Abstract
The thermal decomposition of a heat-not-burn (HNB) tobacco at four temperatures (250–400 °C) was studied via thermogravimetric analysis (TGA) and Multi-shot pyrolizer experiments (Py-GC/MS), and the effect of four potential additives, USY Beta and beta zeolites and Silica Lovel 6000 and SBA-15 silicates at three concentrations (5, 15 and 25% w/w) under an inert and oxidative atmosphere was analyzed. Different techniques were applied showing that the presence of the additives modifies the decomposition processes (TGA). Py-GC/MS showed that these tobaccos generate large amounts of Nicotine and Glycerine. Acid compounds are the most abundant compounds under an inert atmosphere, while Oxygenated compounds predominate under an oxidative atmosphere. In both atmospheres, Furans and Aromatics present in a significant abundance at high temperatures. The additives used reduce both the number and the concentration of most of the compounds generated, especially at high temperatures and concentrations. Moreover, SBA-15 shows good aptitudes to reduce the formation of some individual compounds included in the FDA’s HPHC list, such as Acetone and Acetaldehyde. Finally, smoking experiments corroborated that all additives produce marked reductions in TPM, i.e., the majority fraction obtained, and in practically all the compounds generated. Phenol, a toxicant compound that was detected in a significant amount, is also markedly reduced. SBA-15 is the material that presents a major reduction in the TPM and the principal compounds generated. These results may be of great interest for further reducing the toxicity of smoking this type of heat-not-burn tobacco product.
Keywords:
HNB tobacco; Py-GC/MS; USY; beta; Silica Lovel 6000; SBA-15; harm reduction; smoking experiments 1. Introduction
The tobacco plant (Nicotiana tabacum) was cultivated first by the natives of Mesoamerica and South America. Christopher Columbus introduced it in Europe and in the mid-sixteenth century, sailors and adventurers began to popularize its use. In 1881, the first machine capable of producing cigarettes was patented, and its consumption became very popular during the World Wars.
The negative health effects of tobacco were not initially known; many European doctors prescribed tobacco for its healing properties. At the beginning of the twentieth century, articles began to appear in scientific and medical journals that addressed the harmful effects of tobacco on health [1,2]. Today it is known that cigarette smoke contains more than 8000 different compounds [3], resulting from the distillation, evaporation, combustion, pyrolysis and pyrosynthesis that take place during smoking. At least 250 of these compounds are harmful, including hydrogen cyanide, carbon monoxide and ammonia, and up to now, it has been confirmed that about 70 of these compounds have carcinogenic activity in humans [4].
Nowadays, the tobacco industries are looking for new tobacco-related products that are less harmful to health. This is how electronic cigarettes or electronic Nicotine delivery systems (ENDS) were born. These devices are battery powered, deliver Nicotine without burning tobacco [5,6,7] and use liquids that contain propylene glycol and/or Glycerin, flavorings and commonly also Nicotine to produce an aerosol that is inhaled similar to smoke from conventional cigarettes [5,6,7,8]. Since their first appearance in the early 2000s, e-cigarettes have steadily and rapidly evolved from “first generation” disposable e-cigarette devices to today’s “fourth generation” that use replaceable cartridges (“pods”).
Heat-without-burn devices, also called “heat not burn” (HNB) tobacco products represent another type of ENDS. These devices contain a heating element that heats a tobacco rod to around 350 °C, well below the 800–900 °C of a conventional cigarette [9,10,11]. The aerosols generated have 5–10%, and even lower levels, of toxic substances than in conventional cigarette smoke [12,13]. Different studies have confirmed that the concentration of compounds produced is lower than from conventional tobacco [6,7], but the toxic compounds are not completely removed from the heated tobacco aerosol [12,14,15].
The interest in HNB cigarettes has grown, with an increase in the publications on this topic. These works range from the study of the economic impact on the market [16,17,18] to the study of different compounds produced in smoke when smoking at low temperatures, in light of the Nicotine levels [9,14,19] and emissions in HNB tobacco or the harmful and potentially harmful constituents (HPTC) in mainstream and secondary emissions [20,21,22,23].
Swapna et al. [24] recently published a very interesting review which concluded that overall, toxic chemicals in HNB aerosols appear to be lower than in cigarette smoke. Nevertheless, the concentration of more than twenty harmful and potentially harmful constituents have been reported to be higher in HNB aerosols than in burning. Moreover, several toxic compounds not detected in cigarette smoke are also reported in HNB aerosols.
Consequently, further studies reduce the toxicity of this HNB smoking practice by using catalysts, adsorbents, or any other additive which appears to be a very interesting alternative. In this sense, the use of mesoporous silica materials, zeolites, as well as other adsorbents have proven to be effective in reducing the emissions and smokers’ intake of the many toxicants present in tobacco smoke. Our research group studied the effect of such materials when added to tobacco, obtaining very interesting results [25,26] in good agreement with those obtained by other researchers [27]. Nevertheless, the effect of this type of material on HNB tobaccos is much less studied, to the point that we are not aware of any publications with this objective, except those of our group [28], as well as another by Xiao-Fang Li et al. [29]. So, we started a research program with the objective of studying the effect of this type of additive on the products evolved when heating HNB tobaccos in the corresponding HNB devices. In a previous work [28], the decomposition compounds generated at 300 °C were studied in the presence of various materials with a 25% w/w concentration. In view of the good results obtained, in this work, the temperature sweep has been expanded to cover the gradient suffered by HNB tobacco in its commercial devices, and the effect of these silica materials has been analyzed at various concentrations, lower than the one first studied. In particular, the present work includes the study of the effect of four additives, namely two zeolites (USY and beta) and two silicates (Silica Lovel 6000 and SBA-15), mixed with an HNB tobacco at different concentrations (5, 15 and 25% w/w) with the aim of analyzing how the compounds generated are modified to further reduce the toxicity of this type of tobacco.
2. Materials and Methods
2.1. Additives
USY zeolite was provided by the company, GRACE-Davison (Barcelona, Spain). Beta zeolite was provided by Süd-Chemie (Barcelona, Spain), and Silica Lovel 6000 (SiLo) was supplied by PPG Silica Products (Barcelona, Spain). SBA-15 silicate was synthesized according to the procedure described by Zhang et al. [30]. The textural properties were obtained from the N2 adsorption isotherms at 77 K, measured in an automatic Quantachrome AUTOSORB-6 (Boynton Beach, FL, USA). The surface area was obtained according to the BET method; the pore size distributions were obtained applying the BJH model with pores with a cylindrical geometry. The total pore volume was determined from the N2 adsorbed at P/P0 = 0.965. The SiO2/AlO2 ratio was determined via X-ray fluorescence (XRF).
Heet “amber selection” tobacco was selected for this study and was acquired from a tobacco shop in the area. This tobacco is a sort of reconstituted tobacco, including a large amount of Glycerine as an aerosol-generating compound. According to Xiao-fang Li [29], the composition of a tobacco of this type contains 12.18% water, 3.69% Propylene Glycol, 19.58% Glycerine, 2.89% Nicotine, 36.79% Cellulose, 6.85% Lignin and inorganic matter. The tobacco was mixed with the additives with various concentrations: 5, 15 and 25% w/w. Part of the sheet obtained in the mixtures was subsequently sieved through a 300 µm sieve to be analyzed via thermogravimetric analysis (TGA) and flash pyrolysis (Py-GC/MS), and the rest of the sheet was employed in smoke experiments.
2.2. Experimental
The tobacco was ground and mixed with the four studied additives at three concentrations (i.e., 5, 15 and 25% w/w) in the presence of water to obtain a homogeneous paste. This paste was dried at 35 °C for 24–48 h to obtain thin sheets of the resulting mixtures. The sheets were subsequently crushed and sieved through a 300 µm sieve to obtain a homogenously sized sample.
The decomposition of Heet tobacco in the absence and presence of the different additives was performed in a thermobalance provided by Mettler Toledo (Galdakao, Spain), model TGA/DSC1 under inert and oxidative atmospheres (N2 and synthetic air). Two types of experiments were carried out. The first ones were carried out under dynamic conditions (total decomposition) and 4–5 mg of Heet tobacco and Heet tobacco + additive mixtures were placed into an alumina crucible and heated from 30 to 700 °C at a heating rate of 35 °C/min. The second type of experiments simulated the iQOS device conditions, heating the samples from 30 to 350 °C at 150 °C/min (maximum heating rate of the experimental equipment). The samples were maintained at this temperature for 10 min. A flow of 80 mL/min (STP) of N2 (inert atmosphere) or air (oxidative atmosphere) was employed. All the TGA experiments were duplicated to ensure the reproducibility of the weight loss curves which were practically identical.
A multi-shot pyrolizer provided by Frontier Laboratories Ltd. (Koriyama, Japan), model EGA/Py-3030D, which was attached directly to a chromatograph, model 6890N, with a mass spectrometry detector, model 5973, provided by Agilent Technologies (Barcelona, Spain) was employed to analyze the compounds generated at the different temperatures during the decomposition of the Heet tobacco. About 400 μg of the sample was heated at four temperatures close to the range of the commercial smoking devices, i.e., 250, 300, 350 and 400 °C for 1 min, under an inert atmosphere (helium) and oxidative atmosphere (synthetic air). The reaction products were injected into a separation column (HP-5MS UI, 30 m × 0.25 mm i.d. × 0.25 μm film thickness, provided by Agilent Technologies Spain) with a split ratio of 50:1 (column flow rate: 2 mL/min) using helium as the carrier gas. The generated compounds were detected via MS (the temperature of the GC/MS transfer line, the MS source and the MS Quad were 280, 230 and 150 °C, respectively). The mass spectrometer was operated in electron-impact mode at 70 eV at a scan range of 15–350 amu. The compounds were identified by reference to the National Institute of Standards and Technology library, USA (NIST 08) and/or Wiley Registry of Mass Spectral Data, 7th Edition library (Wiley7n). The experiments were replicated three times to assure the reproducibility of the results. The peak area was normalized by dividing it by the mass of the tobacco analyzed. The experiments were duplicated. Chromatograms obtained in the replicas were very coincident (deviations lower than 5% for the peaks representing areas higher than 2% of the total area and lower than 10% for the rest of the peaks analyzed).
Smoking experiments were performed following the ISO/TR 19478-1:2014 [25]. The smoking machine was designed and built by the research group. It consisted of five steel tubes, surrounded by five resistances, where the nodes are inserted. The resistance controlled the temperature inside the tube. Five cigarettes were simultaneously smoked, and 8 puffs were always taken in each experiment. Each cigarette contained 0.29 g of the previously prepared sheet. At least two replicates were carried out, being the dispersion of the results lower than 10% for most compounds. The temperature of the smoking experiments was 300 °C, and the experiments were performed in duplicate to validate the tendencies obtained in the results. The gaseous fraction of tobacco smoke was collected in a Tedlar bag and analyzed via CG/TCD (CO and CO2) and GC/FID (the rest of the compounds in the not condensed fraction) in a chromatograph supplied by Agilent Technologies, model 6890N, using a GS-GASPRO column (provided by Agilent Technologies). The total particulate matter (TPM) condensed in the trap located before the Tedlar bag was extracted using isopropanol following the ISO4387 standard [25] and analyzed via GC/MS in an Agilent Technologies chromatography, model 6890N, chromatographer with a HP-5-MS column. The identification of the different compounds was performed by comparison with the NIST08 and Wiley7 MS library.
3. Results and Discussion
3.1. Characterization of Additives
The results of the characterization of the additives studied are shown in Table 1. As can be seen, SiLo and SBA-15 have higher pore sizes than the zeolites. The presence of aluminum atoms in the crystalline structure of USY and Beta zeolites gives it a negative defect of charge that must be compensated by protons generating acidity on these materials. The pore volume of the silicates is much larger than those of the zeolites.

Table 1.
Characteristics of the additives studied.
3.2. Thermogravimetric Analysis
Figure 1 shows the remaining mass curve and the time derivative of the mass curves versus temperature obtained from the Heet tobacco in the experiments carried out under dynamic conditions with the four catalysts. Figure 1a,b show the results for an inert atmosphere and Figure 1c,d show those for an oxidative atmosphere. Under an inert atmosphere, Heet tobacco (black continuous line) presents the following decomposition steps: elimination of moisture (Tª ≤ 100 °C), with a weight loss of around 10%; evaporation of volatiles in the range 120–240 °C, principally formed by Glycerine and Nicotine, assuming around a 23% mass loss for the Heet tobacco under both atmospheres [31]; two overlapped processes in the range 220–375 °C, due to decomposition of hemicellulose and cellulose, respectively, with a weight loss of around 32%, and finally, the pyrolysis of lignin that occurs in a wide range of temperatures at around 450 °C (weight loss of around 12%). A little process appears at high temperatures (650 °C) due to the dehydrogenation and aromatization of char and/or decomposition of endogenous inorganic compounds. Heet tobacco is a commercial reconstituted tobacco, manufactured by Philip Morris International, the real composition of which is unknown, and which involves small changes in its composition between commercial batches. TGA analysis allows us to obtain an initial idea of its composition, volatiles and organic material, and as can be seen, the results for these samples are similar to those reported in the literature [29]. Under an oxidative atmosphere (Figure 1c), the same steps are observed under 400 °C (pyrolysis zone), but the decomposition of hemicellulose and cellulose (220–375 °C) appears in a single overlapping peak. At high temperatures, a significant peak due to the combustion of the carbonaceous residue can be observed.
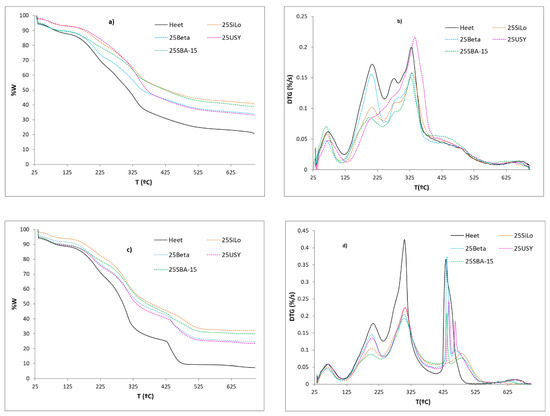
Figure 1.
TG and DTG curves vs. temperature of Heet tobacco/additive mixtures under dynamic conditions: (a,b) inert atmosphere, (c,d) oxidative atmosphere.
In the presence of the additives, the peak due to the loss of Glycerine (120–240 °C) decreases, being SBA-15, which is the material that shows the largest reduction in both atmospheres. The small Glycerine molecule size (10 A) may penetrate the large pores of the SBA-15 catalyst which is also the one with the largest surface area. Beta zeolite produces almost no effect at low temperatures. Under an inert atmosphere, the intensity of the peaks associated with the decomposition of hemicellulose and cellulose decreases too, except when using UYS zeolite, where the high acidity of this zeolite produces a marked increase in the intensity of the decomposition of the cellulose. Again, SBA-15 is the material showing larger reductions for these peaks. Under an air atmosphere, the peak due to the combustion of the material (430–550 °C) decreases notably in the presence of the additives, and moves towards higher temperatures, being again the SBA-15, i.e., the additive which decreases the intensity of this peak more. SBA-15 splits the peak of combustion into a sharp peak and a soft peak at high temperatures. SiLo produces two sharp peaks. The behavior of Beta zeolite is similar to that of USY and that of Silo is similar to that of SBA-15. The two zeolites produce, in both atmospheres, a lower residue than expected, thus favoring the evolution of volatiles, whereas the two silica materials produce almost the expected residue. However, this range of temperature exceeds by far that which is normally used by HNB devices which operate at well below 400 °C.
Figure 2 shows the remaining mass and the mass time derivative curves obtained in the isothermal conditions simulating those of the iQOS device for the four catalysts. As can be seen, the first two peaks mainly due to evaporation of moisture and the evaporation of Glycerine are very similar in both atmospheres and also have a similar appearance to that obtained in the dynamic experiments (Figure 1). However, the decomposition of the rest of the tobacco markedly changes. In the first place, it can be observed that the main peak under the two atmospheres is that corresponding to the Glycerine and other volatiles such as Nicotine, i.e., the second peak, whereas the decomposition peak of the organic matter associated with tobacco (cellulose and lignin) is quite reduced but is still assumed to make a significant contribution to the decomposition process. In addition, the wide peak due to the partial decomposition of these tobacco components is more marked under an oxidative atmosphere, which could indicate a greater generation of the decomposition products. Under both atmospheres, the addition of the catalysts and especially of SAB-15 and SiLo provoke less intense peaks, both for the elimination of Glycerine and for the partial decomposition of the tobacco components, especially under an air atmosphere.
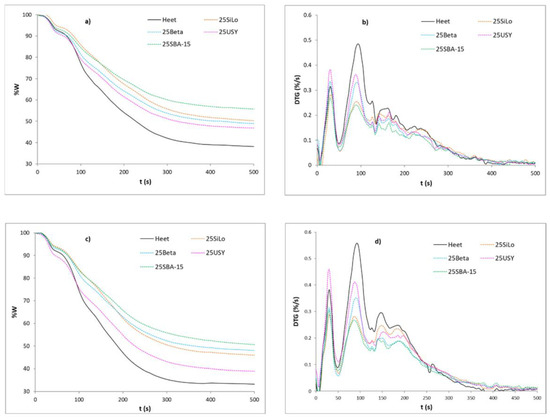
Figure 2.
TG and DTG curves vs. temperature of Heet tobacco/additive mixtures under isothermal conditions: (a,b) inert atmosphere, (c,d) oxidative atmosphere.
The residue obtained in both atmospheres is very high (around 50–60%, except for USY), showing that the decomposition of the sample is only partial in these types of experiments, as is the case in the HNB smoking devices. Beta zeolite is the material causing the largest differences under an air atmosphere of the peak corresponding to the decomposition of the tobacco components, and together with SBA-15 and SiLo yield higher residues than expected. This behavior of Beta zeolite under this isothermal condition was not expected from the results of the dynamic experiments. It can be concluded that the presence of the catalysts, especially SBA-15, results in a lower intensity of the Glycerine and Nicotine evolution peaks, as well as the tobacco decomposition peaks as a result first of the adsorption of these molecules and those evolved from the decomposition of the tobacco components on the pore structure of these materials at the relatively low temperature of the experiment as well as the catalytic effect. This effect would be in good agreement with what has been observed by other authors, i.e., [27,32]. Lin et al. [27] remarked on the ability of SBA-15 and NaY zeolite to remove the particulate matter of tobacco and TSNAs, observing a certain selectivity of the SBA-15 due to its fiber-like morphology that favors the adsorption of the TSNAs between its fibers. Calabuig [32] studied the effect of SBA-15 in contact with tobacco by using different techniques, observing a clear effect of this material that is capable of adsorbing and reducing the compounds generated in tobacco smoke. When studying the effect of the catalyst on the thermal behavior of tobacco tars previously obtained and mixed with it, it was concluded that the main effect of the catalysts is favoring the cracking of the tars at temperatures relatively low for the conventional smoking of tobacco cigarettes. Thus, the adsorption of the products evolved on the catalysts, as well as this catalytic effect on their cracking, would likely explain the mechanism of the catalysts on this type of process.
3.3. Analysis of the Products of Decomposition of Heet Tobacco and Its Mixtures with the Four Catalysts Studied Under Inert and Oxidative Atmospheres:Effect of Catalyst Concentration and Temperature
Four temperatures between 250 and 400 °C were studied under an inert atmosphere (He) and oxidative atmosphere (air) in EGA Py/GC/MS equipment to identify and semi quantify the products obtained in the decomposition of Heet tobacco as explained in the Experimental section.
The pyrograms obtained in EGA Py/GC/MS present many peaks. Tables S1 and S2 of the Supplementary Materials show the results under both atmospheres for the decomposition of Heet tobacco (retention time, assigned compound, the match quality according to the Wyley library and the peak area per mg of sample). The pyrograms of Heet tobacco obtained at the four temperatures studied under both atmospheres are shown in Figure 3 (Figure 3a He atmosphere, Figure 3b Air atmosphere). Three significant peaks/zones can be observed. The first zone, at times less than 2 min, is mainly due to the formation of CO2 and water and other low-molecular-weight compounds. The second zone, between 9 and 14 min, where a wide peak is observed, is mainly due to Glycerine, and at around 13.6 min, a sharp and intense Nicotine peak appears. The rest of the pyrogram is made up of many small peaks. A first inspection of these figures reveals that under a He atmosphere, the evolution of Nicotine and Glycerine does not depend on the temperature, whereas a series of small peaks in all the chromatograms appear to increase their intensity as the temperature increases. Under an inert atmosphere, according to Marcilla et al. [33], the decomposition of Nicotine starts at much higher temperatures. Under an air atmosphere, the decomposition is more marked for all compounds (the chromatograms present more peaks and higher intensity, and larger total areas than under a He atmosphere) and the amounts of Glycerine and Nicotine both decrease with temperature, and the increase in the intensity of the secondary peaks is more marked than under a He atmosphere.
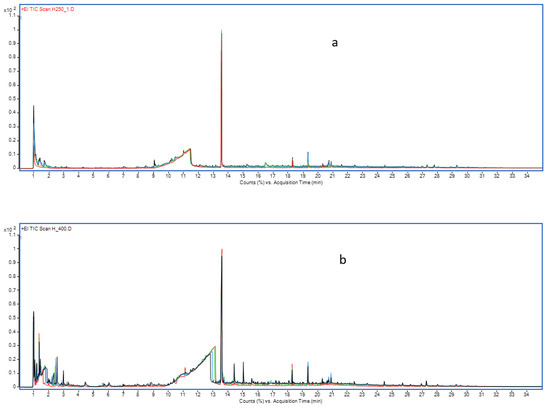
Figure 3.
Pyrograms obtained in the decomposition of Heet tobacco at 250 (red line), 300 (green curve) 350 (blue line) and 400 °C (black line) under: (a) inert atmosphere and (b) oxidative atmosphere.
Figure 3 shows how at 400 °C, the number of peaks and/or their abundance is higher than at 250 °C. In addition, although the abundance of Glycerine and Nicotine at 250 and 400 °C under an inert atmosphere is very similar, under an oxidative atmosphere, the peaks of these compounds decrease with the temperature due to the greater reactivity of the oxidative atmosphere that decomposes these compounds.
Tables S1–S10 of the Supplementary Materials show the compounds identified (with a probability higher than 80%, in most cases more than 90%, and a contribution to the total area greater than 0.5%) obtained in the decomposition of Heet tobacco in the absence and presence of each one of the four additives under inert and oxidative atmospheres as a function of the concentration of additives and temperature. Likewise, the Supplementary Materials shows the chromatograms obtained for all the additives at the three concentrations studied in inert and oxidative atmospheres (Figures S1–S32).
Under both atmospheres, the majority of compounds in the decomposition of Heet tobacco (Tables S1 and S2) are Glycerine, Nicotine, CO2 and H2O, and it should be noted that the quantities obtained in an oxidizing atmosphere are much greater than those obtained under an inert atmosphere. With respect to the Glycerine and the Nicotine, it can be observed that under an inert atmosphere, the quantity of Glycerine obtained increases with the temperature, while the amount of Nicotine increases between 250 and 350 °C but at 400 °C decreases, which would indicate that part of it breaks down into new products, for example, Myosmine, Nicotyrine, Pyridine, Ammonium cyanide, CO and CO2 [27,33]. However, under an oxidizing atmosphere, it seems that both compounds decrease with increasing temperature due to the high reactivity of this atmosphere. As expected, the abundance of CO2 and water increases with the temperature and is much higher in air, as a consequence of the combustion reactions that take place. These results are in good agreement with previous works [33,34,35].
Among the rest of the compounds under an inert atmosphere when pyrolyzing Heet tobacco (Table S1), at 250 °C, 2,3-Dihydro-3,5-Dihydroxy-6-Methyl-4H-Pyran-4-one stands out. This compound can be formed by sugar–amino acid pyrolysis (Amadori reaction) at temperatures below 300 °C [36]. Moreover, when the temperature increases, the abundance of this compound decreases and that of other smaller molecules such as Acetic Acid, Quinic Acid and 1-Hydroxy-2-Propanone increases. It should be noted that five Acid compounds appear among the major compounds, Acetic Acid being the most abundant. The formation of Acids can be due to the dissociation of the O-acetyl groups linked to the main chain of xylan [37] due to the decomposition of hemicellulose. Nevertheless, only a small portion of hemicellulose seems to decompose at 250 °C.
In an oxidative atmosphere (Table S2), the distribution of products obtained in the decomposition of Heet tobacco changes. For example, among the most abundant compounds, there are several Oxygenated compounds such as Acetone, 2,3-Butanedione and Acetaldehyde, which are typical compounds generated in the decomposition of sugars (fructose, glucose and sucrose) contained in tobacco [38]. In addition, the number of acidic compounds decreases, and the number of nitrogenous compounds increases, such as Nicotyrine and Myosmine, which are products of the breakdown of Nicotine. Woodward et al. [39] reported that Myosmine can be formed through Nicotine dehydrogenation to N-methyl myosmine, followed by its demethylation, and Kisaki et al. [40] suggested that in an air atmosphere, the formation of pseudo-oxynicotine takes place followed by the loss of water and cycling to yield N-Methylmiosmine that is decomposed to Nicotyrine and N-Methylnicotinamide.
In this section, SBA-15 and USY were selected for the analysis of the different tendencies obtained in the EGA Py/GC/MS experiments, since the behavior of zeolites is similar between them, and SiLo presents an analogous behavior to that of SBA-15. Figure 4 shows the total area of the pyrogram referring to the mass of tobacco and Heet tobacco mixed with 5, 15 and 25% w/w of SBA-15 and USY at the four temperatures studied under both atmospheres. As expected, there is a greater delivery of mass as the temperature increases in both atmospheres, being much higher in the oxidative atmosphere. As can be seen in Figure 4, when the additives are included in both atmospheres, the tendency is very similar, as commented on for Heet tobacco, and the total area increases with the temperature, the presence of the additives reduces the total area obtained and, consequently, the amount of the compounds generated at the four temperatures in comparison to Heet tobacco. This area reduction caused by the additives is larger for SBA-15 at all temperatures (followed by SiLo, not represented in Figure 4), than for USY (and beta catalysts), and the reduction increases with the concentration of the material. This behavior must be related to the larger pore size and surface area of SBA-15 and SiLo, and also the higher acidity may explain the better behavior of the USY zeolite (Table 1), since at this relatively low temperature the main effect is likely to be the adsorption of the different molecules on the catalyst surface, in addition to a catalytic effect at the highest temperatures tested.
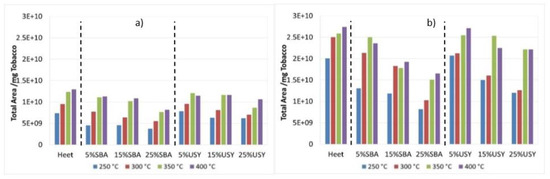
Figure 4.
Total area obtained in EGA Py/GC/MS in the decomposition of Heet tobacco in presence of SBA-15 and USY at various concentrations and temperatures: (a) inert atmosphere and (b) oxidative atmosphere.
The different compounds identified have been grouped by chemical family into Oxygenated compounds (Alcohols, Aldehydes and Ketones), Acids, Furans, Aromatics, Nitrogenous and Aliphatics to facilitate the analysis. It should be mentioned that Acids and Furans have been grouped in a separate category from Oxygenated compounds due to their abundance (Acids) and to the high toxicity associated with them (Furans). In addition, Glycerine was not included in the Oxygenated family and Nicotine not included in the Nitrogenous one and have been discussed separately for better analysis. Figure 5 shows the results for the experiments carried out under an inert atmosphere, while Figure 6 shows the corresponding results under an oxidative atmosphere. Different peaks have been ascribed to long-chain Aliphatics (≥C20H42) in the corresponding tables, since the library shows the same result. As already mentioned, peaks with a low identification match are listed as Not Assigned compounds.
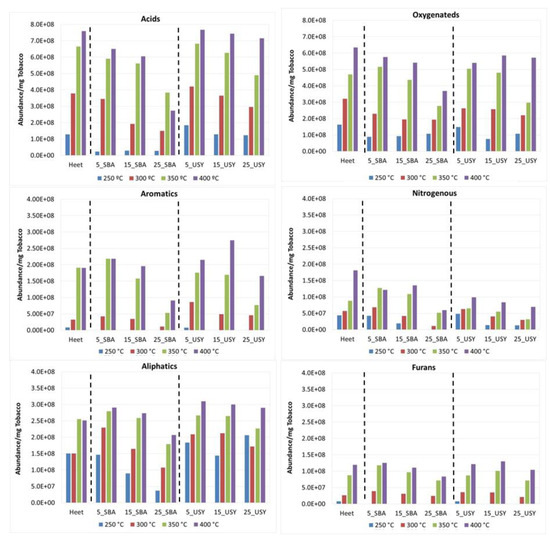
Figure 5.
Abundance of the chemical families obtained in the decomposition of Heet tobacco in the presence of various concentrations of additives SBA-15 and USY under inert atmosphere.
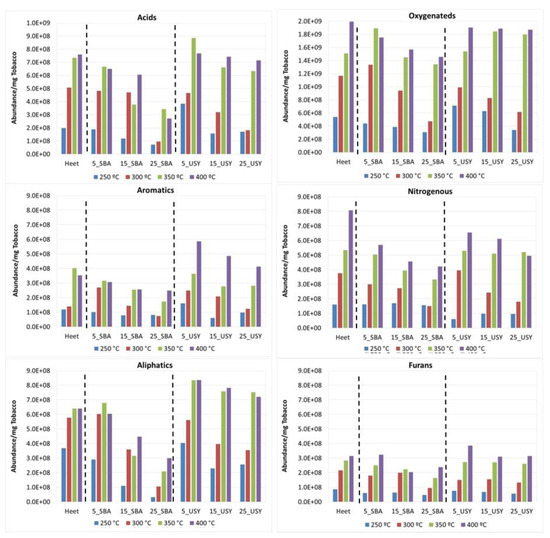
Figure 6.
Abundance of the chemical families obtained in the decomposition of Heet tobacco in the presence of various concentrations of additives SBA-15 and USY under oxidative atmosphere.
Under an inert atmosphere (Figure 5) at 250 °C, the most abundant compounds in the decomposition of the Heet tobacco (first series) were the Oxygenated ones, followed by Aliphatic and Acid compounds. When the temperature increases, the abundance of all families increases, mainly the Acid family, which becomes the most abundant family, followed by the Oxygenated compounds. The Aliphatic family increases with the temperature but in a minor proportion. Under an oxidative atmosphere (Figure 6), the abundance of all the families in Heet tobacco decomposition increases with the temperature except that of the Acids, which does not depend on the type of atmosphere. Under an oxidative atmosphere, the Oxygenated compounds are the most abundant at all temperatures, reaching a proportion of nearly double that of the second family of compounds (Acids). At 400 °C, a significant increase in the formation of nitrogenous compounds can be observed. In both atmospheres between 250 and 300 °C, the families of Furans and Aromatics are the least abundant, but at high temperatures their abundance increases considerably. These families are made up of compounds considered very toxic and harmful, so they must be taken into account despite their lower abundance.
The observed trend for the compounds generated in the decomposition of Heet tobacco with the USY and SBA-15 grouped by family under an inert atmosphere can be observed in Figure 5. As in the case of Heet tobacco, in the presence of the catalysts, the Acids are the most abundant compounds, followed by Oxygenated compounds. For these families, the two additives present reductions at all temperatures. These reductions increase with the concentration of the additives and are more significant in the presence of the material, SBA-15, being SBA-15 at 25% w/w the mixture that presents the highest reductions. The following more abundant family is that of Aliphatic compounds. For this family, only SBA-15 at 25% reductions with respect to the Heet tobacco can be observed at all temperatures, while for other concentrations and temperatures, this is not a clear trend. The families with a minor contribution were nitrogenous and Furan compounds. It should be mentioned that in the case of nitrogenous compounds, USY provides the greatest reductions, more than SBA-15. Furan compounds show reductions in the presence of SBA-15 that increases when the temperature and concentration of the additive increases. Aromatics are not much affected by the presence of the catalysts except for the mixture containing 25% of SBA-15 where they are fairly reduced.
The evolution of the compounds generated in the presence of the additives at the four temperatures under an oxidative atmosphere can be seen in Figure 6. As mentioned before when discussing the effect of the temperature and atmosphere in Heet tobacco, under an oxidative atmosphere, a clear increase in the generation of the different compounds is observed, being highest at the higher temperatures. As in the inert atmosphere, at increasing additive concentrations, higher reductions are observed. Again, SBA-15 is the additive that presents higher reductions for all families. USY zeolite shows at 350 and 400 °C, an increase in the abundance of all the families considered with respect to those obtained when studying sole Heet tobacco, except for the nitrogenous one. These increases occur at the three concentrations studied, being major at low concentrations.
As in the case of Heet tobacco alone, Glycerine, Nicotine, CO2 and water are the most abundant compounds (Tables S3–S10). Both additives reduce the presence of Nicotine with respect to the Heet tobacco. SBA-15 provides similar reductions at the different concentrations and temperatures, while USY highly reduces the Nicotine amount at high concentrations and temperatures. Both CO2 and water increase with temperature but not specially with the catalyst concentration for all the catalyst. Under an inert atmosphere (Table S4), for SBA-15, the next most abundant compound at a low temperature (250 °C) was 2,3-Dihydro-3,5-Dihydroxy-6-Methyl-4H-Pyran-4-one, but at 400 °C, Acetic Acid, Quinic Acid and 1-Hydroxy-2-Propanone were the more abundant compounds. Bazerra et al. [41] studied the flash pyrolysis of Glycerine and found that a significant formation of Oxygenated products, such as organic acids, esters, alcohols, ketones, aldehydes and ether, would indicate that these major compounds are the result of the decomposition of Glycerine. A significant formation of Oxygenated and Acid compounds was observed too in the decomposition of Heet tobacco, but in the case of USY (Table S3), the Acids are always the most abundant compounds, such as Quinic Acid and n-Hexadecanoic Acid at 250 °C, while at 400 °C, also a high formation of Acetic Acid and 1-Hydroxy-2-Propanone can be observed.
Under an oxidative atmosphere, again, Glycerine, Nicotine, CO2 and water are the more abundant compounds (Table S7 (USY) and Table S8 (SBA-15)). CO2 and water increase with the temperature but as in an inert atmosphere, there is no clear effect of the catalyst concentration. Glycerine always shows lower values than in Heet tobacco and decreases its concentration as the additive concentration increases, especially for SBA-15. Nicotine also behaves similarly in an inert atmosphere presenting lower values than Heet tobacco but is seemingly independent of temperatures or concentrations of SBA-15, while clear reductions can be observed with USY, especially at high temperatures and concentrations. In the presence of SBA-15 (Table S8) at 250 °C, it can be observed that Acetone and 2,3-Butanedione are reduced with the presence of SBA-15. When the concentration increases, most of the compounds are reduced. This is in concordance with the work of Asensio et al. [34] when studying the decomposition of Nicotine at high temperatures. They found that Nicotine decomposes, favoring the formation of nitrogenous compounds such as Myosmine and Nicotyrine, whose generation was increased in the presence of additives such as SBA-15. The different compounds increase their abundance when the temperature increases, this increase being a major factor for the formation of Acetaldehyde and Acetic Acid. When Heet tobacco was in contact with USY (Table S7) at 250 °C, the principal compounds were similar to those obtained when using SBA-15, but an increase was observed in the formation of Acetaldehyde and n-Hexadecanoic Acid, and a decrease in the formation of Acetone. When the temperature increases, for all the concentrations, the four main compounds are 2,3-Butanedione, Acetone, Acetaldehyde and n-Hexadecanoic Acid.
It should be mentioned that Acetone and Acetaldehyde are included in the FDA’s HPHC list. Acetone is considered as a respiratory toxicant, and Acetaldehyde is a carcinogen, respiratory toxicant and is addictive, and it is listed by the international agent for Research on Cancer (IARC) within group 2B (possibly carcinogenic to humans) and carcinogens. As can be seen in Figure 7, all the concentrations of SBA-15 reduce the formation of Acetone and Acetaldehyde at a high temperature (400 °C), while at lower temperatures (250–300 °C), the reduction in this compound is observed only at the highest SBA-15 concentration (25% w/w). Moreover, USY material generates practically the same quantity of Acetaldehyde at all temperatures and concentrations as Heet tobacco, and only reductions in the formation of Acetone can be observed at 250 °C and 300 °C at the three concentrations, being higher at the highest concentration, while at high temperatures (350 and 400 °C), an increase in its formation can be observed.
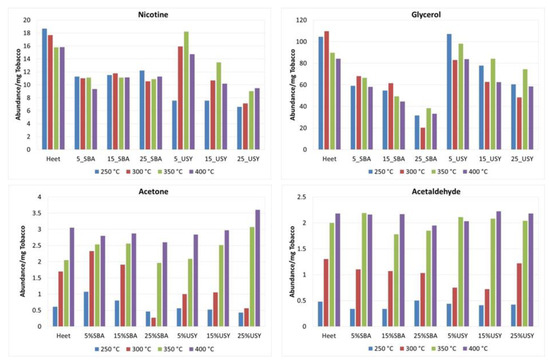
Figure 7.
Evolution with the temperature of the yield of Nicotine, Glycerine, Acetone and Acetaldehyde in oxidative atmosphere.
The FDA is proposing to add 19 chemicals to its list of harmful and potentially harmful components (HPHCs) in tobacco products. The 19 chemicals are specific to electronic Nicotine delivery systems, such as e-cigarettes and e-liquids [42]. Among these 19 compounds are 2,3 Butanedione, Acetic Acid and Glycerine, all considered respiratory toxicants. These compounds were reduced by the addition of SBA-15, at all the temperatures, and they present major reductions at high concentrations of SBA-15. USY material presents, in general, minor reductions compared to SBA-15. Again, the good results observed for SBA-15 could be related to its larger pore size and therefore greater adsorption capacity.
3.4. Smoking Experiments
Smoking experiments were carried out for tobacco and tobacco with three concentrations of the four additives studied. The samples were smoked at 300 °C and the not condensed fraction and the TPM obtained were analyzed. Table 2 shows the yield obtained in the TPM, Nicotine, Glycerine, the sum of gases analyzed by GC/FID, as well as CO measured by GC/TCD. As can be seen, SBA-15 presents reductions in TPM, Nicotine and Glycerine, for all the concentrations, and the reduction increases when the quantity of SBA-15 increases. In addition, a high reduction can be observed for Glycerine which is in accordance with the TGA and Py-GC/MS results. The other additives do not show a reduction in TPM at low concentrations (5% w/w) and present a major reduction when the concentration of the additive increases. In general, SiLo is the additive that shows better reduction after SBA-15. Nicotine was reduced only slightly in the presence of additives, and this reduction is somewhat greater in the presence of Beta zeolite and USY at high concentrations. The total gases tend to increase in the presence of this type of material, but the quantity of gases produced is very low. In the material balance, the total gases correspond to 0.1% of all the compounds generated during the smoking process. The CO produced, determined in an independent analysis via GC/TCD and separate from the general gases that are determined via GC/FID, is more relevant than the total gases and it does show reductions, especially for 25% USY.

Table 2.
Yield obtained in different fractions in smoking experiments.
Tables S11 and S12 in the Supplementary Materials show the compounds retained and analyzed in the tramps (TPM) calculated as µg of compound/cigarette, and Table 3 shows the 16 major compounds detected. As can be seen, Glycerine and Nicotine are the principal compounds generated followed by two acids, Acid n-Hexadecanoic and Acid 9.12.15-Octadecatrienoic. SBA-15 and SiLo reduce them in different proportions at three concentrations, but zeolites favor the formation of the acid when the concentration of additive increases. In general, it can be observed that SBA-15 at 25% w/w is the system that provokes major reductions followed by Silo and Beta. Inside the TPM fraction analyzed, a dangerous product was detected with Nicotine, i.e., phenol. Phenol is a respiratory and cardiovascular toxicant compound and Nicotine (the addictive component) is considered a developmental toxicant. The four additives reduced these compounds, and the reduction increases with their concentration, where SBA-15 presents better reductions for phenol, and USY for Nicotine.

Table 3.
Main compounds obtained in the decomposition of Heet tobacco in smoking experiments alone and in presence of different concentrations of SBA-15, USY, SiLo and Beta (µg compound/cigarette).
Table 4 presents the sum of all the compounds of Tables S11 and S12 grouped by chemical family, where Oxygenated compounds Glycerine do not appear, and Nicotine are not included in nitrogenous compounds. As can be seen, Oxygenated compounds are the more abundant compounds generated in the decomposition of Heet tobacco, followed by Aliphatic and Acid compounds. Moreover, a significant quantity of Furan compounds was formed. This family, as was mentioned in the last section, was collected separately from Oxygenated compounds due to the high toxicity that they usually present, and the Acids were separated due to the high proportion in which they are formed.

Table 4.
Total compounds obtained in the decomposition of Heet tobacco grouped by chemical family (µg compound/cigarette).
Figure 8 shows the reductions obtained in the different families in the presence of the four additives. As can be seen, Acids and Aliphatics are the family of compounds that showed high reductions followed by Oxygenated compounds, and in general, the reduction increases with the concentration. Moreover, silicates show more reduction than zeolites, and SBA-15 is the additive with better results/reductions, but Beta zeolite presents good decreases in Acids and Oxygenated compounds but in a minor proportion compared to silicates. Finally, it can be mentioned that USY only presents reductions at high concentrations and provokes a major formation of more of the families at low concentrations. Silicates are the only additives that show reductions in the Furan compounds, and again, SBA-15 presents the better results.
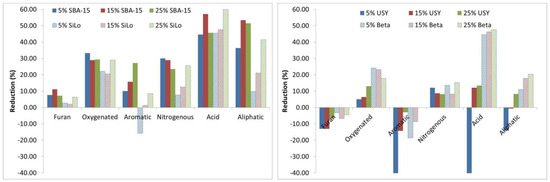
Figure 8.
Reductions in the chemical families obtained in smoking experiments in presence of the four additives.
4. Conclusions
In the TGA experiments carried out simulating the smoking of Heet tobacco under the conditions of the electronic devices, the decomposition of tobacco components still make a significant contribution to the total mass loss, especially in an air atmosphere. All the additives at 25% w/w provoked a significant reduction in the Glycerine decomposition, being much more significant in the case of SBA-15 in both atmospheres.
The results obtained in the study in Py-GC/MS show that Heet tobacco generates large amounts of Nicotine and Glycerine at all the temperatures under both atmospheres. In general, the most abundant families in the pyrolysis of Heet tobacco are Acids followed by Oxygenated compounds, while in an oxidative atmosphere, Oxygenated compounds are double the abundance of Acids (which are not dependent on the atmosphere type). Despite the low toxicity claimed for HNB tobaccos, a significant number of Aromatics and Furans can still be observed, especially at high temperatures (more than 350 °C in an inert atmosphere and 300 °C in an oxidative atmosphere), wherein a significant number of the compounds of these families are considered toxic, and some of them may be carcinogenic. In addition, under an oxidative atmosphere, significant amounts of Acetaldehyde and Acetone can be observed when studying sole Heet Tobacco, which are classified on the FDA’s HPHC list.
When Heet tobacco was mixed with SBA-15 or USY under both atmospheres, the quantity of Glycerine was significantly decreased, especially with SBA-15 while Nicotine was reduced by a small amount in the case of SBA-15 and was significantly reduced in the presence of USY. The effect of USY is unclear for most of the families of compounds, but USY seems especially appropriate to reduce the nitrogenous compounds in an inert atmosphere. With SBA-15, Acids and Oxygenated compounds decrease with the concentration of the additive at the four temperatures. Also, in both atmospheres and at all temperatures, SBA at 25% is able to reduce the amount of the harm due to Aromatics, Furans, Acetaldehyde and Acetone at all temperatures in respect of the Heet tobacco, while at lower contents of catalyst, these compounds are reduced only at the highest temperatures.
The smoking experiments confirm the trend observed in flash pyrolysis, showing how SBA-15 presents better results in reducing the TPM, and reduces all the principal compounds generated in the decomposition of Heet tobacco, at the three concentrations. Moreover, SiLo and Beta zeolite present good reductions in TPM and more of the major compounds studied while USY especially reduces Nicotine. Additionally, both materials reduce the phenol, one of the major compounds obtained during smoking that stands out for its high toxicity. Phenol appears in significant yields, but all additives reduced its formation although this reduction does not seem to be related to concentration.
Therefore, it can be concluded that especially the SBA-15 catalyst is a very interesting candidate to be added to HNB tobaccos to further reduce the amount of compounds generated and, consequently, their toxicity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemengineering8060125/s1, Table S1. Compounds obtained in the decomposition of Heet tobacco at different temperatures; Inert atmosphere (He); Table S2. Compounds obtained in the decomposition of Heet tobacco at different temperatures; Oxidative atmosphere (Air); Table S3. Compounds obtained in the decomposition of Heet tobacco mixed with 5, 15 and 25% w/w of USY- at different temperatures; Inert atmosphere (He); Table S4. Compounds obtained in the decomposition of Heet tobacco mixed with 5, 15 and 25% w/w of SBA-15 at different temperatures; Inert atmosphere (He); Table S5. Compounds obtained in the decomposition of Heet tobacco mixed with 5, 15 and 25% w/w of Beta at different temperatures; Inert atmosphere (He); Table S6. Compounds obtained in the decomposition of Heet tobacco mixed with 5, 15 and 25% w/w of SiLo at different temperatures; Inert atmosphere (He); Table S7. Compounds obtained in the decomposition of Heet tobacco and Heet tobacco mixed with 5, 15 and 25% w/w of USY at different temperatures; Oxidative atmosphere (Air); Table S8. Compounds obtained in the decomposition of Heet tobacco mixed with 5, 15 and 25% w/w of SBA-15 at different temperatures; Oxidative atmosphere (Air); Table S9. Compounds obtained in the decomposition of Heet tobacco mixed with 5, 15 and 25% w/w of SiLo at different temperatures; Oxidative atmosphere (Air); Table S10. Compounds obtained in the decomposition Heet tobacco mixed with 5, 15 and 25% w/w of Beta at different temperatures; Oxidative atmosphere (Air); Table S11. Compounds (µg compound/cigarette) obtained in in smoking experiments of Heet tobacco alone and in presence of different concentrations of USY and SBA-15; Table S12. Compounds (µg compound/cigarette) obtained in the smoking experiments of Heet tobacco in presence of different concentrations of SiLo and Beta at 300 °C; Figure S1. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of USY at 250 °; Figure S2. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SiLo at 250 °C; Figure S3. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SBA-15 at 250 °C; Figure S4. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of BETA at 250 °C; Figure S5. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of USY at 300 °C; Figure S6. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SiLo at 300 °C; Figure S7. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SBA-15 at 300 °C; Figure S8. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of BETA at 300 °C; Figure S9. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of USY at 350 °C; Figure S10. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SiLo at 350 °C; Figure S11. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SBA-15 at 350 °C; Figure S12. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of BETA at 350 °C; Figure S13. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of USY at 400 °C; Figure S14. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SiLo at 400 °C; Figure S15. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SBA-15 at 400 °C; Figure S16. He atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of BETA at 400 °C; Figure S17. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of USY at 250 °C; Figure S18. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SiLo at 250 °C; Figure S19. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SBA-15 at 250 °C; Figure S20. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of BETA at 250 °C; Figure S21. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of USY at 300 °C; Figure S22. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SiLo at 300 °C; Figure S23. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SBA-15 at 300 °C; Figure S24. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of BETA at 300 °C; Figure S25. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of USY at 350 °C; Figure S26. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SiLo at 350 °C; Figure S27. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SBA-15 at 350 °C; Figure S28. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of BETA at 350 °C; Figure S29. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of USY at 400 °C; Figure S30. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SiLo at 400 °C; Figure S31. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of SBA-15 at 400 °C; Figure S32. Air atmosphere chromatograms of Het tobacco (black line) and Heet tobacco mixtures with 5% (red line), 15% (blue line and 25% (green line) of BETA at 400 °C.
Author Contributions
D.B.: visualization, methodology, investigation, validation, data curation, writing—original draft preparation; A.M.: conceptualization, methodology, supervision, writing—reviewing and editing, project administration; C.F.: resources and methodology; M.I.B.: writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital” (IDIFEDER 2018/009 and PROMETEO2020/093).
Data Availability Statement
All data relevant to the study are included in the article or uploaded as Supplementary Materials. Any additional information can be requested by email to the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Proctor, R.N. The History of the Discovery of the Cigarette-lung Cancer Link: Evidentiary Traditions, Corporate Denial, Global Toll. Tob. Control. 2012, 21, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P. Paul Hahn Issues Reassurance on Cigarettes’ Use. United States Tob. J 1953. Bates No. MNAT 00016506-00016507. [Google Scholar]
- Schaller, J.P.; Keller, D.; Poget, L.; Pratte, P.; Kaelin, E.; McHugh, D.; Cudazzo, G.; Smart, D.; Tricker, A.R.; Gautier, L.; et al. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical Composition, Genotoxicity, Cytotoxicity, and Physical Properties of the Aerosol. Regul. Toxicol. Pharm. 2016, 81, S27–S47. [Google Scholar] [CrossRef] [PubMed]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014. [Google Scholar] [PubMed]
- Barua, R.S.; Rigotti, N.A.; Benowitz, N.L.; Cummings, K.M.; Jazayeri, M.A.; Morris, P.B.; Ratchford, E.V.; Sarna, L.; Stecker, E.C.; Wiggins, B.S. ACC expert consensus decision pathway on tobacco cessation treatment: A report of the american college of cardiology task force on clinical expert consensus documents. J. Am. Coll. Cardiol. 2018, 72, 3332–3365. [Google Scholar] [CrossRef] [PubMed]
- Etter, J.F.; Bullen, C.; Flouris, A.D.; Laugesen, M.; Eissenberg, T. Electronic nicotine delivery systems: A research agenda. Tob. Control. 2011, 20, 243–248. [Google Scholar] [CrossRef]
- Hajek, P.; Etter, J.F.; Benowitz, N.; Eissenberg, T.; McRobbie, H. Electronic cigarettes: Review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction 2014, 109, 1801–1810. [Google Scholar] [CrossRef]
- Orellana-Barrios, M.A.; Payne, D.; Mulkey, Z.; Nugent, K. Electronic cigarettes—A narrative review for clinicians. Am. J. Med. 2015, 128, 674–681. [Google Scholar] [CrossRef]
- Auer, R.; Concha-Lozano, N.; Jacot-Sadowski, I.; Cornuz, J.; Berthet, A. Heat-not-burn tobacco cigarettes: Smoke by any other name. JAMA Intern. Med. 2017, 177, 1050–1052. [Google Scholar] [CrossRef]
- Liu, X.; Lugo, A.; Spizzichino, L.; Tabuchi, T.; Pacifici, R.; Gallus, S. Heat-not-burn tobacco products: Concerns from the Italian experience. Tob. Control. 2019, 28, 113–114. [Google Scholar] [CrossRef]
- Paumgartten, F. Heat-not-burn and electronic cigarettes: Truths and untruths about harm reduction. Rev. Assoc. Med. Bras. 2018, 64, 104–105. [Google Scholar] [CrossRef]
- Jaccard, G.; Taffin Djoko, D.; Moennikes, O.; Jeannet, C.; Kondylis, A.; Belushkin, M. Comparative assessment of HPHC yields in the Tobacco Heating System THS2.2 and commercial cigarettes. Regul. Toxicol. Pharmacol. 2017, 90, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buratto, R.; Correia, D.; Parel, M.; Crenna, M.; Bilger, M.; Debrick, A. Determination of eight carbonyl compounds in aerosols trapped in phosphate buffer saline solutions to support in vitro assessment studies. Talanta 2018, 184, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Znyk, M.; Jurewicz, J.; Kaleta, D. Exposure to Heated Tobacco Products and Adverse Health Effects, a Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6651. [Google Scholar] [CrossRef] [PubMed]
- Pacitto, A.; Stabile, L.; Scungio, M.; Rizza, V.; Buonanno, G. Characterization of airborne particles emitted by an electrically heated tobacco smoking system. Environ. Pollut. 2018, 240, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.N.; Djurdjevic, S.; Weitkunat, R.; Baker, G. Estimating the population health impact of introducing a reduced-risk tobacco product into Japan. The efect of direring assumptions, and some comparisons with the U.S. Regul. Toxicol. Pharmacol. 2018, 100, 92–104. [Google Scholar] [CrossRef]
- Czoli, C.D.; White, C.M.; Reid, J.L.; OConnor, R.J.; Hammond, D. Awareness and interest in IQOS heated tobacco products among youth in Canada, England and the USA. Tob. Control. 2019, 29, 89–95. [Google Scholar] [CrossRef]
- Marynak, K.L.; Wang, T.W.; King, B.A.; Shete, S. Awareness and Ever Use of “Heat-Not-Burn” Tobacco Products Among U.S. Adults. Am. J. Prev. Med. 2017, 55, 551–554. [Google Scholar] [CrossRef]
- Bekki, K.; Inaba, Y.; Uchiyama, S.; Kunugita, N. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J. UOEH 2017, 39, 201–207. [Google Scholar] [CrossRef]
- Forster, M.; Fiebelkorn, S.; Yurteri, C.; Mariner, D.; Liu, C.; Wright, C.; McAdam, K.; Murphy, J.; Proctor, C. Assessment of novel tobacco heating product THP1.0. Part 3: Comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharmacol. 2018, 93, 14–33. [Google Scholar] [CrossRef]
- Poynton, S.; Sutton, J.; Goodall, S.; Margham, J.; Forster, M.; Scott, K.; Liu, C.; McAdam, K.; Murphy, J.; Proctor, C. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 1): Product operation and preliminary aerosol chemistry assessment. Food Chem. Toxicol. 2017, 106, 522–532. [Google Scholar] [CrossRef]
- Protano, C.; Manigrasso, M.; Avino, P.; Sernia, S.; Vitali, M. Second-hand smoke exposure generated by new electronic devices (IQOS® and e-cigs) and traditional cigarettes: Submicron particle behaviour in human respiratory system. Ann. Ig. 2016, 28, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Manigrasso, M.; Avino, P.; Vitali, M. Second-hand smoke generated by combustion and electronic smoking devices used in real scenarios: Ultrafine particle pollution and age-related dose assessment. Environ. Int. 2017, 107, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Rahman, M.; Johanson, G.; Palmberg, L.; Ganguly, K. Heated Tobacco Products: Insights into Composition and Toxicity. Toxics 2023, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Marcilla, A.; Gómez-Siurana, A.; Berenguer, D.; Martínez-Castellanos, I.; Beltran, M. Reduction of Tobacco Smoke Components Yields by Zeolites and Synthesized Al-MCM-41. Microporous Mesoporous Mater. 2012, 161, 14–24. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez-Siurana, A.; Martínez-Castellanos, I.; Beltran, M.I.; Berenguer, D. Reduction of tobacco smoke components yield in commercial cigarette brands by addition of HUSY, NaY and Al-MCM-41 to the cigarette rod. Toxicol. Rep. 2015, 2, 152–164. [Google Scholar] [CrossRef]
- Lin, W.G.; Zhou, Y.; Cao, Y.; Zhou, S.L.; Wan, M.M.; Wang, Y.; Zhu, J.H. Applying heterogeneous catalysis to health care: In situ elimination of tobacco-specific nitrosamines (TSNAs) in smoke by molecular sieves. Catal. Today 2013, 212, 52–61. [Google Scholar] [CrossRef]
- Marcilla, A.; Berenguer, D.; Martinez, I. Effect of the addition of zeolites and silicate compounds on the composition of the smoke generated in the decomposition of Heet tobacco under inert and oxidative atmospheres. J. Anal. Appl. Pyrolysis 2022, 164, 105532. [Google Scholar] [CrossRef]
- Li, X.-f.; Wang, E.-b.; Zhang, Z.; Tian, H.-y.; Xu, Y.-m.; Han, L.; Hao, H.; Xu, H.; Song, J.-y.; Liu, W.-z.; et al. Low temperature catalytic pyrolysis performances of heated tobacco sheets by alkali/alkaline earth metal. J. Anal. Appl. Pyrolysis 2023, 169, 105854. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, Y.; Yang, H.; Meng, Y.; Yu, C.; Tu, B.; Zhao, D. Understanding effect of wall structure on the hydrothermal stability of mesostructured silica SBA-15. J. Phys. Chem. B 2005, 109, 8723–8732. [Google Scholar] [CrossRef]
- Gómez-Siurana, A.; Marcilla, A.; Beltran, M.; Martinez, I.; Berenguer, D.; García-Martínez, R.; Hernández-Selva, T. Thermogravimetric study of the pyrolysis of tobacco and several ingredients used in the fabrication of commercial cigarettes: Effect of the presence of MCM-41. Thermochim. Acta 2011, 523, 161–169. [Google Scholar] [CrossRef]
- Calabiug, E. Pirólisis y Descomposición del Tabaco, Efectos del Uso Catalizadores Mesoporosos SBA-15. Ph.D. Thesis, Universidad de Alicante, Alicante, Spain, 2021. [Google Scholar]
- Marcilla, A.; Beltran, M.I.; Gómez-Siurana, A.; Berenguer, D.; Martínez-Castellanos, I. Nicotine/mesoporous solids interactions at increasing temperatures under inert and air environments. J. Anal. Appl. Pyrolysis 2016, 119, 162–172. [Google Scholar] [CrossRef]
- Asensio, J.; Berenguer, D.; Marcilla, A.; Beltran, M.I. Nicotine fast pyrolysis under inert and air environments. Effect of catalysts. J. Anal. Appl. Pyrolysis 2023, 170, 105899. [Google Scholar] [CrossRef]
- Gómez-Siurana, A.; Marcilla, A.; Beltrán, M.; Berenguer, D.; Martínez-Castellanos, I.; Menargues, S. TGA/FTIR Study of Tobacco and Glycerine-Tobacco Mixtures. Thermochim. Acta 2013, 573, 146–157. [Google Scholar] [CrossRef]
- Baker, R.R. A review of pyrolysis studies to unravel reaction steps in burning tobacco. J. Anal. Appl. Pyrolysis 1987, 11, 555–563. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, S.; Han, T.U.; Park, Y.K.; Watanabe, C. Pyrolysis reaction characteristics of Korean pine (Pinus koraiensis) nut shell. J. Anal. Appl. Pyrolysis 2014, 110, 435–441. [Google Scholar] [CrossRef]
- Talhout, R.; Opperhuizen, A.; van Amsterdam, J.G.C. Sugars as tobacco ingredient: Effects on mainstream smoke composition. Food Chem. Toxicol. 2006, 44, 1789–1798. [Google Scholar] [CrossRef]
- Woodward, C.F.; Eisner, A.; Haines, P.G. Pyrolysis of Nicotine to Myosmine. J. Am. Chem. Soc. 1944, 66, 911–914. [Google Scholar] [CrossRef]
- Kisaki, T.; Ihida, M.; Tamaki, E. Chemistry of the N′-Oxides of Nicotine and Myosmine. Bull. Agric. Chem. Soc. Jpn. 1960, 24, 719–728. [Google Scholar] [CrossRef][Green Version]
- Bezerra Batista, L.M.; Freitas Oliveira, J.L.; Aureliano Bezerra, F.; de Morais Araújo, A.M.; Fernandes Juniora, V.J.; Souza Araujo, A.; Alves, A.P.M.; Duarte Gondim, A. Synthesis, characterization and evaluation of niobium catalysts in the flash pyrolysis of Glycerine. Solid State Sci. 2019, 97, 105977. [Google Scholar] [CrossRef]
- Available online: https://www.federalregister.gov/documents/2019/08/05/2019-16658/harmful-and-potentially-harmful-constituents-in-tobacco-products-established-list-proposed-additions (accessed on 1 May 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).