Iron and Manganese Oxidation States, Bonding Environments, and Mobility in the Mining-Impacted Sediments of Coeur d’Alene Lake, Idaho: Core Experiments
Abstract
:1. Introduction
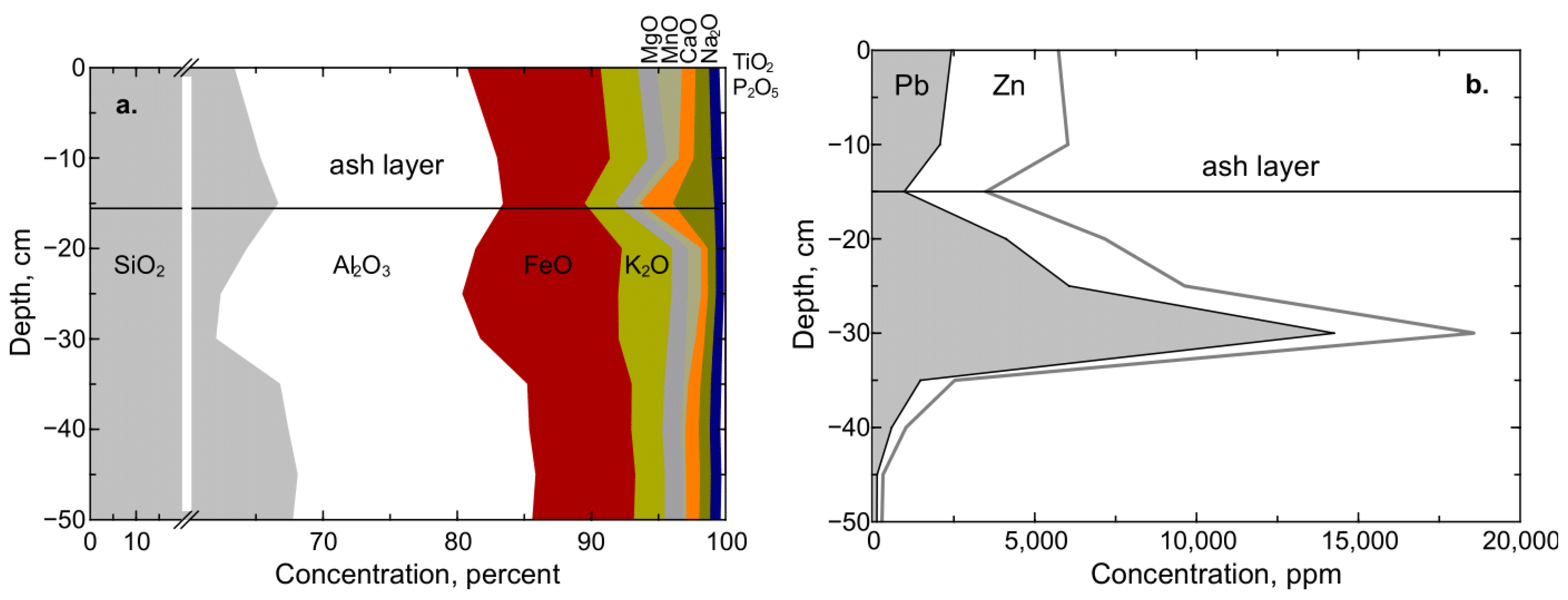
1.1. Legacy Mine Waste Issues, Lakebed Deposition, and Element Mobility
1.2. Lake Environment, Sulfur Cycling, and Iron and Manganese Mobility
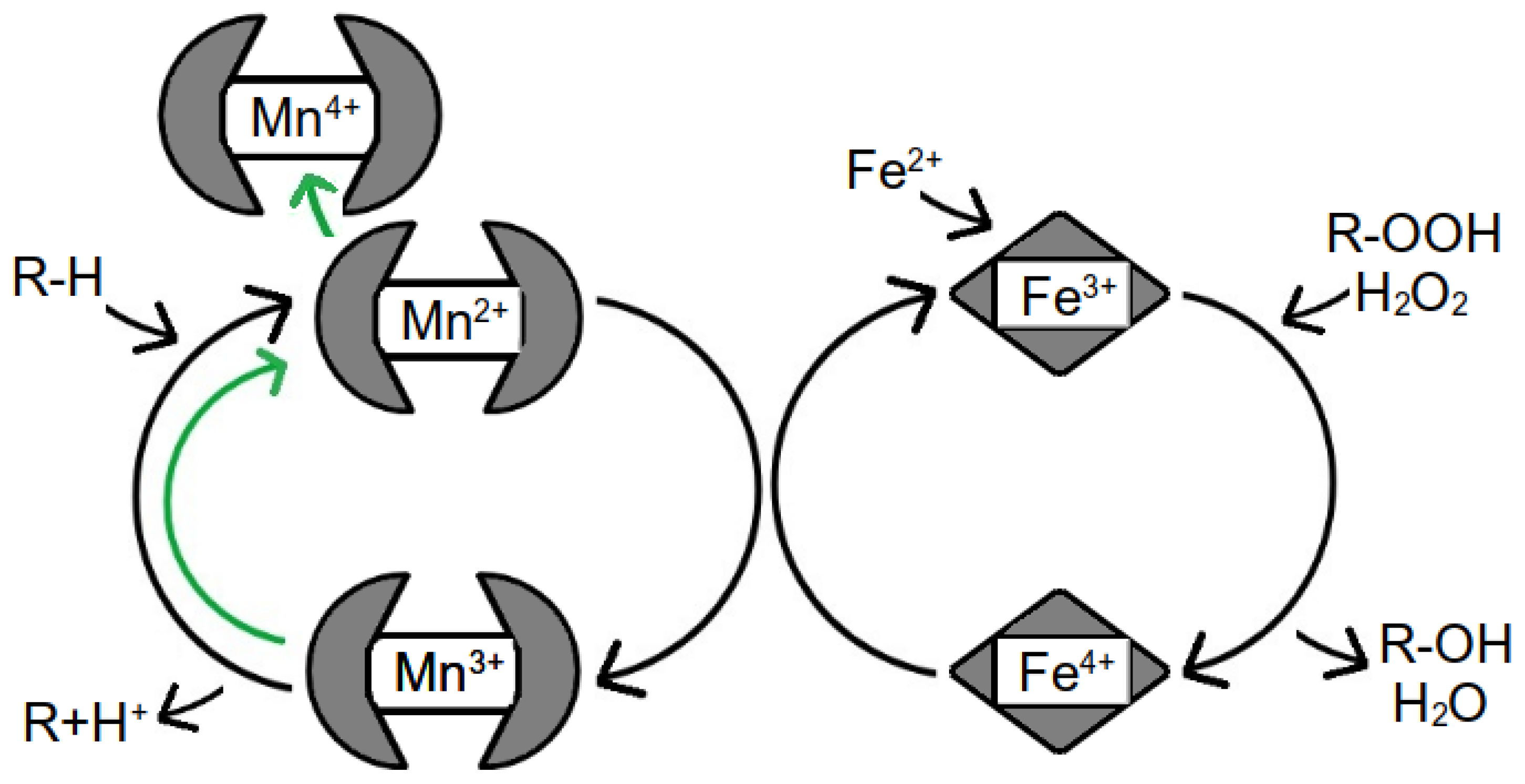
1.3. Lake Environment and Iron and Manganese Cycling
2. Study Area, Materials, and Methods
2.1. Study Design
2.2. Sediment Core Collection
2.3. Algae Collection and Column Loading
2.4. Column Experiment
2.5. X-ray Absorption Spectroscopy
3. Results
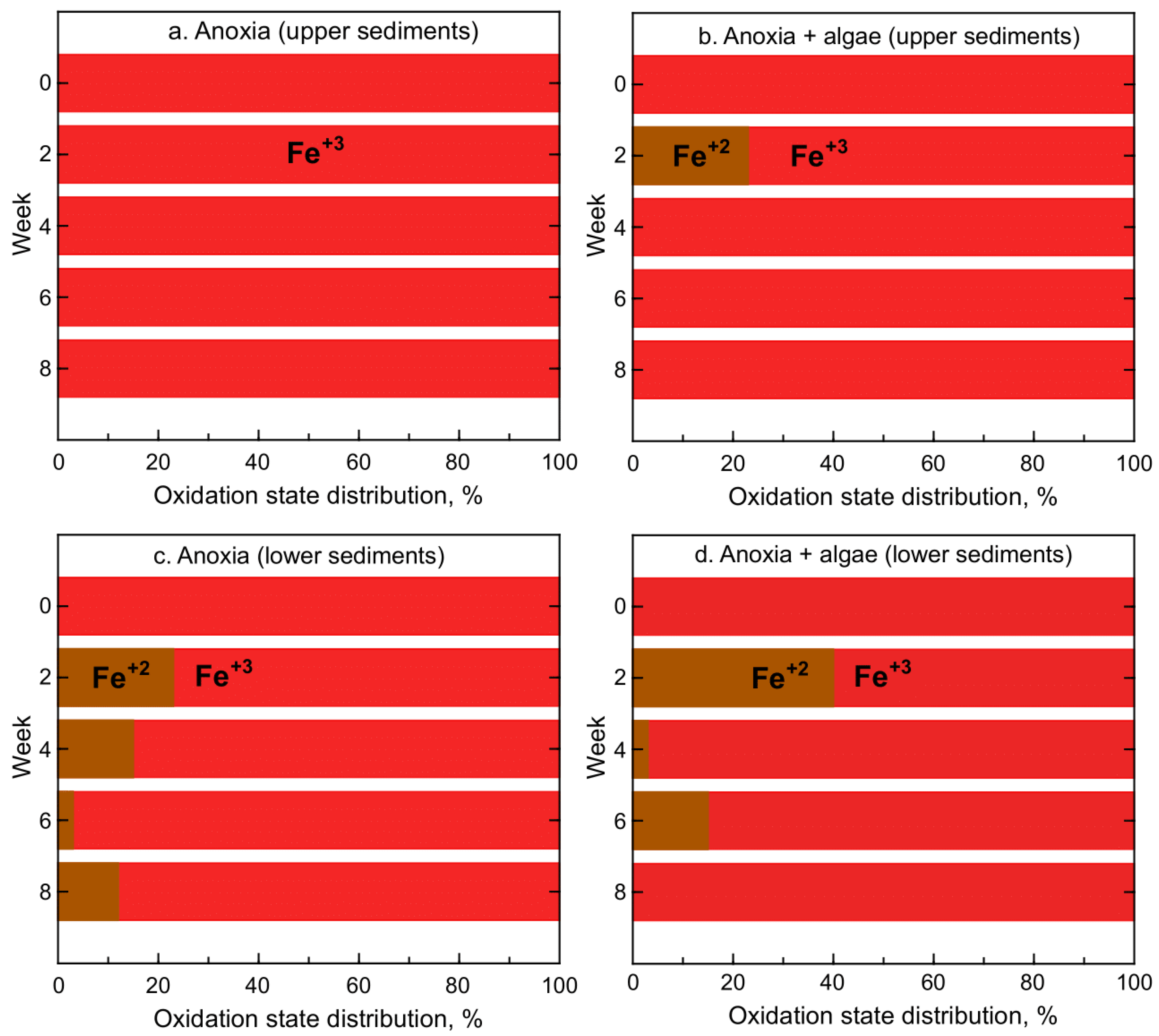
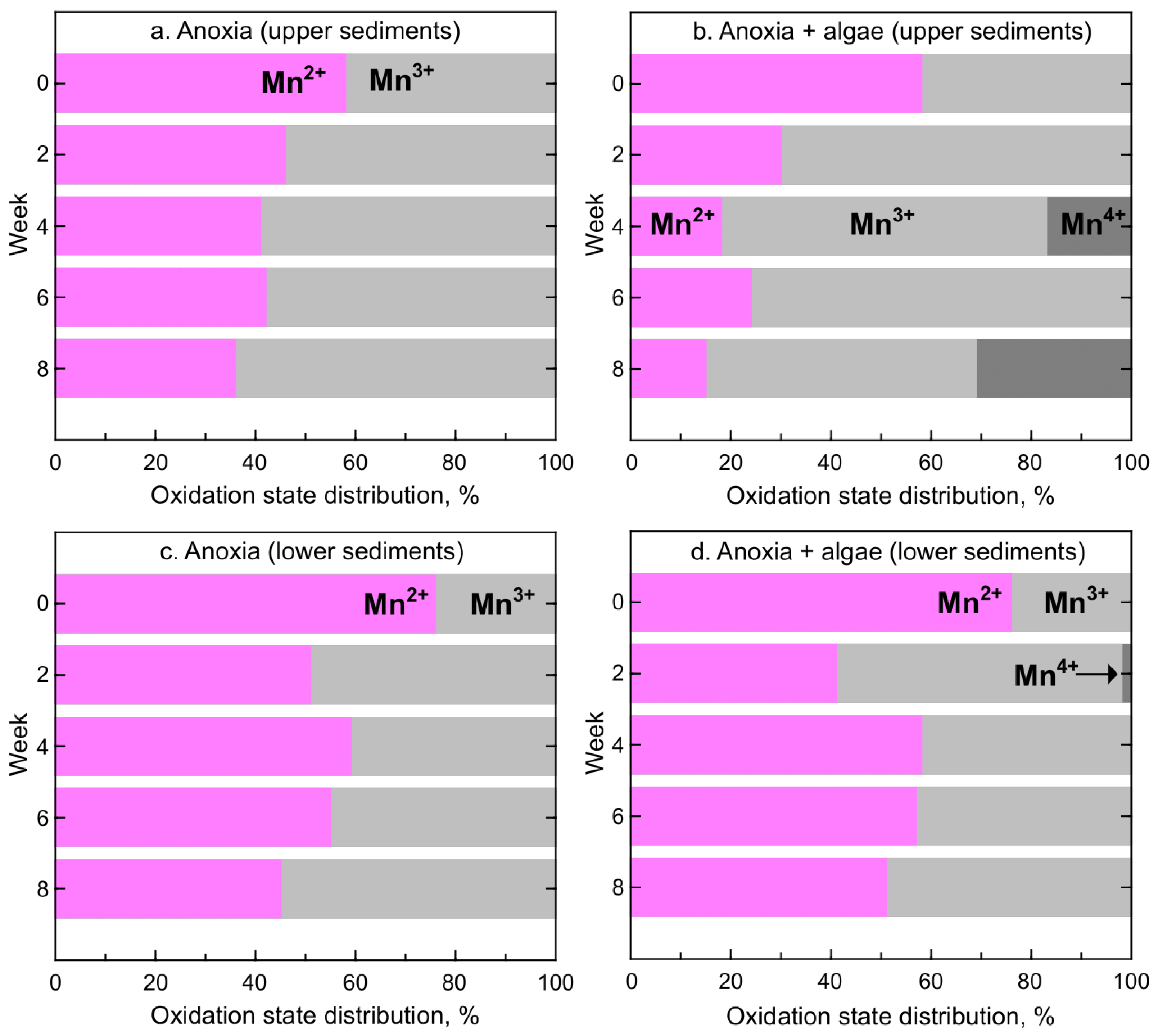
3.1. Iron and Manganese Oxidation State Distribution
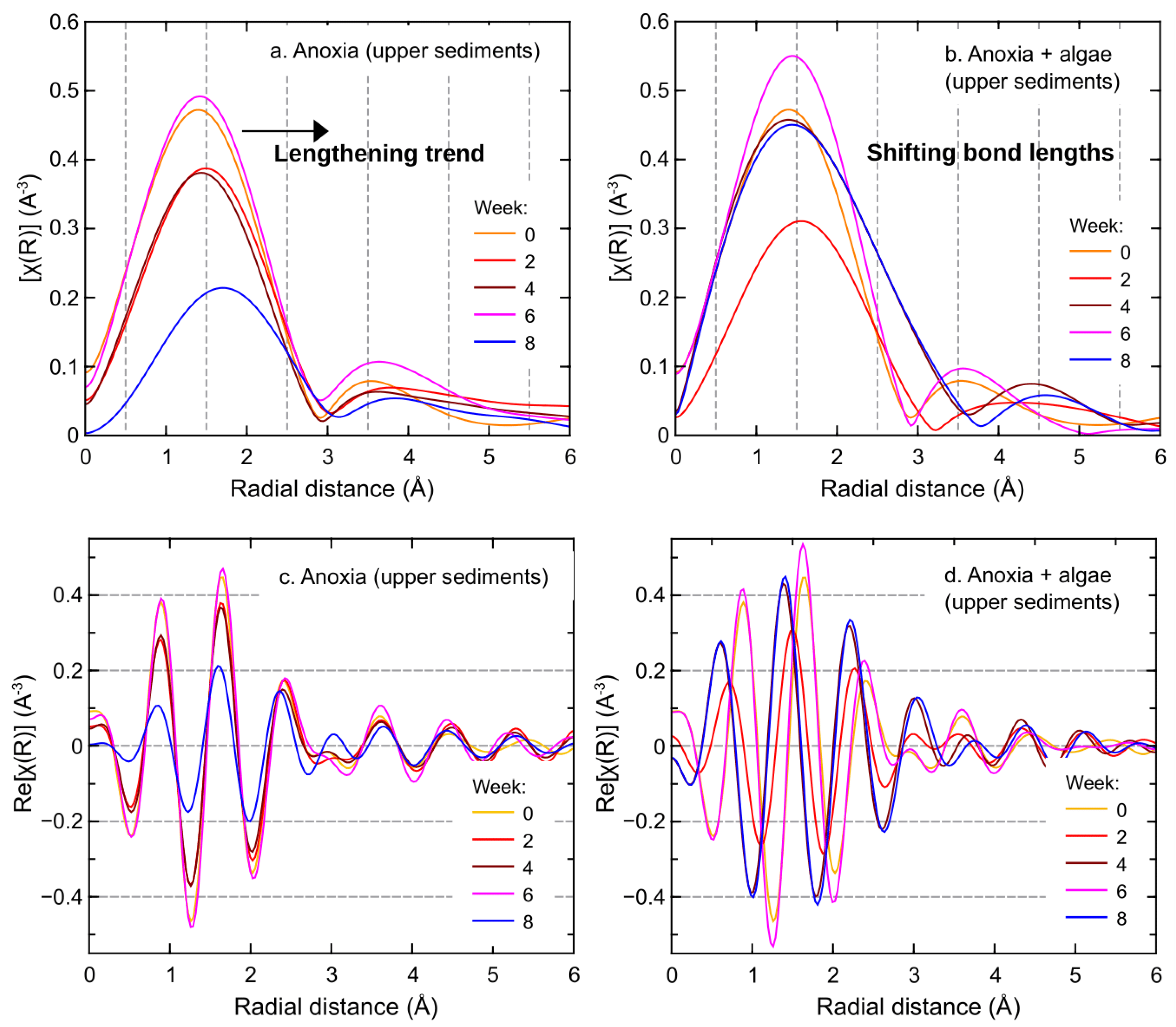
3.2. Upper Sediment Iron and Manganese Bonding Environments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrington, J.M.; LaForce, M.J.; Rember, W.C.; Fendorf, S.E.; Rosenzweig, R.F. Phase Associations and Mobilization of Iron and Trace Elements in Coeur d’Alene Lake, Idaho. Environ. Sci. Technol. 1998, 32, 650–656. [Google Scholar] [CrossRef]
- Horowitz, A.J.; Elrick, K.A.; Robbins, J.A.; Cook, R.B. A Summary of the Effects of Mining and Related Activities on the Sediment-Trace Element Geochemistry of Lake Coeur d’Alene, Idaho, USA. J. Geochem. Explor. 1995, 52, 135–144. [Google Scholar] [CrossRef]
- National Research Council. Superfund and Mining Megasites: Lessons from the Coeur d’Alene River Basin; The National Academies of Sciences, Engineering, Medicine: Washington, DC, USA, 2005; p. 484.
- Ciszewski, D.; Grygar, T.M. A Review of Flood-Related Storage and Remobilization of Heavy Metal Pollutants in River Systems. Water. Air. Soil Pollut. 2016, 227, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozzard, E.; Mayes, W.M.; Potter, H.A.B.; Jarvis, A.P. Seasonal and Spatial Variation of Diffuse (Non-Point) Source Zinc Pollution in a Historically Metal Mined River Catchment, UK. Environ. Pollut. 2011, 159, 3113–3122. [Google Scholar] [CrossRef]
- Huettel, M.; Røy, H.; Precht, E.; Ehrenhauss, S. Hydrodynamical Impact on Biogeochemical Processes in Aquatic Sediments. In The Interactions between Sediments and Water; Kronvang, B., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2003; pp. 231–236. [Google Scholar]
- Krantzberg, G. The Influence of Bioturbation on Physical, Chemical and Biological Parameters in Aquatic Environments: A Review. Environ. Pollut. Ser. Ecol. Biol. 1985, 39, 99–122. [Google Scholar] [CrossRef]
- Schulz-Zunkel, C.; Krueger, F. Trace Metal Dynamics in Floodplain Soils of the River Elbe: A Review. J. Environ. Qual. 2009, 38, 1349–1362. [Google Scholar] [CrossRef]
- Hamilton-Taylor, J.; Davison, W.; Morfett, K. The Biogeochemical Cycling of Zn, Cu, Fe, Mn, and Dissolved Organic C in a Seasonally Anoxic Lake. Limnol. Oceanogr. 1996, 41, 408–418. [Google Scholar] [CrossRef]
- Morfett, K.; Davison, W.; Hamilton-Taylor, J. Trace Metal Dynamics in a Seasonally Anoxic Lake. Environ. Geol. Water Sci. 1988, 11, 107–114. [Google Scholar] [CrossRef]
- Palmer, M.J.; Chételat, J.; Richardson, M.; Jamieson, H.E.; Galloway, J.M. Seasonal Variation of Arsenic and Antimony in Surface Waters of Small Subarctic Lakes Impacted by Legacy Mining Pollution near Yellowknife, NT, Canada. Sci. Total Environ. 2019, 684, 326–339. [Google Scholar] [CrossRef]
- Gadd, G.M. Microbial Influence on Metal Mobility and Application for Bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar] [CrossRef]
- Langman, J.B.; Behrens, D.; Moberly, J.G. Seasonal Formation and Stability of Dissolved Metal Particles in Mining-Impacted, Lacustrine Sediments. J. Contam. Hydrol. 2020, 232, 103655. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.J.; Pedersen, T.F. Seasonal and Interannual Mobility of Arsenic in a Lake Impacted by Metal Mining. Environ. Sci. Technol. 2002, 36, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Wang, S.; Wang, Y. Characteristics of Bioavailable Organic Phosphorus in Sediment and Its Contribution to Lake Eutrophication in China. Environ. Pollut. 2016, 219, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, A.M.; Caraballo, M.A.; Sanchez-Rodas, D.; Nieto, J.M.; Parviainen, A. Dissolved and Particulate Metals and Arsenic Species Mobility along a Stream Affected by Acid Mine Drainage in the Iberian Pyrite Belt (SW Spain). Appl. Geochem. 2012, 27, 1944–1952. [Google Scholar] [CrossRef]
- Smith, K.S.; Huyck, H.L.O. An Overview of the Abundance, Relative Mobility, Bioavailability, and Human Toxicity of Metals. In The Environmental Geochemistry of Mineral Deposits: Part A: Processes, Techniques and Health Issues; Plumlee, G.S., Logsdon, M.J., Filipek, L.F., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 6A, pp. 29–70. ISBN 978-1-62949-013-7. [Google Scholar]
- Wang, F.; Liu, C.; Liang, X.; Wei, Z. Remobilization of Trace Metals Induced by Microbiological Activities near Sediment-Water Interface, Aha Lake, Guiyang. Chin. Sci. Bull. 2003, 48, 2352–2356. [Google Scholar] [CrossRef]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and Bioavailability of Heavy Metals and Metalloids in Soil Environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef] [Green Version]
- Davison, W. Iron and Manganese in Lakes. Earth-Sci. Rev. 1993, 34, 119–163. [Google Scholar] [CrossRef]
- Langman, J.B.; Ali, J.D.; Child, A.W.; Wilhelm, F.M.; Moberly, J.G. Sulfur Species, Bonding Environment, and Metal Mobilization in Mining-Impacted Lake Sediments: Column Experiments Replicating Seasonal Anoxia and Deposition of Algal Detritus. Minerals 2020, 10, 849. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Bookstrom, A.A.; Box, S.E.; Ikramuddin, M. Drainage from Adits and Tailings Piles in the Coeur d’Alene Mining District, Idaho: Sampling, Analytical Methods and Results; US Geological Survey Open-File Report; U.S. Geological Survey: Reston, VA, USA, 1998; p. 19.
- Horowitz, A.J.; Elrick, K.A.; Robbins, J.A.; Cook, R.B. Effect of Mining and Related Activities on the Sediment Trace Element Geochemistry of Lake Coeur d’Alene, Idaho, USA Part II: Subsurface Sediments. Hydrol. Process. 1995, 9, 35–54. [Google Scholar] [CrossRef]
- Paulson, A.J. Biogeochemical Removal of Zn and Cd in the Coeur d’Alene River (Idaho, USA), Downstream of a Mining District. Sci. Total Environ. 2001, 278, 31–44. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Box, S.E.; Bookstrom, A.A.; Hooper, R.L.; Mahoney, J.B. Impacts of Historical Mining in the Coeur d‘Alene River Basin; Bulletin; U.S. Geological Survey: Reston, VA, USA, 2010.
- Woods, P.F. Role of Limnological Processes in Fate and Transport of Nitrogen and Phosphorous Loads Delivered into Coeur d’Alene Lake and Lake Pend Oreille, Idaho, and Flathead Lake, Montana; U.S. Geological Survey: Reston, VA, USA, 2004; p. 44.
- La Force, M.J.; Hansel, C.M.; Fendorf, S. Arsenic Speciation, Seasonal Transformations, and Co-Distribution with Iron in a Mine Waste-Influenced Palustrine Emergent Wetland. Environ. Sci. Technol. 2000, 34, 3937–3943. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Optimization Review Report: Remedial Process Optimization Study: Lake Coeur d’Alene, Bunker Hill Mining and Metallurgical Site, Operable Unit 03, Coeur d’Alene, Kootenai County, Idaho; U.S. Environmental Protection Agency: Coeur d’Alene, ID, USA, 2020; p. 76.
- Wood, M.S.; Beckwith, M.A. Coeur d’Alene Lake, Idaho: Insights Gained from Limnological Studies of 1991–92 and 2004–06; U.S. Geological Survey: Reston, VA, USA, 2008; p. 40.
- Arora, B.; Şengör, S.S.; Spycher, N.F.; Steefel, C.I. A Reactive Transport Benchmark on Heavy Metal Cycling in Lake Sediments. Comput. Geosci. 2015, 19, 613–633. [Google Scholar] [CrossRef]
- Cummings, D.E.; March, A.W.; Bostick, B.; Spring, S.; Caccavo, F.; Fendorf, S.; Rosenzweig, R.F. Evidence for Microbial Fe(III) Reduction in Anoxic, Mining-Impacted Lake Sediments (Lake Coeur d’Alene, Idaho). Appl. Environ. Microbiol. 2000, 66, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of Dissolved Organic Matter on the Environmental Fate of Metals, Nanoparticles, and Colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef]
- Haus, K.L.; Hooper, R.L.; Strumness, L.A.; Mahoney, J.B. Analysis of Arsenic Speciation in Mine Contaminated Lacustrine Sediment Using Selective Sequential Extraction, HR-ICPMS and TEM. Appl. Geochem. 2008, 23, 692–704. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Schäfer, T. Metal Retention and Transport on Colloidal Particles in the Environment. Elements 2005, 1, 205–210. [Google Scholar] [CrossRef]
- Langman, J.B.; Torso, K.; Moberly, J.G. Seasonal and Basinal Influences on the Formation and Transport of Dissolved Trace Metal Forms in a Mining-Impacted Riverine Environment. Hydrology 2018, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Plathe, K.L.; von der Kammer, F.; Hassellöv, M.; Moore, J.N.; Murayama, M.; Hofmann, T.; Hochella, M.F. The Role of Nanominerals and Mineral Nanoparticles in the Transport of Toxic Trace Metals: Field-Flow Fractionation and Analytical TEM Analyses after Nanoparticle Isolation and Density Separation. Geochim. Cosmochim. Acta 2013, 102, 213–225. [Google Scholar] [CrossRef]
- Clark, G.M.; Mebane, C.A. Sources, Transport and Trends for Selected Trace Metals and Nutrients in the Coeur d’Alene and Spokane River Basins, Northern Idaho, 1990–2013; U.S. Geological Survey: Reston, VA, USA, 2014; p. 62.
- Kuwabara, J.S.; Topping, B.R.; Woods, P.F.; Carter, J.L. Free Zinc Ion and Dissolved Orthophosphate Effects on Phytoplankton from Coeur d’Alene Lake, Idaho. Environ. Sci. Technol. 2007, 41, 2811–2817. [Google Scholar] [CrossRef]
- Morra, M.J.; Carter, M.M.; Rember, W.C.; Kaste, J.M. Reconstructing the History of Mining and Remediation in the Coeur d’Alene, Idaho Mining District Using Lake Sediments. Chemosphere 2015, 134, 319–327. [Google Scholar] [CrossRef]
- Woods, P.F.; Beckwith, M.A. Nutrient and Trace-Element Enrichment of Coeur d’Alene Lake, Idaho; U.S. Geological Survey: Reston, VA, USA, 1997; p. 93.
- Harrington, J.M.; Fendorf, S.E.; Rosenzweig, R.F. Biotic Generation of Arsenic(III) in Metal(Loid)-Contaminated Freshwater Lake Sediments. Environ. Sci. Technol. 1998, 32, 2425–2430. [Google Scholar] [CrossRef]
- Pedersen, T.F. A Comment on the Future Environmental Status of Coeur d’Alene Lake, Idaho. Northwest Sci. 1996, 70, 179–182. [Google Scholar]
- Şengör, S.S.; Spycher, N.F.; Ginn, T.R.; Sani, R.K.; Peyton, B. Biogeochemical Reactive–Diffusive Transport of Heavy Metals in Lake Coeur d’Alene Sediments. Appl. Geochem. 2007, 22, 2569–2594. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Box, S.E.; Tonkin, J.W. Modeling Precipitation and Sorption of Elements during Mixing of River Water and Porewater in the Coeur d’Alene River Basin. Environ. Sci. Technol. 2003, 37, 4694–4701. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Blank, R.G. Dissolved and Labile Concentrations of Cd, Cu, Pb, and Zn in the South Fork Coeur d’Alene River, Idaho: Comparisons among Chemical Equilibrium Models and Implications for Biotic Ligand Models. Appl. Geochem. 2008, 23, 3355–3371. [Google Scholar] [CrossRef]
- Gao, Y.; Kan, A.T.; Tomson, M.B. Critical Evaluation of Desorption Phenomena of Heavy Metals from Natural Sediments. Environ. Sci. Technol. 2003, 37, 5566–5573. [Google Scholar] [CrossRef]
- Hoffmann, S.R.; Shafer, M.M.; Armstrong, D.E. Strong Colloidal and Dissolved Organic Ligands Binding Copper and Zinc in Rivers. Environ. Sci. Technol. 2007, 41, 6996–7002. [Google Scholar] [CrossRef]
- Child, A.W.; Moore, B.C.; Vervoort, J.D.; Beutel, M.W. Bioavailability and Uptake of Smelter Emissions in Freshwater Zooplankton in Northeastern Washington, USA Lakes Using Pb Isotope Analysis and Trace Metal Concentrations. Environ. Pollut. 2018, 238, 348–358. [Google Scholar] [CrossRef]
- Farley, M. Eutrophication in Fresh Waters: An International Review. In Encyclopedia of Lakes and Reservoirs; Bengtsson, L., Herschy, R.W., Fairbridge, R.W., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 258–270. ISBN 978-1-4020-4410-6. [Google Scholar]
- Toevs, G.; Morra, M.J.; Winowiecki, L.; Strawn, D.; Polizzotto, M.L.; Fendorf, S. Depositional Influences on Porewater Arsenic in Sediments of a Mining-Contaminated Freshwater Lake. Environ. Sci. Technol. 2008, 42, 6823–6829. [Google Scholar] [CrossRef]
- Bostick, B.C.; Hansel, C.M.; Fendorf, S. Seasonal Fluctuations in Zinc Speciation within a Contaminated Wetland. Environ. Sci. Technol. 2001, 35, 3823–3829. [Google Scholar] [CrossRef]
- Boyle, J. Redox Remobilization and the Heavy Metal Record in Lake Sediments: A Modelling Approach. J. Paleolimnol. 2001, 26, 423–431. [Google Scholar] [CrossRef]
- Boudreau, B.P. Metals and Models: Diagenic Modelling in Freshwater Lacustrine Sediments. J. Paleolimnol. 1999, 22, 227–251. [Google Scholar] [CrossRef]
- Stone, A.T.; Morgan, J.J. Reduction and Dissolution of Manganese(III) and Manganese(IV) Oxides by Organics. 1. Reaction with Hydroquinone. Environ. Sci. Technol. 1984, 18, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Sunda, W.G.; Kieber, D.J. Oxidation of Humic Substances by Manganese Oxides Yields Low-Molecular-Weight Organic Substrates. Nature 1994, 367, 62–64. [Google Scholar] [CrossRef]
- Murray, K.J.; Tebo, B.M. Cr(III) Is Indirectly Oxidized by the Mn(II)-Oxidizing Bacterium Bacillus Sp. Strain SG-1. Environ. Sci. Technol. 2007, 41, 528–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, Y.M.; Lion, L.W.; Ghiorse, W.C.; Shuler, M.L. Production of Biogenic Mn Oxides by Leptothrix Discophora SS-1 in a Chemically Defined Growth Medium and Evaluation of Their Pb Adsorption Characteristics. Appl. Environ. Microbiol. 1999, 65, 175–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nealson, K.H.; Saffarini, D. Iron and Manganese in Anaerobic Respiration: Environmental Significance, Physiology, and Regulation. Annu. Rev. Microbiol. 1994, 48, 311–343. [Google Scholar] [CrossRef]
- Andeer, P.F.; Learman, D.R.; McIlvin, M.; Dunn, J.A.; Hansel, C.M. Extracellular Haem Peroxidases Mediate Mn(II) Oxidation in a Marine Roseobacter Bacterium via Superoxide Production. Environ. Microbiol. 2015, 17, 3925–3936. [Google Scholar] [CrossRef]
- Bargar, J.R.; Tebo, B.M.; Bergmann, U.; Webb, S.M.; Glatzel, P.; Chiu, V.Q.; Villalobos, M. Biotic and Abiotic Products of Mn(II) Oxidation by Spores of the Marine Bacillus Sp. Strain SG-1. Am. Mineral. 2005, 90, 143–154. [Google Scholar] [CrossRef]
- Brouwers, G.-J.; de Vrind, J.P.M.; Corstjens, P.L.A.M.; Cornelis, P.; Baysse, C.; de Vrind-de Jong, E.W. CumA, a Gene Encoding a Multicopper Oxidase is Involved in Mn2+ Oxidation in Pseudomonas Putida GB-1. Appl. Environ. Microbiol. 1999, 65, 1762–1768. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, C.N.; Soldatova, A.V.; Lee, S.-W.; Spiro, T.G.; Tebo, B.M. Mn(II,III) Oxidation and MnO2 Mineralization by an Expressed Bacterial Multicopper Oxidase. Proc. Natl. Acad. Sci. USA 2013, 110, 11731–11735. [Google Scholar] [CrossRef] [PubMed]
- Melton, E.D.; Swanner, E.D.; Behrens, S.; Schmidt, C.; Kappler, A. The Interplay of Microbially Mediated and Abiotic Reactions in the Biogeochemical Fe Cycle. Nat. Rev. Microbiol. 2014, 12, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Sun, X.; Huang, H.; Bai, Y.; Wang, Y.; Luo, H.; Yao, B.; Zhang, X.; Su, X. Oxidation of a Non-Phenolic Lignin Model Compound by Two Irpex Lacteus Manganese Peroxidases: Evidence for Implication of Carboxylate and Radicals. Biotechnol. Biofuels 2017, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villalobos, M.; Toner, B.; Bargar, J.; Sposito, G. Characterization of the Manganese Oxide Produced by Pseudomonas Putida Strain MnB1. Geochim. Cosmochim. Acta 2003, 67, 2649–2662. [Google Scholar] [CrossRef]
- Wong, D.W.S. Structure and Action Mechanism of Ligninolytic Enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, C.A.; Purvine, S.O.; Zink, E.; Wu, S.; Paša-Tolić, L.; Chaput, D.L.; Santelli, C.M.; Hansel, C.M. Mechanisms of Manganese(II) Oxidation by Filamentous Ascomycete Fungi Vary with Species and Time as a Function of Secretome Composition. Front. Microbiol. 2021, 12, 610497. [Google Scholar] [CrossRef]
- Guo, M.; Corona, T.; Ray, K.; Nam, W. Heme and Nonheme High-Valent Iron and Manganese Oxo Cores in Biological and Abiological Oxidation Reactions. ACS Cent. Sci. 2019, 5, 13–28. [Google Scholar] [CrossRef]
- Olivo, G.; Cussó, O.; Borrell, M.; Costas, M. Oxidation of Alkane and Alkene Moieties with Biologically Inspired Nonheme Iron Catalysts and Hydrogen Peroxide: From Free Radicals to Stereoselective Transformations. JBIC J. Biol. Inorg. Chem. 2017, 22, 425–452. [Google Scholar] [CrossRef]
- Sahu, S.; Goldberg, D.P. Activation of Dioxygen by Iron and Manganese Complexes: A Heme and Nonheme Perspective. J. Am. Chem. Soc. 2016, 138, 11410–11428. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Miao, C.; Xia, C.; Lee, Y.-M.; Nam, W.; Sun, W. Mechanistic Insights into the Enantioselective Epoxidation of Olefins by Bioinspired Manganese Complexes: Role of Carboxylic Acid and Nature of Active Oxidant. ACS Catal. 2018, 8, 4528–4538. [Google Scholar] [CrossRef]
- Bryce, C.; Blackwell, N.; Schmidt, C.; Otte, J.; Huang, Y.-M.; Kleindienst, S.; Tomaszewski, E.; Schad, M.; Warter, V.; Peng, C.; et al. Microbial Anaerobic Fe(II) Oxidation—Ecology, Mechanisms and Environmental Implications. Environ. Microbiol. 2018, 20, 3462–3483. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, P.; Shukla, G.; Raj, G.; Ferreira, L.F.R.; Bharagava, R.N. Microbial Manganese Peroxidase: A Ligninolytic Enzyme and Its Ample Opportunities in Research. SN Appl. Sci. 2018, 1, 45. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, X.; Monte-Pérez, I.; Ray, K. Oxidation Reactions with Bioinspired Mononuclear Non-Heme Metal–Oxo Complexes. Angew. Chem. Int. Ed. 2016, 55, 7632–7649. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.C.; Wu, X.; Cho, J.; Cho, K.-B.; Kim, S.H.; Seo, M.S.; Lee, Y.-M.; Kubo, M.; Ogura, T.; Shaik, S.; et al. Water as an Oxygen Source: Synthesis, Characterization, and Reactivity Studies of a Mononuclear Nonheme Manganese(IV) Oxo Complex. Angew. Chem. 2010, 49, 8190–8194. [Google Scholar] [CrossRef] [PubMed]
- Kappler, A.; Bryce, C.; Mansor, M.; Lueder, U.; Byrne, J.M.; Swanner, E.D. An Evolving View on Biogeochemical Cycling of Iron. Nat. Rev. Microbiol. 2021, 19, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Subramanian, V.; Gibbs, R.J. Hydrous FE and MN Oxides—Scavengers of Heavy Metals in the Aquatic Environment. Crit. Rev. Environ. Control. 1984, 14, 33–90. [Google Scholar] [CrossRef]
- Wojtkowska, M. Migration and Forms of Metals in Bottom Sediments of Czerniakowskie Lake. Bull. Environ. Contam. Toxicol. 2013, 90, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Leach, D.L.; Landis, G.P.; Hofstra, A.H. Metamorphic Origin of the Coeur d’Alene Base- and Precious-Metal Veins in the Belt Basin, Idaho and Montana. Geology 1988, 16, 122–125. [Google Scholar] [CrossRef]
- Long, K.R. Production and Disposal of Mill Tailings in the Coeur d‘Alene Mining Region, Shoshone County, Idaho: Preliminary Estimates; Open-File Report; U.S. Geological Survey: Reston, VA, USA, 1998.
- Hickey, P.J.; McDaniel, P.A.; Strawn, D.G. Characterization of Iron- and Manganese-Cemented Redoximorphic Aggregates in Wetland Soils Contaminated with Mine Wastes. J. Environ. Qual. 2008, 37, 2375–2385. [Google Scholar] [CrossRef]
- Ingamells, C.O.; Pitard, F.F. Applied Geochemical Analysis; Chemical Analysis 88; Wiley: New York, NY, USA, 1986; ISBN 0-471-83279-0. [Google Scholar]
- Johnson, W.M.; Maxwell, J.A. Rock and Mineral Analysis, 2nd ed.; Wiley & Sons: New York, NY, USA, 1981; ISBN 978-0-471-02743-0. [Google Scholar]
- Schüler, V.C.O. Chemical Analysis and Sample Preparation. In Modern Methods of Geochemical Analysis; Wainerdi, R.E., Uken, E.A., Eds.; Monographs in Geoscience; Springer US: Boston, MA, USA, 1971; pp. 53–71. ISBN 978-1-4684-1830-9. [Google Scholar]
- Welch, E.B. Should Nitrogen Be Reduced to Manage Eutrophication If It Is Growth Limiting? Evidence from Moses Lake. Lake Reserv. Manag. 2009, 25, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Heap, M.J.; Reuschlé, T.; Farquharson, J.I.; Baud, P. Permeability of Volcanic Rocks to Gas and Water. J. Volcanol. Geotherm. Res. 2018, 354, 29–38. [Google Scholar] [CrossRef]
- Jensen, L.C.; Becerra, J.R.; Escudey, M. Impact of Physical/Chemical Properties of Volcanic Ash-Derived Soils on Mechanisms Involved during Sorption of Ionisable and Non-Ionisable Herbicides; IntechOpen: Rijeka, Croatia, 2018; ISBN 978-1-78984-819-9. [Google Scholar]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alp, E.E.; Mini, S.M.; Ramanathan, M. X-ray Absorption Spectroscopy: EXAFS and XANES—A Versatile Tool to Study the Atomic and Electronic Structure of Materials; Department of Energy, Argonne National Laboratory: Lemont, IL, USA, 1990; pp. 25–36.
- Newville, M. Fundamentals of XAFS. Rev. Mineral. Geochem. 2014, 78, 33–74. [Google Scholar] [CrossRef]
- Sham, T.K. Nanoparticles and Nanowires: Synchrotron Spectroscopy Studies. Int. J. Nanotechnol. 2008, 5, 1194–1246. [Google Scholar] [CrossRef]
- Gaur, A.; Shrivastava, B.D. Speciation Using X-ray Absorption Fine Structure (XAFS). Rev. J. Chem. 2015, 5, 361–398. [Google Scholar] [CrossRef]
- Penner-Hahn, J.E. X-ray Absorption Spectroscopy; eLS: Hong Kong, China, 2005. [Google Scholar] [CrossRef]
- Koningsberger, D.C.; Mojet, B.L.; van Dorssen, G.E.; Ramaker, D.E. XAFS Spectroscopy: Fundamental Principles and Data Analysis. Top. Catal. 2000, 10, 143–155. [Google Scholar] [CrossRef]
- Koningsberger, D.C.; Ramaker, D.E. Applications of X-ray Absorption Spectroscopy in Heterogeneous Catalysis: EXAFS, Atomic XAFS, and Delta XANES. In Handbook of Heterogeneous Catalysis; American Cancer Society: Atlanta, GA, USA, 2008; pp. 774–803. ISBN 978-3-527-61004-4. [Google Scholar]
- van Bokhoven, J.A.; Ressler, T.; de Groot, F.M.F.; Knopp-Gericke, G. Extended X-ray Absorption Fine-Structure Spectroscopy. ChemInform 2005, 36. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Orpen, A.G. Nickel Carbonyl [Ni(CO)4] and Iron Carbonyl [Fe(CO)5]: Molecular Structures in the Solid State. Organometallics 1993, 12, 1481–1483. [Google Scholar] [CrossRef]
- Chen, W.T.; Hsu, C.W.; Lee, J.F.; Pao, C.W.; Hsu, I.J. Theoretical Analysis of Fe K-Edge XANES on Iron Pentacarbonyl. ACS Omega 2020, 5, 4991–5000. [Google Scholar] [CrossRef]
- Hanson, A.W. The Crystal Structure of Iron Pentacarbonyl. Acta Crystallogr. 1962, 15, 930–933. [Google Scholar] [CrossRef]
- Hicks, L.J.; Bridges, J.C.; Gurman, S.J. Ferric Saponite and Serpentine in the Nakhlite Martian Meteorites. Geochim. Cosmochim. Acta 2014, 136, 194–210. [Google Scholar] [CrossRef] [Green Version]
- Sayers, D.E.; Stern, E.A.; Herriott, J.R. Measurement of Fe–S Bond Lengths in Rubredoxin Using Extended X-ray Absorption Fine Structure (EXAFS). J. Chem. Phys. 1976, 64, 427–428. [Google Scholar] [CrossRef]
- Pauling, L. Metal-Metal Bond Lengths in Complexes of Transition Metals. Proc. Natl. Acad. Sci. USA 1976, 73, 4290–4293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, B.; Frazer, B.H.; Belz, A.; Conrad, P.G.; Nealson, K.H.; Haskel, D.; Lang, J.C.; Srajer, G.; De Stasio, G. Multiple Scattering Calculations of Bonding and X-ray Absorption Spectroscopy of Manganese Oxides. J. Phys. Chem. A 2003, 107, 2839–2847. [Google Scholar] [CrossRef]
- Leto, D.F.; Jackson, T.A. Mn K-Edge X-ray Absorption Studies of Oxo- and Hydroxo-Manganese(IV) Complexes: Experimental and Theoretical Insights into Pre-Edge Properties. Inorg. Chem. 2014, 53, 6179–6194. [Google Scholar] [CrossRef]
- Mastelaro, V.R.; Zanotto, E.D. X-ray Absorption Fine Structure (XAFS) Studies of Oxide Glasses—A 45-Year Overview. Materials 2018, 11, 204. [Google Scholar] [CrossRef] [Green Version]
- Luther, G.W. The Role of One- and Two-Electron Transfer Reactions in Forming Thermodynamically Unstable Intermediates as Barriers in Multi-Electron Redox Reactions. Aquat. Geochem. 2010, 16, 395–420. [Google Scholar] [CrossRef]
- LaRowe, D.E.; Carlson, H.K.; Amend, J.P. The Energetic Potential for Undiscovered Manganese Metabolisms in Nature. Front. Microbiol. 2021, 12, 1347. [Google Scholar] [CrossRef]
- Straub, K.L.; Benz, M.; Schink, B. Iron Metabolism in Anoxic Environments at near Neutral PH. FEMS Microbiol. Ecol. 2001, 34, 181–186. [Google Scholar] [CrossRef]
- Bradley, A.S.; Leavitt, W.D.; Johnston, D.T. Revisiting the Dissimilatory Sulfate Reduction Pathway. Geobiology 2011, 9, 446–457. [Google Scholar] [CrossRef]
- Plugge, C.; Zhang, W.; Scholten, J.; Stams, A. Metabolic Flexibility of Sulfate-Reducing Bacteria. Front. Microbiol. 2011, 2, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Barco, R.A.; Emerson, D.; Roden, E.E. Comparative Genomic Analysis of Neutrophilic Iron(II) Oxidizer Genomes for Candidate Genes in Extracellular Electron Transfer. Front. Microbiol. 2017, 8, 1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofrichter, M. Review: Lignin Conversion by Manganese Peroxidase (MnP). Enzyme Microb. Technol. 2002, 30, 454–466. [Google Scholar] [CrossRef]
- Quintanar, L.; Stoj, C.; Taylor, A.B.; Hart, P.J.; Kosman, D.J.; Solomon, E.I. Shall We Dance? How a Multicopper Oxidase Chooses Its Electron Transfer Partner. Acc. Chem. Res. 2007, 40, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Wariishi, H.; Valli, K.; Gold, M.H. Manganese(II) Oxidation by Manganese Peroxidase from the Basidiomycete Phanerochaete Chrysosporium. Kinetic Mechanism and Role of Chelators. J. Biol. Chem. 1992, 267, 23688–23695. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.; Faivre, D. Iron Solubility, Colloids and Their Impact on Iron (Oxyhydr)Oxide Formation from Solution. Earth-Sci. Rev. 2015, 150, 520–530. [Google Scholar] [CrossRef]
- Bennett, B.D.; Gralnick, J.A. Mechanisms of Toxicity by and Resistance to Ferrous Iron in Anaerobic Systems. Free Radic. Biol. Med. 2019, 140, 167–171. [Google Scholar] [CrossRef]
- Schädler, S.; Burkhardt, C.; Hegler, F.; Straub, K.; Miot, J.; Benzerara, K. Formation of Cell-Iron-Mineral Aggregates by Phototrophic and Nitratereducing Anaerobic Fe (II)-Oxidizing Bacteria. Geomicrobiol. J. 2009, 26, 93–103. [Google Scholar] [CrossRef]
- Sundaramoorthy, M.; Kishi, K.; Gold, M.H.; Poulos, T.L. The Crystal Structure of Manganese Peroxidase from Phanerochaete Chrysosporium at 2.06-A Resolution. J. Biol. Chem. 1994, 269, 32759–32767. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Lovley, D.R. Mechanisms for Fe(III) Oxide Reduction in Sedimentary Environments. Geomicrobiol. J. 2002, 19, 141–159. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic Enzymes and Its Mechanisms for Degradation of Lignocellulosic Waste in Environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Chapter One—Insights into Lignin Degradation and Its Potential Industrial Applications. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 82, pp. 1–28. [Google Scholar]
- Bucheli-Witschel, M.; Egli, T. Environmental Fate and Microbial Degradation of Aminopolycarboxylic Acids. FEMS Microbiol. Rev. 2001, 25, 69–106. [Google Scholar] [CrossRef] [PubMed]
- Soldatova, A.V.; Romano, C.A.; Tao, L.; Stich, T.A.; Casey, W.H.; Britt, R.D.; Tebo, B.M.; Spiro, T.G. Mn(II) Oxidation by the Multicopper Oxidase Complex Mnx: A Coordinated Two-Stage Mn(II)/(III) and Mn(III)/(IV) Mechanism. J. Am. Chem. Soc. 2017, 139, 11381–11391. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hao, J.; Elzinga, E.J.; Piotrowiak, P.; Nanda, V.; Yee, N.; Falkowski, P.G. Anoxic Photogeochemical Oxidation of Manganese Carbonate Yields Manganese Oxide. Proc. Natl. Acad. Sci. USA 2020, 117, 22698–22704. [Google Scholar] [CrossRef] [PubMed]
- López-Rayo, S.; Lucena, S.; Lucena, J.J. Chemical Properties and Reactivity of Manganese Chelates and Complexes in Solution and Soils. J. Plant Nutr. Soil Sci. 2014, 177, 189–198. [Google Scholar] [CrossRef]
- Norvell, W.A. Reactions of Metal Chelates in Soils and Nutrient Solutions. In Micronutrients in Agriculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1991; pp. 187–227. ISBN 978-0-89118-878-0. [Google Scholar]
- McKenzie, R. The Adsorption of Lead and Other Heavy Metals on Oxides of Manganese and Iron. Soil Res. 1980, 18, 61–73. [Google Scholar] [CrossRef]
- Dang, D.H.; Lenoble, V.; Durrieu, G.; Omanović, D.; Mullot, J.-U.; Mounier, S.; Garnier, C. Seasonal Variations of Coastal Sedimentary Trace Metals Cycling: Insight on the Effect of Manganese and Iron (Oxy)Hydroxides, Sulphide and Organic Matter. Mar. Pollut. Bull. 2015, 92, 113–124. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, F.; Liu, H.; Qu, J.; Liu, R. Respective Role of Fe and Mn Oxide Contents for Arsenic Sorption in Iron and Manganese Binary Oxide: An X-ray Absorption Spectroscopy Investigation. Environ. Sci. Technol. 2014, 48, 10316–10322. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swanson, G.; Langman, J.B.; Child, A.W.; Wilhelm, F.M.; Moberly, J.G. Iron and Manganese Oxidation States, Bonding Environments, and Mobility in the Mining-Impacted Sediments of Coeur d’Alene Lake, Idaho: Core Experiments. Hydrology 2023, 10, 23. https://doi.org/10.3390/hydrology10010023
Swanson G, Langman JB, Child AW, Wilhelm FM, Moberly JG. Iron and Manganese Oxidation States, Bonding Environments, and Mobility in the Mining-Impacted Sediments of Coeur d’Alene Lake, Idaho: Core Experiments. Hydrology. 2023; 10(1):23. https://doi.org/10.3390/hydrology10010023
Chicago/Turabian StyleSwanson, Gaige, Jeff B. Langman, Andrew W. Child, Frank M. Wilhelm, and James G. Moberly. 2023. "Iron and Manganese Oxidation States, Bonding Environments, and Mobility in the Mining-Impacted Sediments of Coeur d’Alene Lake, Idaho: Core Experiments" Hydrology 10, no. 1: 23. https://doi.org/10.3390/hydrology10010023
APA StyleSwanson, G., Langman, J. B., Child, A. W., Wilhelm, F. M., & Moberly, J. G. (2023). Iron and Manganese Oxidation States, Bonding Environments, and Mobility in the Mining-Impacted Sediments of Coeur d’Alene Lake, Idaho: Core Experiments. Hydrology, 10(1), 23. https://doi.org/10.3390/hydrology10010023






