Applying Geophysical and Hydrogeochemical Methods to Evaluate Groundwater Potential and Quality in Middle Egypt
Abstract
:1. Introduction
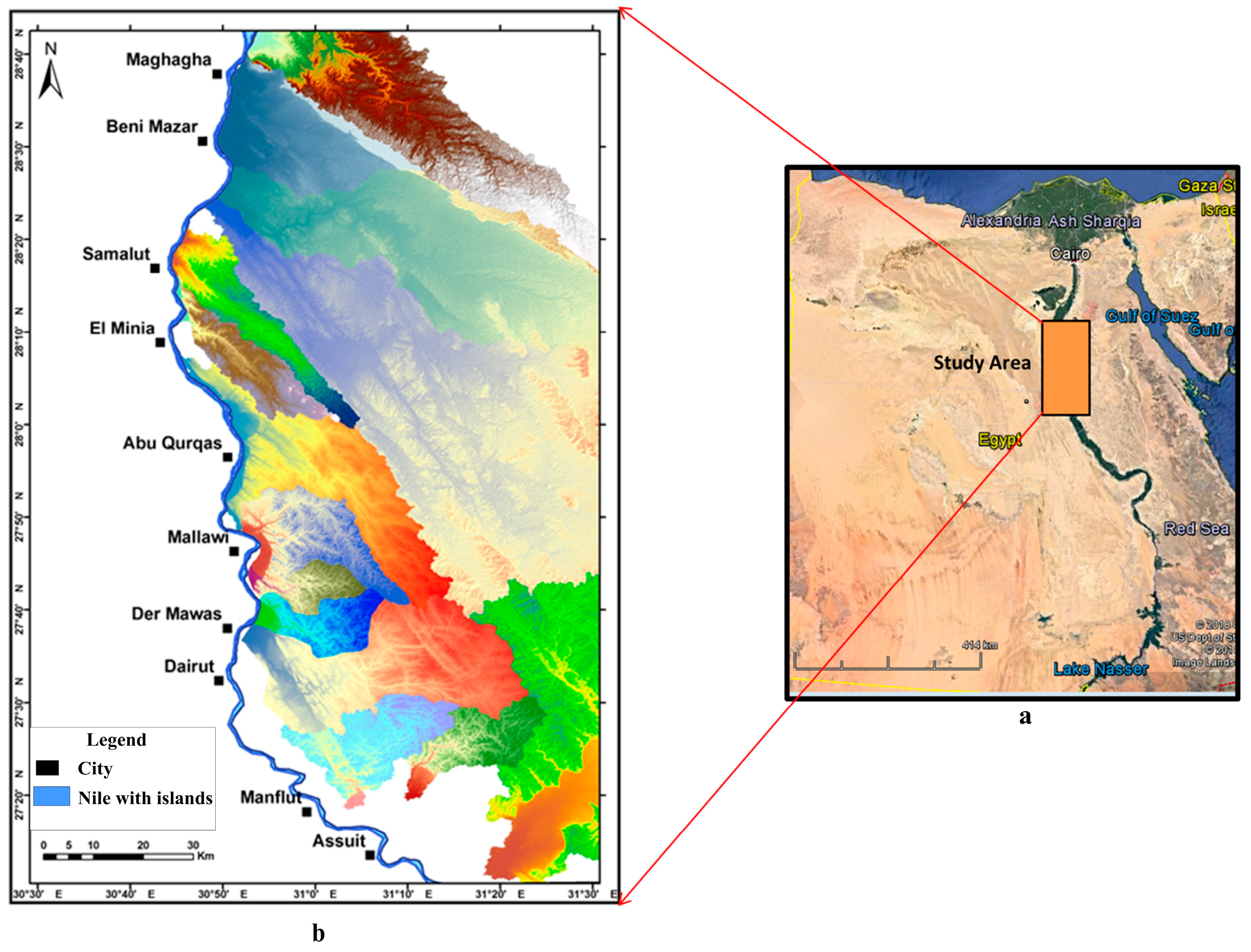
2. Geological Settings
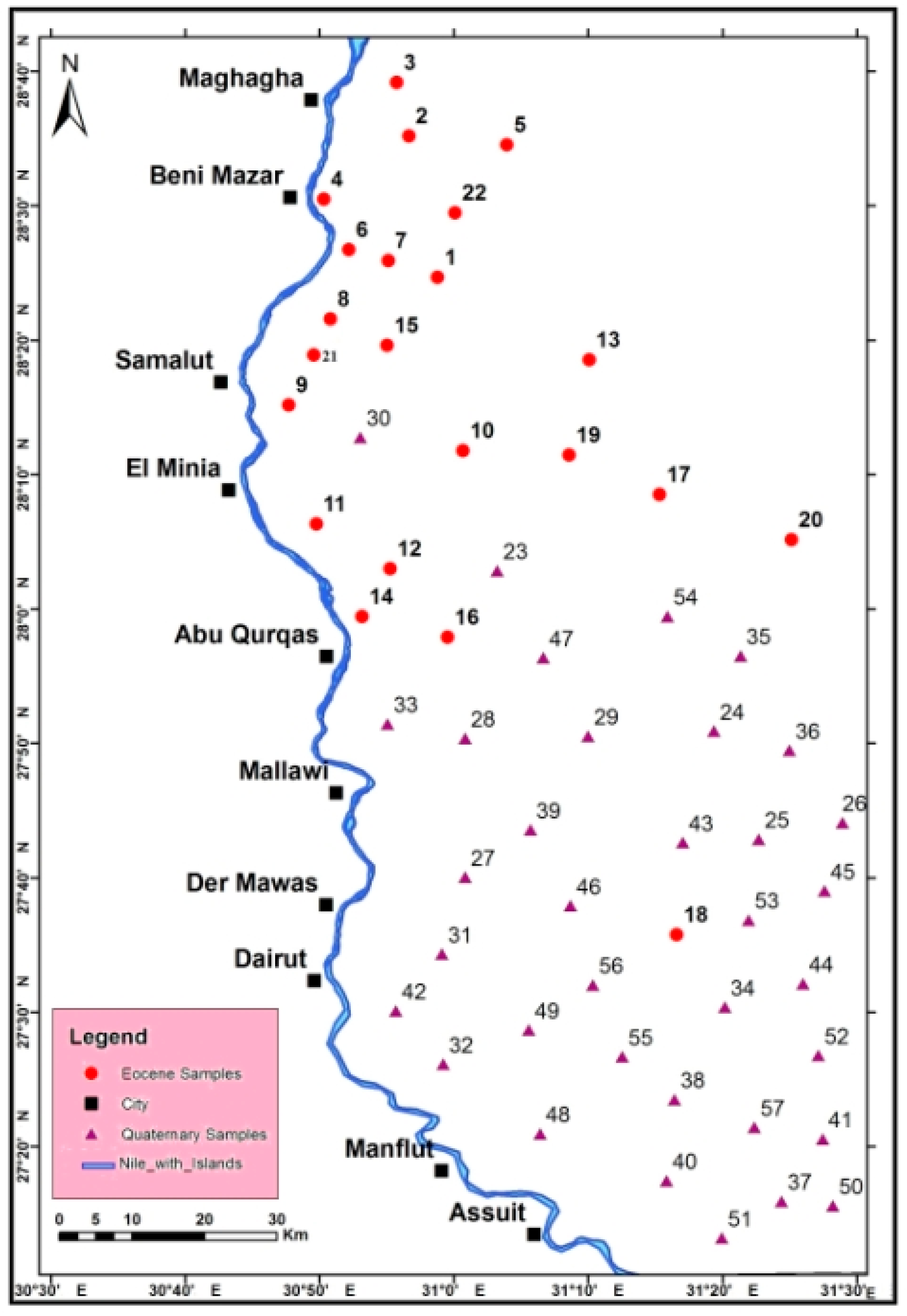
3. Materials and Methods
4. Results and Discussion
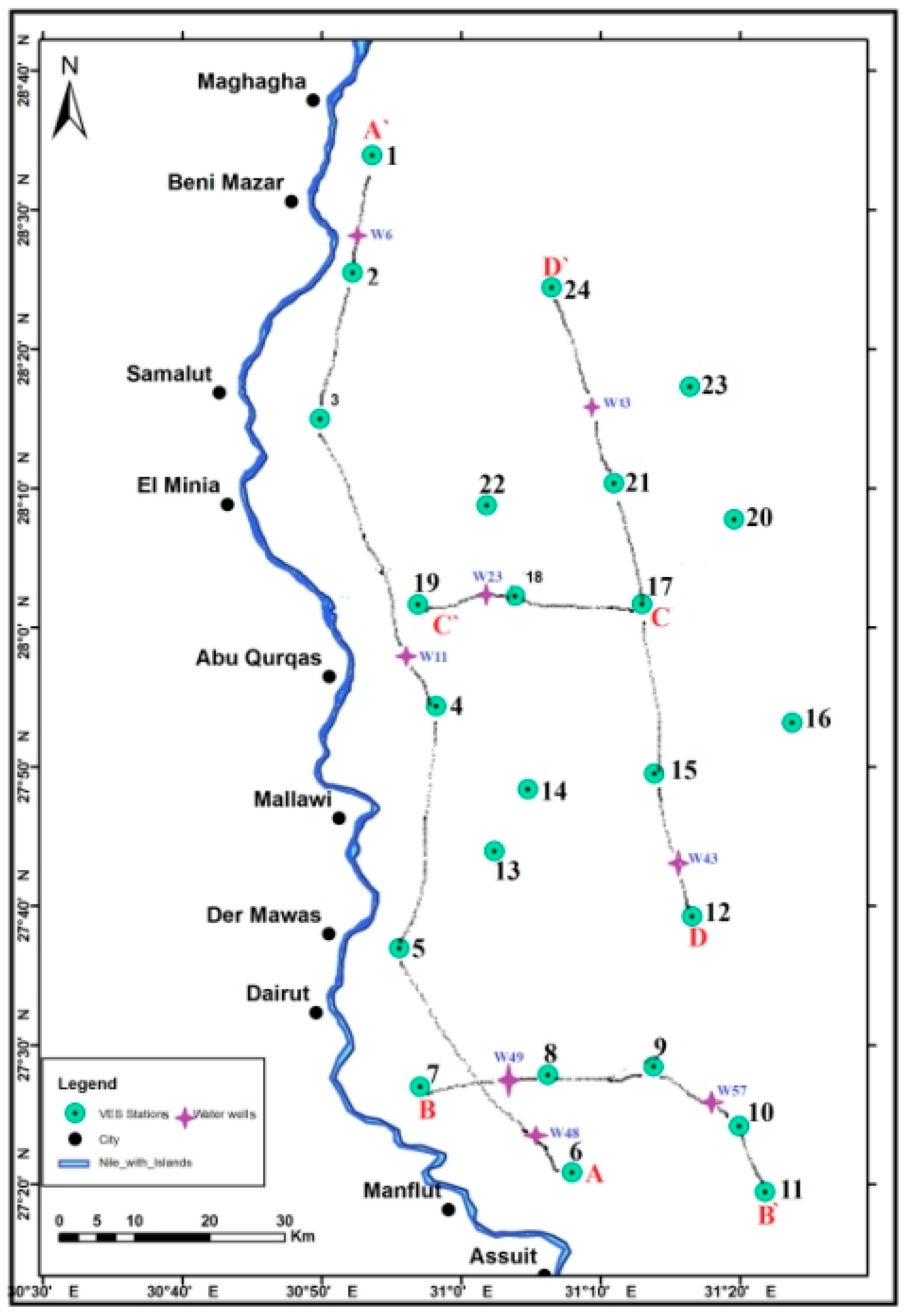
4.1. Electrical Resistivity Studies
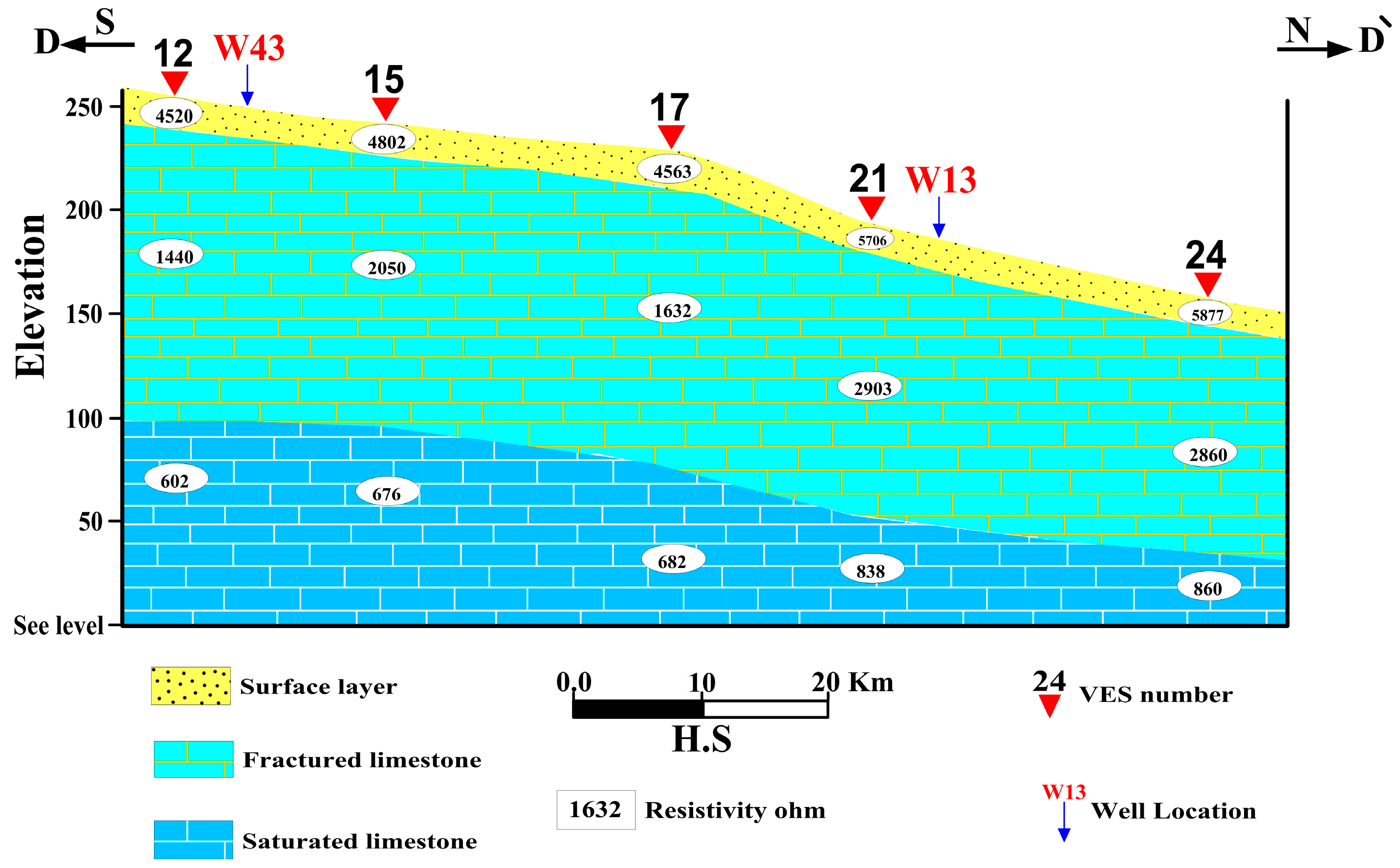
4.1.1. Geoelectrical Cross-Section A-A`
4.1.2. Geoelectrical Cross-Section B-B`
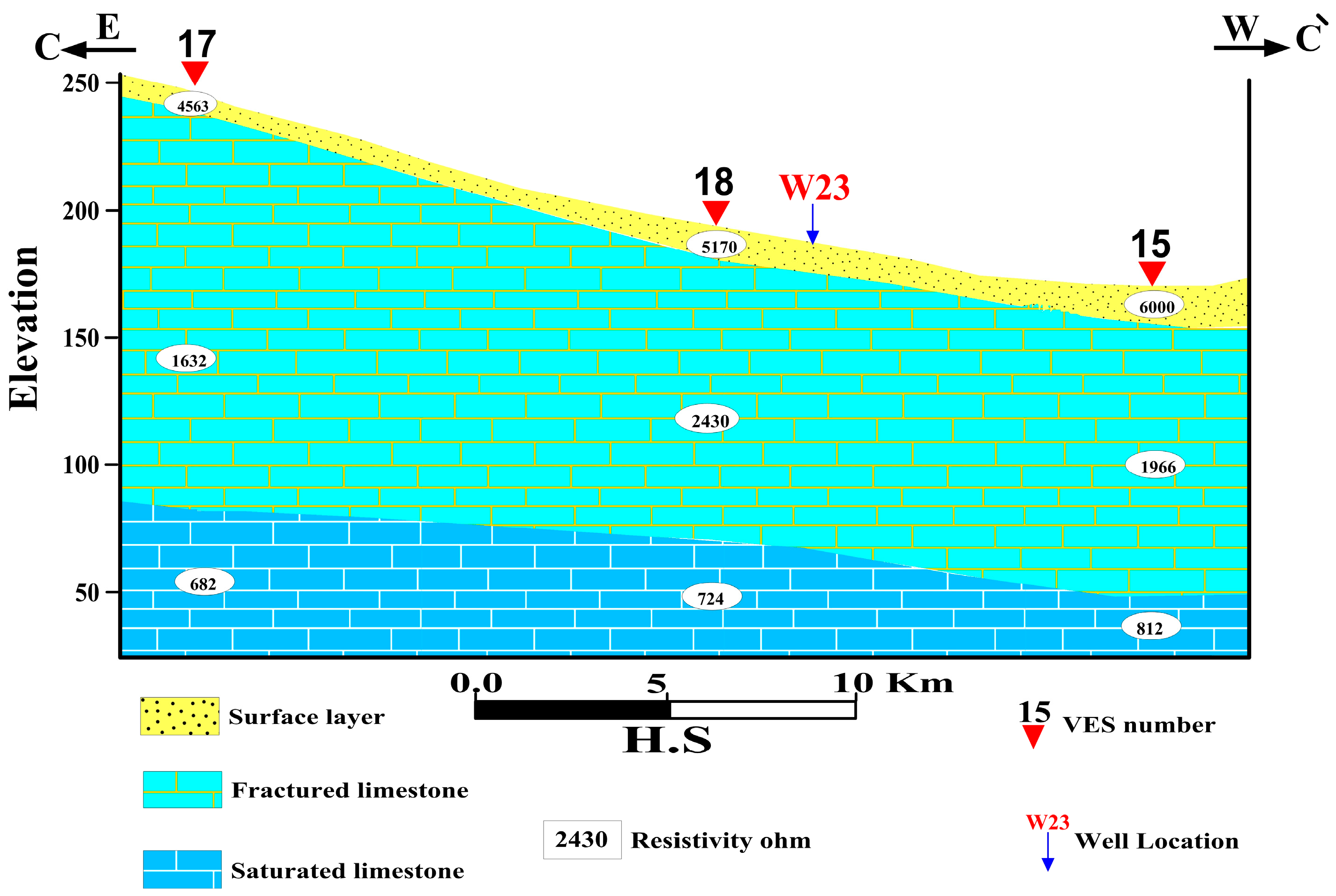
4.1.3. Geoelectrical Cross-Section C-C`
4.1.4. Geoelectrical Cross-Section D-D`
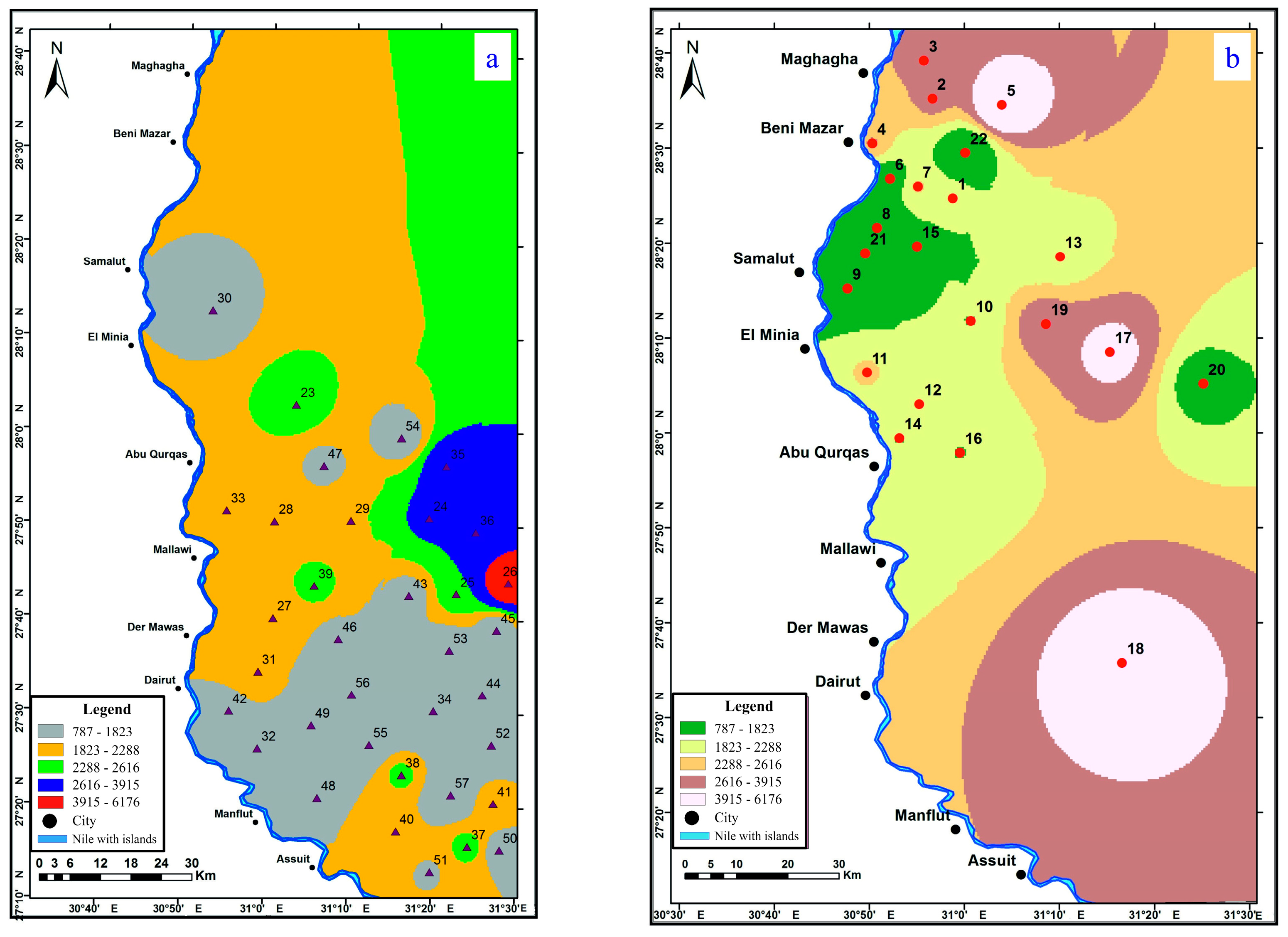
4.2. Groundwater Level
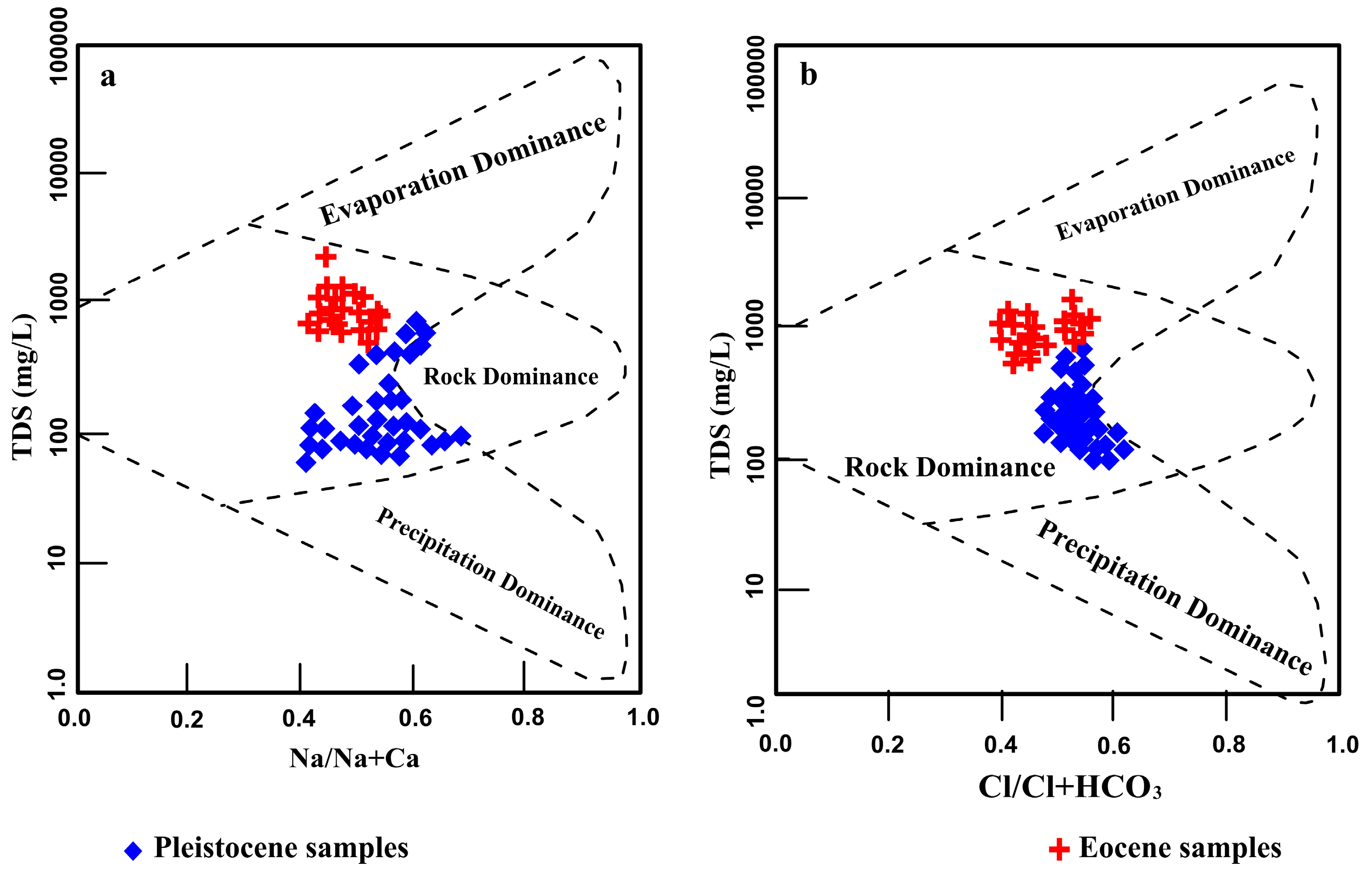
4.3. Hydrogeochemical Properties of Groundwater
4.4. Assessment of Water Quality for Domestic and Drinking Uses
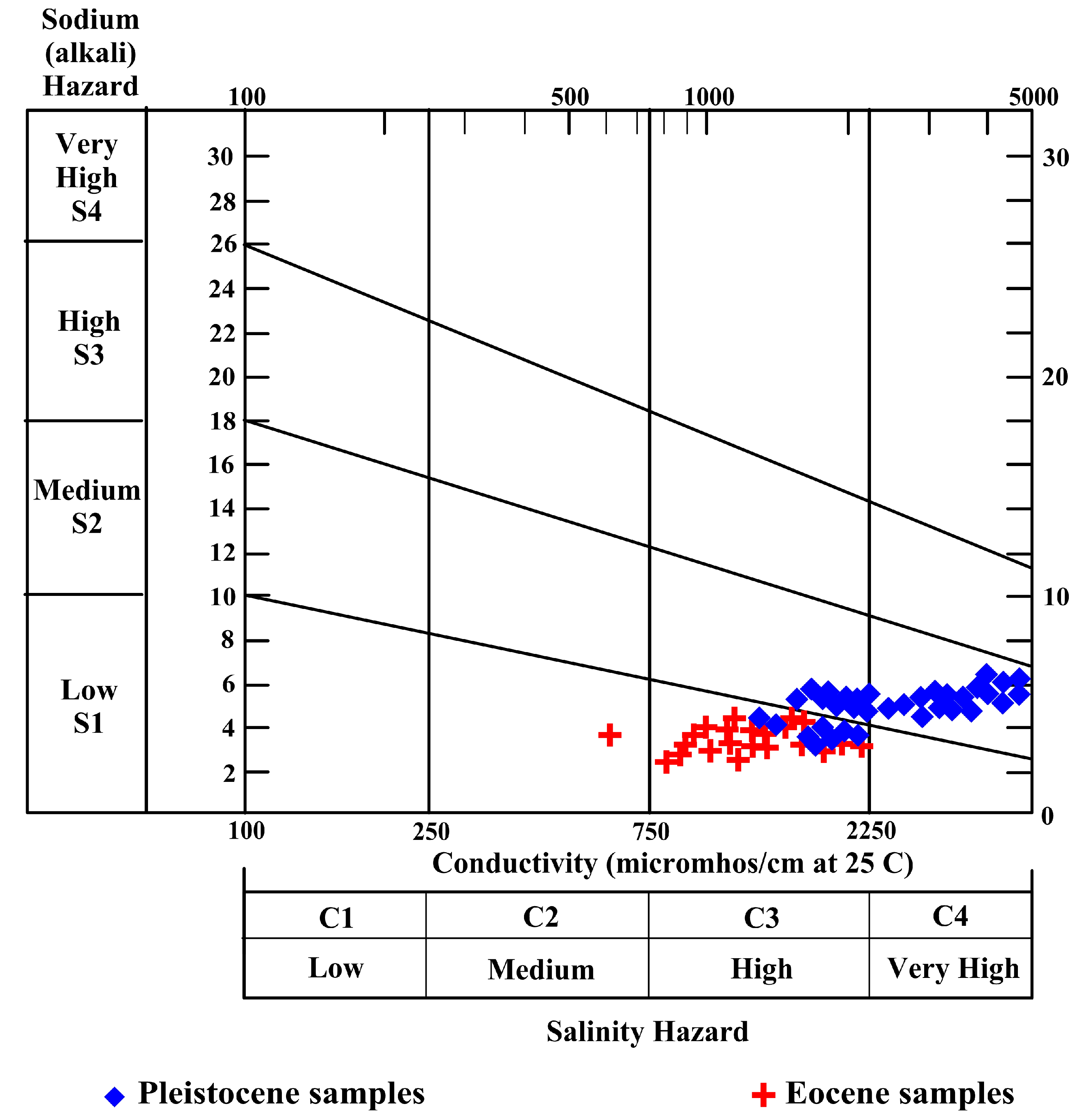
4.5. Evaluation of Water Quality for Irrigation
4.5.1. Sodium Hazard (Na%)
4.5.2. Residual Sodium Carbonate (RSC)
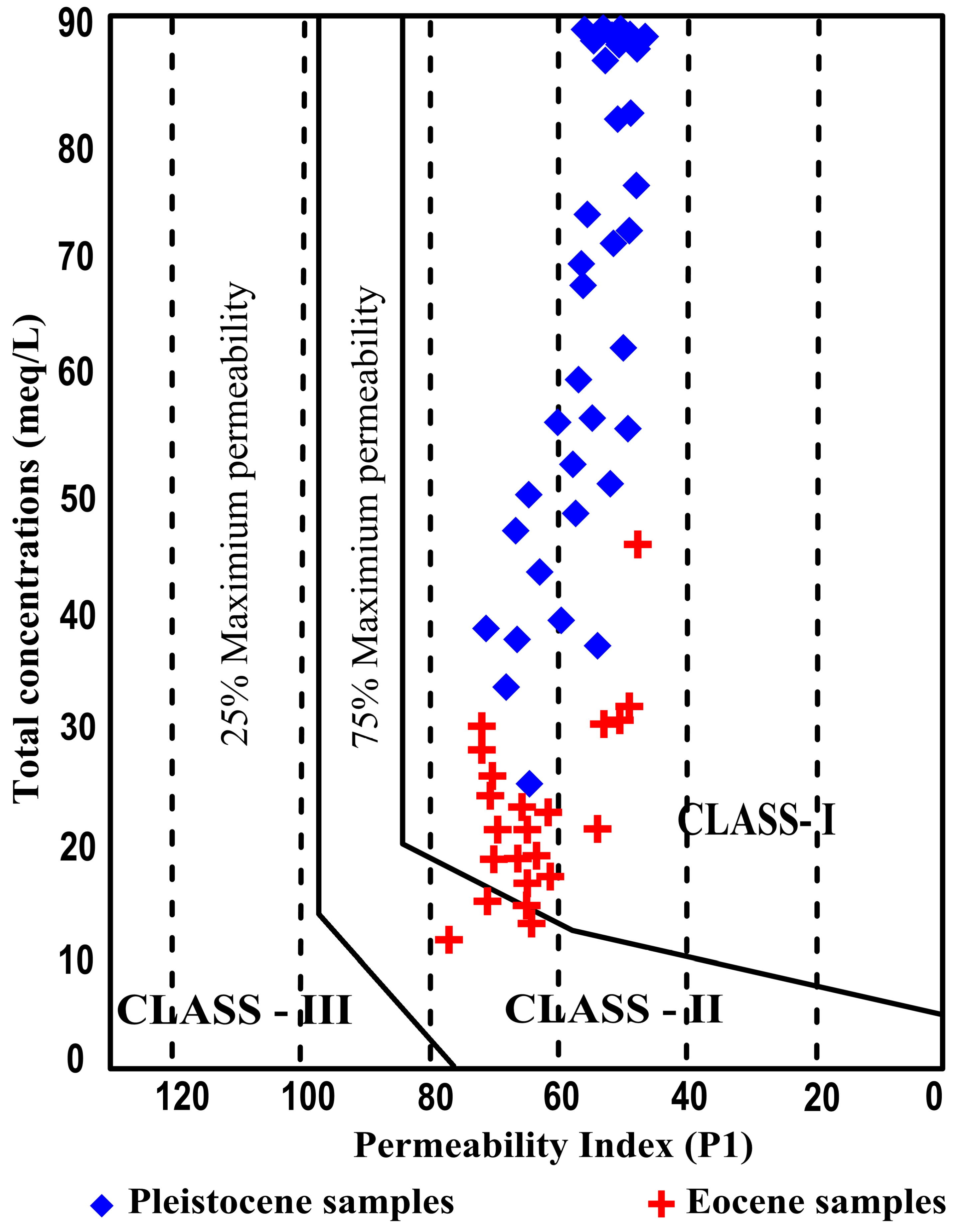
4.5.3. Permeability Index (PI)
4.5.4. Kelly’s Ratio (KR)
4.5.5. Soluble Sodium Percentage (SSP)
4.5.6. Potential Salinity (PS)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidiropoulos, P.; Dalezios, N.R.; Loukas, A.; Mylopoulos, N.; Spiliotopoulos, M.; Faraslis, I.N.; Alpanakis, N.; Sakellariou, S. Quantitative Classification of Desertification Severity for Degraded Aquifer Based on Remotely Sensed Drought Assessment. Hydrology 2021, 8, 47. [Google Scholar] [CrossRef]
- Sidiropoulos, P.; Mylopoulos, N.; Lyra, A.; Tziatzios, G.A.; Loukas, A. Risk analysis framework for the optimum remediation of a contaminated aquifer under uncertainty: Application in Lake Karla aquifer, Thessaly, Greece. Stoch. Environ. Res. Risk Assess 2023, 37, 1281–1302. [Google Scholar] [CrossRef]
- Cedrick, M.M.; Alexander, A.; Nobert, J.; Mbudi, C.N.U.D. Modeling groundwater flow under chaotic urbanization constraints in Kinshasa Capital Region (D.R. Congo). Phys. Chem. Earth 2021, 124, 102985. [Google Scholar] [CrossRef]
- Alexakis, D.E. Applying Factor Analysis and the CCME Water Quality Index for Assessing Groundwater Quality of an Aegean Island (Rhodes, Greece). Geosciences 2022, 12, 384. [Google Scholar] [CrossRef]
- Alexakis, D.; Kiskira, K.; Gamvroula, D.; Emmanouil, C.; Psomopoulos, C. Evaluating toxic element contamination sources in groundwater bodies of two Mediterranean sites. Environ. Sci. Pollut. Res. 2021, 28, 34400–34409. [Google Scholar] [CrossRef]
- Gamvroula, D.E.; Alexakis, D.E. Evaluating the Performance of Water Quality Indices: Application in Surface Water of Lake Union, Washington State-USA. Hydrology 2022, 9, 116. [Google Scholar] [CrossRef]
- Tsitsis, C.; Alexakis, D.E.; Moustris, K.; Gamvroula, D.E. Combining Artificial Neural Network and Driver–Pressure–State–Impact–Response Approach for Evaluating a Mediterranean Lake. Water 2023, 15, 266. [Google Scholar] [CrossRef]
- Alexakis, D.E. Meta-Evaluation of water quality indices. Application into groundwater resources. Water 2020, 12, 1890. [Google Scholar] [CrossRef]
- Alexakis, D.E. Linking DPSIR model and water quality indices to achieve sustainable development goals in groundwater resources. Hydrology 2021, 8, 90. [Google Scholar] [CrossRef]
- Liu, J.; Feng, J.; Gao, Z.; Wang, M.; Li, G.; Shi, M.; Zhanget, H. Hydrochemical characteristics and quality assessment of groundwater for drinking and irrigation purposes in the Futuan River Basin, China. Arab. J. Geosci. 2019, 12, 560. [Google Scholar] [CrossRef]
- Rasul, A.K. Hydrochemistry and quality assessment of Derbendikhan Reservoir, Kurdistan Region, Northeastern Iraq. Arab. J. Geosci. 2019, 12, 312. [Google Scholar] [CrossRef]
- Berhe, B.A. Evaluation of groundwater and surface water quality suitability for drinking and agricultural purposes in Kombolcha town area, eastern Amhara region, Ethiopia. Appl. Water Sci. 2020, 10, 127. [Google Scholar] [CrossRef]
- Al Maliki, A.A.; Abbass, Z.D.; Hussain, H.M.; Al-Ansari, N. Assessment of the groundwater suitability for irrigation near Al Kufa City and preparing the final water quality maps using spatial distribution tools. Environ. Earth Sci. 2020, 79, 330. [Google Scholar] [CrossRef]
- Nagarajan, M.; Gauns, A.; Lalitha, R.; Baskar, M. GIS-based assessment of groundwater quality for drinking and irrigation by water quality index. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 2361–2370. [Google Scholar] [CrossRef]
- Asadi, E.; Isazadeh, M.; Samadianfard, S.; Ramli, M.F.; Mosavi, A.; Nabipou, N.; Shamshirband, S.; Hajnal, E.; Chau, K.W. Groundwater quality assessment for sustainable drinking and irrigation. Sustainability 2020, 12, 177. [Google Scholar] [CrossRef]
- Ismail, E.; El-Sayed, E.; Sakr, S.; Youssef, E. Characteristic of groundwater potentialities in West Nile Valley South, Minia Governorate, Egypt. Arab. J. Geosci. 2017, 10, 521. [Google Scholar] [CrossRef]
- Ismail, E.; Zaki, R.; Rapantova, N.; Licbinska, M.; Sharawi, H. Hydrogeochemical characteristics of the groundwater in the quaternary aquifer of western fringes of El-Minia Governorate, Egypt using an integration of geochemical modeling and geo-statistical techniques. Desalin. Water Treat. 2020, 189, 134–151. [Google Scholar] [CrossRef]
- Ismail, E.; El-Rawy, M. Assessment of groundwater quality in West Sohag, Egypt. Desalin. Water Treat. 2018, 123, 101–108. [Google Scholar] [CrossRef]
- Abd-Elaty, I.; Abd-Elhamid, H.F.; Nezhad, M.M. Numerical analysis of physical barriers systems efficiency in controlling saltwater intrusion in coastal aquifers. Environ. Sci. Pollut. Res. 2019, 26, 35882–35899. [Google Scholar] [CrossRef]
- Snousy, M.G.; Ismail, E.; Zaki, R. Trace element occurrence and distribution problems in the irrigation water at El-Minia district, north Upper Egypt. Arab. J. Geosci. 2019, 12, 582. [Google Scholar] [CrossRef]
- Geriesh, M.H.; Mansour, B.M.H.; Farouk, H. Assessment of drinking water quality along Port Said Canal treatment plants, Suez Canal corridor, Egypt. Arab. J. Geosci. 2019, 12, 738. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Sefelnasr, A.; Ismail, E. Numerical modeling technique for groundwater management in Samalut city, Minia Governorate, Egypt. Arab. J. Geosci. 2019, 12, 124. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Sefelnasr, A.; Ismail, E. Response of the interaction between surface water and groundwater to climate change and proposed megastructure. J. Afr. Earth Sci. 2020, 162, 103723. [Google Scholar] [CrossRef]
- Abdalazem, A.; Gamee, M.; Hamdan, A.; Awad, A.; Mohamed, A. Groundwater quality assessment for irrigation in West Edfu region, Aswan Egypt. Assiut J. Agri. Sci. 2020, 51, 125–149. [Google Scholar]
- Sharaky, A.M.; Abdoun, S.H. Assessment of groundwater quality in Bahariya Oasis, Western Desert, Egypt. Environ. Earth Sci. 2020, 79, 145. [Google Scholar] [CrossRef]
- Abotalib, Z.; Heggy, E.; El Bastawesy, M.; Ismail, E.; Gad, A.; Attwa, M. Groundwater mounding: A diagnostic feature for mapping aquifer connectivity in hyper-arid deserts. Sci. Total Environ. 2021, 801, 149760. [Google Scholar] [CrossRef]
- Snousy, M.G.; Jianhua, W.; Fengmei, S.; Abdelhalim, A.; Ismail, E. Groundwater Quality and Its Regulating Geochemical Processes in Assiut Province, Egypt. Expo. Health 2022, 14, 305–323. [Google Scholar] [CrossRef]
- Abotalib, Z.; Abdelhady, A.A.; Heggy, E.; Salem, S.G.; Ismail, E.; Ali, A.; Khalil, M.M. Irreversible and large-scale heavy metal pollution arising from increased damming and untreated water reuse in the Nile Delta. Earth’s Future 2023, 11, e2022EF002987. [Google Scholar] [CrossRef]
- Ismail, E.; Snousy, M.G.; Alexakis, D.E.; Abdelhalim, A.; Ahmed, M.S.; Elsayed, E. Diagnosis of Groundwater Quality in North Assiut Province, Egypt, for Drinking and Irrigation Uses by Applying Multivariate Statistics and Hydrochemical Methods. Water 2023, 15, 2812. [Google Scholar] [CrossRef]
- Said, R. The Geology of Egypt; Balkema: Rotterdam, The Netherlands, 1990. [Google Scholar]
- Zaki, R. Geochemical Characteristics and Element Associations in Different Rock Types of El Minia District, North Upper Egypt. Sedimentol. Egypt 2011, 13, 349–370. [Google Scholar]
- Gamil, R. Hydro-Geochemical Study for the Water Resources in the Desert Fringes of Assiut Governorate, Egypt. Ph.D. Thesis, Faculty of Science, Assuit University, Asyut, Egypt, 2013. [Google Scholar]
- Tamer, M.A. Hydrogeology of R. E. A. and Some Changes Occurring as a Result of the Construction of Hydrotechnologic Projects. Ph.D. Thesis, Kiev University, Kyiv, Ukraine, 1974. [Google Scholar]
- Piper, A.M. A Graphic Procedure in the Geochemical Interpretation and Analysis of Water Samples; USGS, Water Supply 1953, Paper 1454; US Geological Survey: Reston, VA, USA, 1953. [Google Scholar]
- Richards, L.A. Diagnosis and improvement of saline and alkaline soils. In U.S. Department of Agricultural Handbook; No. 60; US Government Printing Office: Washington, DC, USA, 1954; 160p. [Google Scholar]
- Gibbs, R.J. Mechanisms controlling World’s water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Wilcox, V. Classification and Use of Irrigation Waters; US Department of Agriculture: Washington, DC, USA, 1955; 19p. [Google Scholar]
- Zohdy, A.A.R. A new method for the automatic interpretation of Schlumberger and Wenner sounding curve. Geophysics 1989, 54, 245–253. [Google Scholar] [CrossRef]
- Velpen, V.D.B.; Resist, A.A. Computer Program for the Interpretation of Resistivity Sounding Curves, An ITC.M. Sc., Research Project; ITC: Delft, The Netherlands, 1988. [Google Scholar]
- Davis, P.A.; Greenhalgh, S.A.; Merrick, N.P. Resistivity Sounding Computations with Any Array using a Single Digital Filter. Explor. Geophys. 1980, 11, 54–62. [Google Scholar] [CrossRef]
- Todd, D.K. Groundwater Hydrology, 2nd ed.; Wiley: New York, NY, USA, 1982; 552p. [Google Scholar]
- Paliwal, K.V. Irrigation with Saline Water; Monogram No. 2 (New Series); IARI: New Delhi, India, 1972; 198p. [Google Scholar]
- Joshi, D.M.; Kumar, A.; Agarwal, N. Assessment of the irrigation water quality of River Ganga in Haridwar District India. J. Chem. 2009, 2, 285–292. [Google Scholar]
- Doneen, L.D. Notes on Water Quality in Agriculture; Water Science and Engineering, University of California: Davis, CA, USA, 1964; pp. 101–105. [Google Scholar]
- Kelly, W.P. Use of Saline Irrigation Water. Soil Sci. 1963, 95, 355–391. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; Water Supply, Paper 2254; USGS: Reston, VA, USA, 1985; pp. 23–25. [Google Scholar]
- Sawyer, G.N.; McCarthy, D.L. Chemistry of Sanitary Engineers, 2nd ed.; Mc Graw Hill: New York, NY, USA, 1987; 518p. [Google Scholar]
- Bauder, T.A.; Waskom, R.M.; Davis, J.G. Irrigation Water Quality Criteria; Extension Fact Sheet No. 506; Colorado State University: Fort Collins, CO, USA, 2007; Available online: https://extension.colostate.edu/docs/pubs/crops/00506.pdf (accessed on 17 January 2023).
- Eaton, F.M. Significance of carbonate in Irrigation Water. Soil Sci. 1950, 69, 123–133. [Google Scholar] [CrossRef]
- Ragunath, H.M. Groundwater; Wiley Eastern: New Delhi, India, 1987; 563p. [Google Scholar]
- Domenico, P.A.; Schwartz, F.W. Physical and Chemical Hydrogeology; Wiley: New York, NY, USA, 1990; pp. 410–420. [Google Scholar]
- Kelly, W.P. Permissible composition and concentration of irrigated waters. Proceeding ASCF 1940, 66, 607. [Google Scholar]
- Paliwal, K.V. Effect of gypsum application on the quality of irrigation waters. Madras. Agric. J. 1967, 59, 646–647. [Google Scholar]
- Doneen, L.D. Salination of Soil by Salts in the Irrigation Water. Am. Geophys. Union Trans. 1954, 35, 943–950. [Google Scholar] [CrossRef]
- Doneen, L.D. The influence of crop and soil on percolating waters. In Proceedings of the 1961 Biennial Conference on Groundwater Recharge, Dusseldorf, Germany; 1962; pp. 156–163. [Google Scholar]





| Item | Equation | References |
|---|---|---|
| TH | TH = 2.497 Ca2+ + 4.115 Mg2+ ions in mg/L | [41] |
| SAR | all ions in meq/L | [35] |
| Na (%) | all ions in meq/L | [37] |
| RSC | all ions in meq/L | [35] |
| MH | all ions in meq/L | [42] |
| SSP | SSP = ((Na+ + K+)/(K+ + Na+ + Ca2+ + Mg2+)) × 100 all ions in meq/L | [43] |
| PS | all ions in meq/L | [44] |
| KR | all ions in meq/L | [45] |
| Class. No | EC (μS/cm) | Water Class | Pleistocene Water Samples (%) | Eocene Water Samples (%) |
|---|---|---|---|---|
| 1 | <250 | Excellent | - | - |
| 2 | 251–750 | Good | - | 5 |
| 3 | 751–2000 | Permissible | 23 | 90 |
| 4 | 2001–3000 | Doubtful | 31 | 5 |
| 5 | >3000 | Unsuitable | 46 | - |
| RSC | Water Class | Pleistocene Water Samples (%) | Eocene Water Samples (%) |
|---|---|---|---|
| <1.25 | Safe | - | 5 |
| 1.25–2.5 | suitable | 3 | 59 |
| >2.5 | unsuitable | 97 | 36 |
| Class. No | PI (%) | Water Class | Pleistocene Water Samples (%) | Eocene Water Samples (%) |
|---|---|---|---|---|
| 1 | >75 | Excellent | 100 | 82 |
| 2 | 75–25 | Good | - | 18 |
| 3 | <25 | Unsuitable | - | - |
| SSP (epm) | Water Quality | Pleistocene Water Samples (%) | Eocene Water Samples (%) |
|---|---|---|---|
| <20 | Excellent quality | - | - |
| 20–40 | Good quality | - | 14 |
| 40–80 | Fair | 100 | 86 |
| >80 | Poor | - | - |
| Class. No | PS (epm) | Water Class | Pleistocene Water Samples (%) | Eocene Water Samples (%) |
|---|---|---|---|---|
| 1 | <5 | Excellent to Good | 100 | 82 |
| 2 | 5–10 | Good to Injurious | - | 81 |
| 3 | >10 | Injurious to Unsatisfactory | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, E.; Alexakis, D.E.; Heleika, M.A.; Hashem, M.; Ahmed, M.S.; Hamdy, D.; Ali, A. Applying Geophysical and Hydrogeochemical Methods to Evaluate Groundwater Potential and Quality in Middle Egypt. Hydrology 2023, 10, 173. https://doi.org/10.3390/hydrology10080173
Ismail E, Alexakis DE, Heleika MA, Hashem M, Ahmed MS, Hamdy D, Ali A. Applying Geophysical and Hydrogeochemical Methods to Evaluate Groundwater Potential and Quality in Middle Egypt. Hydrology. 2023; 10(8):173. https://doi.org/10.3390/hydrology10080173
Chicago/Turabian StyleIsmail, Esam, Dimitrios E. Alexakis, Mohamed Abou Heleika, Mohamed Hashem, Mohamed S. Ahmed, Doha Hamdy, and Ahmed Ali. 2023. "Applying Geophysical and Hydrogeochemical Methods to Evaluate Groundwater Potential and Quality in Middle Egypt" Hydrology 10, no. 8: 173. https://doi.org/10.3390/hydrology10080173








