng, C.; Abou-Shady, A. Enhancement of ion transport in porous media by the use of a continuously reoriented electric field. J. Zhejiang Univ. Sci. A 2012, 13, 546–558. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, H.; Zhang, X.; Fan, X.; Qian, Y. In situ bioelectrokinetic remediation of phenol-contaminated soil by use of an electrode matrix and a rotational operation mode. Chemosphere 2006, 64, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shady, A.; El-Araby, H.; El-Harairy, A.; El-Harairy, A. Utilizing the approaching/movement electrodes for optimizing the soil electrokinetic remediation: A comprehensive review. S. Afr. J. Chem. Eng. 2024, 50, 75–88. [Google Scholar] [CrossRef]
- Abou-Shady, A. A critical review of enhanced soil electrokinetics using perforated electrodes, pipes, and nozzles. Int. J. Electrochem. Sci. 2024, 19, 100406. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Eissa, D.; Abdelmottaleb, O.; Hegab, R. New approaches to remediate heavy metals containing polluted soil via improved PCPSS. J. Environ. Chem. Eng. 2018, 6, 1322–1332. [Google Scholar] [CrossRef]
- Abou-Shady, A.M.; Eissa, D.T.; Abdelmottaleb, O.M.; Hegab, R.H. Effect of electrokinetic pollutant removal on the status of some macro and micro elements in saline soil. Alex. Sci. Exch. J. 2018, 39, 65–73. [Google Scholar] [CrossRef]
- Abou-Shady, A.M.; Eissa, D.T.; Abdelmottaleb, O.M.; Hegab, R.H. Behavior of V, Cr, Li, and Ba during electrokinetic pollutant removal: Comparison of PCPSS and VA-PCPSS. Alex. Sci. Exch. J. 2018, 39, 189–196. [Google Scholar] [CrossRef]
- Abou-Shady, A. Reclaiming salt-affected soils using electro-remediation technology: PCPSS evaluation. Electrochim. Acta 2016, 190, 511–520. [Google Scholar] [CrossRef]
- Souza, F.L.; Sáez, C.; Lanza, M.R.V.; Cañizares, P.; Rodrigo, M.A. Removal of chlorsulfuron and 2,4-D from spiked soil using reversible electrokinetic adsorption barriers. Sep. Purif. Technol. 2017, 178, 147–153. [Google Scholar] [CrossRef]
- Guedes, P.; Lopes, V.; Couto, N.; Mateus, E.P.; Pereira, C.S.; Ribeiro, A.B. Electrokinetic remediation of contaminants of emergent concern in clay soil: Effect of operating parameters. Environ. Pollut. 2019, 253, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, S.; Hartog, N. Electrokinetics-enhanced biodegradation of heavy polycyclic aromatic hydrocarbons in soil around iron and steel industries. Electrochim. Acta 2012, 85, 228–234. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Li, X.; Wang, X.; Li, X.; Su, Z.; Zhang, H.; Guo, S. Effects of electrokinetic operation mode on removal of polycyclic aromatic hydrocarbons (PAHs), and the indigenous fungal community in PAH-contaminated soil. J. Environ. Sci. Health Part A 2013, 48, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q. Insights into the remediation of cadmium-pyrene co-contaminated soil by electrokinetic and the influence factors. Chemosphere 2020, 254, 126861. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Santoyo, G.; Cameselle, C.; Bustos, E. Electrokinetic—Enhanced ryegrass cultures in soils polluted with organic and inorganic compounds. Environ. Res. 2017, 158, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Almeira, O.J.; Peng, C.-S.; Abou-Shady, A. Simultaneous removal of cadmium from kaolin and catholyte during soil electrokinetic remediation. Desalination 2012, 300, 1–11. [Google Scholar] [CrossRef]
- Luan, Y.; Xu, J.; Zhou, J.; Wang, H.; Han, F.; Wang, K.; Lv, Y. Migration and removal of labile Cadmium contaminants in paddy soils by electrokinetic remediation without changing soil pH. Int. J. Environ. Res. Public Health 2022, 19, 3812. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Li, D.; Ye, X.; Xu, H.; Yao, G.; Wang, J.; Zhang, Q.; Hu, J.; Gao, N. Enhancement of Cd phytoextraction by hyperaccumulator Sedum alfredii using electrical field and organic amendments. Environ. Sci. Pollut. Res. 2017, 24, 5060–5067. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, J.; Zhu, S.; Wang, Y.; Gao, H. Exchange electrode-electrokinetic remediation of Cr-contaminated soil using solar energy. Sep. Purif. Technol. 2018, 190, 297–306. [Google Scholar] [CrossRef]
- Ma, H.; Duan, Z.; Guo, J.; Zhu, X.; Shi, X.; Zhou, W.; Jiang, M.; Xiong, J.; Li, T. Lead dissociation and redistribution properties of actual contaminated farmland soil after long-term EKAPR treatment. Environ. Geochem. Health 2022, 45, 9507–9524. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.; Hansen, H.K.; Cubillos, M. Electrokinetic remediation using pulsed sinusoidal electric field. Electrochim. Acta 2012, 86, 124–129. [Google Scholar] [CrossRef]
- Rojo, A.; Hansen, H.K.; del Campo, J. Electrodialytic remediation of copper mine tailings with sinusoidal electric field. J. Appl. Electrochem. 2010, 40, 1095–1100. [Google Scholar] [CrossRef]
- Rojo, A.; Hansen, H.K.; Monárdez, O. Electrokinetic remediation of mine tailings by applying a pulsed variable electric field. Miner. Eng. 2014, 55, 52–56. [Google Scholar] [CrossRef]
- Inman, M.E.; Jennings Taylor, E.; Myers, D.L.; Zhou, C. Electrokinetic Soil Remediation Using Novel Electrodes and Modulated Reverse Electric Fields. In Emerging Technologies in Hazardous Waste Management 8; Tedder, D.W., Pohland, F.G., Eds.; Kluwer Academic Publishers: Boca Raton, FL, USA, 2002; pp. 21–28. ISBN 978-0-306-46921-3. [Google Scholar]
- Li, Z.; Yuan, S.; Wan, J.; Long, H.; Tong, M. A combination of electrokinetics and Pd/Fe PRB for the remediation of pentachlorophenol-contaminated soil. J. Contam. Hydrol. 2011, 124, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Fan, R.; Li, T.; Hartog, N.; Li, F.; Yang, X. Synergistic effects of bioremediation and electrokinetics in the remediation of petroleum-contaminated soil. Chemosphere 2014, 109, 226–233. [Google Scholar] [CrossRef]
- Guo, S.; Li, F.; Li, P.; Wang, S.; Zhao, Q.; Li, G.; Wu, B.; Tai, P. Study on Remediation Technologies of Organic and Heavy Metal Contaminated Soils BT. In Twenty Years of Research and Development on Soil Pollution and Remediation in China; Luo, Y., Tu, C., Eds.; Springer: Singapore, 2018; pp. 703–723. ISBN 978-981-10-6029-8. [Google Scholar]
- Li, J.; Li, F.; Tong, M.; Guo, S. Treatment of PAHs contaminated soil in abandoned industrial site using combined method of improved in situ capping and electrokinetic enhanced-bioremediation. J. Hazard. Mater. 2023, 455, 131606. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, T.; Yao, K.; Kasu, C.M.; Zhao, X.; Li, Z.; Gong, J. Consolidation of soft clay by cyclic and progressive electroosmosis using electrokinetic geosynthetics. Arab. J. Geosci. 2022, 15, 1193. [Google Scholar] [CrossRef]
- Mena, E.; Villaseñor, J.; Cañizares, P.; Rodrigo, M.A. Effect of electric field on the performance of soil electro-bioremediation with a periodic polarity reversal strategy. Chemosphere 2016, 146, 300–307. [Google Scholar] [CrossRef]
- Mena, E.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Electrokinetic remediation of soil polluted with insoluble organics using biological permeable reactive barriers: Effect of periodic polarity reversal and voltage gradient. Chem. Eng. J. 2016, 299, 30–36. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, S.; Li, F.; Wu, B.; Yang, X.; Li, X. Coupling electrokinetics with microbial biodegradation enhances the removal of cycloparaffinic hydrocarbons in soils. J. Hazard. Mater. 2016, 320, 591–601. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Ma, J.; Ye, J.; Guo, P.; Li, L. Fate of antibiotic-resistant bacteria and antibiotic resistance genes in the electrokinetic treatment of antibiotic-polluted soil. Chem. Eng. J. 2018, 337, 584–594. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Liu, W.; Long, Y.; Ye, J.; Li, B.; Li, N.; Yan, M.; Zhu, C. Impact of electrokinetic remediation of heavy metal contamination on antibiotic resistance in soil. Chem. Eng. J. 2020, 400, 125866. [Google Scholar] [CrossRef]
- Yang, G.C.C.; Huang, S.-C.; Jen, Y.-S.; Tsai, P.-S. Remediation of phthalates in river sediment by integrated enhanced bioremediation and electrokinetic process. Chemosphere 2016, 150, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shao, Z.; Xu, W.; Zhao, X. Insights into electro-bioremediation of PAH-contaminated soil under polarity reversal conditions: Effect of effective current intensity and soil properties on microbial function. Chem. Eng. J. 2023, 478, 147493. [Google Scholar] [CrossRef]
- Fernández-Cascán, J.; Isidro, J.; Guadaño, J.; Sáez, C.; Rodrigo, M.A. Electrochemically-assisted remediation of silt polluted with aged HCHs. Electrochim. Acta 2023, 464, 142934. [Google Scholar] [CrossRef]
- Crognale, S.; Cocarta, D.M.; Streche, C.; D’Annibale, A. Development of laboratory-scale sequential electrokinetic and biological treatment of chronically hydrocarbon-impacted soils. New Biotechnol. 2020, 58, 38–44. [Google Scholar] [CrossRef]
- Barba, S.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Can electro-bioremediation of polluted soils perform as a self-sustainable process? J. Appl. Electrochem. 2018, 48, 579–588. [Google Scholar] [CrossRef]
- Ramírez, E.M.; Camacho, J.V.; Rodrigo, M.A.; Cañizares, P. Combination of bioremediation and electrokinetics for the in-situ treatment of diesel polluted soil: A comparison of strategies. Sci. Total Environ. 2015, 533, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, S. Effects of soil organic carbon metabolism on electro-bioremediation of petroleum-contaminated soil. J. Hazard. Mater. 2023, 459, 132180. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.; Guo, S.; Li, X.; Xu, Y.; Wang, Y.; Li, X. Effect of polarity-reversal on electrokinetic enhanced bioremediation of Pyrene contaminated soil. Electrochim. Acta 2016, 187, 567–575. [Google Scholar] [CrossRef]
- Fan, R.; Tian, H.; Wu, Q.; Yi, Y.; Yan, X.; Liu, B. Mechanism of bio-electrokinetic remediation of pyrene contaminated soil: Effects of an electric field on the degradation pathway and microbial metabolic processes. J. Hazard. Mater. 2022, 422, 126959. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Guo, S.; Li, T.; Wu, B. Coupling interactions between electrokinetics and bioremediation for pyrene removal from soil under polarity reversal conditions. CLEAN–Soil Air Water 2013, 41, 383–389. [Google Scholar] [CrossRef]
- Li, F.; Guo, S.; Hartog, N.; Yuan, Y.; Yang, X. Isolation and characterization of heavy polycyclic aromatic hydrocarbon-degrading bacteria adapted to electrokinetic conditions. Biodegradation 2016, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, S.; Wang, J.; Zhang, W.; Chen, G.; Wang, H.; Du, J.; Liu, Y.; Naidu, R. Novel Bacillus cereus strain from electrokinetically remediated saline soil towards the remediation of crude oil. Environ. Sci. Pollut. Res. 2018, 25, 26351–26360. [Google Scholar] [CrossRef] [PubMed]
- Harbottle, M.J.; Lear, G.; Sills, G.C.; Thompson, I.P. Enhanced biodegradation of pentachlorophenol in unsaturated soil using reversed field electrokinetics. J. Environ. Manag. 2009, 90, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, C.; Liu, H.; Zhang, Z.; Sun, H. A laboratory feasibility study on a new electrokinetic nutrient injection pattern and bioremediation of phenanthrene in a clayey soil. J. Hazard. Mater. 2010, 184, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Barba, S.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Effect of the polarity reversal frequency in the electrokinetic-biological remediation of oxyfluorfen polluted soil. Chemosphere 2017, 177, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Barba, S.; Villaseñor, J.; Cañizares, P.; Rodrigo, M.A. Strategies for the electrobioremediation of oxyfluorfen polluted soils. Electrochim. Acta 2019, 297, 137–144. [Google Scholar] [CrossRef]
- Barba, S.; Ocaña, H.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Electrobioremediation of oxyfluorfen-polluted soil by means of a fixed-bed permeable biological barrier. Water Air Soil Pollut. 2019, 230, 126. [Google Scholar] [CrossRef]
- Vieira dos Santos, E.; Sáez, C.; Cañizares, P.; Martínez-Huitle, C.A.; Rodrigo, M.A. Reversible electrokinetic adsorption barriers for the removal of atrazine and oxyfluorfen from spiked soils. J. Hazard. Mater. 2017, 322, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, S.; Saez, C.; Cañizares, P.; Rodrigo, M.A. Reversible electrokinetic adsorption barriers for the removal of organochlorine herbicide from spiked soils. Sci. Total Environ. 2018, 640–641, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Z.; Ma, Y.; Li, Y.; Zhu, C.; Li, H. Electrokinetic remediation of antibiotic-polluted soil with different concentrations of tetracyclines. Environ. Sci. Pollut. Res. 2019, 26, 8212–8225. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.; Báez, M.E.; Calzadilla, W.; Aranda, M.; Salazar, R. Removal of chloridazon and its metabolites from soil and soil washing water by electrochemical processes. Electrochim. Acta 2022, 425, 140682. [Google Scholar] [CrossRef]
- Miller de Melo Henrique, J.; Isidro, J.; Sáez, C.; López-Vizcaíno, R.; Yustres, A.; Navarro, V.; Dos Santos, E.V.; Rodrigo, M.A. Enhancing soil vapor extraction with EKSF for the removal of HCHs. Chemosphere 2022, 296, 134052. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, X.; Wang, H.; Qian, Y. Mobilization of phenol and dichlorophenol in unsaturated soils by non-uniform electrokinetics. Chemosphere 2005, 59, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, X.; Wang, H.; Qian, Y. The use of non-uniform electrokinetics to enhance in situ bioremediation of phenol-contaminated soil. J. Hazard. Mater. 2005, 121, 187–194. [Google Scholar] [CrossRef] [PubMed]
- López-Vizcaíno, R.; Yustres, A.; Sáez, C.; Cañizares, P.; Rodrigo, M.A.; Navarro, V. Effect of polarity reversal on the enhanced electrokinetic remediation of 2,4-D-polluted soils: A numerical study. Electrochim. Acta 2017, 258, 414–422. [Google Scholar] [CrossRef]
- Kim, S.S.; Han, S.J. Application of an enhanced electrokinetic ion injection system to bioremediation. Water Air Soil Pollut. 2003, 146, 365–377. [Google Scholar] [CrossRef]
- Kebria, D.Y.; Taghizadeh, M.; Camacho, J.V.; Latifi, N. Remediation of PCE contaminated clay soil by coupling electrokinetics with zero-valent iron permeable reactive barrier. Environ. Earth Sci. 2016, 75, 699. [Google Scholar] [CrossRef]
- Ho, S.V.; Sheridan, P.W.; Athmer, C.J.; Heitkamp, M.A.; Brackin, J.M.; Weber, D.; Brodsky, P.H. Integrated In Situ Soil Remediation Technology: The Lasagna Process. Environ. Sci. Technol. 1995, 29, 2528–2534. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.V.; Athmer, C.; Sheridan, P.W.; Hughes, B.M.; Orth, R.; McKenzie, D.; Brodsky, P.H.; Shapiro, A.; Thornton, R.; Salvo, J.; et al. The Lasagna Technology for In Situ Soil Remediation. 1. Small Field Test. Environ. Sci. Technol. 1999, 33, 1086–1091. [Google Scholar] [CrossRef]
- Ho, S.V.; Athmer, C.; Sheridan, P.W.; Hughes, B.M.; Orth, R.; McKenzie, D.; Brodsky, P.H.; Shapiro, A.M.; Sivavec, T.M.; Salvo, J.; et al. The Lasagna Technology for In Situ Soil Remediation. 2. Large Field Test. Environ. Sci. Technol. 1999, 33, 1092–1099. [Google Scholar] [CrossRef]
- Vidal, J.; Báez, M.E. Behavior of chlorpyrifos and 3, 5, 6-trichloro-2-pyridinol (TCP) in a sodium-dodecyl sulphate-electrokinetic soil washing system. Electrochim. Acta 2023, 445, 141936. [Google Scholar] [CrossRef]
- Pazos, M.; Sanromán, M.A.; Cameselle, C. Improvement in electrokinetic remediation of heavy metal spiked kaolin with the polarity exchange technique. Chemosphere 2006, 62, 817–822. [Google Scholar] [CrossRef]
- Lukman, S.; Mu’azu, N.D.; Essa, M.H.; Usman, A. Optimal removal of cadmium from heavily contaminated saline–sodic soil using integrated electrokinetic-adsorption technique. Arab. J. Sci. Eng. 2015, 40, 1289–1297. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, D.; Wang, Q.; Wang, Y.; Sun, X. Electrokinetic remediation of Cd-contaminated soil using low voltage gradients coupled with array adsorption zone and polarity exchange. Process Saf. Environ. Prot. 2022, 157, 81–91. [Google Scholar] [CrossRef]
- Liu, X.; Zhuang, Y. Removal of Chromium from Contaminated Soil by Electrokinetic Remediation Combined with Adsorption by Anion Exchange Resin and Polarity Reversal. Int. J. Geosynth. Ground Eng. 2024, 10, 2. [Google Scholar] [CrossRef]
- Han, J.-G.; Hong, K.-K.; Kim, Y.-W.; Lee, J.-Y. Enhanced electrokinetic (E/K) remediation on copper contaminated soil by CFW (carbonized foods waste). J. Hazard. Mater. 2010, 177, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; van Doren, J.; Fang, Z.; Li, W. Improvement in electrokinetic remediation of Pb-contaminated soil near lead acid battery factory. Trans. Nonferrous Met. Soc. China 2015, 25, 3088–3095. [Google Scholar] [CrossRef]
- Essa, M.H.; Mu’azu, N.D.; Lukman, S.; Bukhari, A. Application of box-behnken design to hybrid electrokinetic-adsorption removal of mercury from contaminated saline-sodic clay soil. Soil Sediment Contam. Int. J. 2015, 24, 30–48. [Google Scholar] [CrossRef]
- Andrade, D.C.; Đolić, M.B.; Martínez-Huitle, C.A.; dos Santos, E.V.; Silva, T.F.C.V.; Vilar, V.J.P. Coupling electrokinetic with a cork-based permeable reactive barrier to prevent groundwater pollution: A case study on hexavalent chromium-contaminated soil. Electrochim. Acta 2022, 429, 140936. [Google Scholar] [CrossRef]
- Xu, H.; Bai, J.; Yang, X.; Zhang, C.; Yao, M.; Zhao, Y. Lab scale-study on the efficiency and distribution of energy consumption in chromium contaminated aquifer electrokinetic remediation. Environ. Technol. Innov. 2022, 25, 102194. [Google Scholar] [CrossRef]
- Sun, R.; Gong, W.; Chen, Y.; Hong, J.; Wang, Y. Influence of polarity exchange frequency on electrokinetic remediation of Cr-contaminated soil using DC and solar energy. Process Saf. Environ. Prot. 2021, 153, 117–129. [Google Scholar] [CrossRef]
- Lu, P.; Feng, Q.; Meng, Q.; Yuan, T. Electrokinetic remediation of chromium- and cadmium-contaminated soil from abandoned industrial site. Sep. Purif. Technol. 2012, 98, 216–220. [Google Scholar] [CrossRef]
- Zhao, M.; Song, C.; Zhang, F.; Jia, X.; Ma, D. New-style electrokinetic-adsorption remediation of cadmium-contaminated soil using double-group electrodes coupled with chitosan-activated carbon composite membranes. Sci. Total Environ. 2023, 904, 166919. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Guo, X.; Fu, R. Inhibition characteristics of the electrokinetic removal of inorganic contaminants from soil due to evolution of the acidic and alkaline fronts. Process Saf. Environ. Prot. 2021, 155, 343–354. [Google Scholar] [CrossRef]
- Han, S.J.; Kim, S.S. Application of enhanced electrokinetic extraction for lead spiked kaolin. KSCE J. Civ. Eng. 2003, 7, 499–506. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, C.; Zhang, K.; Luo, H.; Luo, J.; Liu, F. Effect of ascorbic acid and combination with fulvic acid on the electrokinetic remediation of paddy soil contaminated by arsenic-containing acid mine drainage. Appl. Geochem. 2023, 152, 105632. [Google Scholar] [CrossRef]
- Acosta Hernández, I.; Muñoz Morales, M.; Fernández Morales, F.J.; Rodríguez Romero, L.; Villaseñor Camacho, J. Removal of heavy metals from mine tailings by in-situ bioleaching coupled to electrokinetics. Environ. Res. 2023, 238, 117183. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.W.; Wang, F.Y.; Huang, Z.H.; Wang, H. Simultaneous removal of 2,4-dichlorophenol and Cd from soils by electrokinetic remediation combined with activated bamboo charcoal. J. Hazard. Mater. 2010, 176, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, L.; Zhang, Q.; Wu, L.; Zhang, J.; Larson, S.L.; Ballard, J.H.; Ma, Y.; Su, Y.; Han, F.X. Coupling Electrokinetics and Phytoremediation to Remove Uranium from Contaminated Soil: A Laboratory Pilot-Scale Study. ACS Earth Space Chem. 2021, 5, 3448–3457. [Google Scholar] [CrossRef]
- Sánchez, V.; López-Bellido, F.J.; Cañizares, P.; Villaseñor, J.; Rodríguez, L. Scaling up the electrokinetic-assisted phytoremediation of atrazine-polluted soils using reversal of electrode polarity: A mesocosm study. J. Environ. Manag. 2020, 255, 109806. [Google Scholar] [CrossRef]
- Medina-Díaz, H.L.; López-Bellido, F.J.; Alonso-Azcárate, J.; Fernández-Morales, F.J.; Rodríguez, L. Comprehensive study of electrokinetic-assisted phytoextraction of metals from mine tailings by applying direct and alternate current. Electrochim. Acta 2023, 445, 142051. [Google Scholar] [CrossRef]
- Mohrazi, A.; Ghasemi-Fasaei, R.; Mojiri, A.; Shirazi, S.S. Investigating an electro-bio-chemical phytoremediation of multi-metal polluted soil by maize and sunflower using RSM-based optimization methodology. Environ. Exp. Bot. 2023, 211, 105352. [Google Scholar] [CrossRef]
- Xu, L.; Dai, H.; Skuza, L.; Wei, S. The effects of different electric fields and electrodes on Solanum nigrum L. Cd hyperaccumulation in soil. Chemosphere 2020, 246, 125666. [Google Scholar] [CrossRef] [PubMed]
- Mulati, H.; Mamat, A.; Ailijiang, N.; Jiang, L.; Li, N.; Hu, Y.; Su, Y. Electrokinetic-assisted phytoremediation of pb-contaminated soil: Influences of periodic polarity reversal direct current field. Sustainability 2023, 15, 8439. [Google Scholar] [CrossRef]
- Rocha, I.M.V.; Silva, K.N.O.; Silva, D.R.; Martínez-Huitle, C.A.; Santos, E. V Coupling electrokinetic remediation with phytoremediation for depolluting soil with petroleum and the use of electrochemical technologies for treating the effluent generated. Sep. Purif. Technol. 2019, 208, 194–200. [Google Scholar] [CrossRef]
- Sánchez, V.; Francisco; López-Bellido, J.; Rodrigo, M.A.; Rodríguez, L. Enhancing the removal of atrazine from soils by electrokinetic-assisted phytoremediation using ryegrass (Lolium perenne L.). Chemosphere 2019, 232, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, Y.; Li, K.; Yuan, X.; Liu, H.; Li, M.; Xu, T.; Zhang, Z.; Johnson, D.M.; Xi, Y. Enhancement of electrokinetic-phytoremediation by Ophiopogon japonicus: Stimulation of electrokinetic on root system and improvement of polycyclic aromatic hydrocarbon degradation. Environ. Sci. Pollut. Res. 2023, 30, 97591–97600. [Google Scholar] [CrossRef] [PubMed]
- Lohner, S.T.; Becker, D.; Mangold, K.-M.; Tiehm, A. Sequential Reductive and Oxidative Biodegradation of Chloroethenes Stimulated in a Coupled Bioelectro-Process. Environ. Sci. Technol. 2011, 45, 6491–6497. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.N.O.; Paiva, S.S.M.; Souza, F.L.; Silva, D.R.; Martínez-Huitle, C.A.; Santos, E. V Applicability of electrochemical technologies for removing and monitoring Pb2+ from soil and water. J. Electroanal. Chem. 2018, 816, 171–178. [Google Scholar] [CrossRef]
- Feijoo, J.; Ottosen, L.M.; Nóvoa, X.R.; Rivas, T.; de Rosario, I. An improved electrokinetic method to consolidate porous materials. Mater. Struct. 2017, 50, 186. [Google Scholar] [CrossRef]
- Bergado, D.T.; Balasubramaniam, A.S.; Patawaran, M.A.B.; Kwunpreuk, W. Electro-osmotic consolidation of soft Bangkok clay with prefabricated vertical drains. Proc. Inst. Civ. Eng. Improv. 2000, 4, 153–163. [Google Scholar] [CrossRef]
- Mwandira, W.; Mavroulidou, M.; Satheesh, A.; Gunn, M.J.; Gray, C.; Purchase, D.; Garelick, J. An electrokinetic-biocementation study for clay stabilisation using carbonic anhydrase-producing bacteria. Environ. Sci. Pollut. Res. 2023, 30, 104916–104931. [Google Scholar] [CrossRef]
- Malekzadeh, M.; Lovisa, J.; Sivakugan, N. An Overview of Electrokinetic Consolidation of Soils. Geotech. Geol. Eng. 2016, 34, 759–776. [Google Scholar] [CrossRef]
- Zhuang, Y. Large scale soft ground consolidation using electrokinetic geosynthetics. Geotext. Geomembranes 2021, 49, 757–770. [Google Scholar] [CrossRef]
- Safdar, M.U.; Mavroulidou, M.; Gunn, M.J.; Purchase, D.; Payne, I.; Garelick, J. Electrokinetic biocementation of an organic soil. Sustain. Chem. Pharm. 2021, 21, 100405. [Google Scholar] [CrossRef]
- Oonnittan, A.; Isosaari, P.; Sillanpää, M. Effect of polarity reversal on hexachlorobenzene removal during electrokinetic Fenton process. J. Environ. Eng. 2013, 139, 1228–1232. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.H. Switching effects of electrode polarity and introduction direction of reagents in electrokinetic-Fenton process with anionic surfactant for remediating iron-rich soil contaminated with phenanthrene. Electrochim. Acta 2011, 56, 8094–8100. [Google Scholar] [CrossRef]
- Yang, G.C.C.; Huang, S.-C.; Wang, C.-L.; Jen, Y.-S. Degradation of phthalate esters and acetaminophen in river sediments using the electrokinetic process integrated with a novel Fenton-like process catalyzed by nanoscale schwertmannite. Chemosphere 2016, 159, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Mu’azu, N.D.; Essa, M.H.; Lukman, S. Response surface modeling of rate of replenishing processing fluids during hybrid electrokinetics-adsorption treatment of saline-sodic soil. Arab. J. Sci. Eng. 2017, 42, 1117–1127. [Google Scholar] [CrossRef]
- Kaniraj, S.R.; Yee, J.H.S. Electro-Osmotic Consolidation Experiments on an Organic Soil. Geotech. Geol. Eng. 2011, 29, 505–518. [Google Scholar] [CrossRef]
- Ho, S.V.; Athmer, C.J.; Sheridan, P.W.; Shapiro, A.P. Scale-up aspects of the LasagnaTM process for in situ soil decontamination. J. Hazard. Mater. 1997, 55, 39–60. [Google Scholar] [CrossRef]
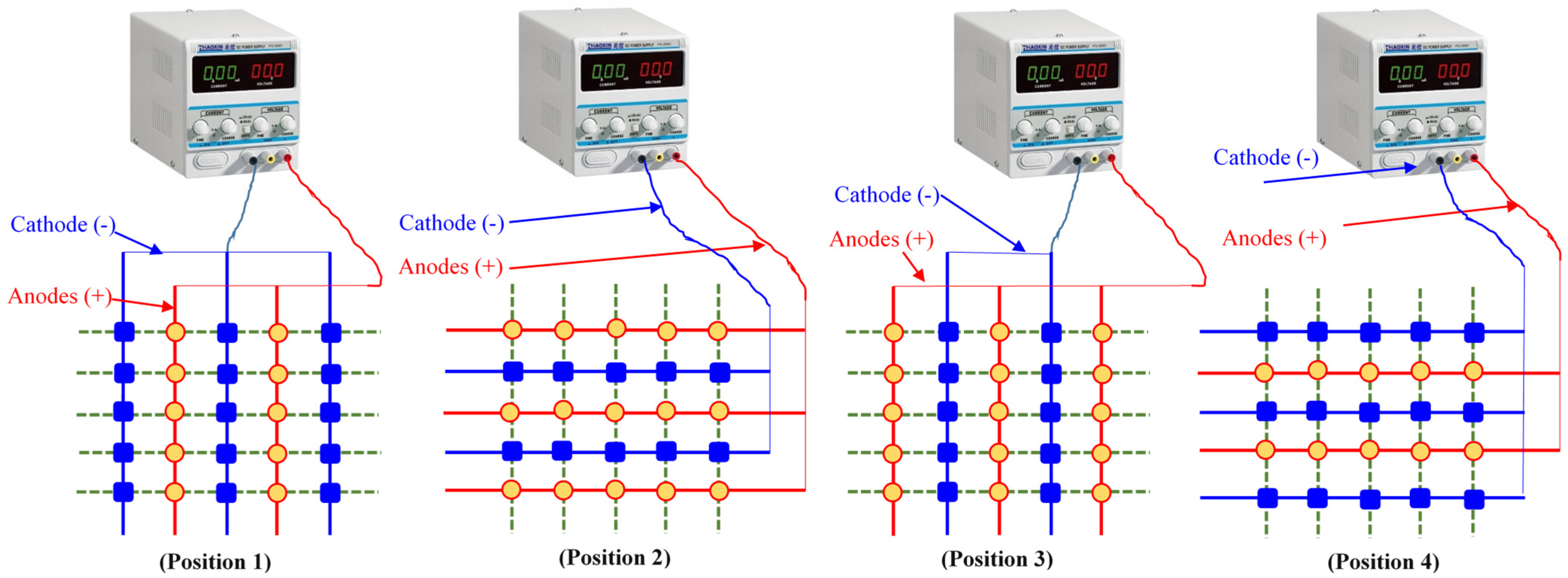
 ), and un-electrified electrodes (○) are shown.
), and un-electrified electrodes (○) are shown.
 ), and un-electrified electrodes (○) are shown.
), and un-electrified electrodes (○) are shown.
| No. | Soils Contaminated with Inorganic and Organic Pollutants | Applied Voltage | Reverse-Polarity Mode | Experimental Period | References |
|---|---|---|---|---|---|
| 1 | 2,4-dichlorophenol- and Cd-contaminated soils |
|
|
| [118] |
| 2 | Cadmium–pyrene-contaminated soils |
|
|
| [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou-Shady, A.; El-Araby, H. Reverse Polarity-Based Soil Electrokinetic Remediation: A Comprehensive Review of the Published Data during the Past 31 Years (1993–2023). ChemEngineering 2024, 8, 82. https://doi.org/10.3390/chemengineering8040082
Abou-Shady A, El-Araby H. Reverse Polarity-Based Soil Electrokinetic Remediation: A Comprehensive Review of the Published Data during the Past 31 Years (1993–2023). ChemEngineering. 2024; 8(4):82. https://doi.org/10.3390/chemengineering8040082
Chicago/Turabian StyleAbou-Shady, Ahmed, and Heba El-Araby. 2024. "Reverse Polarity-Based Soil Electrokinetic Remediation: A Comprehensive Review of the Published Data during the Past 31 Years (1993–2023)" ChemEngineering 8, no. 4: 82. https://doi.org/10.3390/chemengineering8040082
APA StyleAbou-Shady, A., & El-Araby, H. (2024). Reverse Polarity-Based Soil Electrokinetic Remediation: A Comprehensive Review of the Published Data during the Past 31 Years (1993–2023). ChemEngineering, 8(4), 82. https://doi.org/10.3390/chemengineering8040082








