Oxidation of Airborne m-Xylene in Pulsed Corona Discharge: Impact of Water Sprinkling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. PCD Reactor
2.3. Experimental Procedure
3. Results and Discussion
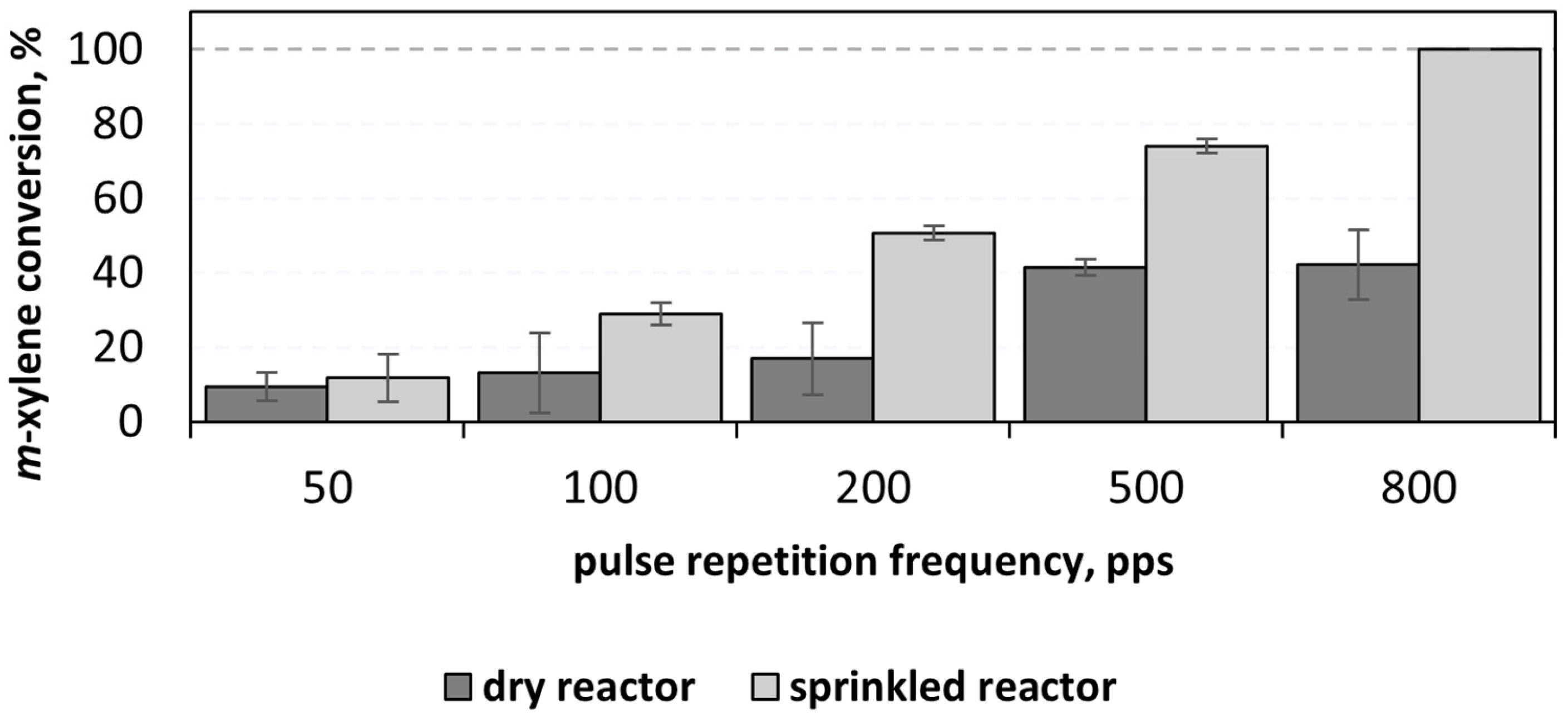
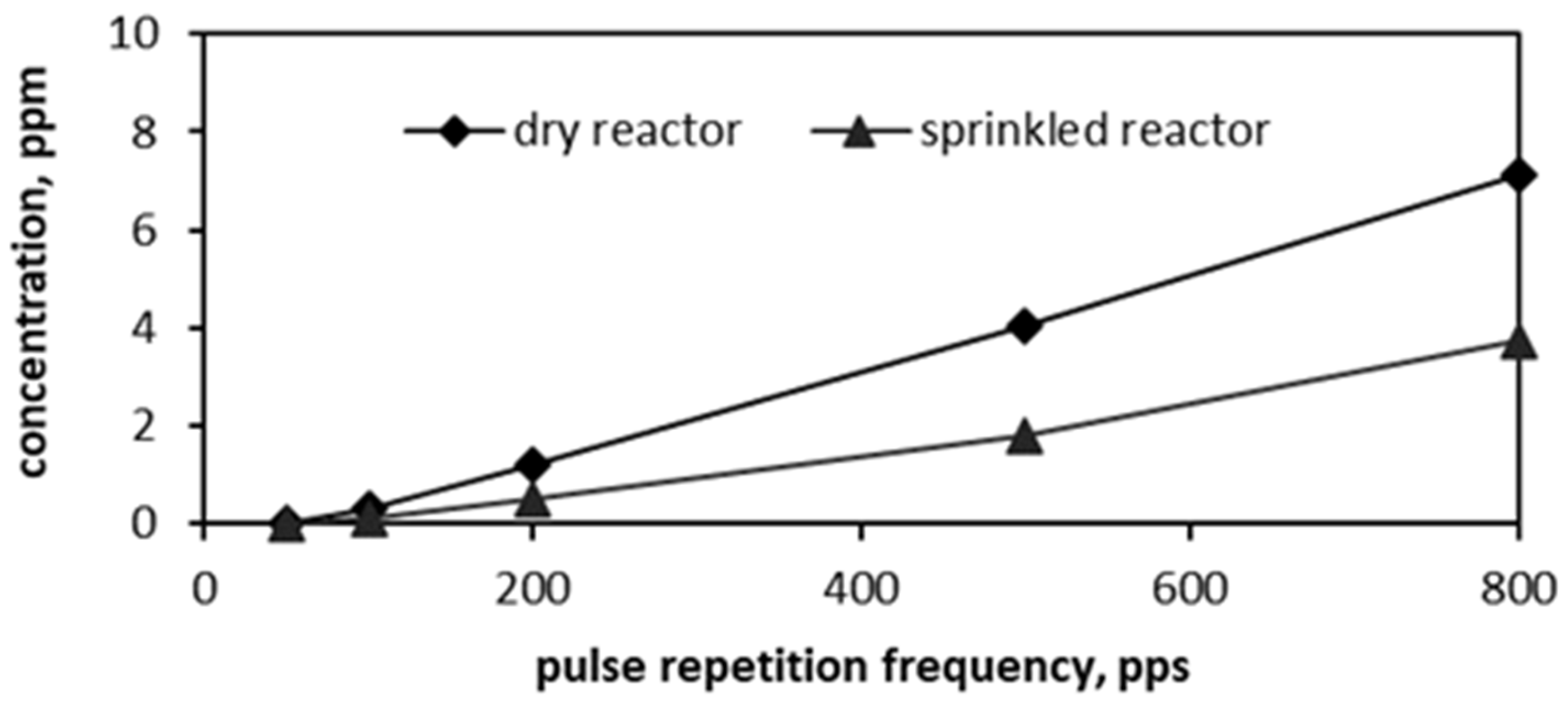
3.1. PCD Oxidation of Airborne m-Xylene
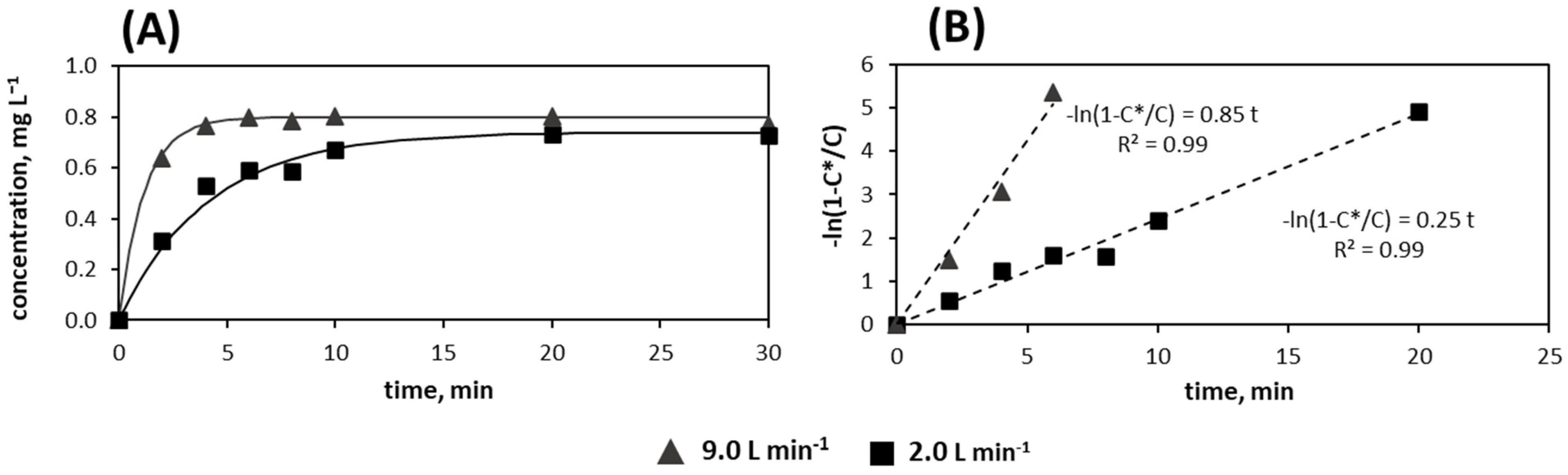
3.2. m-Xylene Absorption and Oxidation in Water
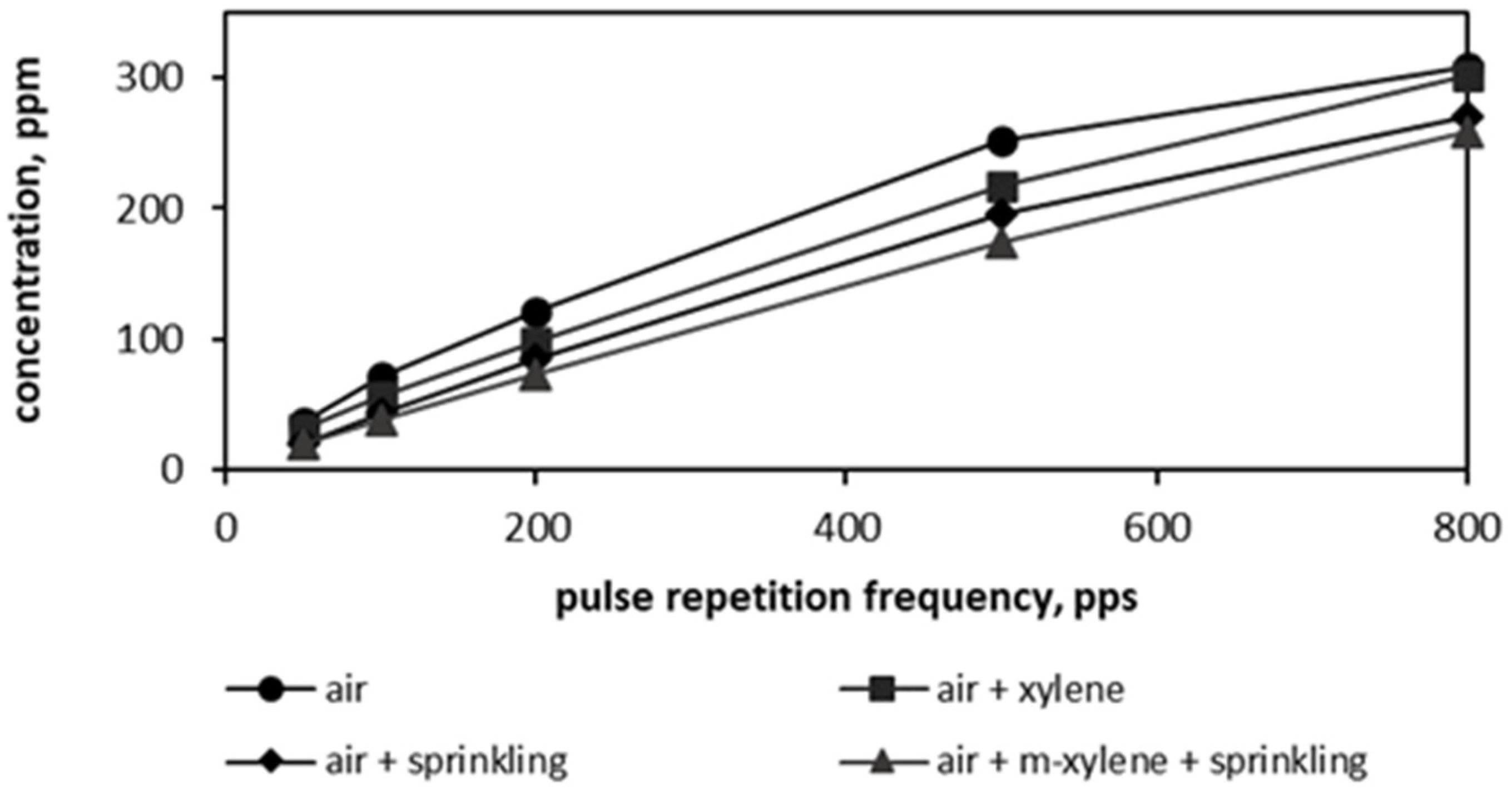
3.3. Ozone Generation in PCD Reactor
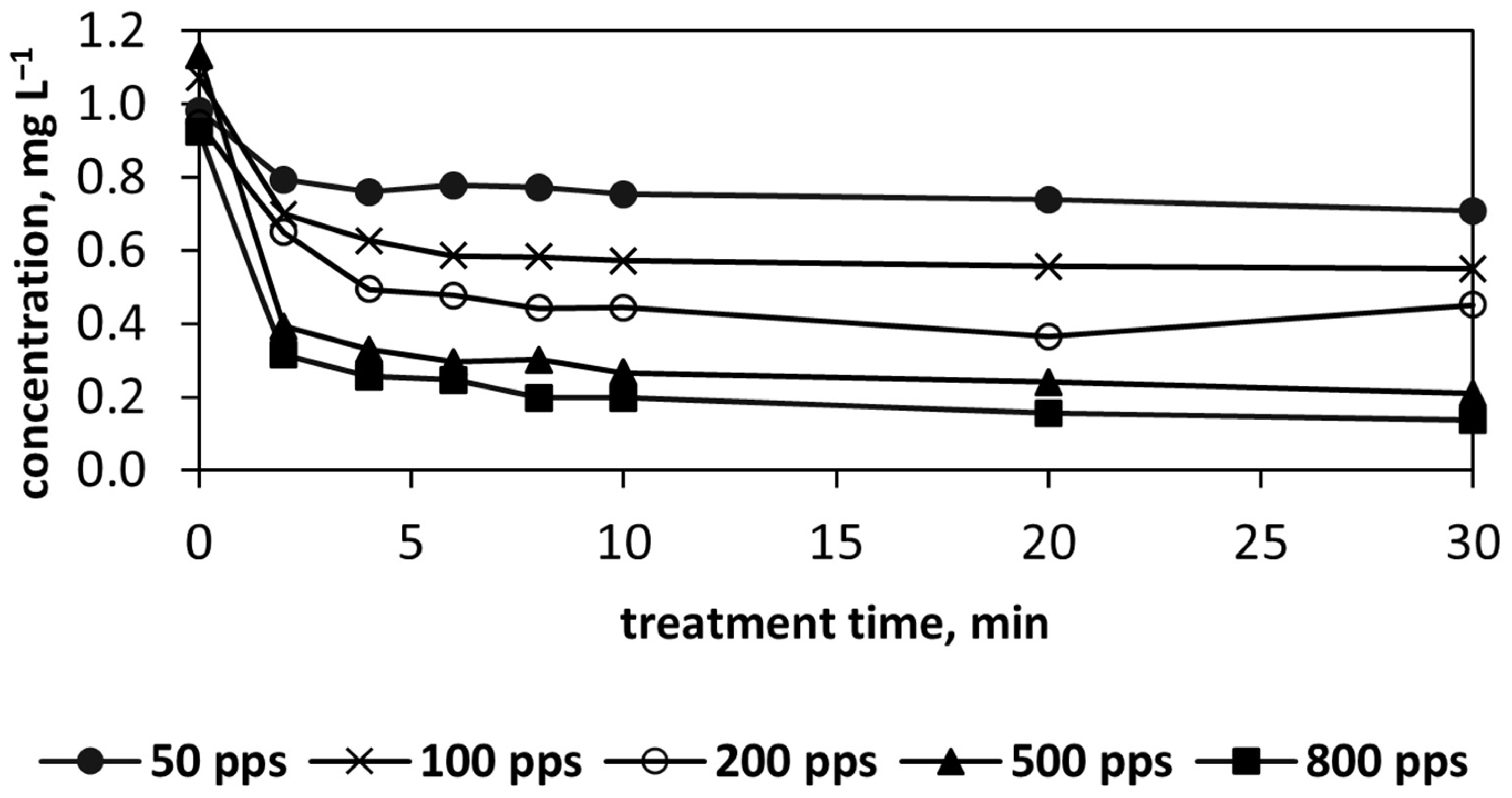
3.4. m-Xylene Oxidation: Impact of Water Circulation Rate
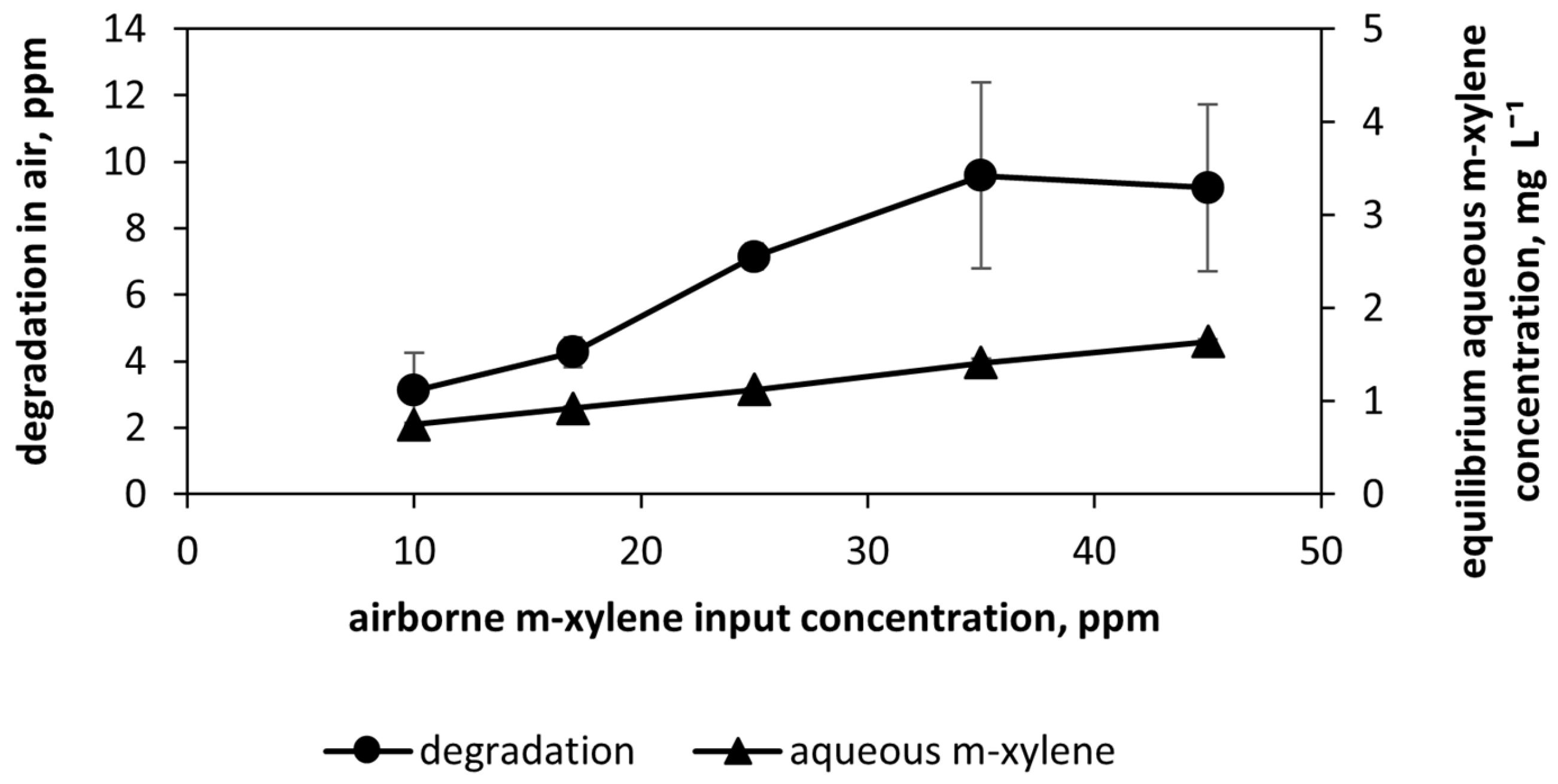
3.5. m-Xylene Oxidation: Impact of Airborne m-Xylene Concentration
3.6. m-Xylene Oxidation: Impact of Sprinkling Water pH
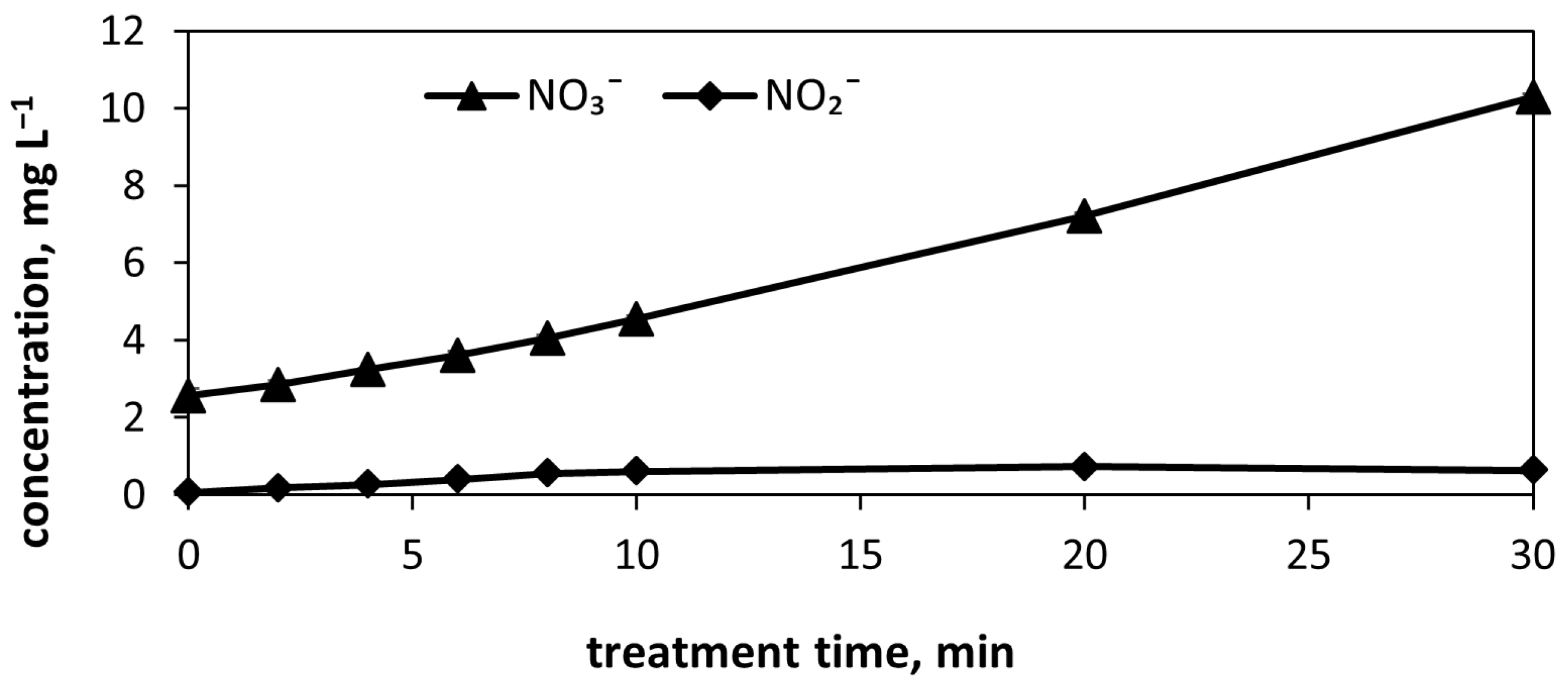
3.7. Nitrogen Oxidation
3.8. Energy Efficiency of m-Xylene Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearson, J.K. European solvent VOC emission inventories based on industry-wide information. Atmos. Environ. 2019, 204, 118–124. [Google Scholar] [CrossRef]
- European Parliament and of the Council of the European Union. Directive (EU) 2016/2284 of the European Parliament and of the Council of 14 December 2016 on the reduction of national emissions of certain atmospheric pollutants, amending Directive 2003/35/EC and repealing Directive 2001/81/EC. Off. J. Eur. Union. 2016. Available online: https://eur-lex.europa.eu/eli/dir/2016/2284/oj (accessed on 15 September 2024).
- Hirota, K.; Sakai, H.; Washio, M.; Kojima, T. Application of Electron Beams for the Treatment of VOC Streams. Ind. Eng. Chem. Res. 2004, 43, 1185–1191. [Google Scholar] [CrossRef]
- Revah, S.; Morgan-Sagastume, J.M. Biotechnology for Odor and Air Pollution Control; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Kornev, I.; Preis, S. Aqueous Benzene Oxidation in Low-Temperature Plasma of Pulsed Corona Discharge. J. Adv. Oxid. Technol. 2016, 19, 284–289. [Google Scholar] [CrossRef][Green Version]
- Du, C.; Gong, X.; Lin, Y. Decomposition of volatile organic compounds using corona discharge plasma technology. J. Air Waste Manag. Assoc. 2019, 69, 879–899. [Google Scholar] [CrossRef]
- Hezami, L.; Nguyen-Tri, P.; Saoud, W.A.; Bouzaza, A.; El Jery, A.; Nguyen, D.D.; Gupta, V.K.; Assadi, A.A. Assadi, Recent progress in air treatment with combined photocatalytic/plasma processes: A review. J. Environ. Manag. 2021, 299, 113588. [Google Scholar] [CrossRef]
- Onga, L.; Kattel-Salusoo, E.; Preis, S.; Dulova, N. Degradation of anti-inflammatory drug dexamethasone by pulsed corona discharge: The effect of peroxycompounds addition. J. Environ. Chem. Eng. 2022, 10, 108042. [Google Scholar] [CrossRef]
- Tikker, P.; Nikitin, D.; Preis, S. Oxidation of aqueous bisphenols A and S by pulsed corona discharge: Impacts of process control parameters and oxidation products identification. Chem. Eng. J. 2022, 438, 135602. [Google Scholar] [CrossRef]
- Sobacchi, M.G.; Saveliev, A.V.; Fridman, A.A.; Gutsol, A.F.; Kennedy, L.A. Experimental Assessment of Pulsed Corona Discharge for Treatment of VOC Emissions. Plasma Chem. Plasma Process. 2003, 23, 347–370. [Google Scholar] [CrossRef]
- Jiang, N.; Lu, N.; Shang, K.; Li, J.; Wu, Y. Innovative approach for benzene degradation using hybrid surface/packed-bed discharge plasmas. Environ. Sci. Technol. 2013, 47, 9898–9903. [Google Scholar] [CrossRef]
- Kask, M.; Krichevskaya, M.; Preis, S.; Bolobajev, J. Oxidation of Aqueous Toluene by Gas-Phase Pulsed Corona Discharge in Air-Water Mixtures Followed by Photocatalytic Exhaust Air Cleaning. Catalysts 2021, 11, 549. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Sato, M.; Ohgiyama, T.; Clements, J.S. Formation of chemical species and their effects on microorganisms using a pulsed high voltage discharge in water. In Proceedings of the 1994 IEEE Industry Applications Society Annual Meeting, Denver, CO, USA, 2–6 October 1994. [Google Scholar] [CrossRef]
- Schiorlin, M.; Marotta, E.; Rea, M.; Paradisi, C. Comparison of toluene removal in air at atmospheric conditions by different corona discharges. Environ. Sci. Technol. 2009, 43, 9386–9392. [Google Scholar] [CrossRef] [PubMed]
- Zoveidavianpoor, M.; Samsuri, A.; Shadizadeh, S.R. Safety, and Environmental Challenges of Xylene in Upstream Petroleum Industry. Energy Environ. 2012, 23, 1339–1352. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Public Health Statement Xylene. 2007. Available online: www.atsdr.cdc.gov/ (accessed on 28 July 2024).
- Niaz, K.; Bahadar, H.; Maqbool, F.; Abdollahi, M. A review of environmental and occupational exposure to xylene and its health concerns. EXCLI J. 2015, 14, 1167–1186. [Google Scholar] [CrossRef]
- European Chemicals Agency, Substance Infocard (Xylene). 2024. Available online: https://Echa.Europa.Eu/et/Substance-Information/-/Substanceinfo/100.014.124 (accessed on 28 July 2024).
- Bolden, A.L.; Kwiatkowski, C.F.; Colborn, T. New look at BTEX: Are ambient levels a problem. Environ. Sci. Technol. 2015, 49, 5261–5276. [Google Scholar] [CrossRef]
- Altof, K.; Krichevskaya, M.; Preis, S.; Tähemaa, T.; Bolobajev, J. Ozone-assisted degradation of 2-methoxyethanol in a prototype plug flow photocatalytic reactor. Chem. Eng. J. 2024, 481, 148488. [Google Scholar] [CrossRef]
- Khadem, A.; Khani, M.R.; Rad, R.H.; Shokri, B.; Rashnoo, S.; Ghobadian, B. Experimental analysis of volatile organic compounds conversion by a dielectric barrier discharge reactor to study the main products: Hydrogen, CO, CO2, NOx and hydrocarbons. Chem. Eng. Process. Process Intensif. 2019, 145, 107660. [Google Scholar] [CrossRef]
- Chen, J.; Xie, Z.; Tang, J.; Zhou, J.; Lu, X.; Zhao, H. Oxidation of toluene by dielectric barrier discharge with photo-catalytic electrode. Chem. Eng. J. 2016, 284, 166–173. [Google Scholar] [CrossRef]
- Carey, J.H. An Introduction to Advanced Oxidation Processes (AOP) for Destruction of Organics in Wastewater. Water Qual. Res. J. 1992, 27, 103466. [Google Scholar] [CrossRef]
- Tikker, P.; Kornev, I.; Preis, S. Oxidation energy efficiency in water treatment with gas-phase pulsed corona discharge as a function of spray density. J. Electrost. 2020, 106, 103466. [Google Scholar] [CrossRef]
- Preis, S.; Panorel, I.C.; Kornev, I.; Hatakka, H.; Kallas, J. Pulsed corona discharge: The role of ozone and hydroxyl radical in aqueous pollutants oxidation. Water Sci. Technol. 2013, 68, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Lukes, P.; Clupek, M.; Babicky, V.; Janda, V.; Sunka, P. Generation of ozone by pulsed corona discharge over water surface in hybrid gas-liquid electrical discharge reactor. J. Phys. D Appl. Phys. 2005, 38, 409–416. [Google Scholar] [CrossRef]
- Kornev, I.; Saprykin, F.; Preis, S. Stability and energy efficiency of pulsed corona discharge in treatment of dispersed high-conductivity aqueous solutions. J. Electrost. 2017, 89, 42–50. [Google Scholar] [CrossRef]
- Ono, R.; Oda, T. Dynamics of ozone and OH radicals generated by pulsed corona discharge in humid-air flow reactor measured by laser spectroscopy. J. Appl. Phys. 2003, 93, 5876–5882. [Google Scholar] [CrossRef]
- Ajo, P.; Kornev, I.; Preis, S. Pulsed Corona Discharge Induced Hydroxyl Radical Transfer Through the Gas-Liquid Interface. Sci. Rep. 2017, 7, 16152. [Google Scholar] [CrossRef]
- Onga, L.; Kattel-Salusoo, E.; Trapido, M.; Preis, S. Oxidation of Aqueous Dexamethasone Solution by Gas-Phased Pulsed Corona Discharge. Water 2022, 14, 467. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar] [CrossRef]
- Ridgway, H.F.; Mohan, B.; Cui, X.; Chua, K.J.; Islam, M.R. Molecular dynamics simulation of gas-phase ozone reactions with sabinene and benzene. J. Mol. Graph. Model. 2017, 74, 241–250. [Google Scholar] [CrossRef]
- Fan, X.; Kang, S.; Li, J.; Zhu, T. Formation of Nitrogen Oxides (N2O, NO, and NO2) in Typical Plasma and Plasma-Catalytic Processes for Air Pollution Control. Water Air Soil Pollut. 2018, 229, 351. [Google Scholar] [CrossRef]
- Sakakura, T.; Murakami, N.; Takatsuji, Y.; Morimoto, M.; Haruyama, T. Contribution of Discharge Excited Atomic N, N2*, and N2+ to a Plasma/Liquid Interfacial Reaction as Suggested by Quantitative Analysis. ChemPhysChem 2019, 20, 1467–1474. [Google Scholar] [CrossRef]
- Preis, S.; Panorel, I.; Coll, S.L.; Kornev, I. Formation of Nitrates in Aqueous Solutions Treated with Pulsed Corona Discharge: The Impact of Organic Pollutants. Ozone Sci. Eng. 2014, 36, 94–99. [Google Scholar] [CrossRef]
- Kočí, K.; Krejčíková, S.; Šolcová, O.; Obalová, L. Photocatalytic decomposition of N2O on Ag-TiO2. Catal. Today 2012, 191, 134–137. [Google Scholar] [CrossRef]
- Shang, K.; Wang, X.; Zhou, X.; Wang, N. Diagnosis of electron temperature in Ar/O2 mixed gas and destruction of toluene/benzene by positive dc discharge plasma. J. Electrost. 2009, 67, 746–750. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altof, K.; Krichevskaya, M.; Preis, S.; Bolobajev, J. Oxidation of Airborne m-Xylene in Pulsed Corona Discharge: Impact of Water Sprinkling. ChemEngineering 2024, 8, 99. https://doi.org/10.3390/chemengineering8050099
Altof K, Krichevskaya M, Preis S, Bolobajev J. Oxidation of Airborne m-Xylene in Pulsed Corona Discharge: Impact of Water Sprinkling. ChemEngineering. 2024; 8(5):99. https://doi.org/10.3390/chemengineering8050099
Chicago/Turabian StyleAltof, Kristen, Marina Krichevskaya, Sergei Preis, and Juri Bolobajev. 2024. "Oxidation of Airborne m-Xylene in Pulsed Corona Discharge: Impact of Water Sprinkling" ChemEngineering 8, no. 5: 99. https://doi.org/10.3390/chemengineering8050099
APA StyleAltof, K., Krichevskaya, M., Preis, S., & Bolobajev, J. (2024). Oxidation of Airborne m-Xylene in Pulsed Corona Discharge: Impact of Water Sprinkling. ChemEngineering, 8(5), 99. https://doi.org/10.3390/chemengineering8050099






