Microplastics in Sludges and Soils: A Comprehensive Review on Distribution, Characteristics, and Effects
Abstract
:1. Introduction
2. Microplastics in Wastewater
| Location | WW Type | MPs in Influent (Particles/L) | MPs in Effluent (Particles/L) | MP Size (µm) | MP Polymer Type | MPs in Sludge (Particle/kg) | SS Treatment Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| China | UW | 8.72 | 6908 | 500–5000 | PE, PET, PP, PAN | N/A | Dewatering | [33] |
| China | UW & IW | 6.55 | 0. 59 | 43–5000 | PE, PP, PS, PP-PE | 7.0 | N/A | [30] |
| China | UW | 12.03 | 0. 59 | 0–5000 | PA, PE, PES, PET, PP, PI, PVC, PAC, PU, others | N/A | N/A | [31] |
| China | UW | 1.75 | 2190.4 | N/A | PE, PET, PP, PAN | N/A | Dewatering | [30] |
| China | 1.2 | 893 | 500–5000 | PA, PE, PET, PVAC, PP, PS, PES, EP, EPM, CN, Acrylic | 1.0 | N/A | [45] | |
| China | MW | 44.07 | 1.97 | 0–5000 | PET, PE, PP, PA | N/A | Dewatering | [47] |
| China | UW | 16 | 8.7 | 500–3500 | PA, PET, PE, PP, PS, PC, PU, PVC | 2.9 | Dewatering | [46] |
| China | UW & IW | 18–890, 8–23 | 6–26, 6–12 | 50–400 | PE, PET, PA, PP, PS, PVC | N/A | N/A | [42] |
| China | N/A | N/A | N/A | 100–5000 | PE, PB, PP | 16.5–38.5 | Anaerobic digestion | [48] |
| Denmark | N/A | N/A | N/A | 10–25, >300 | PA, PP, PE, PU, PS, PES, Acrylic | 13 | N/A | [49] |
| Finland | UMW | 61 | 27 | 20–300 | PE, PET, PP, PMMA, PS, POM | 0.8 | Anaerobic digestion | [34] |
| France | UMW | 244 | 16130 | 20–500 | PS, PE, PP, PET, PA | 2.84 | N/A | [50] |
| Italy | UMW | 2.5 | 113 | 10–5000 | PA, PE, PTU, PP, PS, PU, others | 0.4 | N/A | [35] |
| Iran | MW | 206 | 94 | 1–5000 | N/A | 183 | N/A | [41] |
| Korea | UMW | 10.16–23.75 | 13,200 | 106–300 | N/A | 7.34–13.27 | Thickening, Anaerobic digestion, Dewatering | [51] |
| Spain | UW | 11.1 | 112,000 | 150–5000 | PE, PP | 2.8 | N/A | [38] |
| Spain | UM & IW | 264, 1567 | 39, 131 | 100–5000 | PP, PS, PE, PVC, HDPE, PEMMA | N/A | N/A | [36] |
| Spain | UMW | 236 | 165,000 | 25–5000 | PE, PP, PU, PCL, PET, PS, PMMA | 26 | Dewatering | [37] |
| Thailand | UW | 77 | 10.6 | N/A | PE, PET | N/A | N/A | [40] |
| Thailand | UM | 4–50 | 2–30 | 50–5000 | PA, PE, PET, PVAC, PP, PS, PES, EP, Acrylic, others | 42–214 | N/A | [52] |
| Turkey | 72.6 | 2934 | 250–2000 | N/A | 8.2 | N/A | [53] | |
| UK | UW | 2102.1 | 129.1 | 2–1000 | PA, PE, PET, PS, PVC, PP | 1974 | Anaerobic digestion | [39] |
3. Microplastics in Sludge
3.1. Abundance of Microplastics in Sludge
3.2. Size and Shape Distribution of Microplastics in Sludge
3.3. Composition Distribution of Microplastics in Sludge
3.4. Surface Morphology of Microplastics in Sludge
4. Microplastics in Soil
4.1. Abundance of Microplastics in Soils
4.2. Size and Shape Distribution of Microplastics in Soils
4.3. Composition Distribution of Microplastics in Soils
4.4. Surface Morphology of Microplastics in Soils
5. Statistical Analysis of Microplastic Distribution
6. Effects of Microplastics on Media’s Characteristics
6.1. Effect of Microplastics on Sludge Properties
- Disrupting Floc Formation: MPs might hinder the development of flocs by decreasing the efficacy of coagulants and flocculants used in wastewater treatment [174]. In numerous ways, this can cause the sludge’s settling and dewatering properties to be weak [175]; (a) surface charge: it is one factor that may prevent MPs from forming flocs since it differs from the surface charge of natural sludge particles. (b) Size: Because MPs are much smaller than natural sludge particles, they settle more slowly and are more likely to stay suspended in the liquid phase, where they can help create flocs. (c) Hydrophobicity: MPs tend to cluster together rather than adhere to other sludge particles because they are hydrophobic, which means they repel water. (d) Adsorption: Organic substances and other pollutants in sludge can be absorbed by MPs, which can obstruct the chemical reactions resulting in floc formation. They can bind to the surface of chemicals that help produce flocs, like calcium hydroxide and aluminum sulfate, which lessens the effectiveness of those substances. Due to the decreased ability of these compounds to form strong connections between particles, weaker flocs that are more challenging to remove from the water are produced. MPs can also prevent floc formation as a physical barrier between particles [174,176]. To illustrate, MPs may become entangled in flocs and be unable to settle out of the water adequately. This process causes treated wastewater to be more turbid and decreases the effectiveness of removing suspended solids. According to studies, including MPs in sludge can interfere with floc formation, which can greatly impact how the sludge settles, is dewatered, and is treated [177]. The researchers discovered that the inclusion of MPs decreased the sludge’s settling velocity, indicating that the flocs were less compact and less able to settle. It can be because the MPs interfered with floc formation by giving bacteria a surface to adhere to, preventing the production of larger, more stable flocs. The researchers found that the capillary suction time decreased by approximately 17% for smaller MPs, indicating that a low concentration of MPs can improve sludge dewatering to a degree [84]. Similarly, Qian et al. investigated how MPs affected the dewatering capabilities of employed activated sludge. As the MPs interfered with floc formation, the researchers observed that the presence of MPs hindered the sludge’s ability to be dewatered [178]. Researchers discovered that the MPs prevented extracellular polymeric substances (EPS) from adhering to the sludge flocs, reducing the flocs’ strength and stability and making dewatering more complicated.
- Altering Floc Size and Structure: MPs can change the shape and size of flocs, which affects how dense, porous, and compressible they are, which may impact the effectiveness of sludge dewatering and disposal. Through various methods, MPs can change the size and density of flocs in sludge [179]. (a) Adsorption: MPs can adhere to the surface of flocs, changing their surface characteristics and leading particles to aggregate or disperse, resulting in changes in the floc size and density. (b) Interference with flocculation agents: by striving for binding sites on the floc surface, MPs can inhibit the activity of flocculation agents such as polymers or coagulants. This may result in smaller or less thick flocs and lessen the efficacy of these agents. (c) Physical obstruction: MPs can physically prevent flocs from arising by occupying the space in the sludge matrix. As a result, smaller, less dense flocs may result from the prevention of particles from aggregating to create bigger aggregates [180]. In one such study, Zhang et al. discovered that adding MPs to sludge caused the floc size to expand significantly. The study employed polystyrene MPs and found that they had up to 25% larger average floc sizes. The researchers credited the ability of the MPs to build a scaffold-like structure within the sludge, which improved the size and stability of the flocs, as the cause of this increase [181]. Xu et al.‘s investigation evaluated the MPs’ impact on sludge floc structure. According to the study, the structure of the flocs significantly changed when MPs were added to sludge. The scientists noticed that the MPs gathered within the flocs, forming a more compact and regular structure. The sludge’s settling and dewatering characteristics may vary due to this structural change. It is revealed that the size of sludge flocs decreased by 9.8% and 30.8% when the concentration of MPs was 100 and 300 mg/L, respectively. This suggests that MPs may interfere with forming larger particles in the sludge flocs [84]. Additionally, Zhang et al. concluded that adding MPs to sludge greatly decreased the flocs’ settling velocity. The ability of the MPs to expand and stabilize the flocs, making them more difficult to settle, was identified by the researchers as the cause of this decline [182].
- Reducing Biodegradability: MPs cannot degrade and may end up in sludge over time. As a result, sludge’s biodegradability is lowered, making treatment and disposal more challenging [183]. To begin with, by interfering with the formation and stability of flocs, MPs can decrease the dewaterability of flocs in sludge [84]. Therefore, smaller and weaker flocs that are more challenging to dewater can be the outcome [184]. MPs may potentially have a detrimental effect on the cellular mechanisms involved in floc production [184]. Microorganisms produce EPS, which aids in binding sludge particles together, and these substances are critical for the creation and stability of flocs [185]. However, the adsorption of EPS and other organic molecules by MPs, which decreases their availability for floc formation, can interfere with these activities [185]. The surface area of the sludge particles can also be increased, which can result in increased water absorption and decreased dewaterability [84]. Smaller particles are inclined to absorb water and swelling because they have a higher surface area to volume ratio [186]. Overall, by interfering with both physical and biological processes involved in floc formation and stability, the presence of MPs in sludge can have considerable detrimental effects on its dewaterability.
- Increasing chemical demand: MPs have the potential to raise the chemical demand essential to efficient treatment. To illustrate, by adsorbing organic and inorganic contaminants, MPs can increase the chemical demand in sludge, producing more complex and stable particles. Because of the increased chemical demand, it may be more challenging to treat the sludge using traditional methods because more chemicals must be added to the treatment process. Furthermore, MPs could operate as a source of nutrients for the microorganisms in the sludge, increasing their activity and demand for chemicals [188]. Overall, the presence of MPs in sludge can make treatment more difficult and raise the price of chemical application. The impact of MPs on sludge’s chemical oxygen demand (COD) was studied by Wei et al. According to this study, the COD of sludge increased by 12% when MPs were added. The ability of the MPs to adsorb organic contaminants, which might raise the COD of the sludge, was acknowledged by the researchers as the cause of this increase [189]. Another study by Shi et al. investigated the impact of MPs on the heavy metal content of sludge. According to the research, adding MPs to sludge caused a noticeable rise in the leaching level of heavy metals. The ability of the MPs to adsorb heavy metals, which might add to the overall chemical demand of the sludge, was identified by the researchers as the reason for this rise [190].
- Physical interference: The development and function of microorganisms in sludge can be physically hampered by MPs [186]. They have the potential to block pores and decrease the supply of nutrients, oxygen, and other vital elements needed by microbes [192]. Multiple mechanisms exist for MPs to physically affect the sludge’s microbiological community: MPs can absorb organic and inorganic contaminants, which can change the chemical structure of the sludge and impact the microbial population [192]. They can also combine with other particles in the sludge to produce larger particles, which can settle out of suspension. As a result, microbes may have less access to nutrients and oxygen, which may inhibit their growth and alter their rate of metabolism [193]. In one such investigation, Wei et al., (2020) discovered that adding MPs to sludge significantly increased the viscosity and yield stress of the sludge. Their examination of the pore structure indicated that the presence of MPs resulted in a 12% reduction in porosity. This suggests that the aggregation of MPs may block the cavities in sludge, hindering the formation of granular sludge and potentially impeding the accumulation and movement of MPs into deeper sludge layers. The ability of the MPs to create a network-like structure inside the sludge enhanced its overall viscosity and yield stress, which the researchers attributed to this rise [194].
- Toxicity: MPs have the potential to leak hazardous substances such as plasticizers, flame retardants, antioxidants, light stabilizers, and other compounds utilized in their production [195]. In addition to altering the growth and metabolism of the microbial population in sludge, these chemicals can also cause soil pollution as a result of the sludge amendment [196,197]. It is found that additives generated by MPs can directly damage microbial cells, which can impact microbial activities [198]. According to Wei et al., the main inhibitory mechanism that promotes the breakdown of anaerobic digesting bacteria cell walls is bisphenol A produced by PVC [189]. PVC plastic products used in the medical industry have antibacterial qualities because they contain plasticizers that are selective to types of microbes like Gram-negative bacteria and sulfate-reducing bacteria and are resistant to nitrifying bacteria.
- Biofilm formation: MPs may also promote the development of biofilm, a protective bacterial layer that develops on surfaces. As a result, dangerous bacteria may build up in the sludge, further upsetting the microbial population [199]. To be precise, a procedure known as microbial adhesion allows MPs to create biofilms in the sludge’s microbial community. Bacteria and fungi in the sludge can adhere to the MPs’ surface and create a slimy covering known as a biofilm [200]. The microorganisms can live and develop on the surface of the MP owing to this biofilm’s protective habitat. The development of biofilms on MPs may also be aided by the sludge’s organic matter content. Organic substances give the microorganisms access to nutrition, which can promote their development and adherence to the MP’s surface. A biofilm that has developed on an MP can entice additional bacteria to join the community. As a result, diverse microbial communities may develop on the surface of the MPs, which may impact the MPs’ transport and fate in the environment [201].
- Antibiotic resistance: Antibiotic-resistant bacteria can accumulate in MPs and pass their resistance genes to other microbes in sludge [203]. MPs in the sludge can offer bacteria a surface to adhere to and build biofilms. Bacteria can exchange genetic material within these biofilms, including genes that confer antibiotic resistance. As a result, MPs in sludge may help the microbial community develop increased antibiotic resistance [204]. According to one study by Wang et al., adding MPs to sludge led to a noticeable effect of antibiotic resistance genes (ARGs) in the sludge. The researchers hypothesized that the MPs might have served as a means for the distribution of ARGs, which could have significant consequences on the emergence of antibiotic resistance in the environment [205]. According to a different study by Wang et al., the presence of MPs in sludge significantly increased the amount of mobile genetic elements (MGEs) in the sludge. MGEs, which include ARGs, are genetic elements capable of transferring genetic material between bacteria. According to the researchers, the MPs may have served as a surface for the attachment and transfer of MGEs. This possibility has substantial consequences for the spread of antibiotic resistance in the environment [206].
6.2. Effect of Microplastics on Soil Ecosystems
- Aggregation: By decreasing soil stability and increasing soil erosion, MPs can have an adverse impact on the soil’s ability to aggregate. Aggregates in the soil are crucial for preserving soil stability and structure but can be disrupted by MPs [210]. A rise in soil erosion and topsoil loss could result from this, which would be detrimental to the health and productivity of plants. Additionally, they may have a negative impact on soil aggregation by reducing the activity of soil microbes that are crucial for preserving soil stability and structure [211]. It is worth noting that the presence of MPs in soils may decline soil aggregation by about 15–30% [212]. Chen et al.‘s research revealed that soil aggregation significantly decreased when MPs were added to the soil. The researchers credited this to MPs’ potential to obstruct the natural mechanisms that cause soil aggregation. Particularly, the MPs can physically displace soil aggregates, which lowers their stability and leads to their disintegration. According to the study, the MPs also changed the microbial community in the soil, which impacted soil aggregation [213]. A study by Liu et al. showed that the presence of MPs in soil decreased soil organic carbon and increased bulk density, both of which are signs of soil degradation. Researchers hypothesized that the MPs may have impeded the natural processes that cause soil aggregation, which in turn decreased the stability of soil aggregates and affected soil structure and water retention [214].
- Porosity: The porosity characteristics of soils may be negatively impacted by MPs [210]. They may remain in soil pores, decreasing the soil’s capacity to retain air and water. Reduced soil fertility and plant growth may result from this. MPs may also change the microbial communities in the soil, which may have an additional effect on the soil’s porosity and capacity for nutrient cycling [215]. According to one study by Yu et al., adding MPs to soil caused a noticeable reduction in soil porosity. The scientists explained this by stating that they can block soil pores, limiting the quantity of air and water the soil can hold. The investigation also discovered that MPs changed the soil’s microbial population, which affected soil porosity [216]. The addition of low-density polyethylene (LDPE) MPs to soil led to a reduction in soil porosity by around 3–5% and an increase in soil compaction, according to Qi et al.‘s findings in a related investigation. The researchers hypothesized that MPs may have impeded the natural processes that result in soil aggregation, reducing the stability of soil aggregates and affecting soil structure and water retention [217].
- Bulk Density: By creating more soil compaction, MPs can change the bulk density characteristics of soils [218]. This is because MPs are denser than the majority of naturally occurring soil particles and can fill up the soil’s pore spaces, decreasing the soil’s capacity to store water and air. As a result, the soil has a larger bulk density and is denser. Porosity loss can have a negative effect on plant development and microbial activity by reducing air and water infiltration into the soil [219,220]. According to a study by Qi et al., soils with LDPE-MP concentrations showed a loss of 14% in bulk density [217]. The researchers hypothesized that MPs may have impeded the natural processes that result in soil aggregation, reducing the stability of soil aggregates and affecting soil structure and water retention. Similar findings were obtained by Chai et al. in another investigation. According to the study, soils with MP concentrations had noticeably affected bulk densities [221]. The reason for this, according to the researchers, is that by reducing soil pore space, they may also decrease the amount of air and water that the soil can hold. According to de Souza Machado et al., MPs have a stronger influence on soil parameters than MPs that are only slightly different from the shape of the soil particles. MPs will decrease the bulk density by 2–6% and enhance evapotranspiration of the soil. Plant development and agricultural production may be negatively impacted by the increase in bulk density brought on by the presence of MPs. Compacted soils are less able to store water and have poor aeration, which can cause waterlogging and lessened plant nutrient availability [211].
- Water availability: MPs can also alter the water availability characteristics of soils in other ways. (a) Reduced water holding capacity: MPs can lower the soil’s ability to hold water by clogging pore pores that would otherwise be filled with air or water. Due to this, plant growth may be hindered, and runoff may be exacerbated [218]. (b) Enhanced soil erosion: MPs can exacerbate soil erosion by lowering the stability of soil aggregates. This may result in more sediment being deposited in adjacent water bodies, which may have an even greater effect on water availability. (c) Greater runoff: MPs can potentially produce greater runoff by lowering infiltration rates. As a result, more water evaporates off the soil’s top rather than seeping in. (d) Changed soil structure: MPs can change soil structure by aggregating with other particles. This may impact how water moves through the soil and make it more challenging for plant roots to get to water. Some research revealed that polyester, polyacrylic, and polyethylene have decreased soil water retention capacity (~5%) and decreased bulk density [211,219]. A change in the physical characteristics of the soil may accelerate root growth and exudation since MPs are becoming more widely recognized as a significant emerging contaminant. Accordingly, MP’s presence in soils can reduce the soil’s ability to retain water, and it can be concluded that this might be brought on by modifications in soil structure brought on by the presence of MPs.
- Soil pH: The kind, size, and quantity of MPs in the soil, as well as the type of soil, are all variables that affect how MPs affect soil pH. Studies have revealed that MPs can modify the chemical structure of the soil, changing the pH. They have numerous potential effects on the pH of the soils. Firstly, they can discharge poisons and compounds that change the chemical composition of soil. Many types of plastic contain substances like phthalates and bisphenol A (BPA) that can leak out of the material and enter the soil. By making the soil more or less acidic, these compounds can change the pH of the soil. Second, MPs can absorb and hold water, which may result in changes in moisture levels and ultimately impact pH. They may also lessen the oxygen accessible to soil bacteria, which may affect their capacity to control the pH of the soil [219]. Gharahi et al. investigated the impact of polyethylene on soil pH. The research revealed that polyethylene affects the pH of the soil, which varied between 7.03–7.53 compared to the pH = 7.87 of the control sample, leading to a rise in adsorption of heavy metal ions and this can be attributed to the higher negative charge of the MPs surface in higher pH values, which generates an electrostatic attraction to the metal cation [223]. The effects of different MPs on soil pH are studied by Liu et al. and represent a 2% loss in soil pH when adding MPs [224]. Additionally, Yang et al. studied the impact of MPs on soil pH, and according to the study, MPs raised the soil’s alkalinity by raising its pH. The researchers attributed this impact to the interaction of MPs with soil organic matter, minerals, and various organic and inorganic contaminants [225].
- Nutrients: According to studies, MPs can alter soil pH, decrease microbial activity, and interfere with nutrient cycle mechanisms, which all impact the nutritional characteristics of soils [226]. They can also adsorb minerals, which lessens their availability for plant uptake and has a variety of other effects on soil nutrients; (a) MPs can decrease soil fertility by obstructing the passage of water and air through the soil. This may result in lower plant nutrient uptake and decreased agricultural production. (b) Microbial community alteration: MPs can potentially change the soil microbial community diversity and activity. This may impact nutrient cycle procedures like nitrogen fixation and mineralization. (c) Toxic chemical contamination of soil: MPs can absorb and store harmful substances like pesticides and heavy metals from the environment [227]. These MPs can pollute soil when they enter it with these hazardous compounds, which can harm plant development and health. The circulation system for nutritional components such as nitrogen is powered by microorganisms. The primary processes that link nitrogen transformation and nitrogen cycle are ammunition, nitrogen fixation, nitrification, and denitrification. The correspondence microorganisms must be present for each process [228].
- Organic compounds: Organic compounds in soils can be impacted by MPs in several ways [236]. Some ways that MPs can affect organic compounds in soils include (a) Changing the soil structure: MPs can build up in soil pores and alter the soil’s structure, impacting the amount of nutrients and water plants can access. Changes in microbial activity and decomposition rates may result from this, which may affect the organic molecules found in soil. (b) Adsorption of organic compounds: MPs can absorb organic pollutants like pesticides and herbicides due to their large surface area. As a result, these compounds may be less readily available for microbial or plant uptake. (c) Leaching of organic compounds: MPs can also help organic molecules in soils leach into groundwater or bodies of surface water. Aquatic ecosystems may be impacted, and water resources may become contaminated. (d) Modifying microbial communities: The diversity and composition of the microorganisms inhabiting the soil can be altered by MPs. These microorganisms are crucial in nutrient cycling and decomposition processes [237]. It is worth noting that MPs interact with organic compounds in soils in various complex ways, depending on the type of soil, the size and concentration of the MP, the length of exposure, and the environmental circumstances. Therefore, changes in microbial communities can have implications for plant nutrition and soil health.
7. How Biofouling Influences Microplastics Accumulation and Transport in the Soil and Sludge
8. Conclusions
9. Recommendations on Future Work
- study the effect of MPs on wastewater treatment methods;
- develop standardized sampling and analysis methods to evaluate the fate of MPs in WWTPs or other environmental media;
- evaluate the pollution risks to humans from MPs in sludge-amended soils;
- evaluate the possibility of uptake of MPs by plants;
- assess the effects of MPs on trophic transfer along the food chain;
- analyze the potential risks of degradation byproducts released from MPs;
- study the plastic weathering and transport processes within the soil profile.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| aAP | Aromatic polyamide |
| ABS | Acrylonitrile butadiene styrene |
| AF | Acrylic fibers |
| CA | Cellulose acetate |
| CN | Cellulose nitrate |
| EE | Ethylene ethyl |
| EP | Epoxy resins |
| EPM | Ethylene-propylene polymer |
| EVA | Ethylene-vinyl acetate |
| HDPE | High-density polyethylene |
| IW | Industrial wastewater |
| LDPE | Low-density polyethylene |
| MW | Mixed wastewater |
| SBR | Styrene butadiene rubber |
| SS | Sewage Sludge |
| PA | Polyamide |
| PAC | Polyacrylochloride |
| PAN | Polyacrylonitrile |
| PB | Polybutylene |
| PBM | Polybutylene |
| PC | Polycarbonate |
| PCL | Poly ε-caprolactone |
| PDS | Polydimethyl Siloxane |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PES | Polyester |
| PI | Polyimide |
| PMMA | Polymethyl methacrylate |
| PO | Polyolefin |
| POM | Polyoxymethylene |
| PP | Polypropylene |
| PP-PE | Polypropylene- polyethylene |
| PS | Polystyrene |
| PTU | Polytereurethane |
| PU | Polyurethane |
| PVAC | Polyvinyl acetate |
| PVC | Polyvinylchloride |
| WW | Wastewater |
| WWTP | Wastewater Treatment Plant |
| UW | Urban Wastewater |
References
- Jiang, C.; Yin, L.; Li, Z.; Wen, X.; Luo, X.; Hu, S.; Yang, H.; Long, Y.; Deng, B.; Huang, L. Microplastic pollution in the rivers of the Tibet Plateau. Environ. Pollut. 2019, 249, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Nayebi, B.; Ghalebizade, M.; Niavol, K.P. Removal of Acid Red 131 by peroxi-coagulation using stainless steel and aluminum electrodes: A comparative study. Water Conserv. Sci. Eng. 2021, 6, 201–211. [Google Scholar] [CrossRef]
- Frias, J.P.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Manna, C.; Jain, M. Abundance, interaction, ingestion, ecological concerns, and mitigation policies of microplastic pollution in riverine ecosystem: A review. Sci. Total Environ. 2021, 782, 146695. [Google Scholar] [CrossRef]
- Arab, M.; Danesh, S. A sustainable system for decontamination of cephalexin antibiotic using electrocoagulation technology and response surface methodology. Remediat. J. 2023, 33, 365–378. [Google Scholar] [CrossRef]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Sarkar, B.; Tsang, D.C.; Kirkham, M. Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef]
- Acarer, S. Microplastics in wastewater treatment plants: Sources, properties, removal efficiency, removal mechanisms, and interactions with pollutants. Water Sci. Technol. 2023, 87, 685–710. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, D.; Li, Z.; Zhu, C. Advancement and challenges of microplastic pollution in the aquatic environment: A review. Water Air Soil Pollut. 2018, 229, 140. [Google Scholar] [CrossRef]
- Xu, Z.; Bai, X.; Ye, Z. Removal and generation of microplastics in wastewater treatment plants: A review. J. Clean. Prod. 2021, 291, 125982. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef] [PubMed]
- Lofty, J.; Muhawenimana, V.; Wilson, C.; Ouro, P. Microplastics removal from a primary settler tank in a wastewater treatment plant and estimations of contamination onto European agricultural land via sewage sludge recycling. Environ. Pollut. 2022, 304, 119198. [Google Scholar] [CrossRef]
- Milojevic, N.; Cydzik-Kwiatkowska, A. Agricultural use of sewage sludge as a threat of microplastic (Mp) spread in the environment and the role of governance. Energies 2021, 14, 6293. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Hossain, M.N.; Rahman, M.M.; Afrin, S.; Akbor, M.A.; Siddique, M.A.B.; Malafaia, G. Identification and quantification of microplastics in agricultural farmland soil and textile sludge in Bangladesh. Sci. Total Environ. 2023, 858, 160118. [Google Scholar] [CrossRef]
- Piehl, S.; Leibner, A.; Löder, M.G.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro-and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Fu, C.; Zhou, Y.; Dai, Z.; Li, Y.; Tu, C.; Luo, Y. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- van den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef]
- Bostan, N.; Ilyas, N.; Akhtar, N.; Mehmood, S.; Saman, R.U.; Sayyed, R.; Shatid, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Pandiaraj, S. Toxicity assessment of microplastic (MPs); a threat to the ecosystem. Environ. Res. 2023, 234, 116523. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the environment: Intake through the food web, human exposure and toxicological effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- Büks, F.; Kaupenjohann, M. Global concentrations of microplastic in soils, a review. Soil Discuss. 2020, 2020, 649–662. [Google Scholar] [CrossRef]
- Nayebi, B.; Khurana, P.; Pulicharla, R.; Karimpour, S.; Brar, S.K. Preservation, storage, and sample preparation methods for freshwater microplastics—A comprehensive review. Environ. Sci. Adv. 2023, 2, 1060–1081. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.; Sin, A.; Kim, G.; Khan, S.; Nadagouda, M.N.; Sahle-Demessie, E.; Han, C. Pretreatment methods for monitoring microplastics in soil and freshwater sediment samples: A comprehensive review. Sci. Total Environ. 2023, 871, 161718. [Google Scholar] [CrossRef] [PubMed]
- Stock, F.; Kochleus, C.; Bänsch-Baltruschat, B.; Brennholt, N.; Reifferscheid, G. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—A review. TrAC Trends Anal. Chem. 2019, 113, 84–92. [Google Scholar] [CrossRef]
- Fazey, F.M.; Ryan, P.G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 2016, 210, 354–360. [Google Scholar] [CrossRef]
- Kaiser, D.; Kowalski, N.; Waniek, J.J. Effects of biofouling on the sinking behavior of microplastics. Environ. Res. Lett. 2017, 12, 124003. [Google Scholar] [CrossRef]
- Long, Z.; Pan, Z.; Wang, W.; Ren, J.; Yu, X.; Lin, L.; Lin, H.; Chen, H.; Jin, X. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 2019, 155, 255–265. [Google Scholar] [CrossRef]
- Yang, L.; Li, K.; Cui, S.; Kang, Y.; An, L.; Lei, K. Removal of microplastics in municipal sewage from China’s largest water reclamation plant. Water Res. 2019, 155, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Y. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Xie, Y.; Zhong, S.; Gao, P. Occurrence and removal of microplastics from wastewater treatment plants in a typical tourist city in China. J. Clean. Prod. 2021, 291, 125968. [Google Scholar] [CrossRef]
- Salmi, P.; Ryymin, K.; Karjalainen, A.K.; Mikola, A.; Uurasjärvi, E.; Talvitie, J. Particle balance and return loops for microplastics in a tertiary-level wastewater treatment plant. Water Sci. Technol. 2021, 84, 89–100. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Franco, A.; Arellano, J.; Albendín, G.; Rodríguez-Barroso, R.; Zahedi, S.; Quiroga, J.M.; Coello, M.D. Mapping microplastics in Cadiz (Spain): Occurrence of microplastics in municipal and industrial wastewaters. J. Water Process Eng. 2020, 38, 101596. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef]
- Cunsolo, S.; Williams, J.; Hale, M.; Read, D.S.; Couceiro, F. Optimising sample preparation for FTIR-based microplastic analysis in wastewater and sludge samples: Multiple digestions. Anal. Bioanal. Chem. 2021, 413, 3789–3799. [Google Scholar] [CrossRef]
- Tadsuwan, K.; Babel, S. Microplastic abundance and removal via an ultrafiltration system coupled to a conventional municipal wastewater treatment plant in Thailand. J. Environ. Chem. Eng. 2022, 10, 107142. [Google Scholar] [CrossRef]
- Yahyanezhad, N.; Bardi, M.J.; Aminirad, H. An evaluation of microplastics fate in the wastewater treatment plants: Frequency and removal of microplastics by microfiltration membrane. Water Pract. Technol. 2021, 16, 782–792. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Duan, L.; Zhang, Y.; Zhou, Y.; Sui, Q.; Xu, D.; Qu, H.; Yu, G. Occurrence and distribution of microplastics in domestic, industrial, agricultural and aquacultural wastewater sources: A case study in Changzhou, China. Water Res. 2020, 182, 115956. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Horton, A.A.; Cross, R.K.; Read, D.S.; Jürgens, M.D.; Ball, H.L.; Svendsen, C.; Vollertsen, J.; Johnson, A.C. Semi-automated analysis of microplastics in complex wastewater samples. Environ. Pollut. 2021, 268, 115841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Su, Y.; Zhu, J.; Shi, J.; Huang, H.; Xie, B. Distribution and removal characteristics of microplastics in different processes of the leachate treatment system. Waste Manag. 2021, 120, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Dou, M.; Wang, C.; Li, G.; Jia, R. Abundance and removal characteristics of microplastics at a wastewater treatment plant in Zhengzhou. Environ. Sci. Pollut. Res. 2020, 27, 36295–36305. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Xu, J.; Su, X.; Lu, M.; Wang, Z.; Zhang, Y. Occurrence and characteristics of microplastics in a wastewater treatment plant. Bull. Environ. Contam. Toxicol. 2021, 107, 677–683. [Google Scholar] [CrossRef]
- Kong, W.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A.; Almadiy, A.A.; Thakur, N.; Salama, E.-S. Occurrence, characteristics, and microbial community of microplastics in anaerobic sludge of wastewater treatment plants. Environ. Pollut. 2024, 344, 123370. [Google Scholar] [CrossRef]
- Klemmensen, N.D.; Chand, R.; Blanco, M.S.; Vollertsen, J. Microplastic abundance in sludge-treated fields: Variance and estimated half-life. Sci. Total Environ. 2024, 922, 171394. [Google Scholar] [CrossRef]
- Kazour, M.; Terki, S.; Rabhi, K.; Jemaa, S.; Khalaf, G.; Amara, R. Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill. Mar. Pollut. Bull. 2019, 146, 608–618. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y. Treatment characteristics of microplastics at biological sewage treatment facilities in Korea. Mar. Pollut. Bull. 2018, 137, 1–8. [Google Scholar] [CrossRef]
- Maw, M.M.; Boontanon, N.; Aung, H.K.Z.Z.; Jindal, R.; Fujii, S.; Visvanathan, C.; Boontanon, S.K. Microplastics in wastewater and sludge from centralized and decentralized wastewater treatment plants: Effects of treatment systems and microplastic characteristics. Chemosphere 2024, 361, 142536. [Google Scholar] [CrossRef] [PubMed]
- Vardar, S.; Onay, T.T.; Demirel, B.; Kideys, A.E. Evaluation of microplastics removal efficiency at a wastewater treatment plant discharging to the Sea of Marmara. Environ. Pollut. 2021, 289, 117862. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.; Lee, J.-Y.; Cha, J. Microplastics in soil and freshwater: Understanding sources, distribution, potential impacts, and regulations for management. Sci. Prog. 2022, 105, 00368504221126676. [Google Scholar] [CrossRef] [PubMed]
- Chand, R.; Kohansal, K.; Toor, S.; Pedersen, T.H.; Vollertsen, J. Microplastics degradation through hydrothermal liquefaction of wastewater treatment sludge. J. Clean. Prod. 2022, 335, 130383. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Xing, R.; Xie, S.; Yang, X.; Cui, P.; Lü, J.; Liao, H.; Yu, Z.; Wang, S. Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology. J. Hazard. Mater. 2020, 384, 121271. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- El Hayany, B.; El Fels, L.; Quénéa, K.; Dignac, M.-F.; Rumpel, C.; Gupta, V.K.; Hafidi, M. Microplastics from lagooning sludge to composts as revealed by fluorescent staining-image analysis, Raman spectroscopy and pyrolysis-GC/MS. J. Environ. Manag. 2020, 275, 111249. [Google Scholar] [CrossRef]
- Sujathan, S.; Kniggendorf, A.-K.; Kumar, A.; Roth, B.; Rosenwinkel, K.-H.; Nogueira, R. Heat and bleach: A cost-efficient method for extracting microplastics from return activated sludge. Arch. Environ. Contam. Toxicol. 2017, 73, 641–648. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Di, M.; Li, Z.; Wang, J. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chem. Eng. J. 2019, 362, 176–182. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in sewage sludge: Effects of treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Pittura, L.; Foglia, A.; Akyol, Ç.; Cipolletta, G.; Benedetti, M.; Regoli, F.; Eusebi, A.L.; Sabbatini, S.; Tseng, L.Y.; Katsou, E. Microplastics in real wastewater treatment schemes: Comparative assessment and relevant inhibition effects on anaerobic processes. Chemosphere 2021, 262, 128415. [Google Scholar] [CrossRef]
- Leslie, H.; Brandsma, S.; Van Velzen, M.; Vethaak, A. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hurley, R.; Vogelsang, C.; Nizzetto, L.; Olsen, M. Mapping Microplastics in Sludge; Norwegian Institute for Water Research: Oslo, Norway, 2017. [Google Scholar]
- Hernández-Arenas, R.; Beltrán-Sanahuja, A.; Navarro-Quirant, P.; Sanz-Lazaro, C. The effect of sewage sludge containing microplastics on growth and fruit development of tomato plants. Environ. Pollut. 2021, 268, 115779. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.A.; Iordachescu, L.; Tumlin, S.; Vollertsen, J. A complete mass balance for plastics in a wastewater treatment plant-macroplastics contributes more than microplastics. Water Res. 2021, 201, 117307. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, E.A.; Habibi, M.; Haddad, E.; Hasanin, M.; Angel, D.L.; Booth, A.M.; Sabbah, I. Microplastic distributions in a domestic wastewater treatment plant: Removal efficiency, seasonal variation and influence of sampling technique. Sci. Total Environ. 2021, 752, 141880. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Petroody, S.S.A.; Hashemi, S.H.; van Gestel, C.A. Transport and accumulation of microplastics through wastewater treatment sludge processes. Chemosphere 2021, 278, 130471. [Google Scholar] [CrossRef]
- Vockenberg, T.; Wichard, T.; Ueberschaar, N.; Franke, M.; Stelter, M.; Braeutigam, P. The sorption behaviour of amine micropollutants on polyethylene microplastics–impact of aging and interactions with green seaweed. Environ. Sci. Process. Impacts 2020, 22, 1678–1687. [Google Scholar] [CrossRef]
- El Hayany, B.; El Fels, L.; Ouhdouch, Y.; Hafidi, M. Fate of pathogenic microorganisms during lagooning sludge composting and exploration of bacteriophages as indicator of hygienization. Environ. Technol. Innov. 2021, 21, 101268. [Google Scholar] [CrossRef]
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senthirajah, K.; Lundmark, A.; Rogers, Z.; Suresh, S.; Evans, G.; Palanisami, T. Improved methodology to determine the fate and transport of microplastics in a secondary wastewater treatment plant. Water Res. 2020, 173, 115549. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Silveira, I.T.; Chua, A.; Leusch, F.D. An audit of microplastic abundance throughout three Australian wastewater treatment plants. Chemosphere 2021, 263, 128294. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Manjón, A.; Martínez-Díez, R.; Sol, D.; Laca, A.; Laca, A.; Rancaño, A.; Díaz, M. Long-term occurrence and fate of microplastics in WWTPs: A case study in southwest Europe. Appl. Sci. 2022, 12, 2133. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Ma, J.; An, Y.; Liu, Q.; Yang, S.; Qu, Y.; Chen, H.; Zhao, W.; Tian, Y. Microplastics pollution in the soil mulched by dust-proof nets: A case study in Beijing, China. Environ. Pollut. 2021, 275, 116600. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Ren, H.; Cao, G.; Xie, G.; Xing, D.; Liu, B. Investigation and fate of microplastics in wastewater and sludge filter cake from a wastewater treatment plant in China. Sci. Total Environ. 2020, 746, 141378. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; López-Castellanos, J.; Olmos, S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020, 156, 111211. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; Van Loosdrecht, M.C.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef]
- Üstün, G.E.; Bozdaş, K.; Can, T. Abundance and characteristics of microplastics in an urban wastewater treatment plant in Turkey. Environ. Pollut. 2022, 310, 119890. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, L.; Li, R.; Xu, L.; Shen, Y.; Li, S.; Tu, C.; Wu, L.; Christie, P.; Luo, Y. Microplastics in an agricultural soil following repeated application of three types of sewage sludge: A field study. Environ. Pollut. 2021, 289, 117943. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Zhang, Z.; Yan, Z.; Zhang, Y. Effects of chronic exposure to different sizes and polymers of microplastics on the characteristics of activated sludge. Sci. Total Environ. 2021, 783, 146954. [Google Scholar] [CrossRef]
- Yu, J.T.; Diamond, M.L.; Helm, P.A. A fit-for-purpose categorization scheme for microplastic morphologies. Integr. Environ. Assess. Manag. 2023, 19, 422–435. [Google Scholar] [CrossRef]
- Panno, S.V.; Kelly, W.R.; Scott, J.; Zheng, W.; McNeish, R.E.; Holm, N.; Hoellein, T.J.; Baranski, E.L. Microplastic contamination in karst groundwater systems. Groundwater 2019, 57, 189–196. [Google Scholar] [CrossRef]
- Rosal, R. Morphological description of microplastic particles for environmental fate studies. Mar. Pollut. Bull. 2021, 171, 112716. [Google Scholar] [CrossRef] [PubMed]
- Gesamp, G. Guidelines for the monitoring and assessment of plastic litter in the ocean. GESAMP Rep. Stud. 2019, 99, 130. [Google Scholar]
- Yang, Z.; Li, S.; Ma, S.; Liu, P.; Peng, D.; Ouyang, Z.; Guo, X. Characteristics and removal efficiency of microplastics in sewage treatment plant of Xi’an City, northwest China. Sci. Total Environ. 2021, 771, 145377. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, M.; Tang, Y.; Dai, R.; Mo, L.; Yang, W.; Liang, Y.; Huang, K. Microfibers shed from synthetic textiles during laundry: Flow to wastewater treatment plants or release to receiving waters through storm drains? Process Saf. Environ. Prot. 2022, 168, 689–697. [Google Scholar] [CrossRef]
- Di Bella, G.; Corsino, S.F.; De Marines, F.; Lopresti, F.; La Carrubba, V.; Torregrossa, M.; Viviani, G. Occurrence of Microplastics in Waste Sludge of Wastewater Treatment Plants: Comparison between Membrane Bioreactor (MBR) and Conventional Activated Sludge (CAS) Technologies. Membranes 2022, 12, 371. [Google Scholar] [CrossRef]
- Kukkola, A.; Schneidewind, U.; Haverson, L.; Kelleher, L.; Drummond, J.D.; Sambrook Smith, G.; Lynch, I.; Krause, S. Snapshot Sampling May not Be Enough to Obtain Robust Estimates for Riverine Microplastic Loads. ACS EsT Water 2024, 4, 2309–2319. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Vanoirbeek, J.A.; Van Belleghem, F.; Vleeschouwers, B.; Hoet, P.H.; Ghosh, M. Sampling strategies and analytical techniques for assessment of airborne micro and nano plastics. Environ. Int. 2023, 174, 107885. [Google Scholar] [CrossRef]
- Harley-Nyang, D.; Memon, F.A.; Jones, N.; Galloway, T. Investigation and analysis of microplastics in sewage sludge and biosolids: A case study from one wastewater treatment works in the UK. Sci. Total Environ. 2022, 823, 153735. [Google Scholar] [CrossRef]
- Talukdar, A.; Kundu, P.; Bhattacharya, S.; Dutta, N. Microplastic contamination in wastewater: Sources, distribution, detection and remediation through physical and chemical-biological methods. Sci. Total Environ. 2024, 916, 170254. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D. PE-RT: A New Class of Polyethylene for Industrial Pipes. In Proceedings of the International Conference on Offshore Mechanics and Arctic Engineering, Hamburg, Germany, 4–9 June 2006; pp. 513–521. [Google Scholar]
- Paiva, R.; Wrona, M.; Nerín, C.; Veroneze, I.B.; Gavril, G.-L.; Cruz, S.A. Importance of profile of volatile and off-odors compounds from different recycled polypropylene used for food applications. Food Chem. 2021, 350, 129250. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.-X.; Tang, R.-C.; Zhai, A.-D. Hydrophilic and antibacterial surface functionalization of polyamide fabric by coating with polylysine biomolecule. Prog. Org. Coat. 2020, 142, 105571. [Google Scholar] [CrossRef]
- Tsironi, T.N.; Chatzidakis, S.M.; Stoforos, N.G. The future of polyethylene terephthalate bottles: Challenges and sustainability. Packag. Technol. Sci. 2022, 35, 317–325. [Google Scholar] [CrossRef]
- Turner, A. Foamed polystyrene in the marine environment: Sources, additives, transport, behavior, and impacts. Environ. Sci. Technol. 2020, 54, 10411–10420. [Google Scholar] [CrossRef]
- Prata, J.C. Microplastics in wastewater: State of the knowledge on sources, fate and solutions. Mar. Pollut. Bull. 2018, 129, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mei, Q.; Chen, L.; Zhang, H.; Dong, B.; Dai, X.; He, C.; Zhou, J. Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process. Water Res. 2019, 157, 228–237. [Google Scholar] [CrossRef]
- Keerthika, K.; Padmavathy, P.; Rani, V.; Jeyashakila, R.; Aanand, S.; Kutty, R. Contamination of microplastics, surface morphology and risk assessment in beaches along the Thoothukudi coast, Gulf of Mannar region. Environ. Sci. Pollut. Res. 2022, 29, 75525–75538. [Google Scholar] [CrossRef]
- Abadi, Z.T.R.; Abtahi, B.; Fathi, M.; Mashhadi, N.; Grossart, H.-P. Size, shape, and elemental composition as predictors of microplastic surface erosion. J. Hazard. Mater. 2024, 476, 134961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, P.L.; Biesinger, M.C.; Grifi, M. Plastics and beaches: A degrading relationship. Mar. Pollut. Bull. 2009, 58, 80–84. [Google Scholar] [CrossRef]
- Veerasingam, S.; Saha, M.; Suneel, V.; Vethamony, P.; Rodrigues, A.C.; Bhattacharyya, S.; Naik, B. Characteristics, seasonal distribution and surface degradation features of microplastic pellets along the Goa coast, India. Chemosphere 2016, 159, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ou, Q.; van der Hoek, J.P.; Liu, G.; Lompe, K.M. Photo-oxidation of Micro-and Nanoplastics: Physical, Chemical, and Biological Effects in Environments. Environ. Sci. Technol. 2024, 58, 991–1009. [Google Scholar] [CrossRef]
- Abadi, Z.T.R.; Abtahi, B.; Grossart, H.-P.; Khodabandeh, S. Microplastic content of Kutum fish, Rutilus frisii kutum in the southern Caspian Sea. Sci. Total Environ. 2021, 752, 141542. [Google Scholar] [CrossRef]
- Bullard, J.E.; Zhou, Z.; Davis, S.; Fowler, S. Breakdown and modification of microplastic beads by aeolian abrasion. Environ. Sci. Technol. 2022, 57, 76–84. [Google Scholar] [CrossRef]
- Tajwar, M.; Yousuf Gazi, M.; Saha, S.K. Characterization and spatial abundance of microplastics in the coastal regions of Cox’s Bazar, Bangladesh: An integration of field, laboratory, and GIS techniques. Soil Sediment Contam. Int. J. 2022, 31, 57–80. [Google Scholar] [CrossRef]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes–origin, impact and potential solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- He, S.; Tong, J.; Xiong, W.; Xiang, Y.; Peng, H.; Wang, W.; Yang, Y.; Ye, Y.; Hu, M.; Yang, Z. Microplastics influence the fate of antibiotics in freshwater environments: Biofilm formation and its effect on adsorption behavior. J. Hazard. Mater. 2023, 442, 130078. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastic/nanoplastic toxicity in plants: An imminent concern. Environ. Monit. Assess. 2023, 195, 27. [Google Scholar] [CrossRef]
- Rose, P.K.; Yadav, S.; Kataria, N.; Khoo, K.S. Microplastics and nanoplastics in the terrestrial food chain: Uptake, translocation, trophic transfer, ecotoxicology, and human health risk. TrAC Trends Anal. Chem. 2023, 167, 117249. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C. Fate and Impacts of Microplastics in the Environment: Hydrosphere, Pedosphere, and Atmosphere. Environments 2023, 10, 70. [Google Scholar] [CrossRef]
- Eze, C.G.; Nwankwo, C.E.; Dey, S.; Sundaramurthy, S.; Okeke, E.S. Food chain microplastics contamination and impact on human health: A review. Environ. Chem. Lett. 2024, 22, 1889–1927. [Google Scholar] [CrossRef]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2020, 14, 16–22. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.; Andrady, A.L.; Bornman, J.F.; Aucamp, P.J.; Bais, A.F.; Banaszak, A.T.; Barnes, P.W.; Bernhard, G.H.; Bruckman, L.S.; Busquets, R. Plastics in the environment in the context of UV radiation, climate change and the Montreal Protocol: UNEP Environmental Effects Assessment Panel, Update 2023. Photochem. Photobiol. Sci. 2024, 23, 629–650. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, Y.; Li, W.; Wang, J.; Zhang, X.; He, J.; Li, J.; Ma, Y.; Niu, Z. Co-effects of biofouling and inorganic matters increased the density of environmental microplastics in the sediments of Bohai Bay coast. Sci. Total Environ. 2020, 717, 134431. [Google Scholar] [CrossRef] [PubMed]
- Schell, T.; Hurley, R.; Buenaventura, N.T.; Mauri, P.V.; Nizzetto, L.; Rico, A.; Vighi, M. Fate of microplastics in agricultural soils amended with sewage sludge: Is surface water runoff a relevant environmental pathway? Environ. Pollut. 2022, 293, 118520. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss floodplain soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Pérez-Reverón, R.; González-Sálamo, J.; Hernández-Sánchez, C.; González-Pleiter, M.; Hernández-Borges, J.; Díaz-Peña, F.J. Recycled wastewater as a potential source of microplastics in irrigated soils from an arid-insular territory (Fuerteventura, Spain). Sci. Total Environ. 2022, 817, 152830. [Google Scholar] [CrossRef]
- Reddy, A.S.; Nair, A.T. The fate of microplastics in wastewater treatment plants: An overview of source and remediation technologies. Environ. Technol. Innov. 2022, 28, 102815. [Google Scholar] [CrossRef]
- Gautam, K.; Sadasivam, A. Recent trends in analytical measures of microplastic in soil and toxicopathological risk assessment in earthworms. TrAC Trends Anal. Chem. 2023, 168, 117292. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci. Total Environ. 2019, 694, 133798. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of plastic pollution on ecosystem services, sustainable development goals, and need to focus on circular economy and policy interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616, 1056–1065. [Google Scholar] [CrossRef]
- Franco, A.; Martín-García, A.; Egea-Corbacho, A.; Arellano, J.; Albendín, G.; Rodríguez-Barroso, R.; Quiroga, J.; Coello, M. Assessment and accumulation of microplastics in sewage sludge at wastewater treatment plants located in Cádiz, Spain. Environ. Pollut. 2023, 317, 120689. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tang, W.; Wu, S.; Liu, H.; Yang, C. Fate and effects of microplastics in wastewater treatment processes. Sci. Total Environ. 2021, 757, 143902. [Google Scholar] [CrossRef]
- Koutnik, V.S.; Leonard, J.; Alkidim, S.; DePrima, F.J.; Ravi, S.; Hoek, E.M.; Mohanty, S.K. Distribution of microplastics in soil and freshwater environments: Global analysis and framework for transport modeling. Environ. Pollut. 2021, 274, 116552. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Allen, S.; Luo, X.; Allen, D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci. Total Environ. 2021, 758, 143634. [Google Scholar] [CrossRef]
- Corradini, F.; Casado, F.; Leiva, V.; Huerta-Lwanga, E.; Geissen, V. Microplastics occurrence and frequency in soils under different land uses on a regional scale. Sci. Total Environ. 2021, 752, 141917. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, W.; Lu, S.; Huang, W.; Yuan, Q.; Tian, M.; Lv, W.; He, D. Microplastic pollution in rice-fish co-culture system: A report of three farmland stations in Shanghai, China. Sci. Total Environ. 2019, 652, 1209–1218. [Google Scholar] [CrossRef]
- Chen, S.; Ai, X.; Dong, T.; Li, B.; Luo, R.; Ai, Y.; Chen, Z.; Li, C. The physico-chemical properties and structural characteristics of artificial soil for cut slope restoration in Southwestern China. Sci. Rep. 2016, 6, 20565. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, Q.-L.; An, X.-L.; Yang, X.-R.; Christie, P.; Ke, X.; Wu, L.-H.; Zhu, Y.-G. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- Rillig, M.C.; de Souza Machado, A.A.; Lehmann, A.; Klümper, U. Evolutionary implications of microplastics for soil biota. Environ. Chem. 2018, 16, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the terrestrial ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.K.A.; Raju, S.; Singh, A.; Senathirajah, K.; Bhagwat-Russell, G.; Daggubati, L.; Kandaiah, R.; Palanisami, T. Occurrence and distribution of microplastics in long-term biosolid-applied rehabilitation land: An overlooked pathway for microplastic entry into terrestrial ecosystems in Australia. Environ. Pollut. 2023, 336, 122464. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Fuller, S.G.; Gautam, A. A procedure for measuring microplastics using pressurized fluid extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef]
- Leusch, F.D.; Lu, H.-C.; Perera, K.; Neale, P.A.; Ziajahromi, S. Analysis of the literature shows a remarkably consistent relationship between size and abundance of microplastics across different environmental matrices. Environ. Pollut. 2023, 319, 120984. [Google Scholar] [CrossRef]
- Long, Z.; Pan, Z.; Jin, X.; Zou, Q.; He, J.; Li, W.; Waters, C.N.; Turner, S.D.; do Sul, J.A.I.; Yu, X. Anthropocene microplastic stratigraphy of Xiamen Bay, China: A history of plastic production and waste management. Water Res. 2022, 226, 119215. [Google Scholar] [CrossRef]
- Liu, X.; Tang, N.; Yang, W.; Chang, J. Microplastics pollution in the soils of various land-use types along Sheshui River basin of Central China. Sci. Total Environ. 2022, 806, 150620. [Google Scholar] [CrossRef]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.-M.; Hou, D. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, Y.; Li, Y.; Hu, Q.; Wang, C.; Hu, J.; Zhang, W.; Wang, L.; Zhang, C.; Zhang, H. New insights into the vertical distribution and microbial degradation of microplastics in urban river sediments. Water Res. 2021, 188, 116449. [Google Scholar] [CrossRef] [PubMed]
- Ragoobur, D.; Huerta-Lwanga, E.; Somaroo, G.D. Microplastics in agricultural soils, wastewater effluents and sewage sludge in Mauritius. Sci. Total Environ. 2021, 798, 149326. [Google Scholar] [CrossRef]
- Li, N.-Y.; Qu, J.-H.; Yang, J.-Y. Microplastics distribution and microbial community characteristics of farmland soil under different mulch methods. J. Hazard. Mater. 2023, 445, 130408. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef] [PubMed]
- Golgoli, M.; Khiadani, M.; Shafieian, A.; Sen, T.K.; Hartanto, Y.; Johns, M.; Zargar, M. Microplastics fouling and interaction with polymeric membranes: A review. Chemosphere 2021, 283, 131185. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, S.; Qin, W.; Zhang, C.; Lv, C.; Zou, M. Effects of land use on the distribution of soil microplastics in the Lihe River watershed, China. Chemosphere 2023, 324, 138292. [Google Scholar] [CrossRef]
- Sarkar, S.; Diab, H.; Thompson, J. Microplastic pollution: Chemical characterization and impact on wildlife. Int. J. Environ. Res. Public Health 2023, 20, 1745. [Google Scholar] [CrossRef]
- Gayathri, N.; Prasad, G.; Prabhakaran, V.; Priya, V. Understanding the impact of microplastic contamination on soil quality and eco-toxicological risks in horticulture: A comprehensive review. Case Stud. Chem. Environ. Eng. 2024, 9, 100633. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Leusch, F.D. Systematic assessment of data quality and quality assurance/quality control (QA/QC) of current research on microplastics in biosolids and agricultural soils. Environ. Pollut. 2022, 294, 118629. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Slynkova, N.; Dwyer, J.; Griffith, M.; Fernandes, M.; Jaeger, J.E.; Leusch, F.D. Comprehensive assessment of microplastics in Australian biosolids: Abundance, seasonal variation and potential transport to agroecosystems. Water Res. 2024, 250, 121071. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hernandez-Milian, G. Microplastic extraction from marine vertebrate digestive tracts, regurgitates and scats: A protocol for researchers from all experience levels. Bio-Protoc. 2018, 8, e3087. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.R.; Kim, Y.-N.; Yoon, J.-H.; Dickinson, N.; Kim, K.-H. Plastic contamination of forest, urban, and agricultural soils: A case study of Yeoju City in the Republic of Korea. J. Soils Sediments 2021, 21, 1962–1973. [Google Scholar] [CrossRef]
- Khaldoon, S.; Lalung, J.; Maheer, U.; Kamaruddin, M.A.; Yhaya, M.F.; Alsolami, E.S.; Alorfi, H.S.; Hussein, M.A.; Rafatullah, M. A Review on the Role of Earthworms in Plastics Degradation: Issues and Challenges. Polymers 2022, 14, 4770. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, G.; Feng, F.; Ye, J.; Liao, C.-H.; Wu, C.-H.; Chen, S.-C. Microplastics in the soil environment: Focusing on the sources, its transformation and change in morphology. Sci. Total Environ. 2023, 896, 165291. [Google Scholar] [CrossRef]
- Sørensen, L.; Groven, A.S.; Hovsbakken, I.A.; Del Puerto, O.; Krause, D.F.; Sarno, A.; Booth, A.M. UV degradation of natural and synthetic microfibers causes fragmentation and release of polymer degradation products and chemical additives. Sci. Total Environ. 2021, 755, 143170. [Google Scholar] [CrossRef]
- Kabir, M.S.; Wang, H.; Luster-Teasley, S.; Zhang, L.; Zhao, R. Microplastics in landfill leachate: Sources, detection, occurrence, and removal. Environ. Sci. Ecotechnol. 2023, 16, 100256. [Google Scholar] [CrossRef]
- Sellström, U.; de Wit, C.A.; Lundgren, N.; Tysklind, M. Effect of sewage-sludge application on concentrations of higher-brominated diphenyl ethers in soils and earthworms. Environ. Sci. Technol. 2005, 39, 9064–9070. [Google Scholar] [CrossRef]
- Banerjee, M.; Burton, D.; Depoe, S. Impact of sewage sludge application on soil biological characteristics. Agric. Ecosyst. Environ. 1997, 66, 241–249. [Google Scholar] [CrossRef]
- Pradas del Real, A.E.; Castillo-Michel, H.; Kaegi, R.; Sinnet, B.; Magnin, V.; Findling, N.; Villanova, J.; Carrière, M.; Santaella, C.; Fernández-Martínez, A. Fate of Ag-NPs in sewage sludge after application on agricultural soils. Environ. Sci. Technol. 2016, 50, 1759–1768. [Google Scholar] [CrossRef]
- Koul, B.; Bhat, N.; Abubakar, M.; Mishra, M.; Arukha, A.P.; Yadav, D. Application of Natural Coagulants in Water Treatment: A Sustainable Alternative to Chemicals. Water 2022, 14, 3751. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Timilsina, A.; Adhikari, K.; Yadav, A.K.; Joshi, P.; Ramena, G.; Bohara, K. Effects of microplastics and nanoplastics in shrimp: Mechanisms of plastic particle and contaminant distribution and subsequent effects after uptake. Sci. Total Environ. 2023, 894, 164999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef]
- Qian, J.; He, X.; Wang, P.; Xu, B.; Li, K.; Lu, B.; Jin, W.; Tang, S. Effects of polystyrene nanoplastics on extracellular polymeric substance composition of activated sludge: The role of surface functional groups. Environ. Pollut. 2021, 279, 116904. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Q.; Li, J.; Li, Q.; Xu, H.; Ye, Q.; Wang, Y.; Shu, S.; Zhang, J. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism. Sci. Total Environ. 2021, 752, 141837. [Google Scholar] [CrossRef] [PubMed]
- Shahi, N.K.; Maeng, M.; Kim, D.; Dockko, S. Removal behavior of microplastics using alum coagulant and its enhancement using polyamine-coated sand. Process. Saf. Environ. Prot. 2020, 141, 9–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Yue, J.; Xing, X.; Yang, Z.; Wang, X.; Wang, Q.; Zhang, J. Enhanced removal of polyethylene terephthalate microplastics through polyaluminum chloride coagulation with three typical coagulant aids. Sci. Total Environ. 2021, 800, 149589. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Li, J. The removal of microplastics in the wastewater treatment process and their potential impact on anaerobic digestion due to pollutants association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Zhang, X.; Yang, X.; Niu, S.; Zheng, Z.; Dong, B.; Hur, J.; Dai, X. Aging and mitigation of microplastics during sewage sludge treatments: An overview. Sci. Total Environ. 2024, 922, 171338. [Google Scholar] [CrossRef] [PubMed]
- Zita, A.; Hermansson, M. Effects of ionic strength on bacterial adhesion and stability of flocs in a wastewater activated sludge system. Appl. Environ. Microbiol. 1994, 60, 3041–3048. [Google Scholar] [CrossRef]
- Huang, L.; Jin, Y.; Zhou, D.; Liu, L.; Huang, S.; Zhao, Y.; Chen, Y. A review of the role of extracellular polymeric substances (EPS) in wastewater treatment systems. Int. J. Environ. Res. Public Health 2022, 19, 12191. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Milojevic, N.; Jachimowicz, P. The fate of microplastic in sludge management systems. Sci. Total Environ. 2022, 848, 157466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-T.; Wei, W.; Huang, Q.-S.; Wang, C.; Wang, Y.; Ni, B.-J. Insights into the microbial response of anaerobic granular sludge during long-term exposure to polyethylene terephthalate microplastics. Water Res. 2020, 179, 115898. [Google Scholar] [CrossRef]
- Zöhre, K.; James, A. Effectiveness of microplastics removal in wastewater treatment plants: A critical analysis of wastewater treatment processes. J. Environ. Chem. Eng. 2022, 10, 107831. [Google Scholar]
- Wei, W.; Huang, Q.-S.; Sun, J.; Wang, J.-Y.; Wu, S.-L.; Ni, B.-J. Polyvinyl chloride microplastics affect methane production from the anaerobic digestion of waste activated sludge through leaching toxic bisphenol-A. Environ. Sci. Technol. 2019, 53, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Rong, H.; Li, J.; Zhao, Y.; Ren, L.; Chen, S.; Liu, J.; Mei, M.; Xue, Y.; Wang, T. Mechanistic insights into effect of in-situ microplastics on heavy metals leaching behavior from its dyeing sludge incineration bottom ash. J. Environ. Chem. Eng. 2023, 11, 110089. [Google Scholar] [CrossRef]
- Ma, J.; Gong, Z.; Wang, Z.; Liu, H.; Chen, G.; Guo, G. Elucidating degradation properties, microbial community, and mechanism of microplastics in sewage sludge under different terminal electron acceptors conditions. Bioresour. Technol. 2022, 346, 126624. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Mahmud, A.; Wasif, M.M.; Roy, H.; Mehnaz, F.; Ahmed, T.; Pervez, M.; Naddeo, V.; Islam, M. Aquatic Microplastic Pollution Control Strategies: Sustainable Degradation Techniques, Resource Recovery, and Recommendations for Bangladesh. Water 2022, 14, 3968. [Google Scholar] [CrossRef]
- Wei, W.; Hao, Q.; Chen, Z.; Bao, T.; Ni, B.-J. Polystyrene nanoplastics reshape the anaerobic granular sludge for recovering methane from wastewater. Water Res. 2020, 182, 116041. [Google Scholar] [CrossRef]
- European Commission. Review of Reach with Regard to the Registration Requirements on Polymers 070307/2011/602175/SER/D3 Final Report Part A: Polymers; European Commission DG Environment: Brussels, Belgium, 2012. [Google Scholar]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, H.; Hua, X.; Dang, Y.; Han, Y.; Yu, Z.; Chen, X.; Ding, P.; Li, H. Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 726, 138679. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.M.M.; Hai, F.I.; Lu, W.; Al-Mamun, A.; Dhar, B.R. A review of mechanisms underlying the impacts of (nano) microplastics on anaerobic digestion. Bioresour. Technol. 2021, 329, 124894. [Google Scholar]
- Cholewińska, P.; Moniuszko, H.; Wojnarowski, K.; Pokorny, P.; Szeligowska, N.; Dobicki, W.; Polechoński, R.; Górniak, W. The occurrence of microplastics and the formation of biofilms by pathogenic and opportunistic bacteria as threats in aquaculture. Int. J. Environ. Res. Public Health 2022, 19, 8137. [Google Scholar] [CrossRef]
- Glaser, J. The Importance of Biofilms to the Fate and Effects of Microplastics. In Bacterial Biofilms; IntechOpen: London, UK, 2020. [Google Scholar]
- Yang, Y.; Liu, W.; Zhang, Z.; Grossart, H.-P.; Gadd, G.M. Microplastics provide new microbial niches in aquatic environments. Appl. Microbiol. Biotechnol. 2020, 104, 6501–6511. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Wang, X.; Cheng, T.; Fu, K.; Qin, Z.; Feng, K. Biofilm structural and functional features on microplastic surfaces in greenhouse agricultural soil. Sustainability 2022, 14, 7024. [Google Scholar] [CrossRef]
- Perveen, S.; Pablos, C.; Reynolds, K.; Stanley, S.; Marugán, J. Growth and prevalence of antibiotic-resistant bacteria in microplastic biofilm from wastewater treatment plant effluents. Sci. Total Environ. 2023, 856, 159024. [Google Scholar] [CrossRef]
- Shi, J.; Wang, B.; Li, X.; Su, Y.; Wu, D.; Xie, B. Distinguishing removal and regrowth potential of antibiotic resistance genes and antibiotic resistant bacteria on microplastics and in leachate after chlorination or Fenton oxidation. J. Hazard. Mater. 2022, 430, 128432. [Google Scholar] [CrossRef]
- Wang, Z.; Su, Y.; Zhu, J.; Wu, D.; Xie, B. Size-dependent effects of microplastics on antibiotic resistance genes fate in wastewater treatment systems: The role of changed surface property and microbial assemblages in a continuous exposure mode. Sci. Total Environ. 2022, 851, 158264. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, J.; Dai, H.; Zhao, Y.; Li, D.; Duan, W.; Guo, Y. Microplastics affect the ammonia oxidation performance of aerobic granular sludge and enrich the intracellular and extracellular antibiotic resistance genes. J. Hazard. Mater. 2021, 409, 124981. [Google Scholar] [CrossRef]
- Yang, X.; Bento, C.P.; Chen, H.; Zhang, H.; Xue, S.; Lwanga, E.H.; Zomer, P.; Ritsema, C.J.; Geissen, V. Influence of microplastic addition on glyphosate decay and soil microbial activities in Chinese loess soil. Environ. Pollut. 2018, 242, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Chukwuemeka, I.S.; Li, G.; Mo, Y.; Jacques, K.J. Impacts of microplastics and urbanization on soil health: An urgent concern for sustainable development. Green Anal. Chem. 2024, 8, 100095. [Google Scholar]
- Zhai, Y.; Bai, J.; Chang, P.; Liu, Z.; Wang, Y.; Liu, G.; Cui, B.; Peijnenburg, W.; Vijver, M.G. Microplastics in terrestrial ecosystem: Exploring the menace to the soil-plant-microbe interactions. TrAC Trends Anal. Chem. 2024, 174, 117667. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Liang, X.; Lu, S.; Ren, J.; Zhang, Y.; Han, Z.; Gao, B.; Sun, K. Effects of microplastics on soil carbon pool and terrestrial plant performance. Carbon Res. 2024, 3, 37. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Gerdawischke, M.; Rillig, M.C. Microplastics have shape-and polymer-dependent effects on soil aggregation and organic matter loss—An experimental and meta-analytical approach. Microplast. Nanoplast. 2021, 1, 7. [Google Scholar] [CrossRef]
- Chen, L.; Han, L.; Feng, Y.; He, J.; Xing, B. Soil structures and immobilization of typical contaminants in soils in response to diverse microplastics. J. Hazard. Mater. 2022, 438, 129555. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, F.; Li, X. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, J.; Liu, Y.; Chen, L.; Tao, S.; Liu, W. Distribution characteristics of microplastics in agricultural soils from the largest vegetable production base in China. Sci. Total Environ. 2021, 756, 143860. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Beriot, N.; Gort, G.; Lwanga, E.H.; Gooren, H.; Yang, X.; Geissen, V. Impact of plastic mulch film debris on soil physicochemical and hydrological properties. Environ. Pollut. 2020, 266, 115097. [Google Scholar] [CrossRef]
- Yu, Y.; Battu, A.K.; Varga, T.; Denny, A.C.; Zahid, T.M.; Chowdhury, I.; Flury, M. Minimal impacts of microplastics on soil physical properties under environmentally relevant concentrations. Environ. Sci. Technol. 2023, 57, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Kim, S.W.; An, Y.-J. Soil microplastics inhibit the movement of springtail species. Environ. Int. 2019, 126, 699–706. [Google Scholar] [CrossRef]
- Chai, M.; Li, R.; Li, B.; Wu, H.; Yu, L. Responses of mangrove (Kandelia obovata) growth, photosynthesis, and rhizosphere soil properties to microplastic pollution. Mar. Pollut. Bull. 2023, 189, 114827. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, W.; Duan, C.; Zhu, X.; Wu, H.; Zhang, X.; Fang, L. Microplastics pollution from different plastic mulching years accentuate soil microbial nutrient limitations. Gondwana Res. 2022, 108, 91–101. [Google Scholar] [CrossRef]
- Gharahi, N.; Zamani-Ahmadmahmoodi, R. Effect of plastic pollution in soil properties and growth of grass species in semi-arid regions: A laboratory experiment. Environ. Sci. Pollut. Res. 2022, 29, 59118–59126. [Google Scholar] [CrossRef]
- Liu, S.; Huang, K.; Yuan, G.; Yang, C. Effects of Polyethylene Microplastics and Phenanthrene on Soil Properties, Enzyme Activities and Bacterial Communities. Processes 2022, 10, 2128. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, P.; Adams, C.A.; Zhang, S.; Sun, Y.; Yu, H.; Wang, F. Effects of microplastics on plant growth and arbuscular mycorrhizal fungal communities in a soil spiked with ZnO nanoparticles. Soil Biol. Biochem. 2021, 155, 108179. [Google Scholar] [CrossRef]
- You, J.; Liu, X.; Zhang, B.; Xie, Z.; Hou, Z.; Yang, Z. Seasonal changes in soil acidity and related properties in ginseng artificial bed soils under a plastic shade. J. Ginseng Res. 2015, 39, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, S.; Zhang, M.; Chen, G.; Zhu, T.; Zhang, S.; Teng, Y.; Christie, P.; Luo, Y. Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere 2016, 151, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liu, Q.; Li, J.; Wang, C.; Li, Y.; Zhang, C. Microplastic pollution in marine environment: Occurrence, fate, and effects (with a specific focus on biogeochemical carbon and nitrogen cycles). Microplast. Pollut. 2021, 105–126. [Google Scholar]
- Rong, L.; Zhao, L.; Zhao, L.; Cheng, Z.; Yao, Y.; Yuan, C.; Wang, L.; Sun, H. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 2021, 773, 145640. [Google Scholar] [CrossRef]
- Awet, T.; Kohl, Y.; Meier, F.; Straskraba, S.; Grün, A.-L.; Ruf, T.; Jost, C.; Drexel, R.; Tunc, E.; Emmerling, C. Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil. Environ. Sci. Eur. 2018, 30, 11. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, M.; Liu, G.; Lu, T.; Qu, Q.; Du, B.; Pan, X. Effects of soil residual plastic film on soil microbial community structure and fertility. Water Air Soil Pollut. 2018, 229, 261. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Gao, J.; Wang, F.; Yang, W.; Han, L.; Lin, D.; Min, B.; Zhi, Y.; Grieger, K. Effect of microplastics on ecosystem functioning: Microbial nitrogen removal mediated by benthic invertebrates. Sci. Total Environ. 2021, 754, 142133. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, L.; Zhao, Y.; Shan, J.; Zheng, X.; Xu, G.; Sun, Y.; Wang, F. Effects of microplastics and nitrogen deposition on soil multifunctionality, particularly C and N cycling. J. Hazard. Mater. 2023, 451, 131152. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, Y.; Cui, W.; Sun, Y.; Zhang, S.; Wang, F. Effects of microplastics and carbon nanotubes on soil geochemical properties and bacterial communities. J. Hazard. Mater. 2022, 433, 128826. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.; Angon, P.B.; Roy, A. Effects of microplastics on soil physical, chemical and biological properties. Nat. Hazards Res. 2024. [Google Scholar] [CrossRef]
- Hüffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.C.; Hofmann, T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total Environ. 2019, 657, 242–247. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Lv, J.; Wang, Z.; Peng, Y.; Shang, J.; Wang, X. Microplastic additions alter soil organic matter stability and bacterial community under varying temperature in two contrasting soils. Sci. Total Environ. 2022, 838, 156471. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, X.; Wu, D.; Peng, L.; Fan, C.; Zhang, W.; Li, Q.; Ge, C. Addition of biodegradable microplastics alters the quantity and chemodiversity of dissolved organic matter in latosol. Sci. Total Environ. 2022, 816, 151960. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, E.; Bodor, A.; Kovács, E.; Papp, S.; Kovács, K.; Perei, K.; Feigl, G. Impacts of plastics on plant development: Recent advances and future research directions. Plants 2023, 12, 3282. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Osborn, A.M.; Duhaime, M.B. Microbes on a bottle: Substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS ONE 2016, 11, e0159289. [Google Scholar] [CrossRef]
- De Tender, C.A.; Devriese, L.I.; Haegeman, A.; Maes, S.; Ruttink, T.; Dawyndt, P. Bacterial community profiling of plastic litter in the Belgian part of the North Sea. Environ. Sci. Technol. 2015, 49, 9629–9638. [Google Scholar] [CrossRef]
- Carson, H.S.; Nerheim, M.S.; Carroll, K.A.; Eriksen, M. The plastic-associated microorganisms of the North Pacific Gyre. Mar. Pollut. Bull. 2013, 75, 126–132. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; Chi, J.d.l.A.; Sanchez del Cid, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salánki, T.; van der Ploeg, M. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic in Terrestrial Ecosystems and the Soil? ACS Publications: Washington, WA, USA, 2012. [Google Scholar]
- Rillig, M.C.; Bonkowski, M. Microplastic and soil protists: A call for research. Environ. Pollut. 2018, 241, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, Z.; Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Effect of polyvinyl chloride microplastics on bacterial community and nutrient status in two agricultural soils. Bull. Environ. Contam. Toxicol. 2021, 107, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xu, X.; Yu, H.; Xi, B.; Tan, W. Comparing the long-term responses of soil microbial structures and diversities to polyethylene microplastics in different aggregate fractions. Environ. Int. 2021, 149, 106398. [Google Scholar] [CrossRef] [PubMed]
- Gaylor, M.O.; Harvey, E.; Hale, R.C. Polybrominated diphenyl ether (PBDE) accumulation by earthworms (Eisenia fetida) exposed to biosolids-, polyurethane foam microparticle-, and penta-BDE-amended soils. Environ. Sci. Technol. 2013, 47, 13831–13839. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Seijo, A.; Lourenço, J.; Rocha-Santos, T.; Da Costa, J.; Duarte, A.; Vala, H.; Pereira, R. Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ. Pollut. 2017, 220, 495–503. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and Arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- Wan, L.; Cheng, H.; Liu, Y.; Shen, Y.; Liu, G.; Su, X. Global meta-analysis reveals differential effects of microplastics on soil ecosystem. Sci. Total Environ. 2023, 867, 161403. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Sun, X.; Peng, Y.; Xiao, L. Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tang, J.; Liu, X.; Liu, Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 2020, 256, 113347. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zhou, S.; Zhang, L.; Ding, S. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2021, 93, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Moyal, J.; Dave, P.H.; Wu, M.; Karimpour, S.; Brar, S.K.; Zhong, H.; Kwong, R.W. Impacts of biofilm formation on the physicochemical properties and toxicity of microplastics: A concise review. Rev. Environ. Contam. Toxicol. 2023, 261, 8. [Google Scholar] [CrossRef]
- Van Melkebeke, M.; Janssen, C.; De Meester, S. Characteristics and sinking behavior of typical microplastics including the potential effect of biofouling: Implications for remediation. Environ. Sci. Technol. 2020, 54, 8668–8680. [Google Scholar] [CrossRef]
- Enfrin, M.; Lee, J.; Le-Clech, P.; Dumee, L.F. Kinetic and mechanistic aspects of ultrafiltration membrane fouling by nano-and microplastics. J. Membr. Sci. 2020, 601, 117890. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ingraffia, R.; de Souza Machado, A.A. Microplastic incorporation into soil in agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Chen, L.; Chao, J.; Teng, J.; Wang, Q. Microplastics in soils: A review of possible sources, analytical methods and ecological impacts. J. Chem. Technol. Biotechnol. 2020, 95, 2052–2068. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; Lu, S.; Qiu, R.; Hu, J.; Li, X.; Bigalke, M.; Shi, H.; He, D. A method for extracting soil microplastics through circulation of sodium bromide solutions. Sci. Total Environ. 2019, 691, 341–347. [Google Scholar] [CrossRef]
- Komar, P.D.; Reimers, C. Grain shape effects on settling rates. J. Geol. 1978, 86, 193–209. [Google Scholar] [CrossRef]
- Fiett, P.P. The Effects of Biofouling on Distribution of Microplastics in the Aquatic Environment. Master’s Thesis, Roskilde University, Roskilde, Denmark, 2017. [Google Scholar]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Kooi, M.; Nes, E.H.V.; Scheffer, M.; Koelmans, A.A. Ups and downs in the ocean: Effects of biofouling on vertical transport of microplastics. Environ. Sci. Technol. 2017, 51, 7963–7971. [Google Scholar] [CrossRef]
- Jalón-Rojas, I.; Romero-Ramírez, A.; Fauquembergue, K.; Rossignol, L.; Cachot, J.; Sous, D.; Morin, B. Effects of biofilms and particle physical properties on the rising and settling velocities of microplastic fibers and sheets. Environ. Sci. Technol. 2022, 56, 8114–8123. [Google Scholar] [CrossRef]
- Lobelle, D.; Cunliffe, M. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 2011, 62, 197–200. [Google Scholar] [CrossRef]
- Ryan, P.G. A brief history of marine litter research. Mar. Anthropog. Litter 2015, 1–25. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Quik, J.T.; de Klein, J.J.; Koelmans, A.A. Spatially explicit fate modelling of nanomaterials in natural waters. Water Res. 2015, 80, 200–208. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef]
- Khatmullina, L.; Isachenko, I. Settling velocity of microplastic particles of regular shapes. Mar. Pollut. Bull. 2017, 114, 871–880. [Google Scholar] [CrossRef]
- Waldschläger, K.; Schüttrumpf, H. Effects of particle properties on the settling and rise velocities of microplastics in freshwater under laboratory conditions. Environ. Sci. Technol. 2019, 53, 1958–1966. [Google Scholar] [CrossRef]
- Kaiser, D.; Estelmann, A.; Kowalski, N.; Glockzin, M.; Waniek, J.J. Sinking velocity of sub-millimeter microplastic. Mar. Pollut. Bull. 2019, 139, 214–220. [Google Scholar] [CrossRef]
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319. [Google Scholar] [CrossRef]
- Guo, Z.; Li, P.; Yang, X.; Wang, Z.; Lu, B.; Chen, W.; Wu, Y.; Li, G.; Zhao, Z.; Liu, G. Soil texture is an important factor determining how microplastics affect soil hydraulic characteristics. Environ. Int. 2022, 165, 107293. [Google Scholar] [CrossRef]
- Fazey, F.M.; Ryan, P.G. Debris size and buoyancy influence the dispersal distance of stranded litter. Mar. Pollut. Bull. 2016, 110, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Leiser, R.; Wu, G.-M.; Neu, T.R.; Wendt-Potthoff, K. Biofouling, metal sorption and aggregation are related to sinking of microplastics in a stratified reservoir. Water Res. 2020, 176, 115748. [Google Scholar] [CrossRef] [PubMed]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, Y.; Xu, L.; Shi, W.; Wang, F.; Leblanc, G.A.; Cui, S.; An, L.; Lei, K. Investigation of the microplastics pro fi le in sludge from China’s largest Water reclamation plant using a feasible. J. Hazard. Mater. 2020, 388, 122067. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.; Nor’en, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; Swedish Environmental Research Institute: Stockholm, Sweden, 2014. [Google Scholar]
- Hongprasith, N.; Kittimethawong, C.; Lertluksanaporn, R.; Eamchotchawalit, T.; Kittipongvises, S.; Lohwacharin, J. IR microspectroscopic identification of microplastics in municipal wastewater treatment plants. Environ. Sci. Pollut. Res. 2020, 27, 18557–18564. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Brandes, E.; Henseler, M.; Kreins, P. Identifying hot-spots for microplastic contamination in agricultural soils—A spatial modelling approach for Germany. Environ. Res. Lett. 2021, 16, 104041. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Kim, B.-H.; Kim, K.-H. Distribution of microplastics in soil by types of land use in metropolitan area of Seoul. Appl. Biol. Chem. 2024, 67, 15. [Google Scholar] [CrossRef]
- Wang, K.; Min, W.; Flury, M.; Gunina, A.; Lv, J.; Li, Q.; Jiang, R. Impact of long-term conventional and biodegradable film mulching on microplastic abundance, soil structure and organic carbon in a cotton field. Environ. Pollut. 2024, 356, 124367. [Google Scholar] [CrossRef]
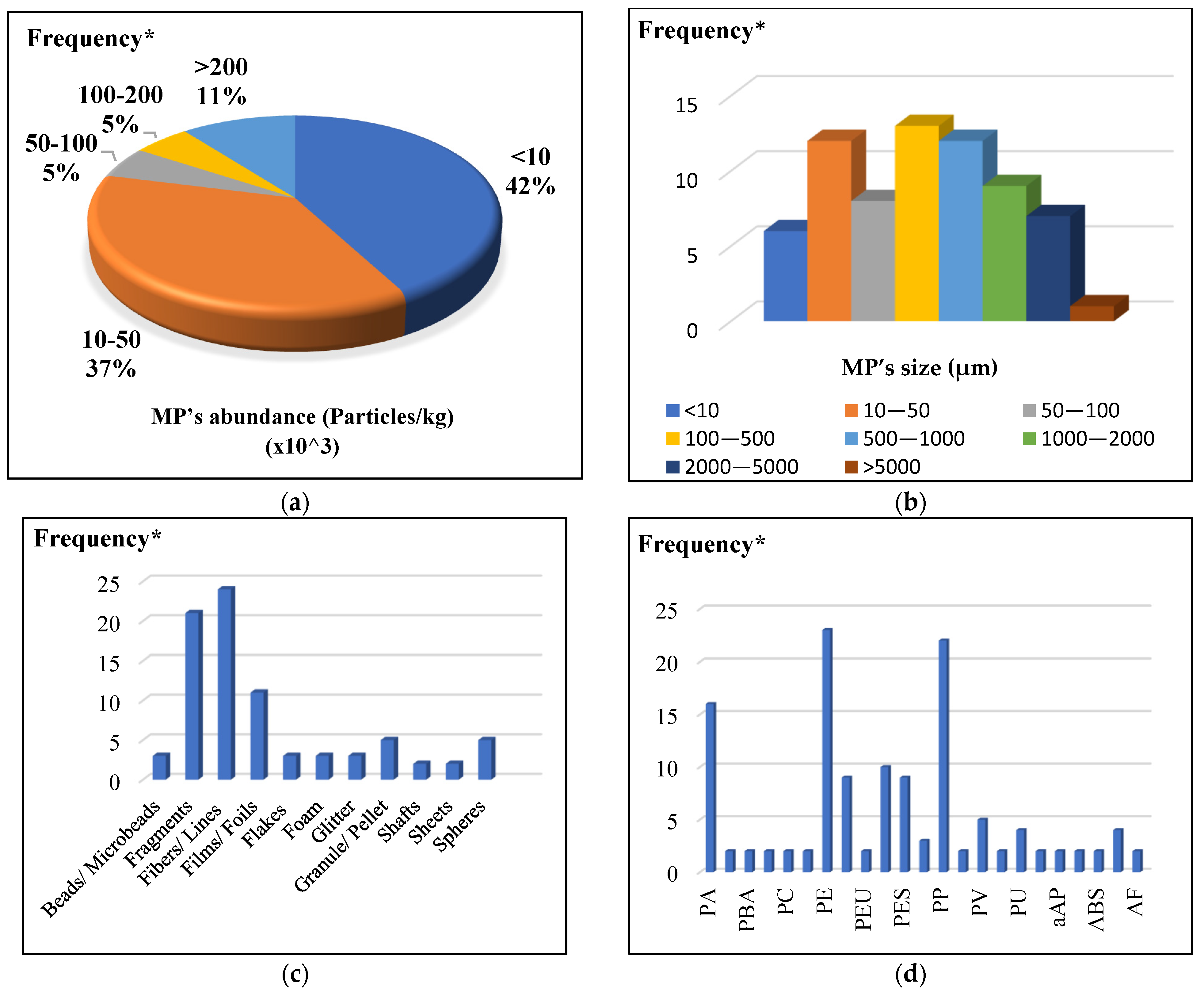

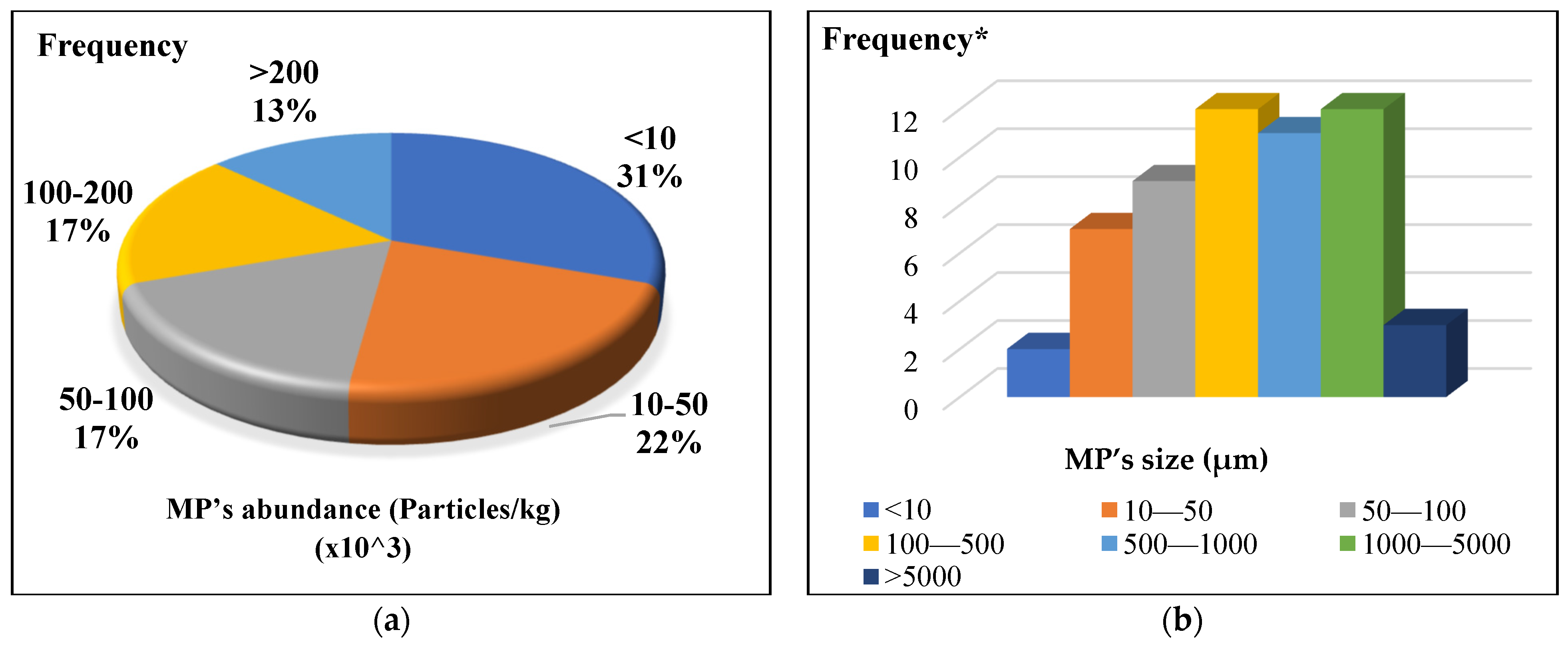

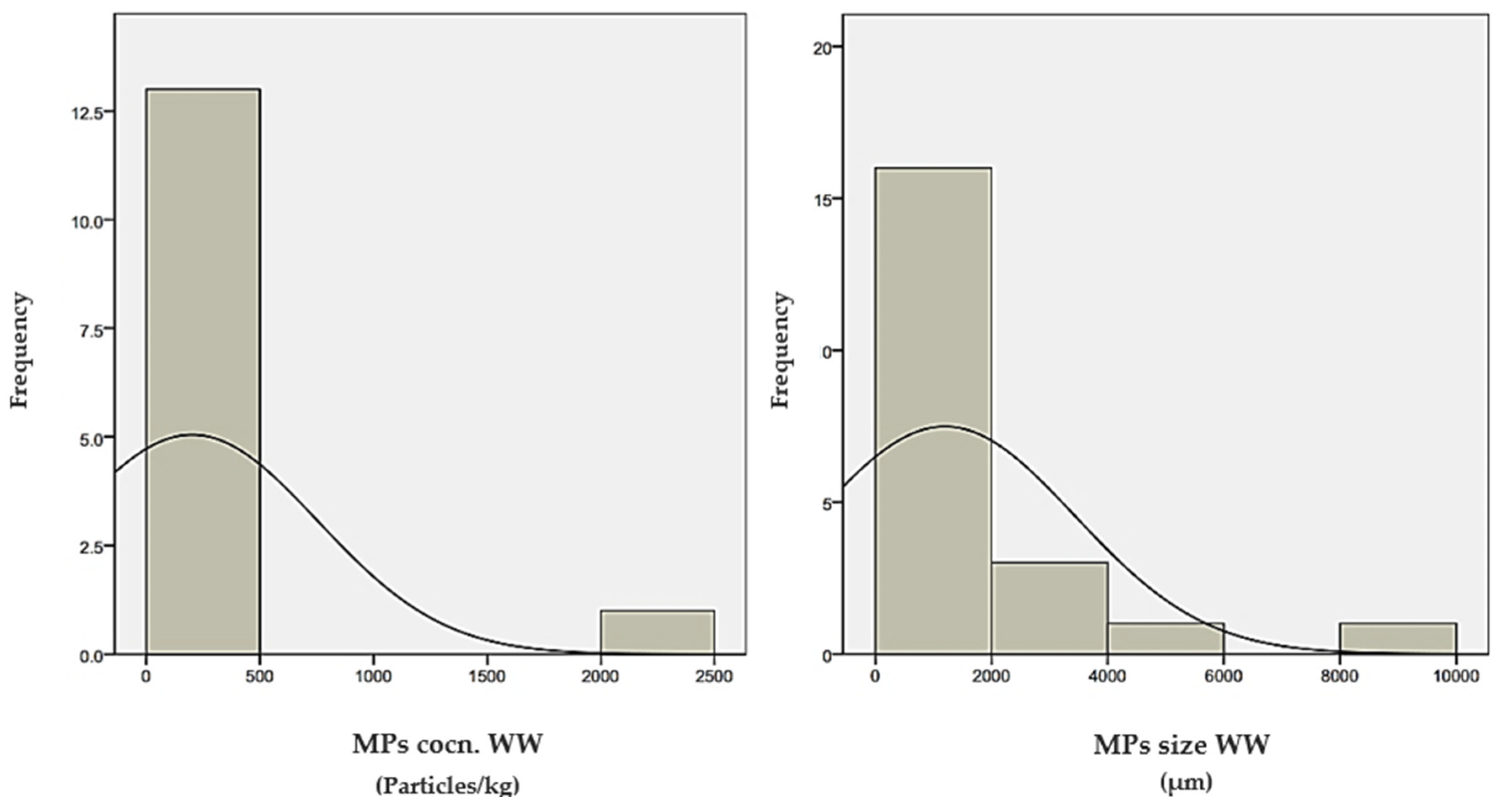
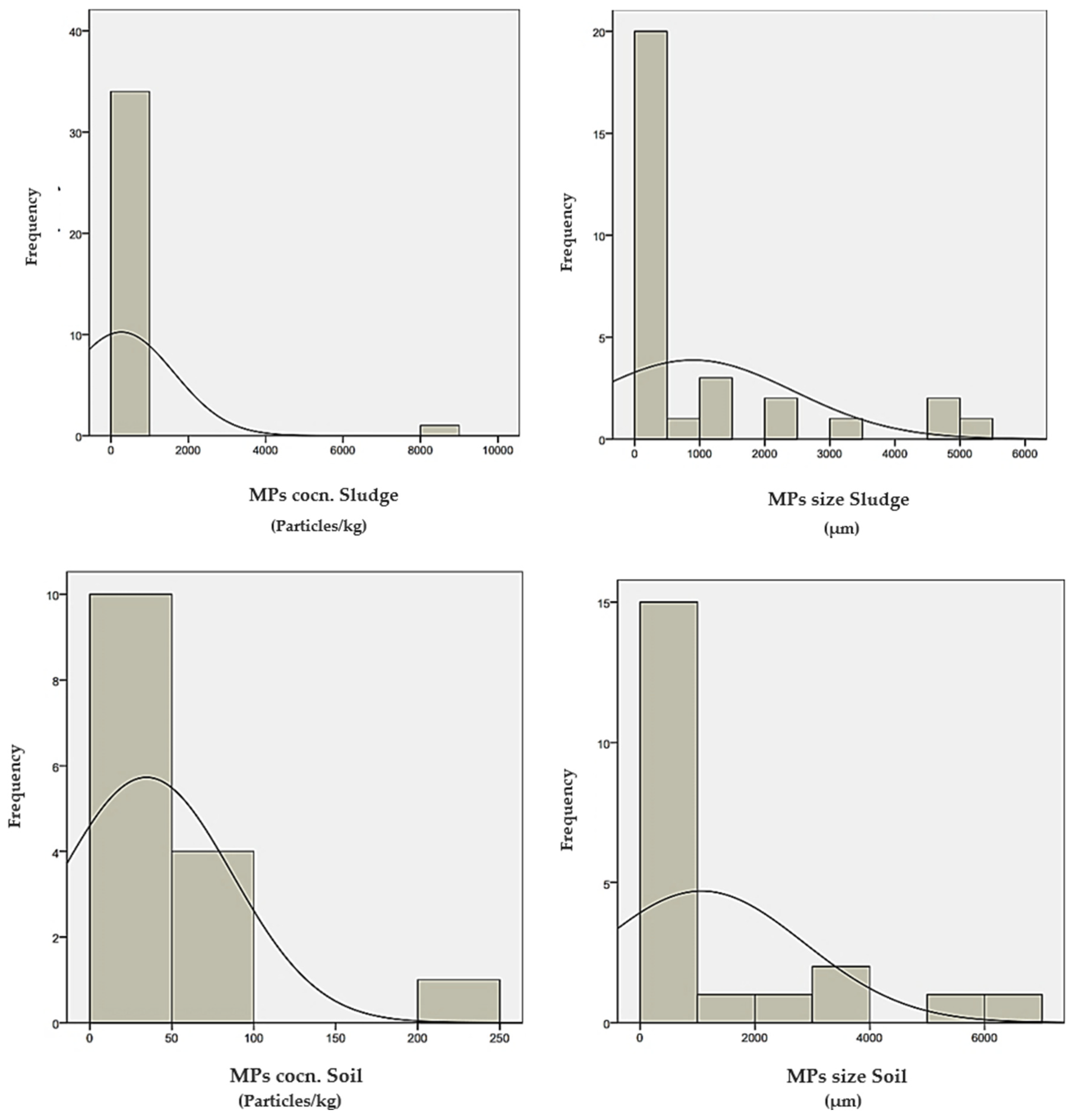
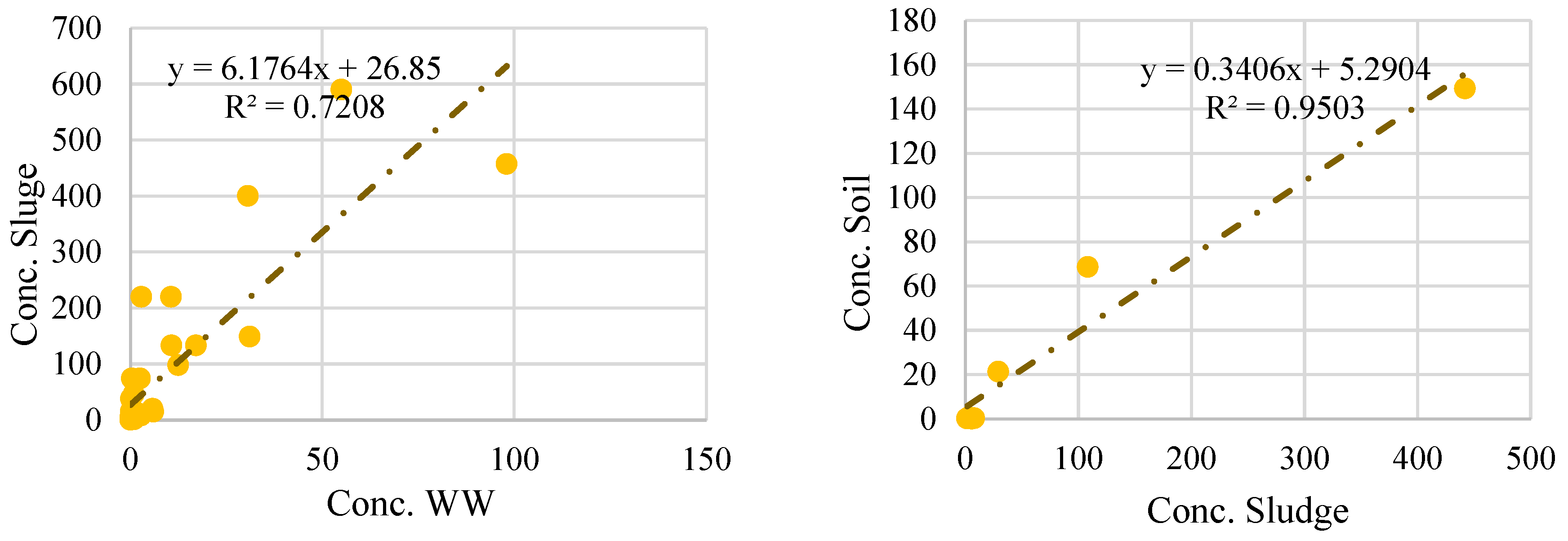

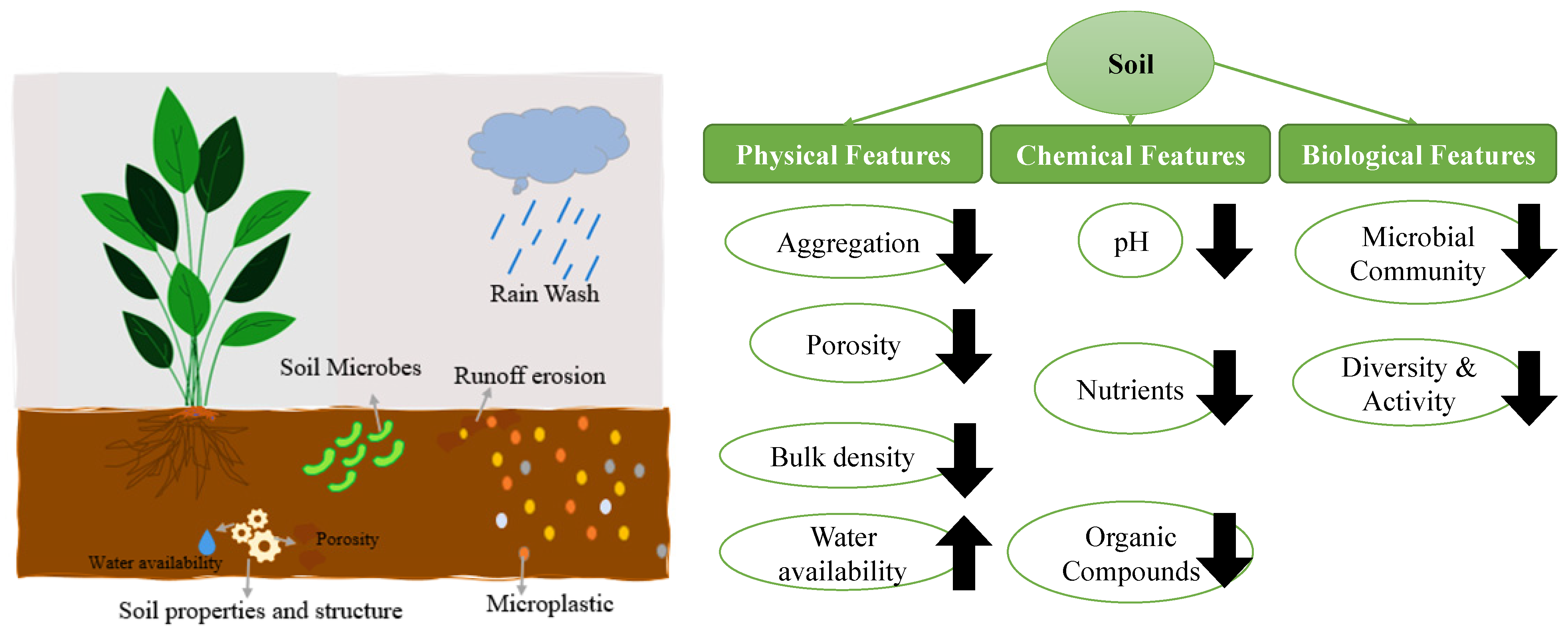

| Descriptive Statistics | MPs’ Conc. in WW (Particles/kg) | MPs’ Conc. in Sludges (Particles/kg) | MPs’ Conc. in Soils (Particles/kg) | MPs’ Size in WW (µm) | MPs’ Size in Sludges (µm) | MPs’ Size in Soils (µm) |
|---|---|---|---|---|---|---|
| Mean | 201 | 271 | 34.6 | 1199 | 899 | 1072 |
| Median | 14 | 10 | 18 | 150 | 66 | 125 |
| Standard Deviation | 553 | 1365 | 52 | 2238 | 1547 | 1785 |
| Kurtosis | 13.2 | 34.7 | 7.5 | 7.2 | 2.7 | 2.4 |
| Skewness | 3.6 | 5.9 | 2.5 | 2.6 | 2 | 1.9 |
| Range | 2101 | 8100 | 200 | 9000 | 5000 | 6000 |
| Minimum | 1 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 2101 | 8100 | 200 | 9000 | 5000 | 6000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arab, M.; Yu, J.; Nayebi, B. Microplastics in Sludges and Soils: A Comprehensive Review on Distribution, Characteristics, and Effects. ChemEngineering 2024, 8, 86. https://doi.org/10.3390/chemengineering8050086
Arab M, Yu J, Nayebi B. Microplastics in Sludges and Soils: A Comprehensive Review on Distribution, Characteristics, and Effects. ChemEngineering. 2024; 8(5):86. https://doi.org/10.3390/chemengineering8050086
Chicago/Turabian StyleArab, Maliheh, Jimmy Yu, and Behnam Nayebi. 2024. "Microplastics in Sludges and Soils: A Comprehensive Review on Distribution, Characteristics, and Effects" ChemEngineering 8, no. 5: 86. https://doi.org/10.3390/chemengineering8050086







