Physicochemical Factors Affecting the Rheology and Stability of Peach Puree Dispersions
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Percentage of Total Dissolved Solids TDS%
2.3. Rheological Behavior
2.3.1. Sample Preparation for Rheological Measurements
2.3.2. Rheological Measurements
2.3.3. Rheological Calculations
2.4. Particle Size
2.5. Zeta-Potential
2.6. Mechanical and Ultrasonic Homogenization Process
2.7. Determination of Sedimentation Phenomena
2.8. Statistical Analysis
3. Results
3.1. Rheological Properties of Peach Puree
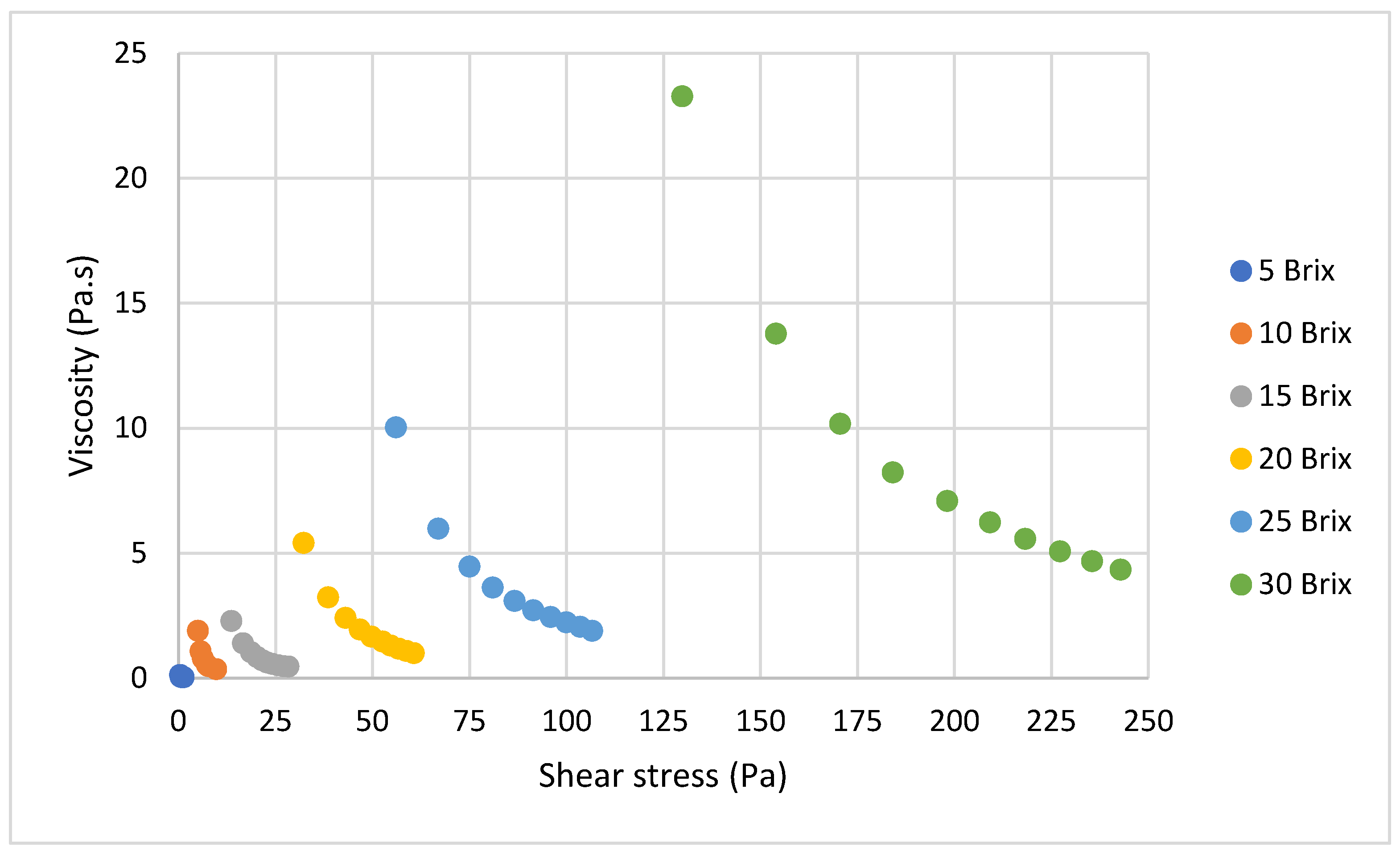
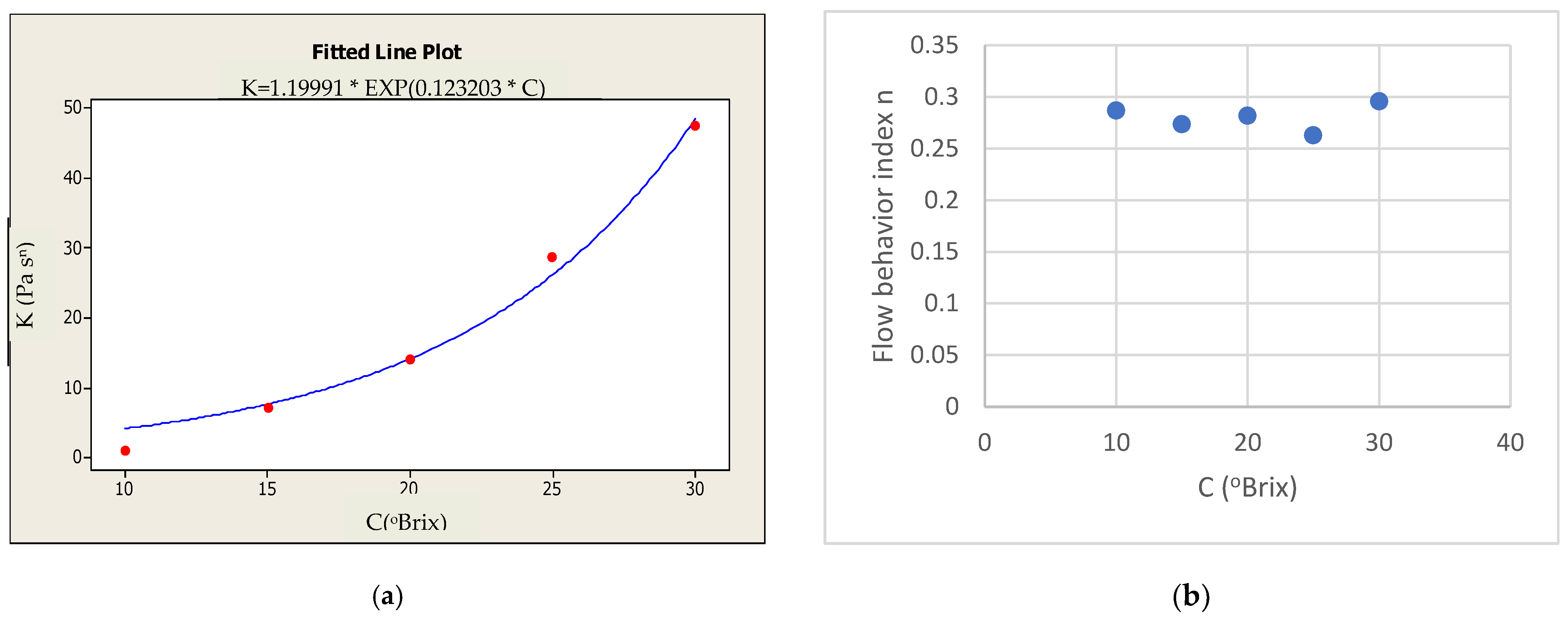
3.1.1. Effect of Soluble Solids Content
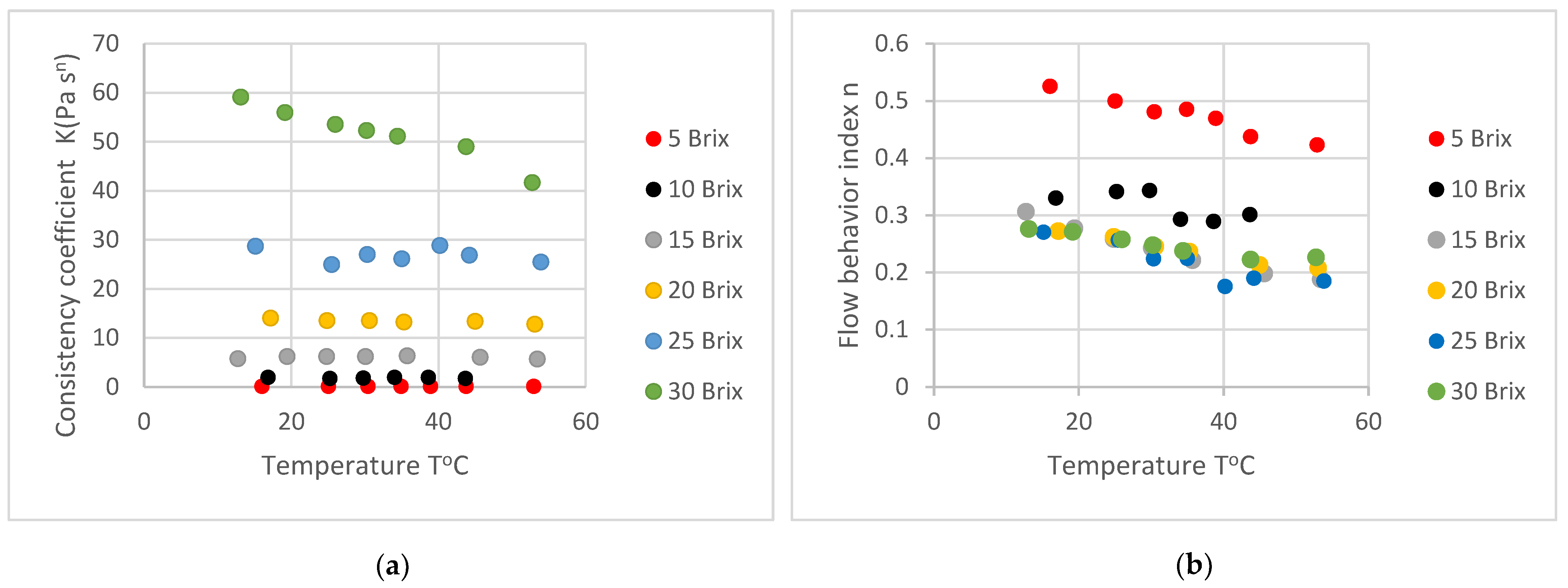
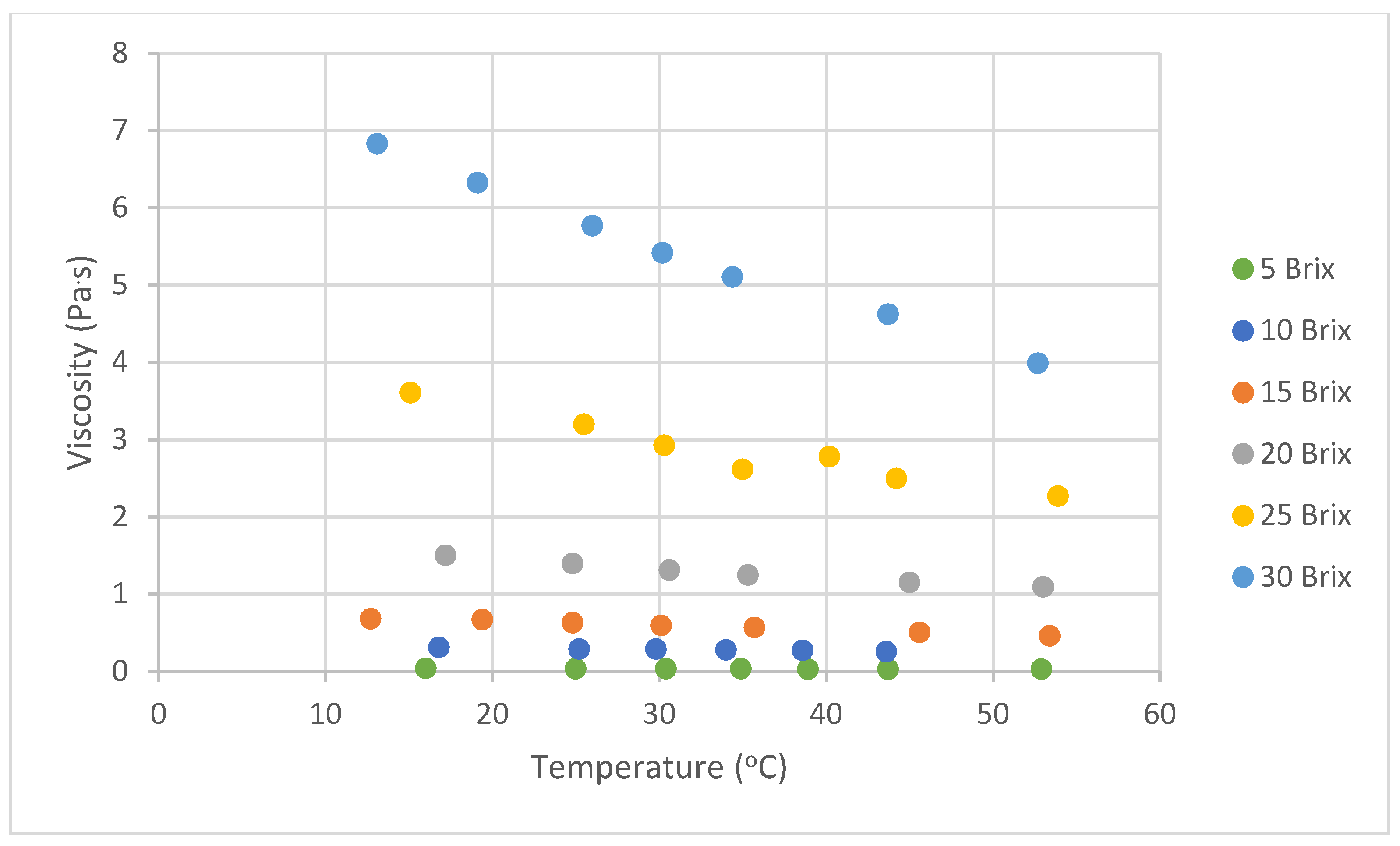
3.1.2. Effect of Temperature
3.2. Particle Size and Zeta-Potential
3.3. Effect of Mechanical and Ultrasonic Homogenization
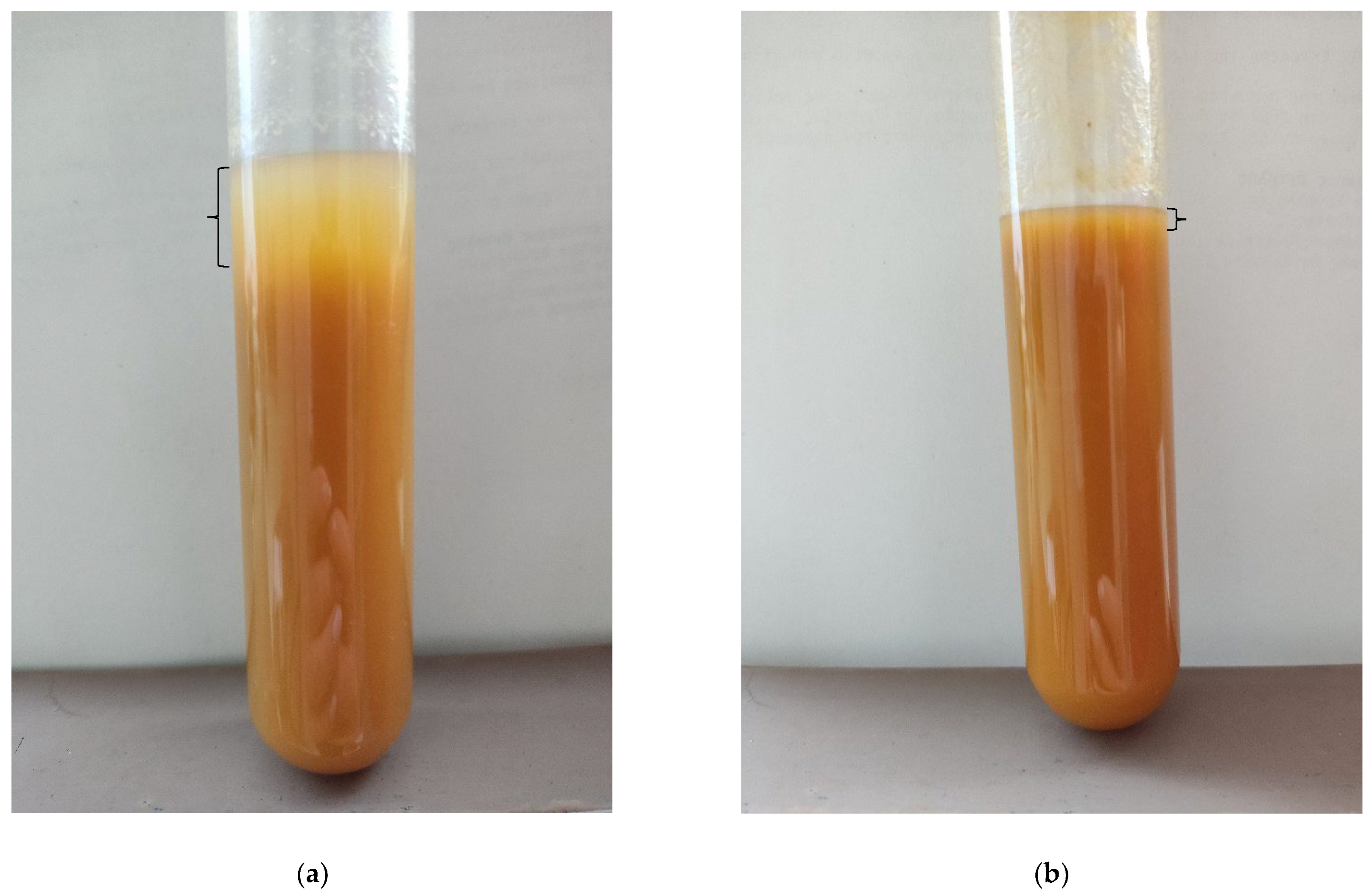
3.4. Sedimentation Kinetics
4. Discussion
4.1. Rheological Properties of Peach Puree
4.1.1. Effect of Soluble Solids Content
4.1.2. Effect of Temperature
4.2. Particle Size and Zeta-Potential
4.3. Mechanical and Ultrasonic Homogenization
4.4. Analysis of Sedimentation Phenomena
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goula, A.M.; Adamopoulos, K.G. Rheological Models of Kiwifruit Juice for Processing Applications. J. Food Process. Technol. 2011, 2, 1–106. [Google Scholar] [CrossRef]
- Álvarez, E.; Cancela, M.A.; Delgado-Bastidas, N.; Maceiras, R. Rheological Characterization of Commercial Baby Fruit Purees. Int. J. Food Prop. 2008, 11, 321–329. [Google Scholar] [CrossRef]
- Wani, S.; Bakshi, R.A.; Khan, Z.S.; Fayaz, S.; Muzaffar, K.; Dar, B.N. Physiochemical, Sensorial and Rheological Characteristics of Puree Developed from Kashmiri Peaches: Influence of Sugar, KMS and Storage Conditions. Heliyon 2021, 7, e07781. [Google Scholar] [CrossRef]
- More, A. Global Peach Puree Sales Market. Available online: https://dataintelo.com/report/global-peach-puree-sales-market/ (accessed on 12 December 2023).
- Zou, B.; Xu, Y.J.; Wu, J.J.; Yu, Y.S.; Xiao, G.S. Phenolic Compounds Participating in Mulberry Juice Sediment Formation during Storage. J. Zhejiang Univ. Sci. B 2017, 18, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Sinchaipanit, P.; Kerr, W.L. Effect of Reducing Pulp-Particles on the Physical Properties of Carrot Juice. Int. Food Res. J. 2007, 14, 205–214. [Google Scholar]
- Balestra, F.; Cocci, E.; Marsilio, G.; Rosa, M.D. Physico-Chemical and Rheological Changes of Fruit Purees during Storage. Procedia Food Sci. 2011, 1, 576–582. [Google Scholar] [CrossRef]
- Colin-Henrion, M.; Cuvelier, G.; Renard, C.M.G.C. Texture of Pureed Fruit and Vegetable Foods. Stewart Postharvest Rev. 2007, 5, 3. [Google Scholar] [CrossRef]
- Abson, R. Factors Contributing to the Rheology of Tomato Puree. Ph.D. Thesis, University of Nottingham, Bonington Campus, Loughborough, UK, 2012. [Google Scholar]
- Massa, A.; GonzÁLez, C.; Maestro, A.; Labanda, J.; Ibarz, A. Rheological Characterization of Peach Purees. J. Texture Stud. 2010, 41, 532–548. [Google Scholar] [CrossRef]
- de Sousa Luz, A.; Berto, M.I. Rheological Behavior of Concentrated Peach Puree and Its Dilutions in a Stationary State/Comportamento Reológico Em Estado Estacionário de Purê de Pêssego Concentrado e Suas Diluições. Braz. J. Dev. 2021, 7, 80697–80715. [Google Scholar] [CrossRef]
- Espinosa, L.; To, N.; Symoneaux, R.; Renard, C.M.G.C.; Biau, N.; Cuvelier, G. Effect of Processing on Rheological, Structural and Sensory Properties of Apple Puree. Procedia Food Sci. 2011, 1, 513–520. [Google Scholar] [CrossRef]
- Krokida, M.K.; Maroulis, Z.B.; Saravacos, G.D. Rheological Properties of Fluid Fruit and Vegetable Puree Products: Compilation of Literature Data. Int. J. Food Prop. 2001, 4, 179–200. [Google Scholar] [CrossRef]
- Sharoba, A.M.; Bahlol, H.; El-Mansy, H.A.; Senge, B. Rheological and Mechanical Properties of Apricot Fruit. In Proceedings of the 3rd International Conference for Food Science and Technology, Cairo, Egypt, 22–24 February 2005. [Google Scholar]
- Ditchfield, C.; Tadini, C.C.; Singh, R.; Toledo, R.T. Rheological Properties of Banana Puree at High Temperatures. Int. J. Food Prop. 2004, 7, 571–584. [Google Scholar] [CrossRef]
- Ibarz, A.; Falguera, V.; Garvín, A. Rheological and Thixotropic Behavior of Banana (Musa cavendishii) Puree. Afinidad 2010, 67, 415–420. [Google Scholar]
- Maceiras, R.; Álvarez, E.; Cancela, M.A. Rheological Properties of Fruit Purees: Effect of Cooking. J. Food Eng. 2007, 80, 763–769. [Google Scholar] [CrossRef]
- Osorio, O.; Martínez-Navarrete, N.; Moraga, G.; Carbonell, J.V. Effect of Thermal Treatment on Enzymatic Activity and Rheological and Sensory Properties of Strawberry Purees. Food Sci. Technol. Int. 2008, 14 (Suppl. 5), 103–108. [Google Scholar] [CrossRef]
- Gundurao, A.; Ramaswamy, H.S.; Ahmed, J. Effect of Soluble Solids Concentration and Temperature on Thermo-Physical and Rheological Properties of Mango Puree. Int. J. Food Prop. 2011, 14, 1018–1036. [Google Scholar] [CrossRef]
- Benítez, E.I.; Genovese, D.B.; Lozano, J.E. Effect of Typical Sugars on the Viscosity and Colloidal Stability of Apple Juice. Food Hydrocoll. 2009, 23, 519–525. [Google Scholar] [CrossRef]
- Dahdouh, L.; Wisniewski, C.; Ricci, J.; Vachoud, L.; Dornier, M.; Delalonde, M. Rheological Study of Orange Juices for a Better Knowledge of Their Suspended Solids Interactions at Low and High Concentration. J. Food Eng. 2016, 174, 15–20. [Google Scholar] [CrossRef]
- Ibarz, A.; Gonzalez, C.; Esplugas, S. Rheology of Clarified Fruit Juices. III: Orange Juices. J. Food Eng. 1994, 21, 485–494. [Google Scholar] [CrossRef]
- Bot, F.; Calligaris, S.; Cortella, G.; Nocera, F.; Peressini, D.; Anese, M. Effect of High Pressure Homogenization and High Power Ultrasound on Some Physical Properties of Tomato Juices with Different Concentration Levels. J. Food Eng. 2017, 213, 10–17. [Google Scholar] [CrossRef]
- Laaksonen, O.; Mäkilä, L.; Jokinen, M.; Metz, T.; Kallio, H.; Yang, B. Impact of Storage on Sensory Quality of Blackcurrant Juices Prepared with or without Enzymatic Treatment at Industrial Scale. Eur. Food Res. Technol. 2020, 246, 2611–2620. [Google Scholar] [CrossRef]
- Cao, X.; Bi, X.; Huang, W.; Wu, J.; Hu, X.; Liao, X. Changes of Quality of High Hydrostatic Pressure Processed Cloudy and Clear Strawberry Juices during Storage. Innov. Food Sci. Emerg. Technol. 2012, 16, 181–190. [Google Scholar] [CrossRef]
- Dundar Kirit, B.; Akyıldız, A. Rheological Properties of Thermally or Non-Thermally Treated Juice/Nectar/Puree: A Review. J. Food Process. Preserv. 2022, 46, e17075. [Google Scholar] [CrossRef]
- Kubo, M.; Atribst, A.A.L.; Augusto, P. High Pressure Homogenization in Fruit and Vegetable Juice and Puree Processing: Effects on Quality, Stability and Phytochemical Profile. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Rojas, M.L.; Leite, T.S.; Cristianini, M.; Alvim, I.D.; Augusto, P.E.D. Peach Juice Processed by the Ultrasound Technology: Changes in Its Microstructure Improve Its Physical Properties and Stability. Food Res. Int. 2016, 82, 22–33. [Google Scholar] [CrossRef]
- Pochapski, D.J.; Carvalho dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Taylor, R.A.; Abu-Nada, E. On the Colloidal and Chemical Stability of Solar Nanofluids: From Nanoscale Interactions to Recent Advances. Phys. Rep. 2020, 867, 1–84. [Google Scholar] [CrossRef]
- Mo, S.; Shao, X.; Chen, Y.; Cheng, Z. Increasing Entropy for Colloidal Stabilization. Sci. Rep. 2016, 6, 36836. [Google Scholar] [CrossRef]
- Salehi, F. Physicochemical Characteristics and Rheological Behaviour of Some Fruit Juices and Their Concentrates. J. Food Meas. Charact. 2020, 14, 2472–2488. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Zhang, W.; Zhang, L.; Zhou, L.; Cai, S.; Hu, X.; Yi, J. Evaluation of Quality Changes of Differently Formulated Cloudy Mixed Juices during Refrigerated Storage after High Pressure Processing. Curr. Res. Food Sci. 2021, 4, 627–635. [Google Scholar] [CrossRef]
- Zhu, D.; Kou, C.; Wei, L.; Xi, P.; Lv, C.; Cao, X.; Liu, H. Effects of High Pressure Homogenization on the Stability of Cloudy Apple Juice. IOP Conf. Ser. Earth Environ. Sci. 2019, 358, 022059. [Google Scholar] [CrossRef]
- Ferrari, G.; Mohapatra, D.; Prabhakar, P.K.; Freitas, J.; de São José, J.F.B. Stability Parameters during Refrigerated Storage and Changes on the Microstructure of Orange-Carrot Blend Juice Processed by High-Power Ultrasound. Front. Sustain. Food Syst. 2022, 6, 891662. [Google Scholar]
- Zhang, W.; Li, Y.; Jiang, Y.; Hu, X.; Yi, J. A Novel Strategy to Improve Cloud Stability of Orange-Based Juice: Combination of Natural Pectin Methylesterase Inhibitor and High-Pressure Processing. Foods 2023, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Mitschka, P. Simple Conversion of Brookfield RVT Readings into Viscosity Functions. Rheol. Acta 1982, 21, 207–209. [Google Scholar] [CrossRef]
- Briggs, J.L.; Steffe, J.F. Research/Application Note Using Brookfield Data and the Mitschka Method to Evaluate Power Law Foods. J. Texture Stud. 1997, 28, 517–522. [Google Scholar] [CrossRef]
- Diamante, L.; Umemoto, M. Rheological Properties of Fruits and Vegetables: A Review. Int. J. Food Prop. 2015, 18, 1191–1210. [Google Scholar] [CrossRef]
- Phaokuntha, S.; Poonlarp, P.B.; Pongsirikul, I. Rheological Properties of Mango Puree and Process Development of Mango Sheet. Acta Hortic. 2014, 1024, 373–380. [Google Scholar] [CrossRef]
- López-Esparza, R.; Balderas Altamirano, M.A.; Pérez, E.; Gama Goicochea, A. Importance of Molecular Interactions in Colloidal Dispersions. Adv. Condens. Matter Phys. 2015, 2015, 683716. [Google Scholar] [CrossRef]
- Sato, Y.; Kawabuchi, S.; Irimoto, Y.; Miyawaki, O. Effect of Water Activity and Solvent-Ordering on Intermolecular Interaction of High-Methoxyl Pectins in Various Sugar Solutions. Food Hydrocoll. 2004, 18, 527–534. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Sierakowski, M.R.; Vidal, J.R.M.B.; Masson, M.L. Influence of Temperature on the Rheological Behavior of Whole Araçá Pulp (Psidium cattleianum Sabine). LWT-Food Sci. Technol. 2006, 39, 427–431. [Google Scholar] [CrossRef]
- Dak, M.; Verma, R.C.; Jaaffrey, S.N.A. Effect of Temperature and Concentration on Rheological Properties of “Kesar” Mango Juice. J. Food Eng. 2007, 80, 1011–1015. [Google Scholar] [CrossRef]
- Wei, S.; Li, X.; Shen, Y.; Zhang, L.; Wu, X. Study on Microscopic Mechanism of Nano-Silicon Dioxide for Improving Mechanical Properties of Polypropylene. Mol. Simul. 2020, 46, 468–475. [Google Scholar] [CrossRef]
- Wu, X.-J.; Wang, Y.; Yang, W.; Xie, B.-H.; Yang, M.-B.; Dan, W. A Rheological Study on Temperature Dependent Microstructural Changes of Fumed Silica Gels in Dodecane. Soft Matter 2012, 8, 10457–10463. [Google Scholar] [CrossRef]
- Branca, C.; Magazù, S.; Maisano, G.; Migliardo, F.; Migliardo, P.; Romeo, G. α,α-Trehalose/Water Solutions. 5. Hydration and Viscosity in Dilute and Semidilute Disaccharide Solutions. J. Phys. Chem. B 2001, 105, 10140–10145. [Google Scholar] [CrossRef]
- Lerbret, A.; Bordat, P.; Affouard, F.; Guinet, Y.; Hédoux, A.; Paccou, L.; Prévost, D.; Descamps, M. Influence of Homologous Disaccharides on the Hydrogen-Bond Network of Water: Complementary Raman Scattering Experiments and Molecular Dynamics Simulations. Carbohydr. Res. 2005, 340, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.L.; Mills, T.B.; Norton, I.T.; Norton-Welch, A.B. The Effect of Sugars on Agar Fluid Gels and the Stabilisation of Their Foams. Food Hydrocoll. 2019, 87, 371–381. [Google Scholar] [CrossRef]
- Nakatuka, Y.; Yoshida, H.; Fukui, K.; Matuzawa, M. The Effect of Particle Size Distribution on Effective Zeta-Potential by Use of the Sedimentation Method. Adv. Powder Technol. 2015, 26, 650–656. [Google Scholar] [CrossRef]
- Georgiadis, N.; Ritzoulis, C.; Sioura, G.; Kornezou, P.; Vasiliadou, C.; Tsioptsias, C. Contribution of Okra Extracts to the Stability and Rheology of Oil-in-Water Emulsions. Food Hydrocoll. 2011, 25, 991–999. [Google Scholar] [CrossRef]
- Manoj, P.; Fillery-Travis, A.J.; Watson, A.D.; Hibberd, D.J.; Robins, M.M. Characterization of a Depletion-Flocculated Polydisperse Emulsion. I. Creaming Behavior. J. Colloid Interface Sci. 1998, 207, 283–293. [Google Scholar] [CrossRef]
- Vallath, A.; Shanmugam, A. Study on Model Plant Based Functional Beverage Emulsion (Non-Dairy) Using Ultrasound—A Physicochemical and Functional Characterization. Ultrason. Sonochem. 2022, 88, 106070. [Google Scholar] [CrossRef]


| Rheological Models | Equation | References |
|---|---|---|
| Power-law | [13,17] | |
| Herschel-Bulkley | [13,17] | |
| Bingham | [39,40] | |
| Casson | [2,7] |
| Sucrose Stock Solution (° Brix) | Reconstituted °Brix 1/1 Mix with: | |
|---|---|---|
| Non Concentrated Puree 10° Brix | Concentrated Puree 30° Brix | |
| 0 | 5 | 15 |
| 5 | 7.5 | 17.5 |
| 10 | 10 | 20 |
| 15 | 12.5 | 22.5 |
| 20 | 15 | 25 |
| 25 | 17.5 | 27.5 |
| 30 | 20 | 30 |
| Brix | Particle Size D [3,2] μm | Zeta Potential (mV) | Mobility (μ/s)/(V/cm) |
|---|---|---|---|
| 5 Brix | 8.8 ± 4.7 | 0 ± 1.6 | 0 ± 0.1 |
| 10 Brix | 7.4 ± 3.4 | −1.1 ± 1.4 | −0.1 ± 0.1 |
| 15 Brix | 8.3 ± 4.3 | 0 ± 1.6 | 0 ± 0.1 |
| 20 Brix | 10.1 ± 5.9 | 0.1 ± 1.4 | 0 ± 0.1 |
| 25 Brix | 9.5 ± 5.7 | −0.3 ± 2.7 | 0 ± 0.2 |
| 30 Brix | 8.4 ± 4.9 | −1.5 ± 3.3 | −0.1 ± 0.3 |
| Processing | Particle Size D [3,2] μm | Zeta Potential (mV) |
|---|---|---|
| Mechanical homogenization | 1.8 ± 1.0 | −8.8 ± 1.1 |
| Ultrasonic homogenization | 1.9 ± 1.3 | −7.6 ± 0.7 |
| (a) Sucrose Content (°Brix) Non-Concentrated Puree | Viscosity (mPa·s) | (b) Sucrose Content (° Brix) Concentrated Puree | Viscosity (mPa·s) |
|---|---|---|---|
| 5 | 167 ± 3.1 B | 15 | 201 ± 3.1 BC |
| 7.5 | 232 ± 3.6 A | 17.5 | 204 ± 4.5 BC |
| 10 | 126 ± 5.1 D | 20 | 148 ± 7.2 D |
| 12.5 | 167 ± 4.6 B | 22.5 | 221 ± 5.9 A |
| 15 | 145 ± 5.0 C | 25 | 216 ± 3.9 AB |
| 17.5 | 88 ± 3.7 E | 27.5 | 161 ± 3.8 D |
| 20 | 133 ± 2.5 CD | 30 | 200 ± 2.5 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyroglou, S.; Ritzoulis, C.; Theocharidou, A.; Vareltzis, P. Physicochemical Factors Affecting the Rheology and Stability of Peach Puree Dispersions. ChemEngineering 2024, 8, 119. https://doi.org/10.3390/chemengineering8060119
Kyroglou S, Ritzoulis C, Theocharidou A, Vareltzis P. Physicochemical Factors Affecting the Rheology and Stability of Peach Puree Dispersions. ChemEngineering. 2024; 8(6):119. https://doi.org/10.3390/chemengineering8060119
Chicago/Turabian StyleKyroglou, Smaro, Christos Ritzoulis, Athina Theocharidou, and Patroklos Vareltzis. 2024. "Physicochemical Factors Affecting the Rheology and Stability of Peach Puree Dispersions" ChemEngineering 8, no. 6: 119. https://doi.org/10.3390/chemengineering8060119
APA StyleKyroglou, S., Ritzoulis, C., Theocharidou, A., & Vareltzis, P. (2024). Physicochemical Factors Affecting the Rheology and Stability of Peach Puree Dispersions. ChemEngineering, 8(6), 119. https://doi.org/10.3390/chemengineering8060119






